Appendix D
Bond dissociation energies of hydrocarbons
The bond dissociation energies that follow are taken from the review of McMillan and Golden [Ann Rev Phys Chem 33, 493 (1982)]. The reader should refer to this publication for the methods of determining the values presented, their uncertainty, and the original sources. In the tables presented, all bond energies and heats of formation are in kilojoules per mole. The values listed in the first column are the heats of formation at 298 K for the reference radical and those above the column heading (in parentheses) for the associated radical. Thus, the tables presented are a source of not only bond energies but also heats of formation of radicals.
McMillan and Golden employ the commonly invoked uncertainty of 5 kJ/mol (1 kcal/mol) for dissociation energies, and most of the uncertainties for the heats of formation fall in the same range. The reader is urged to refer to the McMillan and Golden reference for the specific uncertainty values. Table D1–Table D6.
Table D1
Bond Dissociation Energies of Alkanesa
| ΔHf0(R) | R | (218) H | CH3 | C2H5 | i-C3H7 | t-C4H9 | (329) C6H5 | (200) PhCH2 |
| 147 | CH3 | 440 | 378 | 359 | 359 | 352 | 426 | 317 |
| 108 | C2H5 | 411 | 359 | 344 | 339 | 331 | 408 | 300 |
| 88 | n-C3H7 | 410 | 362 | 344 | 336 | 330 | 409 | 302 |
| 76 | i-C3H7 | 398 | 359 | 339 | 331 | 316 | 401 | 298 |
| 54 | s-C4H9 | 400 | 356 | 335 | 328 | – | – | – |
| 36 | t-C4H9 | 390 | 352 | 331 | 316 | 298 | – | 291 |
| 36 | CH2C(CH3)3 | 418 | 346 | 346 | – | – | – | – |
| 280 | Cyclopropyl | 445 | – | – | – | – | – | – |
| 214 | Cyclopropyl-methyl | 408 | – | – | – | – | – | – |
| 214 | Cyclobutyl | 404 | – | – | – | – | – | – |
| 102 | Cyclopentyl | 395 | – | – | – | – | – | – |
| 58 | Cyclohexyl | 400 | – | – | – | – | – | – |
| 51 | Cycloheptyl | 387 | – | – | – | – | – | – |
Table D2
Bond Dissociation Energies of Alkenes, Alkynes, and Aromatics
| ΔHf0(R) | R | H | CH3 |
| 295 | 460 | 421 | |
| 329 |  | 464 | 426 |
| 565 | 552 | 526 | |
| −548 | •C6F5 | 477 | – |
| 164 | 361 | 311 | |
| 126 |  | 358 | 305 |
| 127 |  | 345 | – |
| 77 |  | 323 | 285 |
| 38 |  | 319 | – |
| 40 |  | 326 | – |
| – |  | 371 | – |
| 161 | 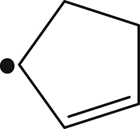 | 344 | – |
| Table Continued | |||

| ΔHf0(R) | R | H | CH3 |
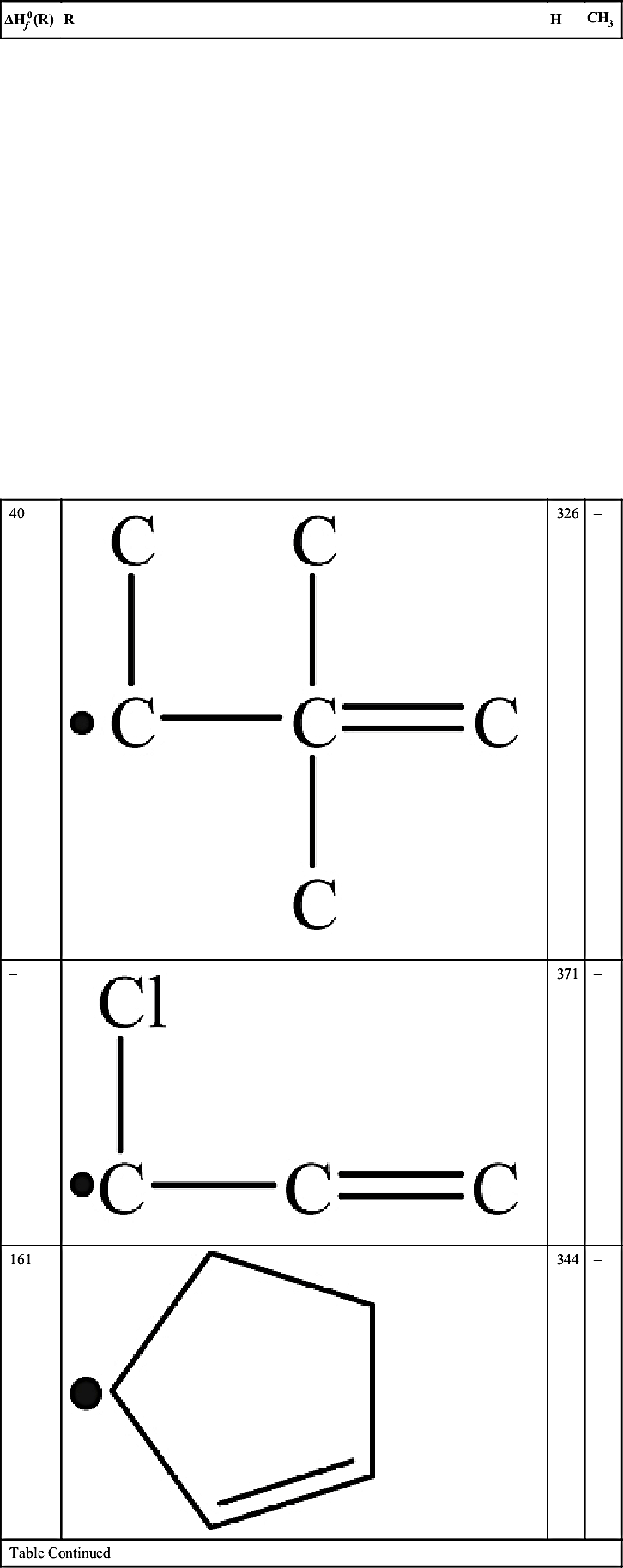
| 205 | 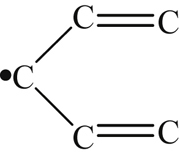 | 318 | – |
| 205 | – | ||
| 242 | 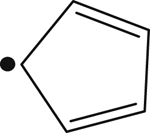 | 297 | – |
