| A | n | E | ||
| NH Reactions | ||||
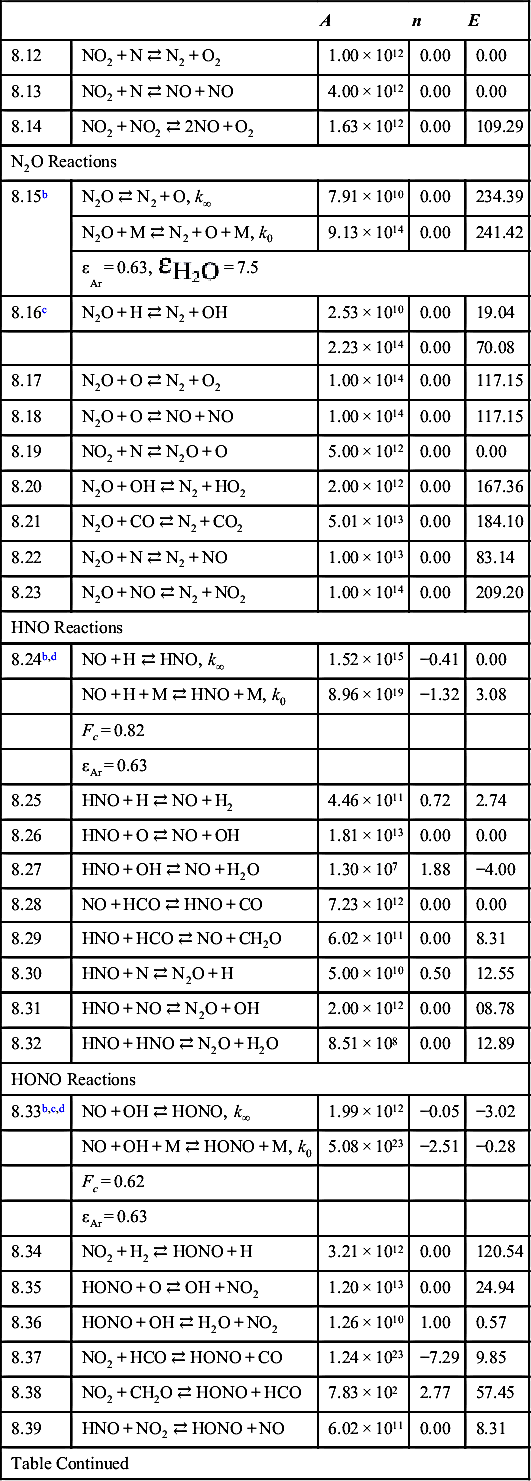
| 8.40 | NH + M ⇄ N + H + M | 2.65 × 1014 | 0.00 | 315.93 |
| 8.41 | N + HO2 ⇄ NH + O2 | 1.00 × 1013 | 0.00 | 8.37 |
| 8.42 | NH + O2 ⇄ NO + OH | 7.60 × 1010 | 0.00 | 6.40 |
| 8.43 | NH + O2 ⇄ HNO + O | 3.89 × 1013 | 0.00 | 74.85 |
| 8.44 | N + H2 ⇄ NH + H | 1.60 × 1014 | 0.00 | 105.19 |
| 8.45 | NH + O ⇄ N + OH | 3.72 × 1013 | 0.00 | 0.00 |
| 8.46 | NH + O ⇄ NO + H | 5.50 × 1013 | 0.00 | 0.00 |
| 8.47 | NH + OH ⇄ N + H2O | 5.00 × 1011 | 0.50 | 8.37 |
| 8.48 | NH + OH ⇄ HNO + H | 2.00 × 1013 | 0.00 | 0.00 |
| 8.49 | NH + N ⇄ N2 + H | 3.00 × 1013 | 0.00 | 0.00 |
| 8.50 | NH + NO ⇄ N2 + OH | 2.16 × 1013 | −0.23 | 0.00 |
| 8.51c | NH + NO ⇄ N2O + H | 2.94 × 1014 | −0.40 | 0.00 |
| −2.16 × 1013 | −0.23 | 0.00 | ||
| 8.52 | HNO + N ⇄ NH + NO | 1.00 × 1013 | 0.00 | 8.37 |
| 8.53 | NH + NO2 ⇄ HNO + NO | 1.00 × 1011 | 0.50 | 16.74 |
| 8.54 | NH + NH ⇄ N2 + H + H | 5.10 × 1013 | 0.00 | 0.00 |
| NH2 Reactions | ||||
| 8.55 | NH2 + M ⇄ NH + H + M | 3.98 × 1023 | −2.00 | 382.44 |
| 8.56 | NH2 + H ⇄ NH + H2 | 7.20 × 105 | 2.32 | 6.65 |
| 8.57 | NH2 + O ⇄ HNO + H | 6.63 × 1014 | −0.50 | 0.00 |
| 8.58 | NH2 + O ⇄ NH + OH | 6.75 × 1012 | 0.00 | 0.00 |
| 8.59 | NH2 + OH ⇄ NH + H2O | 4.00 × 1012 | 2.00 | 4.18 |
| 8.60 | NH2 + O2 ⇄ HNO + OH | 1.78 × 1012 | 0.00 | 62.34 |
| 8.61c | NH2 + NO ⇄ N2 + H2O | 1.30 × 1016 | −1.25 | 0.00 |
| −2.80 × 1013 | −0.55 | 0.00 | ||
| 8.62 | NH2 + NO ⇄ N2O + H2 | 5.00 × 1013 | 0.00 | 103.09 |
| 8.63 | NH2 + NO ⇄ HNO + NH | 1.00 × 1013 | 0.00 | 167.36 |
| 8.64 | NH2 + NO2 ⇄ N2O + H2O | 2.84 × 1018 | −2.20 | 0.00 |
| NH3 Reactions | ||||
| 8.65 | NH3 + M ⇄ NH2 + H + M | 2.20 × 1016 | 0.00 | 391.08 |
| 8.66 | NH3 + H ⇄ NH2 + H2 | 6.38 × 105 | 2.39 | 42.68 |
| 8.67 | NH3 + O ⇄ NH2 + OH | 9.40 × 106 | 1.94 | 27.03 |
| 8.68 | NH3 + OH ⇄ NH2 + H2O | 2.04 × 106 | 2.04 | 2.37 |
| 8.69 | NH2 + HO2 ⇄ NH3 + O2 | 3.00 × 1011 | 0.00 | 92.05 |
| 8.70 | NH2 + NH2 ⇄ NH3 + NH | 5.00 × 1013 | 0.00 | 41.84 |
| Table Continued | ||||

| A | n | E | ||
| NNH Reactions | ||||
| 8.71 | NNH + M ⇄ N2 + H + M | 1.00 × 1014 | 0.00 | 12.47 |
| 8.72 | NNH + H ⇄ N2 + H2 | 1.00 × 1014 | 0.00 | 0.00 |
| 8.73 | NNH + OH ⇄ N2 + H2O | 5.00 × 1013 | 0.00 | 0.00 |
| 8.74 | NH2 + NO ⇄ NNH + OH | 2.80 × 1013 | −0.55 | 0.00 |
| 8.75 | NNH + NO ⇄ HNO + N2 | 5.00 × 1013 | 0.00 | 0.00 |
| 8.76 | NNH + NH ⇄ N2 + NH2 | 5.00 × 1013 | 0.00 | 0.00 |
