Appendix E
Flammability limits in air
The data presented in Table E1 are for fuel gases and vapors and are taken almost exclusively from Zabetakis [U.S. Bur Mines Bulletin 627 (1965)]. The conditions are for the fuel−air mixture at 25 °C and 1 atm unless otherwise specified. As noted in the text, most fuels have a rich limit at approximately ϕ = 3.3 and a lean limit at approximately ϕ = 0.5. The fuels which vary most from the rich limit are those that are either very tightly bound as ammonia is or which can decompose as hydrazine or any monopropellant does. Additional sources of flammability limit data can be found in the Ignition Handbook by Babrauskas (Fire Science Publishers, Issaquah, WA 2003) and from Kuchta [U.S. Bur Mines Bulletin 680 (1985)].
There can also be a flammability limit associated with dust clouds. The flammability limits of combustible dusts are reported as the minimum explosion concentrations. The upper explosion limits for dust clouds have not been determined due to experimental difficulties. In the 14th edition of the Fire Protection Handbook (National Fire Protection Association (NFPA), Boston, MA, 1975), numerous results from the U.S. Bureau of Mines reports are listed. These results were obtained with dusts of 74 μm or smaller. It should be noted that variations in minimum explosive concentrations will occur with change in particle diameter, that is, the minimum explosive concentration is lowered as the diameter of the particle decreases. Other conditions that affect this limit are sample purity, oxygen concentration, strength of ignition source, turbulence, and uniformity of the dispersion. The NFPA tabulation is most extensive and includes data for dusts from agricultural materials, carbonaceous matter, chemicals, drugs, dyes, metals, pesticides, and various plastic resins and molding compounds. Except for metal dusts, it is rather remarkable that most materials have a minimum explosive concentration in the range 0.03–0.05 kg/m3. It should be noted, however, that the variation according to the specific compound can range from 0.01 to 0.50 kg/m3. For a specific value the reader should refer to the NFPA handbook.
Table E1
Flammability Limits of Fuel Gases and Vapors in Air at 25 °C and 1 atm
| Fuel | Lean Limit | Rich Limit | ||
| Vol. % | Vol. % | |||
| Acetal | 1.6 | 10 | ||
| Acetaldehyde | 4.0 | 0.52 | 6 | 0 |
| Acetic acid | 5.4 (100 °C) | |||
| Acetic anhydride | 2.7 (47 °C) | 10 (75 °C) | ||
| Acetanilide | 1.0 (calc) | |||
| Acetone | 2.6 | 0.52 | 13 | 2.6 |
| Acetophenone | 1.1 (calc) | |||
| Acetylacetone | 1.7 (calc) | |||
| Acetyl chloride | 5.0 (calc) | |||
| Acetylene | 2.5 | 100 | ||
| Acrolein | 2.8 | 31 | ||
| Acrylonitrile | 3.0 | |||
| Acetone cyanohydrin | 2.2 | 12 | ||