| 1977 | 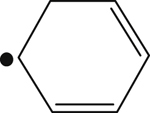 | 305 | – |
| 271 |  | 305 | – |
| 440 | 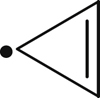 | 379 | – |
| 200 |  | 368 | 317 |
| 253 |  | 356 | 305 |
| 338 | 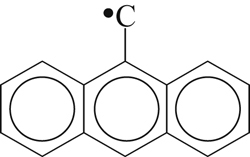 | 342 | 283 |
| 311 | 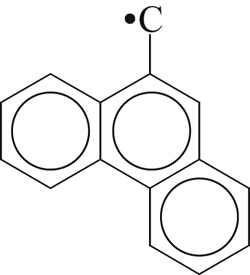 | 356 | 305 |
| 169 | 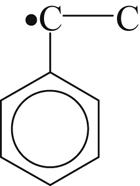 | 357 | 312 |
| Table Continued | |||
| ΔHf0(R) | R | H | CH3 |
| 139 |  | 353 | 308 |
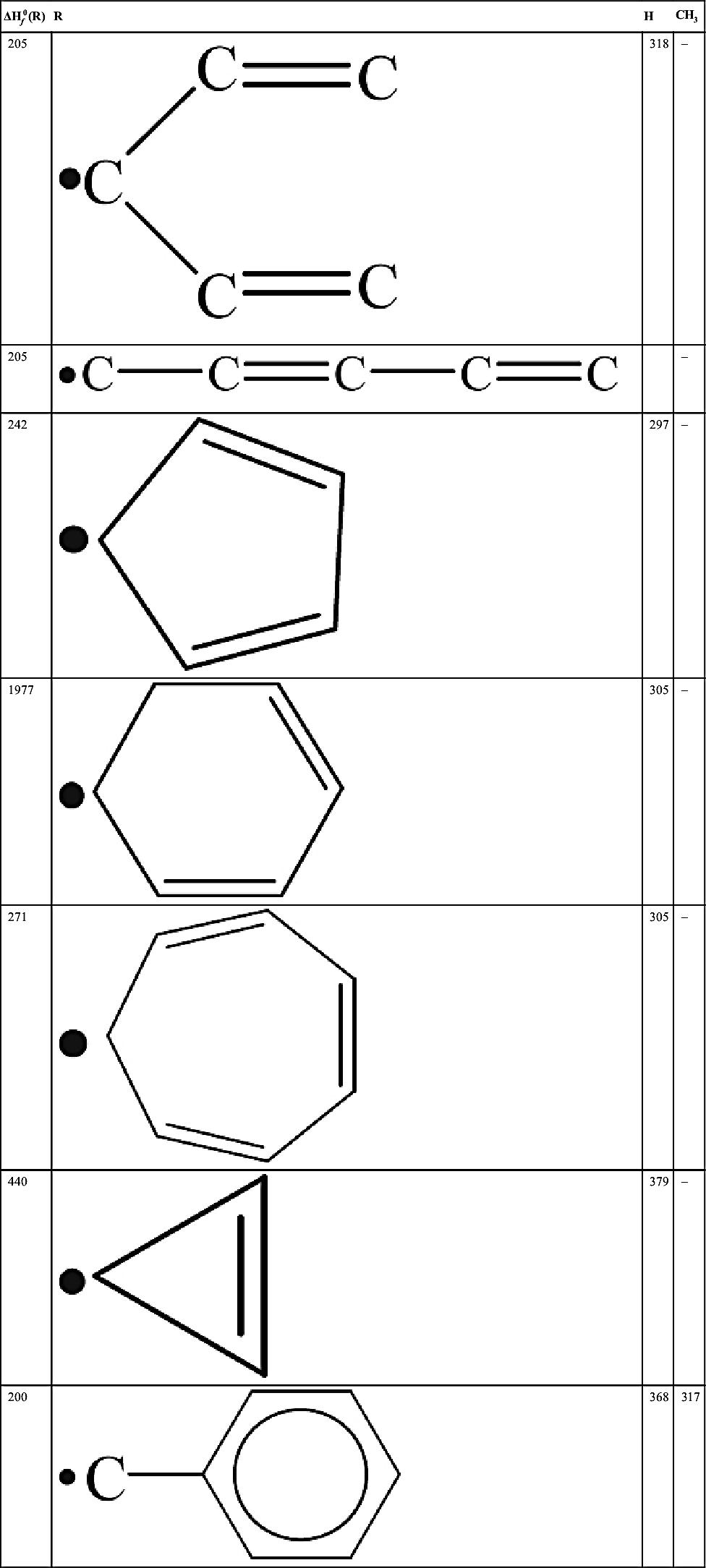
| – |  | 362 | 314 |
| – | 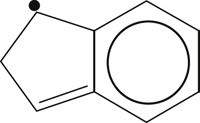 | 351 | – |
| 341 | 374 | 318 | |
| 294 | 365 | 308 | |
| 273 |  | 365 | 321 |
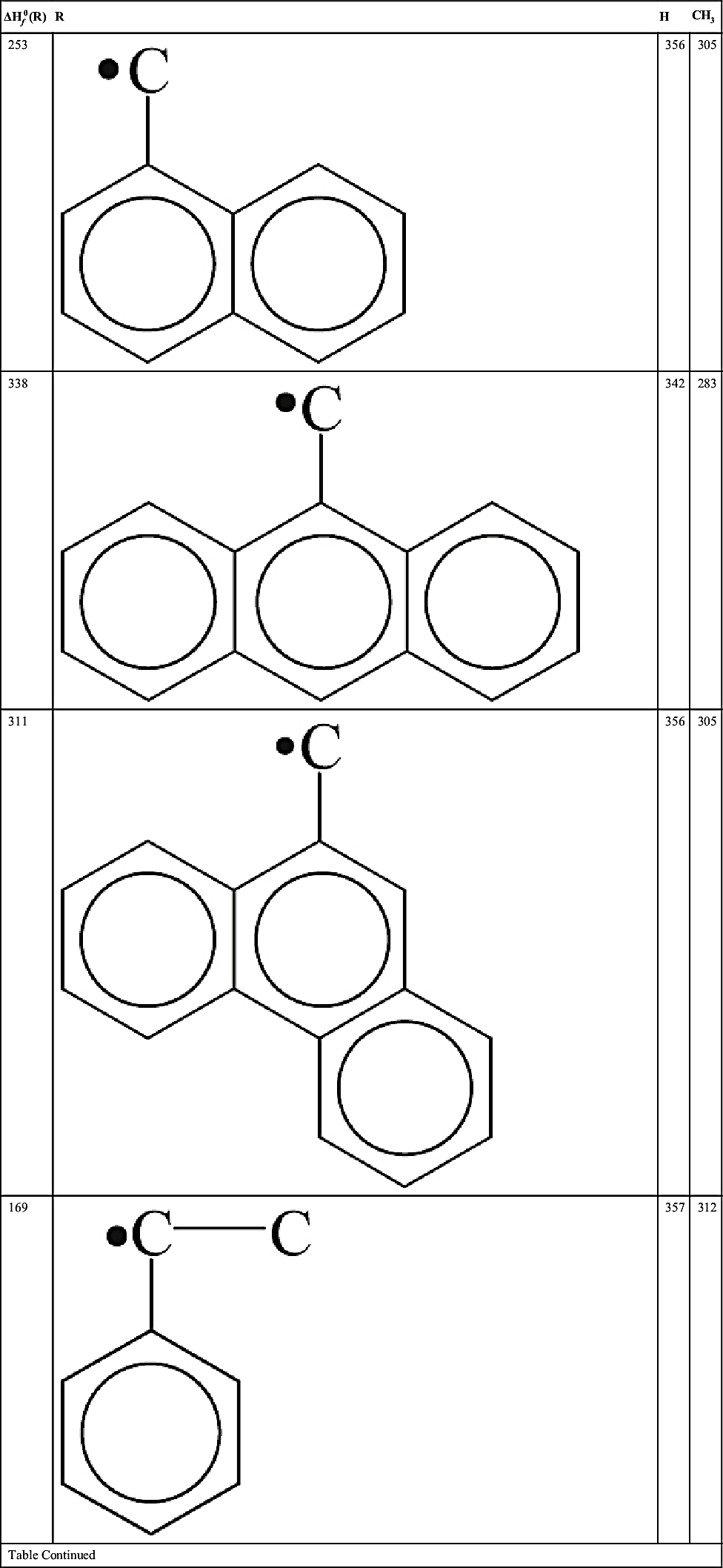
| 222 | 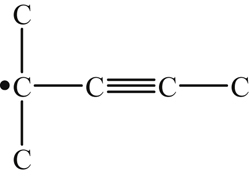 | 344 | 303 |
| 257 |  | 339 | 296 |
| 295 | 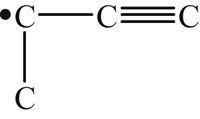 | 348 | 305 |
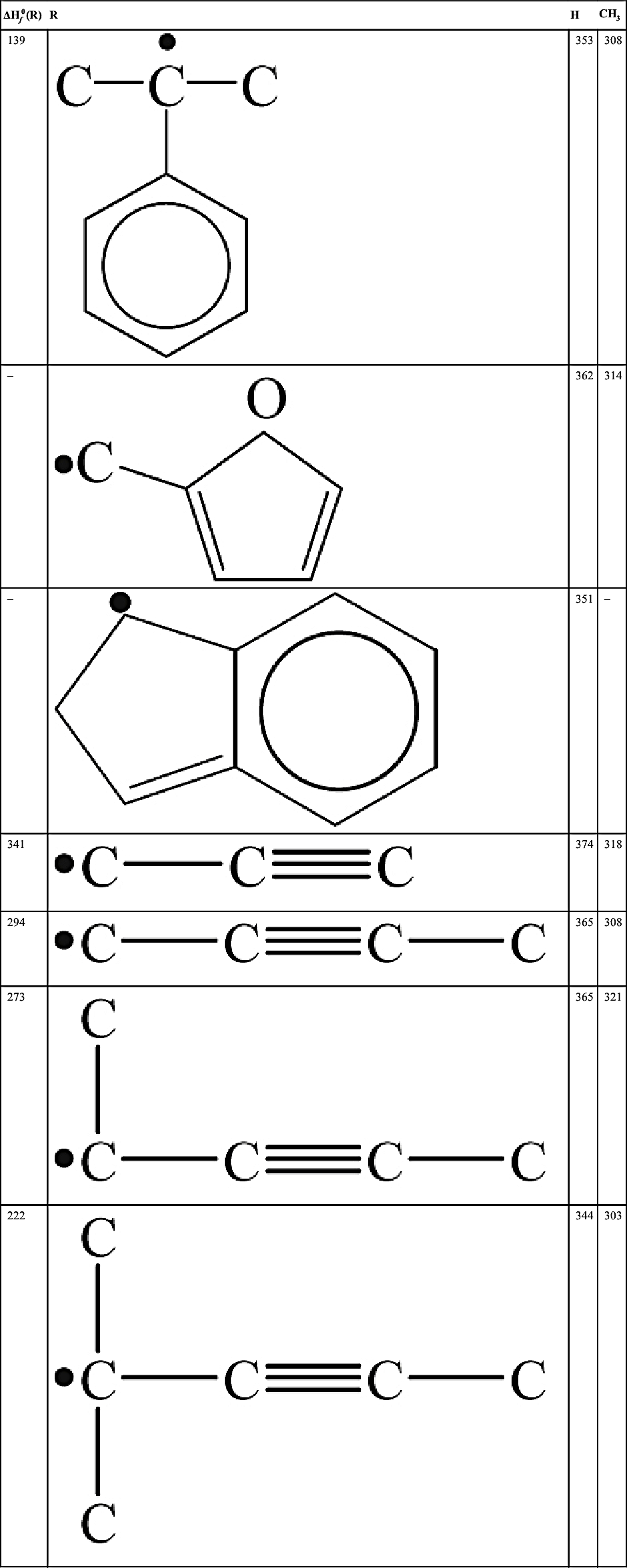

Table D3
Bond Dissociation Energies of C/H/O Compounds
| Oxygen-Centered Radicals | ||||||
| ΔHf0(R) | R1 | (218) H | R1 | (39) OH | (147) CH3 | (108) C2H5 |
| 39 | OH | 498 | 213 | 213 | 386 | 383 |
| 18 | OCH3 | 437 | 157 | – | 349 | 342 |
| −17 | OC2H5 | 436 | 159 | – | 346 | 344 |
| −41 | O-n-C3H7 | 433 | 155 | – | 343 | – |
| −63 | O-n-C4H9 | 431 | – | – | – | – |