| 8.77 | NNH + NH2 ⇄ N2 + NH3 | 5.00 × 1013 | 0.00 | 0.00 |
| N2H2 Reactions | ||||
| 8.78 | N2H2 + M ⇄ NNH + H + M | 1.00 × 1016 | 0.00 | 207.94 |
| 8.79 | N2H2 + M ⇄ NH + NH + M | 3.16 × 1016 | 0.00 | 415.89 |
| 8.80 | N2H2 + H ⇄ NNH + H2 | 1.00 × 1013 | 0.00 | 4.16 |
| 8.81 | NH + NH2 ⇄ N2H2 + H | 3.16 × 1013 | 0.00 | 4.16 |
| 8.82 | N2H2 + O ⇄ NNH + OH | 1.00 × 1011 | 0.50 | 0.00 |
| 8.83 | N2H2 + OH ⇄ NNH + H2O | 1.00 × 1013 | 0.00 | 8.33 |
| 8.84 | NH2 + NH2 ⇄ N2H2 + H2 | 3.98 × 1013 | 0.00 | 49.79 |
| 8.85 | N2H2 + HO2 ⇄ NNH + H2O2 | 1.00 × 1013 | 0.00 | 8.33 |
| 8.86 | NNH + NNH ⇄ N2H2 + N2 | 1.00 × 1013 | 0.00 | 41.59 |
| 8.87 | N2H2 + NH ⇄ NNH + NH2 | 1.00 × 1013 | 0.00 | 4.16 |
| 8.88 | N2H2 + NH2 ⇄ NNH + NH3 | 1.00 × 1013 | 0.00 | 16.61 |
| N2H3 Reactions | ||||
| 8.89 | N2H3 + M ⇄ N2H2 + H + M | 1.00 × 1016 | 0.00 | 207.94 |
| 8.90 | N2H3 + M ⇄ NH2 + NH + M | 1.00 × 1016 | 0.00 | 174.47 |
| 8.91 | N2H3 + H ⇄ NH2 + NH2 | 1.58 × 1012 | 0.00 | 0.00 |
| 8.92 | N2H3 + H ⇄ NH + NH3 | 1.00 × 1011 | 0.00 | 0.00 |
| 8.93 | N2H3 + H ⇄ N2H2 + H2 | 1.00 × 1012 | 0.00 | 8.33 |
| 8.94 | N2H3 + O ⇄ N2H2 + OH | 3.16 × 1011 | 0.5 | 0.00 |
| 8.95 | N2H3 + O ⇄ NNH + H2O | 3.16 × 1011 | 0.50 | 0.00 |
| 8.96 | N2H3 + OH ⇄ N2H2 + H2O | 1.00 × 1013 | 0.00 | 8.33 |
| 8.97 | N2H3 + HO2 ⇄ N2H2 + H2O2 | 1.00 × 1013 | 0.00 | 8.33 |
| 8.98 | NH3 + NH2 ⇄ N2H3 + H2 | 7.94 × 1011 | 0.50 | 90.37 |
| 8.99 | N2H2 + NH2 ⇄ NH + N2H3 | 1.00 × 1011 | 0.50 | 141.42 |
| 8.100 | N2H3 + NH2 ⇄ N2H2 + NH3 | 1.00 × 1011 | 0.50 | 0.00 |
| 8.101 | N2H2 + N2H2 ⇄ NNH + N2H3 | 1.00 × 1013 | 0.00 | 41.59 |
| N2H4 Reactions | ||||
| 8.102 | N2H4 + M ⇄ NH2 + NH2 + M | 4.00 × 1015 | 0.00 | 171.13 |
| 8.103 | N2H4 ⇄ + M ⇄ N2H3 + H + M | 1.00 × 1015 | 0.00 | 266.10 |
| Table Continued | ||||
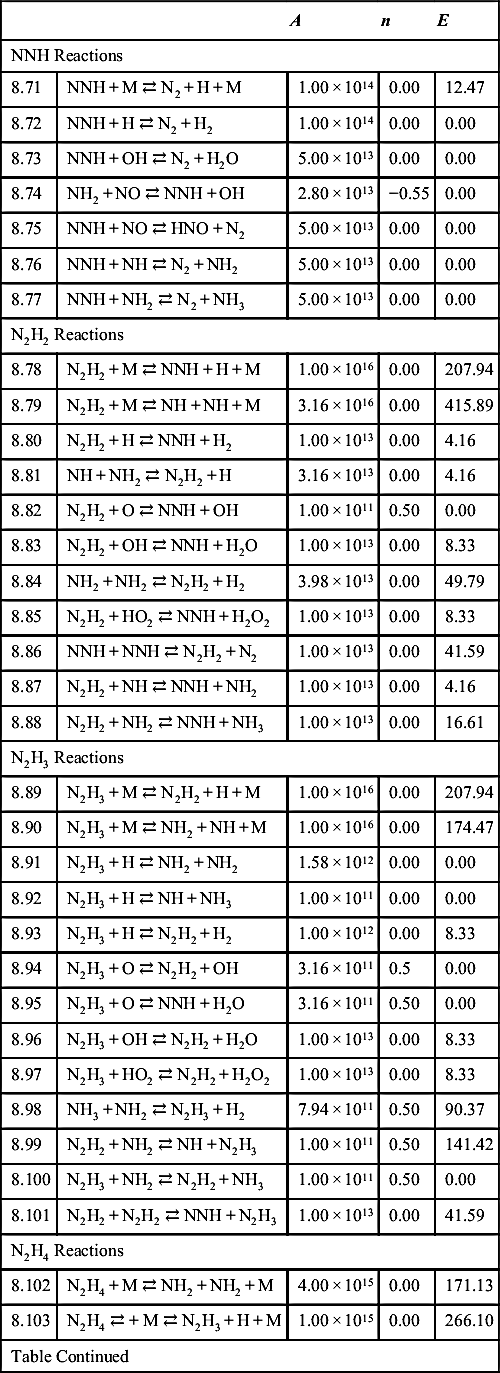
| A | n | E | ||
| 8.104 | N2H4 + H ⇄ N2H3 + H2 | 1.29 × 1013 | 0.00 | 10.46 |
| 8.105 | N2H4 + H ⇄ NH2 + NH3 | 4.46 × 109 | 0.00 | 12.97 |
| 8.106 | N2H4 + O ⇄ N2H2 + H2O | 6.31 × 1013 | 0.00 | 4.98 |
| 8.107 | N2H4 + O ⇄ N2H3 + OH | 2.51 × 1012 | 0.00 | 4.98 |
| 8.108 | N2H4 ⇄ + OH ⇄ N2H3 + H2O | 3.98 × 1013 | 0.00 | 0.00 |
| 8.109 | N2H4 + HO2 ⇄ N2H3 + H2O2 | 3.98 × 1013 | 0.00 | 8.33 |
| 8.110 | N2H4 + NH ⇄ NH2 + N2H3 | 1.00 × 1012 | 0.00 | 8.33 |
| 8.111 | N2H4 + NH2 ⇄ N2H3 + NH3 | 3.98 × 1011 | 0.50 | 8.33 |
| 8.112 | N2H3 + N2H2 ⇄ N2H4 ⇄ + NNH | 1.00 × 1013 | 0.00 | 41.59 |
