Chemical kinetics
Abstract
Chemical reaction types, reaction orders, and the law of mass action that describes the rates of reactions are presented. The Arrhenius rate constant expression and methods for analysis of rate constants, including transition state and recombination rate theories, are discussed as well as the effects of pressure and temperature. Chain reactions are introduced, and steady-state and partial equilibrium approximations are applied for their analysis. The mathematical formalism for large reaction mechanisms is illustrated through the development of the governing equations for coupled thermal and chemical reacting systems without mass diffusion. Finally, sensitivity and rate-of-production analyses are discussed for mechanism analysis as well as methods for mechanism reduction.
Keywords
Arrhenius rate constant; Chemical reactions; Partial equilibrium and steady-state assumptions; Pressure-dependent reactions; Rate-of-production analysis; Reaction rates; Sensitivity analysis; Transition state theory2.1. Introduction
2.2. Rates of Reactions and Their Temperature Dependence
∑nj=1ν′jMj⇄∑nj=1ν″jMj
 (2.1)
(2.1)
H+H+H→H2+H
![]()
n=2,M1=H,M2=H2;
![]()
v′1=3,v″1=1,v″2=1
![]()
RR∼∏nj=1(Mj)ν′j,RR=k∏nj=1(Mj)ν′j
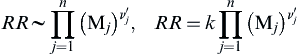 (2.2)
(2.2)
d(Mi)dt=[ν″i−ν′i]RR=[ν″i−ν′i]k∏nj=1(Mj)ν′j
 (2.3)
(2.3)
2.2.1. The Arrhenius Rate Expression
A+B→C+D
![]() (2.4)
(2.4)
O+N2→NO+N
![]()
−RR=d(A)dt=−k(A)(B)=d(B)dt=−d(C)dt=−d(D)dt
![]() (2.5)
(2.5)
RR=ZABexp(−E/RT)
![]() (2.6)
(2.6)
ZAB=(A)(B)σ2AB[8πkBT/μ]1/2
![]() (2.7)
(2.7)
ZAB=Z′AB(A)(B)
![]() (2.8)
(2.8)
RR=Z′AB(A)(B)exp(−E/RT)
![]()
k=Z′ABexp(−E/RT)=Z″ABT1/2exp(−E/RT)
![]() (2.9)
(2.9)
k=Z″ABT1/2[exp(−E/RT)]℘
![]() (2.10)
(2.10)
k=constT1/2exp(−E/RT)=Aexp(−E/RT)
![]() (2.11)
(2.11)
2.2.2. Transition State and Recombination Rate Theories

A+BC⇄(ABC)#→AB+C
![]() (2.12)
(2.12)
K#=(ABC)#(A)(BC)
 (2.13)
(2.13)
K#=(QT)#(QT)A(QT)BCexp(−ERT)
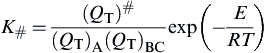 (2.14)
(2.14)
Qvib=∏i[1−exp(−hνi/kBT)]−1
![]() (2.15)
(2.15)
limν→0[1−exp(−hν/kBT)]−1=(kBT/hν)
![]() (2.16)
(2.16)
{(ABC)#/[(A)(BC)]}={[(QT−1)#(kBT/hν)]/[(QT)A(QT)BC]}×exp(−E/RT)
![]() (2.17)
(2.17)
ν(ABC)#={[(A)(BC)(kBT/h)(QT−1)#]/[(QT)A(QT)BC]}×exp(−E/RT)
![]() (2.18)
(2.18)
k=(kBT/h)[(QT−1)#/(QT)A(QT)BC]exp(−E/RT)
![]() (2.19)
(2.19)
A⇄A#→products
![]() (2.20)
(2.20)
Qvib,A=[1−exp(−hνA/kBT)]−1
![]() (2.21)
(2.21)
k=(kBT/h)[1−exp(−hνA/kBT)]exp(−E/RT)
![]() (2.22)
(2.22)
k=(kBT/h)exp(−E/RT)
![]() (2.23)
(2.23)
k=ATnexp(−E/RT)
![]() (2.24)
(2.24)
k∼exp(C/T)1/3
![]() (2.25)
(2.25)
H+H+M→H2+M
![]() (2.26)
(2.26)
d(H2)/dt=k(H)2(M)
![]() (2.27)
(2.27)
2.3. Simultaneous Interdependent Reactions
H2+I2kf⇄kb2HI
![]() (2.28)
(2.28)
d(HI)/dt=2kf(H2)(I2)−2kb(HI)2
![]() (2.29)
(2.29)
2kf(H2)eq(I2)eq−2kb(HI)2eq=0
![]() (2.30)
(2.30)
kfkb=(HI)2eq(H2)eq(I2)eq≡Kc
 (2.31)
(2.31)
d(HI)dt=2kf(H2)(I2)−2kfKc(HI)2
![]() (2.32)
(2.32)
2.4. Chain Reactions
d(HBr)dt=k′exp(H2)(Br2)1/21+k″exp[(HBr)/(Br2)]
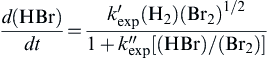 (2.33)
(2.33)
(i)M+Br2ki→2Br·+M}chaininitiatingstep(ii)Br·+H2kii→HBr+H·(iii)H·+Br2kiii→HBr+Br·(iv)H·+HBrkiv→H2+Br·}chaincarryingorpropagatingsteps(v)M+2Br·kv→Br2+M}chainterminatingstep