| −52 | O-i-C3H7 | 438 | 158 | – | 346 | – |
| −69 | O-s-C4H9 | 441 | 152 | – | – | – |
| −91 | O-t-C4H9 | 440 | 159 | – | 348 | – |
| – | O-t-C5H11 | – | 164 | – | – | – |
| – | OCH2C(CH3)3 | 428 | 152 | 194 | – | – |
| 48 | OC6H5 | 362 | – | – | 267 | 264 |
| – | OCF3 | – | 193 | – | – | – |
| – | OC(CF3)3 | – | 149 | – | – | – |
| 10 | O2H | 365 | – | – | – | – |
| −208 | O2CCH3 | 443 | 127 | – | – | 346 |
| −228 | O2CC2H5 | 445 | 127 | – | – | – |
| −249 | O2C-n-C3H7 | 443 | 127 | – | – | – |

| Carbon-Centered Radicals | |||||
| ΔHf0(R) | R1 | (218) H | (147) CH3 | (329) C6H5 | R1 |
| 37 | CHO | 364 | 345 | 403 | 286 |
| −24 | COCH3 | 360 | 340 | 391 | 282 |
| 72 | COCH=CH2 | 364 | – | – | – |
| −43 | COC2H5 | 366 | 337 | 395 | – |
| 109 | COC6H5 | 364 | 343 | 377 | 278 |
| – | COCF3 | 381 | – | – | – |
| −24 | CH2COCH3 | 411 | 361 | – | – |
| −70 | CH(CH3)COCH3 | 386 | – | – | – |
| −26 | CH2OH | 393 | – | 403 | 336 |
| −64 | CH(OH)CH3 | 389 | – | – | 355 |
| −111 | C(OH)(CH3)2 | 381 | – | – | – |
| −12 | CH2OCH3 | 389 | 361 | – | – |
| −18 | Tetrahydrofuran–2–yl | 385 | – | – | – |
| 0.0 | CH(OH)CH=CH2 | 341 | – | – | – |
| −169 | COOCH3 | 388 | – | – | – |
| −70 | CH2OCOC6H5 | 419 | – | – | – |
| −223 | COOH–CH2C6H5 | 280 | – | – | – |