| 8.113 | N2H4 + N2H2 ⇄ N2H3 + N2H3 | 2.50 × 1010 | 0.50 | 124.68 |
| NO3 Reactions | ||||
| 8.114b,d | NO2 + O ⇄ NO3, k∞ | 1.33 × 1013 | 0.00 | 0.00 |
| NO2 + O + M ⇄ NO3 + M, k0 | 1.49 × 1028 | −4.08 | 10.32 | |
| Fc = 0.79–1.8 × 10−4T | ||||
| εAr = 0.63 | ||||
| 8.115 | NO2 + NO2 ⇄ NO3 + NO | 9.64 × 109 | 0.73 | 87.53 |
| 8.116 | NO3 + H ⇄ NO2 + OH | 6.00 × 1013 | 0.00 | 0.00 |
| 8.117 | NO3 + O ⇄ NO2 + O2 | 1.00 × 1013 | 0.00 | 0.00 |
| 8.118 | NO3 + OH ⇄ NO2 + HO2 | 1.40 × 1013 | 0.00 | 0.00 |
| 8.119 | NO3 + HO2 ⇄ NO2 + O2 + OH | 1.50 × 1012 | 0.00 | 0.00 |
| 8.120 | NO3 + NO2 ⇄ NO + NO2 + O2 | 5.00 × 1010 | 0.00 | 12.30 |
| HNO3 Reactions | ||||
| 8.121b,d | NO2 + OH ⇄ HNO3, k∞ | 2.41 × 1013 | 0.00 | 0.00 |
| NO2 + OH + M ⇄ HNO3 + M, k0 | 6.42 × 1032 | −5.49 | 9.83 | |
| Fc = 0.725–2.5 × 10−4T | ||||
| εAr = 0.63 | ||||
| 8.122 | NO + HO2 + M ⇄ HNO3 + M | 2.23 × 1012 | −3.50 | 9.20 |
| 8.123 | HNO3 + OH ⇄ NO2 + HO2 | 1.03 × 1010 | 0.00 | −5.19 |
| 8.124 | NO3 + HO2 ⇄ HNO3 + O2 | 5.60 × 1011 | 0.00 | 0.00 |
Allen MT, Yetter RA, Dryer FL. The decomposition of nitrous oxide at 1.5 ≤ P ≤ 10.5 atm and 1103 ≤ T ≤ 1173 K. Int J Chem Kinet 1995;27:883–909. Allen MT, Yetter RA, Dryer FL. High pressure studies of moist carbon monoxide/nitrous oxide kinetics. Combust Flame 1997;109:449–70.
Table C9
HCl/NxOy/CO/H2/O2 Mechanisma
| A | n | E | ||
| HCl and Cl Reactions | ||||
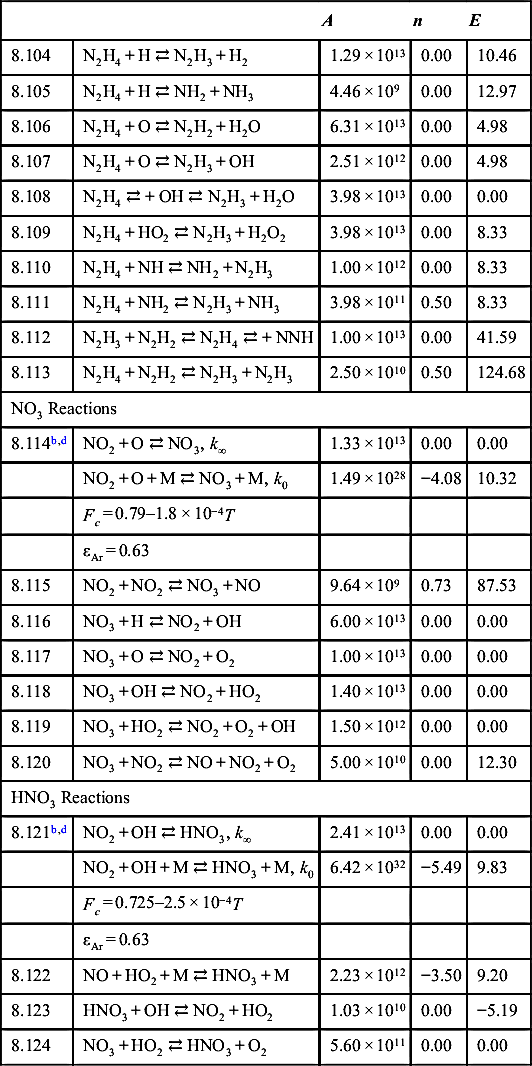
| 9.1 | Cl + H + M ⇄ HCl + M | 7.20 × 1021 | −2.00 | 0.00 |
| 9.2 | Cl + HO2 ⇄ HCl + O2 | 1.08 × 1013 | 0.00 | −1.38 |
| 9.3 | HCl + H ⇄ Cl + H2 | 1.69 × 1013 | 0.30 | 17.32 |
| 9.4 | HCl + O ⇄ Cl + OH | 3.37 × 103 | 2.87 | 14.67 |
| 9.5 | HCl + OH ⇄ Cl + H2O | 2.71 × 107 | 1.65 | −0.93 |
| 9.6 | Cl + H2O2 ⇄ HCl + HO2 | 6.62 × 1012 | 0.00 | 8.16 |
| 9.7 | Cl + HCO ⇄ HCl + CO | 1.00 × 1014 | 0.00 | 0.00 |
| 9.8 | Cl + HNO ⇄ HCl + NO | 9.00 × 1013 | 0.00 | 4.16 |
| 9.9 | Cl + HONO ⇄ HCl + NO2 | 5.00 × 1013 | 0.00 | 0.00 |
| Cl2 Reactions | ||||
| 9.10 | Cl + Cl + M ⇄ Cl2 + M | 4.68 × 1014 | 0.00 | −7.53 |
| 9.11 | Cl2 + H ⇄ Cl + HCl | 8.59 × 1013 | 0.00 | 4.90 |
| ClO Reactions | ||||
| 9.12 | ClO + O ⇄ Cl + O2 | 5.70 × 1013 | 0.00 | 1.52 |
| 9.13 | Cl + HO2 ⇄ ClO + OH | 2.42 × 1013 | 0.00 | 9.62 |
| 9.14 | ClO + CO ⇄ Cl + CO2 | 6.03 × 1011 | 0.00 | 30.96 |
| 9.15 | Cl2 + O ⇄ ClO + Cl | 2.52 × 1012 | 0.00 | 11.38 |
| 9.16 | ClO + NO ⇄ Cl + NO2 | 3.85 × 1012 | 0.00 | 0.59 |
| HOCl Reactions | ||||