H·+O2→·OH+·O·
![]()
·O·+H2→·OH+H·
![]()

d(HBr)dt=kii(Br)(H2)+kiii(H)(Br2)−kiv(H)(HBr)
![]() (2.34)
(2.34)
d(H)dt=kii(Br)(H2)−kiii(H)(Br2)−kiv(H)(HBr)≅0
![]() (2.35)
(2.35)
d(Br)dt=2ki(Br2)(M)−kii(Br)(H2)+kiii(H)(Br2)+kiv(H)(HBr)−2kv(Br)2(M)≅0
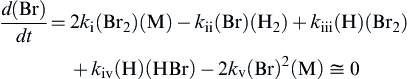 (2.36)
(2.36)
(Br)=(ki/kv)1/2(Br2)1/2
![]() (2.37)
(2.37)
(H)=kii(ki/kv)1/2(H2)(Br2)1/2kiii(Br2)+kiv(HBr)
 (2.38)
(2.38)
d(HBr)dt=2kii(ki/kv)1/2(H2)(Br2)1/21+[kiv(HBr)/kiii(Br2)]
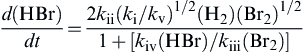 (2.39)
(2.39)
k′exp=2kii(ki/kv)1/2,k″exp=kiv/kiii
![]()
2.5. Pseudo-First-Order Reactions and the “Falloff” Range
A+Mkf⇄kbA∗+M
![]() (2.40)
(2.40)
A∗kp→products
![]() (2.41)
(2.41)
d(A)dt=−kf(A)(M)+kb(A∗)(M)
![]() (2.42)
(2.42)
d(A∗)dt=kf(A)(M)−kb(A∗)(M)−kp(A∗)≅0
![]() (2.43)
(2.43)
(A∗)=kf(A)(M)kb(M)+kp
 (2.44)
(2.44)
−1(A)d(A)dt=kfkp(M)kb(M)+kp=kdiss
 (2.45)
(2.45)
kdiss,∞≡kfkpkb=Kkp
![]() (2.46)
(2.46)
kdiss,0≡kf(M)
![]() (2.47)
(2.47)
(kdiss,0/kdiss,∞)=kb(M)/kp
![]() (2.48)
(2.48)
kdisskdiss,∞=kb(M)kb(M)+kp=kb(M)/kp[kb(M)/kp]+1
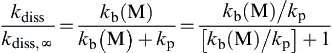 (2.49)
(2.49)
kdisskdiss,∞=kdiss,0/kdiss,∞1+(kdiss,0/kdiss,∞)
 (2.50)
(2.50)
kdiss=0.5kdiss,∞
![]() (2.51)
(2.51)
A+B→D
![]() (2.52)
(2.52)
d(A)dt=−d(D)dt=−k(A)(B)
![]() (2.53)
(2.53)
d(A)dt=−d(D)dt=−k′(A)
![]() (2.54)
(2.54)
2.6. The Partial Equilibrium Assumption
CO+OH→CO2+H
![]() (2.55)
(2.55)
d(CO2)dt=−d(CO)dt=k(CO)(OH)
![]() (2.56)
(2.56)
12H2+12O2⇄OH,H2+12O2⇄H2O
![]()
K2C,f,OH=(OH)2eq(H2)eq(O2)eq,KC,f,H2O=(H2O)eq(H2)eq(O2)1/2eq
 (2.57)
(2.57)
(OH)eq=(H2O)1/2(O2)1/4[K2C,f,OH/KC,f,H2O]1/2
![]() (2.58)
(2.58)
d(CO2)dt=−d(CO)dt=k[K2C,f,OH/KC,f,H2O]1/2(CO)(H2O)1/2(O2)1/4
![]() (2.59)
(2.59)
2.7. Pressure Effect in Fractional Conversion
d(A)dt=−k(A)n
![]() (2.60)
(2.60)
XA=(A)/(M)
![]() (2.61)
(2.61)
(M)dXAdt=−k((M)XA)n
![]() (2.62)
(2.62)
dXAdt=−kXnA(M)n−1
![]() (2.63)
(2.63)
(dXA/dt)∼Pn−1
![]() (2.64)
(2.64)
2.8. Chemical Kinetics of Large Reaction Mechanisms
∑nj=1ν′jiMj⇄∑nj=1ν″jiMji,=1,…,m
 (2.65)
(2.65)
qi=kf,i∏nj=1(Mj)ν′ji−kb,i∏nj=1(Mj)ν″ji
 (2.66)
(2.66)
˙ωji=[ν″ji−ν′ji]qi=νjiqi
![]() (2.67)
(2.67)
˙ωj=∑mi=1νjiqi
 (2.68)
(2.68)
d(Mj)dt=˙ωjj=1,…,n
![]() (2.69)
(2.69)
(Mj)t=0=(Mj)0j=1,…,n
![]() (2.70)
(2.70)
2.8.1. Sensitivity Analysis
∂(Mj)/∂αi
![]()
d(Mj)dt=fj[(Mj),ˉα]j=1,…,n
![]() (2.71)
(2.71)
∂∂t(∂(Mj)∂αi)=∂fj∂αi+∑ns=1∂fj∂(Ms)∂(Ms)∂αii=1,…,m
 (2.72)
(2.72)
αi(Mj)∂(Mj)∂αi=∂ln(Mj)∂lnαi
 (2.73)
(2.73)
αi∂(Mj)∂αi=∂(Mj)∂lnαi
![]() (2.74)
(2.74)
2.8.2. Rate-of-Production Analysis
Cpji=max(νji,0)qi∑mi=1max(νji,0)qi
 (2.75)
(2.75)
Cdji=min(νji,0)qi∑mi=1min(νji,0)qi
 (2.76)
(2.76)
2.8.3. Coupled Thermal and Chemical Reacting Systems
m=∑nj=1mj
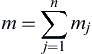 (2.77)
(2.77)
dmjdt=V˙ωjMWj
![]() (2.78)
(2.78)
Yj=mjm=mj∑ns=1ms
![]() (2.79)
(2.79)
Xj=njn=nj∑ns=1ns
![]() (2.80)
(2.80)
Yj=XjMWjMW
![]() (2.81)
(2.81)
MW=∑nj=1XjMWj=1∑nj=1(Yj/MWj)
 (2.82)
(2.82)
dYjdt=˙ωjMWjρj=1,…,n
![]() (2.83)
(2.83)
ρ=∑nj=1(Mj)MWj