| 248 | (C6H5)2CH–COOH | 249 | – | – | – |
| — | C6H5CH2CO–CH2C6H5 | 274 | – | – | – |
| 200 | C6H5CH2–OH | 340 | – | – | – |
| 48 | C6H5CO–F3 | 309 | – | – | – |

Table D4
Bond Dissociation Energies of Sulfur-Containing Compounds
| R1–R2 | D2980 | ΔHf0(R) |
| HS–H | 381 | 141 |
| CH3S–H | 379 | 139 |
| RS–H | 381 | – |
| CH3–SH | 310 | – |
| C2H5–SH | 295 | – |
| t-Bu–SH | 286 | – |
| C6H5–SH | 362 | – |
| CH3S–CH3 | 323 | – |
| CH3S–C2H5 | 307 | – |
| CH3S–n-C3H7 | 309 | – |
| PhS–H | 349 | – |
| PhS–CH3 | 290 | 230 |
| PhCH2–SCH3 | 257 | – |
| CS–S | 431 | 272 |
| OS–O | 544 | – |
| CH3SO2–CH3 | 279 | – |
| CH3SO2–CH2CH=CH3 | 208 | – |
| CH3SO2–CH2C6H5 | 221 | – |
| RS2–H | 293 | – |
| RS2–CH3 | 238 | – |
| HS–SH | 276 | – |
| RS–SR | 301 | – |
Table D5
Bond Dissociation Energies of Nitrogen-Containing Compounds
| Amines and Nitriles | |||||||
| ΔHf0(R) | R | (218) H | (147) CH3 | (108) C2H5 | (200) PhCH2 | (329) C6H5 | (189) NH2 |
| 185 | NH2 | 449 | 355 | 341 | 297 | 427 | 275 |
| 177 | NHCH3 | 418 | 344 | 334 | 287 | 421 | 268 |
| 145 | N(CH3)2 | 383 | 316 | 303 | 260 | 390 | 247 |
| 237 | NHC6H5 | 368 | 299 | 289 | – | 339 | 219 |
| 233 | N(CH3)C6H5 | 366 | 296 | – | – | – | – |
| 33 | NF2 | 317 | – | – | – | – | – |