| 9.17 | HOCl ⇄ Cl + OH | 1.76 × 1020 | −3.01 | 237.32 |
| 9.18 | HOCl ⇄ ClO + H | 8.12 × 1014 | −2.09 | 392.00 |
| 9.19 | HOCl ⇄ HCl + OH | 9.55 × 1013 | 0.00 | 31.88 |
| 9.20 | ClO + H2 ⇄ HOCl + H | 6.03 × 1011 | 0.00 | 59.00 |
| 9.21 | HOCl + O ⇄ ClO + OH | 6.03 × 1012 | 0.00 | 18.28 |
| 9.22 | HOCl + OH ⇄ ClO + H2O | 1.81 × 1012 | 0.00 | 4.14 |
| 9.23 | HCO + ClO ⇄ HOCl + CO | 3.16 × 1013 | 0.00 | 0.00 |
| 9.24 | HOCl + Cl ⇄ Cl2 + OH | 1.81 × 1012 | 0.00 | 1.09 |
| 9.25 | HOCl + Cl ⇄ ClO + HCl | 7.62 × 1012 | 0.00 | 0.75 |
| CClO Reactions | ||||
| 9.26 | COCl + M ⇄ Cl + CO + M | 1.30 × 1014 | 0.00 | 33.47 |
| 9.27 | COCl + O2 ⇄ ClO + CO2 | 7.94 × 1010 | 0.00 | 13.81 |
| 9.28 | COCl + H ⇄ HCl + CO | 1.00 × 1014 | 0.00 | 0.00 |
| 9.29 | COCl + O ⇄ ClO + CO | 1.00 × 1014 | 0.00 | 0.00 |
| Table Continued | ||||

| A | n | E | ||
| 9.30 | COCl + O ⇄ Cl + CO2 | 1.00 × 1013 | 0.00 | 0.00 |
| 9.31 | COCl + OH ⇄ HOCl + CO | 3.30 × 1012 | 0.00 | 0.00 |
| 9.32 | COCl + Cl ⇄ Cl2 + CO | 4.00 × 1014 | 0.00 | 3.35 |
| NOCl Reactions | ||||
| 9.33b | NOCl + M ⇄ Cl + NO + M | 2.51 × 1015 | 0.00 | 133.47 |
| 9.34 | NOCl + H ⇄ HCl + NO | 4.60 × 1013 | 0.00 | 3.73 |
| 9.35 | NOCl + O ⇄ ClO + NO | 5.00 × 1012 | 0.00 | 12.55 |
| 9.36 | NOCl + Cl ⇄ Cl2 + NO | 2.41 × 1013 | 0.00 | 0.00 |
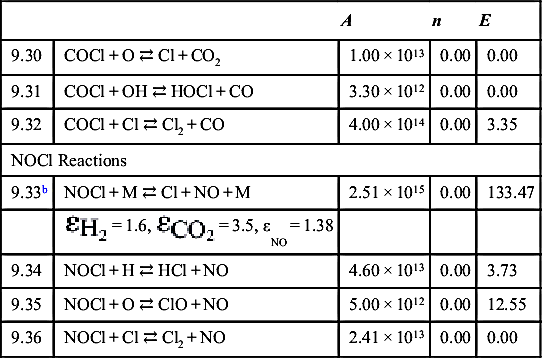
Table C10
O3/NxOy/CO/H2/O2 Mechanisma
| A | n | E | ||
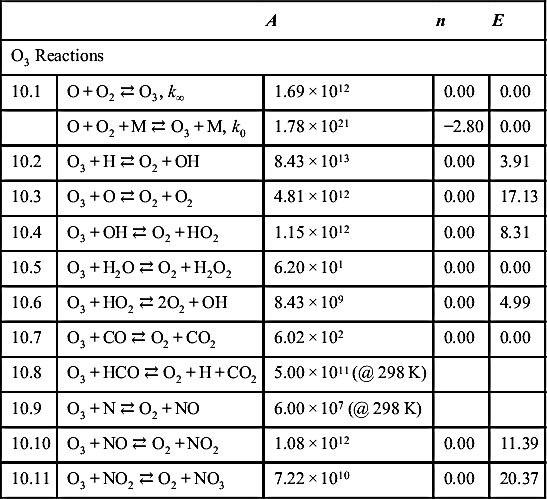
| O3 Reactions | ||||
| 10.1 | O + O2 ⇄ O3, k∞ | 1.69 × 1012 | 0.00 | 0.00 |
| O + O2 + M ⇄ O3 + M, k0 | 1.78 × 1021 | −2.80 | 0.00 | |
| 10.2 | O3 + H ⇄ O2 + OH | 8.43 × 1013 | 0.00 | 3.91 |
| 10.3 | O3 + O ⇄ O2 + O2 | 4.81 × 1012 | 0.00 | 17.13 |
| 10.4 | O3 + OH ⇄ O2 + HO2 | 1.15 × 1012 | 0.00 | 8.31 |
| 10.5 | O3 + H2O ⇄ O2 + H2O2 | 6.20 × 101 | 0.00 | 0.00 |
| 10.6 | O3 + HO2 ⇄ 2O2 + OH | 8.43 × 109 | 0.00 | 4.99 |
| 10.7 | O3 + CO ⇄ O2 + CO2 | 6.02 × 102 | 0.00 | 0.00 |
| 10.8 | O3 + HCO ⇄ O2 + H + CO2 | 5.00 × 1011 (@ 298 K) | ||
| 10.9 | O3 + N ⇄ O2 + NO | 6.00 × 107 (@ 298 K) | ||
| 10.10 | O3 + NO ⇄ O2 + NO2 | 1.08 × 1012 | 0.00 | 11.39 |
| 10.11 | O3 + NO2 ⇄ O2 + NO3 | 7.22 × 1010 | 0.00 | 20.37 |
Atkinson R, Baulch DL, Cox RA, Hampson Jr RF, Kerr JA, Troe J. Evaluated kinetic and photochemical data for atmospheric chemistry. Supplement IV. IUPAC subcommittee on gas kinetic data evaluation for atmospheric chemistry. J Phys Chem Ref Data 1992;21(No. 6):1125–568.
