Problems
(Those with an asterisk require a numerical solution.)
1. A stoichiometric fuel–air mixture flowing in a Bunsen burner forms a well-defined conical flame. The mixture is then made leaner. For the same flow velocity in the tube, how does the cone angle change for the leaner mixture? That is, does the cone angle become larger or smaller than the angle for the stoichiometric mixture? Explain.
2. Sketch a temperature profile that would exist in a one-dimensional laminar flame. Superimpose on this profile a relative plot of what the rate of energy release would be through the flame as well. Below the inflection point in the temperature profile, large amounts of HO2 are found. Explain why. If the flame was due to a first-order, one-step decomposition reaction, could rate data be obtained directly from the existing temperature profile?
3. In which of the two cases would the laminar flame speed be greater: (1) oxygen in a large excess of a wet equimolar CO–CO2 mixture or (2) oxygen in a large excess of a wet equimolar CO–N2 mixture? Both cases are ignitable, contain the same amount of water, and have the same volumetric oxygen–fuel ratio. Discuss the reasons for the selection made.
4. A gas mixture is contained in a soap bubble and ignited by a spark in the center so that a spherical flame spreads radially through the mixture. It is assumed that the soap bubble can expand. The growth of the flame front along a radius is followed by some photographic means. Relate the velocity of the flame front as determined from the photographs to the laminar flame speed as defined in the text. If this method were used to measure flame speeds, what would be its advantages and disadvantages?
5. On what side of stoichiometric would you expect the maximum flame speed of hydrogen–air mixtures? Why?
6. A laminar flame propagates through a combustible mixture in a horizontal tube 3 cm in diameter. The tube is open at both ends. Owing to buoyancy effects, the flame tilts at a 45° angle to the normal and is planar. Assume the tilt is a straight flame front. The normal laminar flame speed for the combustible mixture is 40 cm/s. If the unburned gas mixture has a density of 0.0015 g/cm3, what is the mass burning rate of the mixture in grams per second under this laminar flow condition?
7. The flame speed for a combustible hydrocarbon–air mixture is known to be 30 cm/s. The activation energy of such hydrocarbon reactions is generally assumed to be 160 kJ/mol. The true adiabatic flame temperature for this mixture is known to be 1600 K. An inert diluent is added to the mixture to lower the flame temperature to 1450 K. Since the reaction is of second order, the addition of the inert can be considered to have no other effect on any property of the system. Estimate the flame speed after the diluent is added.
8. A horizontal long tube 3 cm in diameter is filled with a mixture of methane and air in stoichiometric proportions at 1 atm and 27 °C. The column is ignited at the left end and a flame propagates at uniform speed from left to right. At the left end of the tube there is a convergent nozzle that has a 2-cm diameter opening. At the right end there is a similar nozzle 0.3 cm in diameter at the opening. Calculate the velocity of the flame with respect to the tube in centimeters per second. Assume the following:
a. The effect of pressure increase on the burning velocity can be neglected; similarly, the small temperature increase due to adiabatic compression has a negligible effect.
b. The entire flame surface consumes combustible gases at the same rate as an ideal one-dimensional flame.
c. The molecular weight of the burned gases equals that of the unburned gases.
d. The flame temperature is 2100 K.
e. The normal burning velocity for the stoichiometric mixture is 40 cm/s.
Hint: Assume that the pressure in the burned gases is essentially 1 atm. In calculating the pressure in the cold gases, make sure the value is correct to many decimal places.