| 469 | N3 | 385 | – | – | – | – | – |
| 149 | CH2NH2 | 390 | 344 | 332 | 285 | 390 | – |
| 126 | CH2NH(CH3) | 364 | 320 | – | – | 367 | – |
| 109 | CH2N(CH3)2 | 351 | 308 | – | – | 352 | – |
| 435 | CN | 518 | 510 | 495 | – | 548 | – |
| 245 | CH2CN | 389 | 340 | 322 | – | – | – |
| 209 | CH(CH3)CN | 376 | 330 | – | – | – | – |
| 167 | C(CH3)2CN | 362 | 313 | – | – | – | – |
| 249 | C(CH3)(CN)C6H5 | – | 251 | – | – | – | |

| Nitro and Nitroso Compounds and Nitrates | ||||
| ΔHf0(R) | R | (90) NO | (33) NO2 | (71) ONO2 |
| 218 | H | – | 328 | 423 |
| 39 | OH | 206 | 207 | 163 |
| 147 | CH3 | 167 | 254 | – |
| 108 | i-C3H5 | – | 245 | – |
| 76 | i-C3H7 | 153 | 247 | – |
| 36 | t-C4H9 | 165 | 245 | – |
| −467 | CF3 | 179 | – | – |
| 79 | CCl3 | 134 | – | – |
| 331 | C6H5 | 213 | 298 | – |
| −548 | C6F5 | 208 | – | – |
| – | C(NO2)R2 | – | 204 | – |
| – | C(NO2)2R | – | 183 | – |
| – | C(NO2)3 | – | 169 | – |
| – | RO | 171 | 170 | – |
| 33 | NO2 | 41 | 57 | – |

Table D6
Bond Dissociation Energies of Halocarbons
| ΔHf0(R) | R | (218) H | (147) CH3 | (79) F | (121) Cl | (112) Br | (107) I | (−465) CF3 |
| 147 | CH3 | 440 | 378 | 460 | 354 | 297 | 239 | 425 |
| 108 | C2H5 | 411 | – | 451 | 334 | 284 | 223 | – |