Table C11
SOx/NxOy/CO/H2/O2 Mechanisma
| A | n | E | ||
| S and SO Reactions | ||||
| 11.1b | SO + M ⇄ S + O + M | 4.00 × 1014 | 0.00 | 448.96 |
| 11.2 | S + OH ⇄ SO + H | 4.00 × 1013 | 0.00 | 0.00 |
| 11.3 | S + O2 ⇄ SO + O | 5.20 × 106 | 1.81 | −4.99 |
| SO2 Reactions | ||||
| 11.4b,c | SO + O ⇄ SO2, k∞ | 3.20 × 1013 | 0.00 | 0.00 |
| SO + O + M ⇄ SO2 + M, k0 | 1.21 × 1021 | −1.54 | 0.00 | |
| α = 0.8, T∗∗∗ = 1.0 × 10−30,T∗ = 1.0 × 10+30 | ||||
| 11.5 | SO + OH ⇄ SO2 + H | 1.10 × 1017 | −1.35 | 0.00 |
| 11.6 | SO + O2 ⇄ SO2 + O | 7.60 × 103 | 2.37 | 12.47 |
| 11.7 | SO2 + CO ⇄ SO + CO2 | 2.70 × 1012 | 0.00 | 202.03 |
| 11.8 | SO + NO2 ⇄ SO2 + NO | 8.40 × 1012 | 0.00 | 0.00 |
| 11.9 | SO + SO ⇄ SO2 + S | 2.00 × 1012 | 0.00 | 16.63 |
| SO3 Reactions | ||||
| 11.10b | SO2 + O ⇄ SO3, k∞ | 9.20 × 1010 | 0.00 | 9.98 |
| SO2 + O + M ⇄ SO3 + M, k0 | 4.00 × 1028 | −4.00 | 21.97 | |
| 11.11 | SO2 + OH ⇄ SO3 + H | 4.90 × 102 | 2.69 | 99.58 |
| 11.12 | SO3 + O ⇄ SO2 + O2 | 2.00 × 1012 | 0.00 | 83.14 |
| 11.13 | SO2 + NO2 ⇄ SO3 + NO | 6.30 × 1012 | 0.00 | 112.97 |
| 11.14 | SO3 + SO ⇄ SO2 + SO2 | 1.00 × 1012 | 0.00 | 41.57 |
| SH Reactions | ||||
| 11.15 | SH + O2 ⇄ SO + OH | 1.90 × 1013 | 0.00 | 74.83 |
| 11.16 | S + H2 ⇄ SH + H | 1.40 × 1014 | 0.00 | 80.65 |
| 11.17 | SH + O ⇄ SO + H | 1.00 × 1014 | 0.00 | 0.00 |
| 11.18 | SH + OH ⇄ S + H2O | 1.00 × 1013 | 0.00 | 0.00 |
| H2S Reactions | ||||
| 11.19b | H2S + M ⇄ S + H2 + M | 1.60 × 1024 | −2.61 | 372.50 |
| 11.20 | H2S + H ⇄ SH + H2 | 1.20 × 107 | 2.10 | 2.93 |
| 11.21 | H2S + O ⇄ SH + OH | 7.50 × 107 | 1.75 | 12.14 |
| 11.22 | H2S + OH ⇄ SH + H2O | 2.70 × 1012 | 0.00 | 0.00 |
| 11.23 | H2S + S ⇄ SH + SH | 8.30 × 1013 | 0.00 | 30.76 |
| Table Continued | ||||

| A | n | E | ||
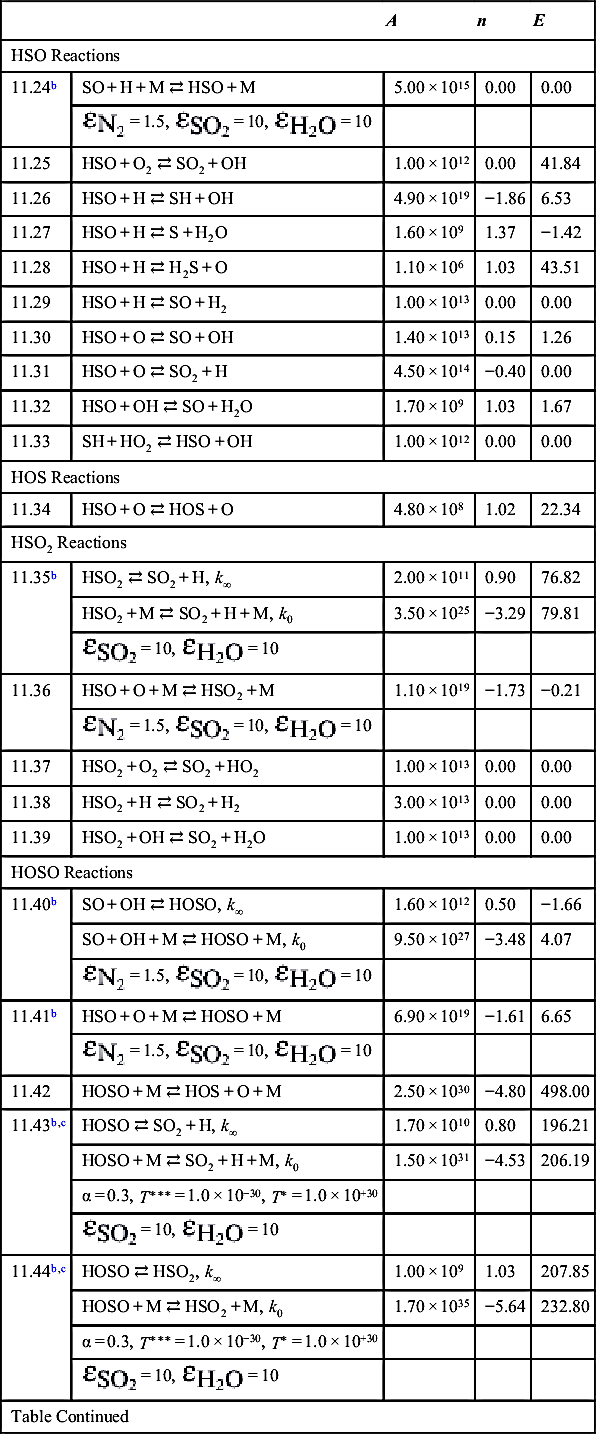
| HSO Reactions | ||||
| 11.24b | SO + H + M ⇄ HSO + M | 5.00 × 1015 | 0.00 | 0.00 |
| 11.25 | HSO + O2 ⇄ SO2 + OH | 1.00 × 1012 | 0.00 | 41.84 |
| 11.26 | HSO + H ⇄ SH + OH | 4.90 × 1019 | −1.86 | 6.53 |
| 11.27 | HSO + H ⇄ S + H2O | 1.60 × 109 | 1.37 | −1.42 |
| 11.28 | HSO + H ⇄ H2S + O | 1.10 × 106 | 1.03 | 43.51 |
| 11.29 | HSO + H ⇄ SO + H2 | 1.00 × 1013 | 0.00 | 0.00 |
| 11.30 | HSO + O ⇄ SO + OH | 1.40 × 1013 | 0.15 | 1.26 |