9. A continuous-flow stirred reactor operates off the decomposition of gaseous ethylene oxide fuel. If the fuel injection temperature is 300 K, the volume of the reactor is 1500 cm3, and the operating pressure is 20 atm, calculate the maximum rate of heat evolution possible in the reactor. Assume that the ethylene oxide follows homogeneous first-order reaction kinetics and that values of the reaction rate constant k are
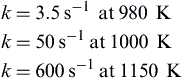
Develop any necessary rate data from these values. You are given that the adiabatic decomposition temperature of gaseous ethylene oxide is 1300 K. The heat of formation of gaseous ethylene oxide at 300 K is 50 kJ/mol. The overall reaction is
![]()
10. What are the essential physical processes that determine the flammability limit?
11. You want to measure the laminar flame speed at 273 K of a homogeneous gas mixture by the Bunsen burner tube method. If the mixture to be measured is 9% natural gas in air, what size would you make the tube diameter? Natural gas is mostly methane. The laminar flame speed of the mixture can be taken as 34 cm/s at 298 K. Other required data can be found in standard reference books.
12. A ramjet has a flame stabilized in its combustion chamber by a single rod whose diameter is 1.25 cm. The mass flow of the unburned fuel air mixture entering the combustion chamber is 22.5 kg/s, which is the limiting amount that can be stabilized at a combustor pressure of 3 atm for the cylindrical configuration. The ramjet is redesigned to fly with the same fuel–air mixture and a mass flow rate twice the original mass flow in the same size (cross-section) combustor. The inlet diffuser is such that the temperature entering the combustor is the same as in the original case, but the pressure has dropped to 2 atm. What is the minimum size rod that will stabilize the flame under these new conditions?
13. A laminar flame propagates through a combustible mixture at 1 atm pressure, has a velocity of 50 cm/s and a mass burning rate of 0.1 g/s cm2. The overall chemical reaction rate is second-order in that it depends only on the fuel and oxygen concentrations. Now consider a turbulent flame propagating through the same combustible mixture at a pressure of 10 atm. In this situation the turbulent intensity is such that the turbulent diffusivity is 10 times the laminar diffusivity. Estimate the turbulent flame propagation and mass burning rates.
14. Discuss the difference between explosion limits and flammability limits. Why is the lean flammability limit the same for both air and oxygen?
15. Explain briefly why halogen compounds are effective in altering flammability limits.
16. ∗Determine the effect of hydrogen addition on the laminar flame speed of a stoichiometric methane–air mixture. Vary the fuel mixture concentration from 100% CH4 to a mixture of 50% CH4 and 50% H2 in increments of 10% H2, maintaining a stoichiometric mixture. Plot the laminar flame speed as a function of percent H2 in the initial mixture and explain the trends. Using the temperature profile, determine how the flame thickness varies with H2 addition. The laminar flame speeds can be evaluated using the freely propagating laminar premixed code of CHEMKIN (or an equivalent code). A useful reaction mechanism for methane oxidation is GRI-Mech3.0 (developed from support by the Gas Research Institute) and can be downloaded from the Website http://www.me.berkeley.edu/gri-mech/version30/text30.html#thefiles.
17. ∗Calculate the laminar burning velocity as a function of pressure at 0.25, 1, and 3 atm of a stoichiometric methane–air mixture. Discuss the results and compare the values with the experimental measurements in Table F3. The laminar premixed flame code of CHEMKIN (or an equivalent code) may be used with the Gas Research Institute reaction mechanism for methane oxidation (http://www.me.berkeley.edu/gri-mech/version30/text30.html#thefiles).
18. ∗The primary zone of a gas-turbine combustor is modeled as a perfectly stirred reactor. The volume of the primary zone is estimated to be 1.5 × 103 cm3. The combustor operates at a pressure of 10 atm with an air inlet temperature of 500 K. For a stoichiometric methane–air mixture, determine the minimum residence time (maximum flow rate) at which blowout occurs. Also determine the fuel-lean and fuel-rich mixture equivalence ratios at which blowout occurs for a reactor residence time equal to twice the time of that determined above. Compare these values with the lean and rich flammability limits given in Appendix E for a methane–air mixture. The perfectly stirred reactor codes of CHEMKIN (or an equivalent code) may be used with the Gas Research Institute reaction mechanism for methane oxidation (http://www.me.berkeley.edu/gri-mech/verson30/text30.hml#thefiles).
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.