| 76 | i-C3H7 | 398 | 359 | 446 | 338 | 286 | 224 | – |
| 200 | CH2C6H5 | 368 | 317 | – | 302 | 241 | 202 | – |
| −33 | CH2F | 418 | 498 | 357 | 357 | – | – | 396 |
| −248 | CHF2 | 423 | 400 | 527 | – | 289 | – | – |
| −467 | CF3 | 446 | 425 | 546 | 361 | 295 | 230 | 413 |
| 118 | CH2Cl | 422 | – | – | 335 | – | – | – |
| 101 | CHCl2 | 414 | – | – | 325 | – | – | – |
| 79 | CCl3 | 401 | – | 426 | 306 | 231 | – | – |
| −269 | CF2Cl | 425 | – | 515 | 318 | 270 | – | – |
| −96 | CFCl2 | – | – | 460 | 305 | – | – | – |
| 174 | CH2Br | 427 | – | – | – | – | – | – |
| 227 | CHBr2 | 434 | – | – | – | – | – | – |
| – | CBr3 | 402 | – | – | – | 235 | – | – |
| −893 | C2F5 | 430 | – | 531 | 346 | 287 | 214 | – |
| – | n-C3F7 | 435 | – | – | – | 278 | 208 | – |
| – | i-C3F7 | 431 | – | – | – | 274 | – | – |
| −303 | CF2CH3 | 416 | – | 522 | – | – | 218 | – |
| −517 | CH2CF3 | 446 | – | 458 | – | – | 236 | – |
| – | CHClCF3 | 426 | – | – | – | 275 | – | – |
| – | CClBrCF3 | 404 | – | – | – | 251 | – | – |
| 435 | CN | 518 | 510 | 470 | 422 | 367 | 303 | 561 |
| 39 | OH | 498 | 387 | – | 251 | 234 | 234 | – |
| 331 | C6H5 | 466 | 428 | 526 | 400 | 337 | 274 | – |
| −548 | C6F5 | 487 | – | – | 383 | – | 277 | – |

..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.