| 11.31 | HSO + O ⇄ SO2 + H | 4.50 × 1014 | −0.40 | 0.00 |
| 11.32 | HSO + OH ⇄ SO + H2O | 1.70 × 109 | 1.03 | 1.67 |
| 11.33 | SH + HO2 ⇄ HSO + OH | 1.00 × 1012 | 0.00 | 0.00 |
| HOS Reactions | ||||
| 11.34 | HSO + O ⇄ HOS + O | 4.80 × 108 | 1.02 | 22.34 |
| HSO2 Reactions | ||||
| 11.35b | HSO2 ⇄ SO2 + H, k∞ | 2.00 × 1011 | 0.90 | 76.82 |
| HSO2 + M ⇄ SO2 + H + M, k0 | 3.50 × 1025 | −3.29 | 79.81 | |
| 11.36 | HSO + O + M ⇄ HSO2 + M | 1.10 × 1019 | −1.73 | −0.21 |
| 11.37 | HSO2 + O2 ⇄ SO2 + HO2 | 1.00 × 1013 | 0.00 | 0.00 |
| 11.38 | HSO2 + H ⇄ SO2 + H2 | 3.00 × 1013 | 0.00 | 0.00 |
| 11.39 | HSO2 + OH ⇄ SO2 + H2O | 1.00 × 1013 | 0.00 | 0.00 |
| HOSO Reactions | ||||
| 11.40b | SO + OH ⇄ HOSO, k∞ | 1.60 × 1012 | 0.50 | −1.66 |
| SO + OH + M ⇄ HOSO + M, k0 | 9.50 × 1027 | −3.48 | 4.07 | |
| 11.41b | HSO + O + M ⇄ HOSO + M | 6.90 × 1019 | −1.61 | 6.65 |
| 11.42 | HOSO + M ⇄ HOS + O + M | 2.50 × 1030 | −4.80 | 498.00 |
| 11.43b,c | HOSO ⇄ SO2 + H, k∞ | 1.70 × 1010 | 0.80 | 196.21 |
| HOSO + M ⇄ SO2 + H + M, k0 | 1.50 × 1031 | −4.53 | 206.19 | |
| α = 0.3, T∗∗∗ = 1.0 × 10−30, T∗ = 1.0 × 10+30 | ||||
| 11.44b,c | HOSO ⇄ HSO2, k∞ | 1.00 × 109 | 1.03 | 207.85 |
| HOSO + M ⇄ HSO2 + M, k0 | 1.70 × 1035 | −5.64 | 232.80 | |
| α = 0.3, T∗∗∗ = 1.0 × 10−30, T∗ = 1.0 × 10+30 | ||||
| Table Continued | ||||
| A | n | E | ||
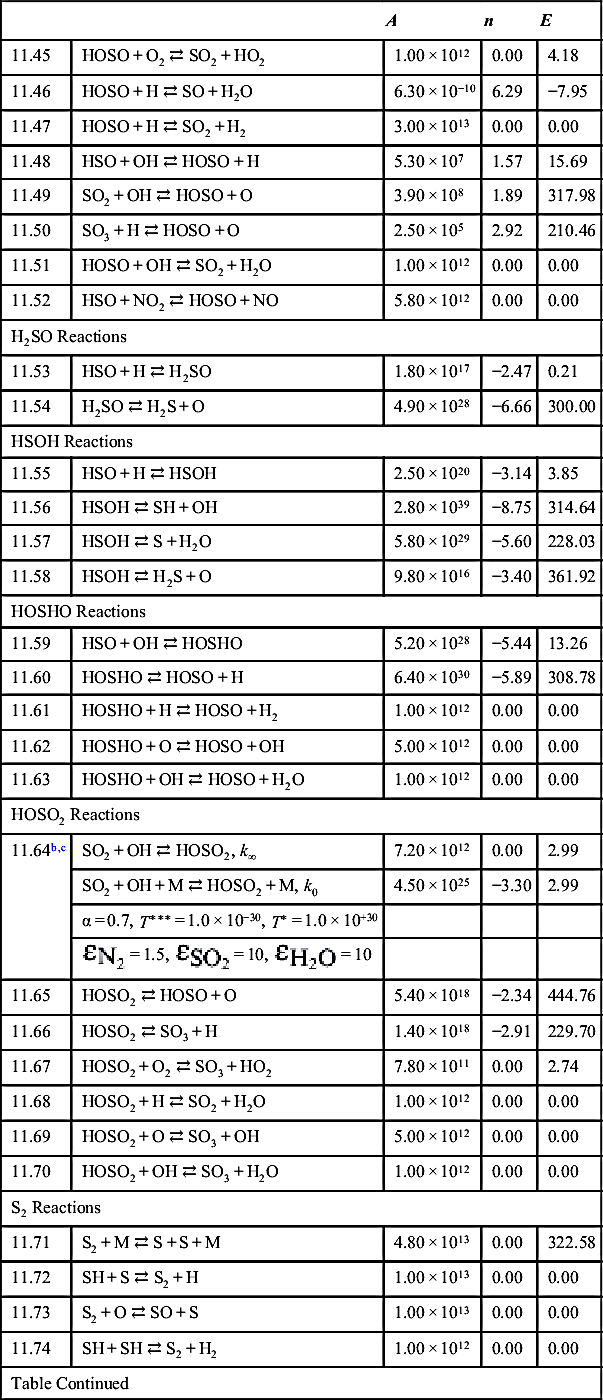
| 11.45 | HOSO + O2 ⇄ SO2 + HO2 | 1.00 × 1012 | 0.00 | 4.18 |
| 11.46 | HOSO + H ⇄ SO + H2O | 6.30 × 10−10 | 6.29 | −7.95 |
| 11.47 | HOSO + H ⇄ SO2 + H2 | 3.00 × 1013 | 0.00 | 0.00 |
| 11.48 | HSO + OH ⇄ HOSO + H | 5.30 × 107 | 1.57 | 15.69 |
| 11.49 | SO2 + OH ⇄ HOSO + O | 3.90 × 108 | 1.89 | 317.98 |
| 11.50 | SO3 + H ⇄ HOSO + O | 2.50 × 105 | 2.92 | 210.46 |
| 11.51 | HOSO + OH ⇄ SO2 + H2O | 1.00 × 1012 | 0.00 | 0.00 |
| 11.52 | HSO + NO2 ⇄ HOSO + NO | 5.80 × 1012 | 0.00 | 0.00 |
| H2SO Reactions | ||||
| 11.53 | HSO + H ⇄ H2SO | 1.80 × 1017 | −2.47 | 0.21 |
| 11.54 | H2SO ⇄ H2S + O | 4.90 × 1028 | −6.66 | 300.00 |
| HSOH Reactions | ||||
| 11.55 | HSO + H ⇄ HSOH | 2.50 × 1020 | −3.14 | 3.85 |
| 11.56 | HSOH ⇄ SH + OH | 2.80 × 1039 | −8.75 | 314.64 |
| 11.57 | HSOH ⇄ S + H2O | 5.80 × 1029 | −5.60 | 228.03 |
| 11.58 | HSOH ⇄ H2S + O | 9.80 × 1016 | −3.40 | 361.92 |
| HOSHO Reactions | ||||