dh=0
![]()
h=∑nj=1Yjhj
 (2.84)
(2.84)
dhdt=∑nj=1(Yjdhjdt+hjdYjdt)
 (2.85)
(2.85)
dhj=cp,jdT
![]() (2.86)
(2.86)
dhdt=∑nj=1Yjcp,jdTdt+∑nj=1hjdYjdt=0
 (2.87)
(2.87)
∑nj=1cp,jYj=cp
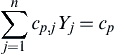 (2.88)
(2.88)
dTdt=−∑nj=1hj˙ωjMWjρcp
 (2.89)
(2.89)
2.8.4. Mechanism Simplification
Problems
(1)CH3CHOk1→0.5CH3˙CO+0.5˙CH3+0.5CO+0.5H2
![]()
(2)CH3˙COk2→˙CH3+CO
![]()
(3)˙CH3+CH3CHOk3→CH4+CH3˙CO
![]()
(4)˙CH3+CH3˙COk4→minorproducts
![]()
d(O3)/dt=kexp[(O3)2/(O2)]
![]()
2O3→3O2
![]()
O3+Mk1→O2+O+M
![]()
O+O2+Mk2→O3+M
![]()
O+O3k3→2O2
![]()
k=2.2×1014exp(−39,360K/T)(molcm3)−1s−1

| T°(K) | Kp |
| 1500 | 0.00323 |
| 1750 | 0.00912 |
| 2000 | 0.0198 |
| 2250 | 0.0364 |
| 2500 | 0.0590 |
| 2750 | 0.0876 |
A+B→C+D
![]()
H2+OHkf→H2O+H
![]()
Cl2+M⇄Cl+Cl+M
![]()
H2+M⇄H+H+M
![]()
H+Cl2⇄HCl+Cl
![]()
Cl+H2⇄HCl+H
![]()
H+Cl+M⇄HCl+M
![]()
N2+O⇄NO+N
![]()
N+O2⇄NO+O
![]()
O2+M⇄O+O+M
![]()