| 11.59 | HSO + OH ⇄ HOSHO | 5.20 × 1028 | −5.44 | 13.26 |
| 11.60 | HOSHO ⇄ HOSO + H | 6.40 × 1030 | −5.89 | 308.78 |
| 11.61 | HOSHO + H ⇄ HOSO + H2 | 1.00 × 1012 | 0.00 | 0.00 |
| 11.62 | HOSHO + O ⇄ HOSO + OH | 5.00 × 1012 | 0.00 | 0.00 |
| 11.63 | HOSHO + OH ⇄ HOSO + H2O | 1.00 × 1012 | 0.00 | 0.00 |
| HOSO2 Reactions | ||||
| 11.64b,c | SO2 + OH ⇄ HOSO2, k∞ | 7.20 × 1012 | 0.00 | 2.99 |
| SO2 + OH + M ⇄ HOSO2 + M, k0 | 4.50 × 1025 | −3.30 | 2.99 | |
| α = 0.7, T∗∗∗ = 1.0 × 10−30, T∗ = 1.0 × 10+30 | ||||
| 11.65 | HOSO2 ⇄ HOSO + O | 5.40 × 1018 | −2.34 | 444.76 |
| 11.66 | HOSO2 ⇄ SO3 + H | 1.40 × 1018 | −2.91 | 229.70 |
| 11.67 | HOSO2 + O2 ⇄ SO3 + HO2 | 7.80 × 1011 | 0.00 | 2.74 |
| 11.68 | HOSO2 + H ⇄ SO2 + H2O | 1.00 × 1012 | 0.00 | 0.00 |
| 11.69 | HOSO2 + O ⇄ SO3 + OH | 5.00 × 1012 | 0.00 | 0.00 |
| 11.70 | HOSO2 + OH ⇄ SO3 + H2O | 1.00 × 1012 | 0.00 | 0.00 |
| S2 Reactions | ||||
| 11.71 | S2 + M ⇄ S + S + M | 4.80 × 1013 | 0.00 | 322.58 |
| 11.72 | SH + S ⇄ S2 + H | 1.00 × 1013 | 0.00 | 0.00 |
| 11.73 | S2 + O ⇄ SO + S | 1.00 × 1013 | 0.00 | 0.00 |
| 11.74 | SH + SH ⇄ S2 + H2 | 1.00 × 1012 | 0.00 | 0.00 |
| Table Continued | ||||
| A | n | E | ||
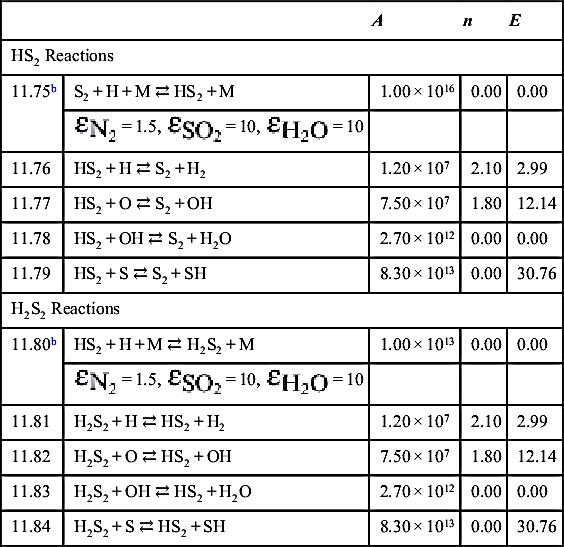
| HS2 Reactions | ||||
| 11.75b | S2 + H + M ⇄ HS2 + M | 1.00 × 1016 | 0.00 | 0.00 |
| 11.76 | HS2 + H ⇄ S2 + H2 | 1.20 × 107 | 2.10 | 2.99 |
| 11.77 | HS2 + O ⇄ S2 + OH | 7.50 × 107 | 1.80 | 12.14 |
| 11.78 | HS2 + OH ⇄ S2 + H2O | 2.70 × 1012 | 0.00 | 0.00 |
| 11.79 | HS2 + S ⇄ S2 + SH | 8.30 × 1013 | 0.00 | 30.76 |
| H2S2 Reactions | ||||
| 11.80b | HS2 + H + M ⇄ H2S2 + M | 1.00 × 1013 | 0.00 | 0.00 |
| 11.81 | H2S2 + H ⇄ HS2 + H2 | 1.20 × 107 | 2.10 | 2.99 |
| 11.82 | H2S2 + O ⇄ HS2 + OH | 7.50 × 107 | 1.80 | 12.14 |
| 11.83 | H2S2 + OH ⇄ HS2 + H2O | 2.70 × 1012 | 0.00 | 0.00 |
| 11.84 | H2S2 + S ⇄ HS2 + SH | 8.30 × 1013 | 0.00 | 30.76 |
The combustion chemistry group at Lawrence Livermore National Laboratory (LLNL) has available detailed mechanisms for hydrogen and various hydrocarbons (e.g., ethanol, dimethyl ether, dimethyl carbonate, CH4, C2H4, C2H6, C3H6, C3H8, nC4H10, methyl butanoate, methyl formate, heptane, iso-octane, and cyclohexane) (http://www-pls.llnl.gov/?url=science_and_technology-chemistry-combustion).
Other examples include the Leeds University and Eötvös University CH4/SOx/NOx mechanisms (http://garfield.chem.elte.hu/Combustion/methane.htm), the University of Galway Combustion Chemistry Center mechanisms (http://c3.nuigalway.ie/mechanisms.html), the Princeton University mechanisms (F.L. Dryer) (http://www.princeton.edu/∼combust/database/other.html), the University of California–San Diego mechanisms (http://web.eng.ucsd.edu/mae/groups/combustion/mechanism.html), and the University of Southern California Combustion Kinetics Laboratory mechanisms (http://ignis.usc.edu/Mechanisms/Modelrelease.html).
The NIST Chemical Kinetics Model Database Web site (http://kinetics.nist.gov/kinetics/index.jsp) is a good resource for chemical kinetic models, thermochemical property data, and elementary rate coefficients. The book Gas-Phase Combustion Chemistry, edited by W.C. Gardiner, Jr. (Springer-Verlag, New York, NY, 1999), also lists many detailed mechanisms for different fuels that are available in technical papers and from the Internet.
Critical reviews of reaction rate data are constantly appearing in the literature and are an important source for mechanism construction. Some examples of reaction rate constant reviews are given below.
1. Baulch DL, Bowman CT, Cobos CJ, Cox RA, Just Th, Kerr JA, Pilling MJ, Stocker D, Troe J, Tsang W, Walker RW, Warnatz J. Evaluated kinetic data for combustion modeling: supplement II. J Phys Chem Ref Data 2005;34:757. Baulch DL, Cobos C, Cox RA, Frank P, Hayman G, Just Th, Kerr JA, Murrells T, Murrells MJ, Pilling MJ, Troe J, Walker RW, Warnatz J. Supplement I. J Phys Chem Ref Data 1994;23:847. Baulch DL, Cobos CJ, Cox RA, Esser C, Frank P, Just Th, Kerr JA, Pilling MJ, Troe J, Walker RW, Warnatz J. Evaluated kinetic data for combustion modeling. J Phys Chem Ref Data 1992;21:411.
2. Gardiner Jr WC. Gas-phase combustion chemistry. NY: Springer-Verlag; 1999. Dean AM, Bozzelli JW. Chapter 2. Combustion chemistry of nitrogen. Hynes AJ, Wine PH. Chapter 3. Kinetics and mechanism of the oxidation of gaseous sulfur compounds. Senkan SM. Survey of rate coefficients in the C–H–Cl–O system. Burcat A, Gardiner Jr WC. Chapter 5. Ideal gas thermochemical data for combustion and air pollution use.
3. Tsang W, Herron JT. Chemical kinetic data base for propellant combustion I. Reactions involving NO, NO2, HNO, HNO2, HCN, and N2O. J Phys Chem Ref Data 1991;20:609. Tsang W. II. Reactions involving CN, NCO, and HNCO. J Phys Chem Ref Data 1992;21:750.
4. Tsang W, Hampson RF. Chemical kinetic data for combustion chemistry. Part 1. Methane and related compounds. J Phys Chem Ref Data 1986;15:1087. Tsang W. Part 2. Methanol. J Phys Chem Ref Data 1987;16:471. Tsang W. Part 3. Propane. J Phys Chem Ref Data 1988;17:887. Tsang W. Part 4. Isobutane. J Phys Chem Ref Data 1990;19:1. Part 5. Propene. J Phys Chem Ref Data 1991;20:221.
6. Cohen N, Westberg KR. Chemical kinetic data sheets for high temperature reactions. Part I. J Phys Chem Ref Data 1983;12:531. Part II. J Phys Chem Ref Data 1991;20:1211.
7. Atkinson R, Baulch DL, Cox RA, Hampson Jr RF, Kerr JA, Rossi MJ, Troe J. Evaluated kinetic and photochemical data for atmospheric chemistry. Supplement VI. IUPAC subcommittee on gas kinetic data evaluation for atmospheric chemistry. J Phys Chem Ref Data 1997;26:1329. Atkinson R, Baulch DL, Cox RA, Hampson Jr RF, Kerr JA, Troe J. Supplement IV. IUPAC subcommittee on gas kinetic data evaluation for atmospheric chemistry. J Phys Chem Ref Data 1992;21:1125. Supplement III. J Phys Chem Ref Data 1989:18:881. Supplement II. J Phys Chem Ref Data 1984;13:1259. Supplement I. J Phys Chem Ref Data 1982;11:327. J Phys Chem Ref Data 1980;9:295.
In order to take advantage of the developing cyber infrastructure, the Process Information Model (PrIMe) has been introduced for developing predictive models of chemical reaction systems based on the scientific collaboratory paradigm. PrIMe makes use of advances in computer science that allow assembly and manipulation of large amounts of combustion chemistry data that may be distributed over different sources using Web-based computer networks. PrIMe consists of a data depository, a data library, and a set of computer-based tools for processing and assembling the data. More information can be found at http://primekinetics.org.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.