| Adipic acid | 1.6 (calc) | |||
| Aldol | 2.0 (calc) | |||
| Allyl alcohol | 2.5 | 18 | ||
| Allyl amine | 2.2 | 22 | ||
| Allyl bromide | 2.7 (calc) | |||
| Allyl chloride | 2.9 | |||
| o-Aminodiphenyl | 0.66 | 4.1 | ||
| Ammonia | 15 | 0.69 [0.63] | 28 | 1.3 [1.4] |
| n-Amyl acetate | 1.0 (100 °C) | 0.51 | 7.1 (100 °C) | 3.3 |
| n-Amyl alcohol | 1.4 (100 °C) | 0.51 | 10 (100 °C) | |
| t-Amyl alcohol | 1.4 (calc) | |||
| n-Amyl chloride | 1.6 (50 °C) | 8.6 (100 °C) | ||
| t-Amyl chloride | 1.5 (85 °C) | |||
| n-Amyl ether | 0.7 (calc) | |||
| Amyl nitrite | 1.0 (calc) | |||
| n-Amyl propionate | 1.0 (calc) | |||
| Amylene | 1.4 | 8.7 | ||
| Aniline | 1.2 (140 °C) | 8.3 (140 °C) | ||
| Anthracene | 0.65 (calc) | |||
| n-Amyl nitrate | 1.1 | |||
| Benzene | 1.3 (100 °C) | 0.48 | 7.9 (100 °C) | 2.9 |
| Benzyl benzoate | 0.7 (calc) | |||
| Benzyl chloride | 1.2 (calc) | |||
| Bicyclohexyl | 0.65 (100 °C) | 5.1 (150 °C) | ||
| Biphenyl | 0.70 (110 °C) | |||
| Table Continued | ||||
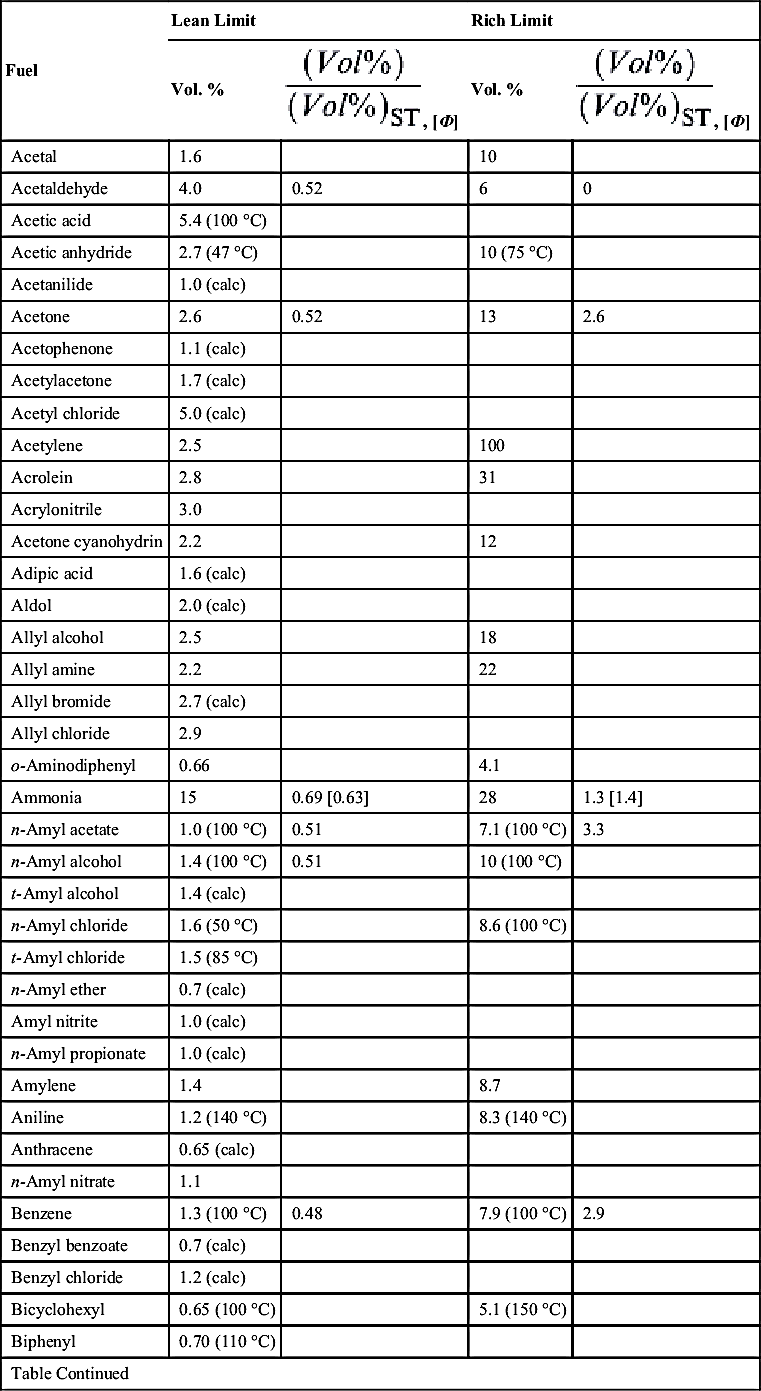
| Fuel | Lean Limit | Rich Limit | ||
| Vol. % | Vol. % | |||
| 2-Biphenyl amine | 0.8 (calc) | |||
| Bromobenzene | 1.6 (calc) | |||
| Butadiene (1,3) | 2.0 | 0.54 | 12 | 3.3 |
| n-Butane | 1.8 | 0.58 [0.57] | 8.4 | 2.7 [2.8] |
| 1,3-Butandiol | 1.9 (calc) | |||
| Butene-1 | 1.6 | 0.50 | 10 | 2.9 |
| Butene-2 | 1.7 | 0.53 | 9.7 | 2.9 |
| n-Butyl acetate | 1.4 (50 °C) | 0.55 | 8.0 (100 °C) | 3.1 |
| n-Butyl alcohol | 1.7 (100 °C) | 0.5 | 12 (100 °C) | |
| s-Butyl alcohol | 1.7 (100 °C) | 9.8 (100 °C) | ||
| t-Butyl alcohol | 1.9 (100 °C) | 9.0 (100 °C) | ||
| t-Butyl amine | 1.7 (100 °C) | 8.9 (100 °C) | ||
| n-Butyl benzene | 0.82 (100 °C) | 5.8 (100 °C) | ||
| s-Butyl benzene | 0.77 (100 °C) | 5.8 (100 °C) | ||
| t-Butyl benzene | 0.77 (100 °C) | 5.8 (100 °C) | ||
| n-Butyl bromide | 2.5 (100 °C) | |||
| Butyl cellosolve | 1.1 (150 °C) | 11 (175 °C) | ||
| n-Butyl chloride | 1.8 | 10 (100 °C) | ||
| n-Butyl formate | 1.7 | 0.54 | 8.2 | 2.6 |
| n-Butyl stearate | 0.3 (calc) | |||
| Butyric acid | 2.1 (calc) | |||
| γ-Butyrolactone | 2.0 (150 °C) | |||
| Carbon disulfide | 1.3 | 0.2 | 50 | 7.7 |
| Carbon monoxide | 12.5 | 74 | ||
| Chlorobenzene | 1.4 | |||
| m-Cresol | 1.1 (150 °C) | |||
| Crotonaldehyde | 2.1 | 16 (60 °C) | ||
| Cumene | 0.88 (100 °C) | 0.51 | 6.5 (100 °C) | 3.8 |
| Cyanogen | 6.6 | |||
| Cyclobutane | 1.8 (calc) | 0.56 | ||
| Cycloheptane | 1.1 (calc) | 0.56 | 6.7 (calc) | 3.4 |
| Cyclohexane | 1.3 | 0.57 | 7.8 | 3.4 |
| Cyclohexanol | 1.2 (calc) | |||
| Cyclohexene | 1.2 (100 °C) | |||
| Cyclohexyl acetate | 1.0 (calc) | |||
| Cyclopentane | 1.5 (calc) | 0.55 | ||
| Cyclopropane | 2.4 | 0.54 | 10.4 | 2.3 |
| Cymene | 0.85 (100 °C) | 0.56 | 6.5 (100 °C) | 3.6 |
| Table Continued | ||||
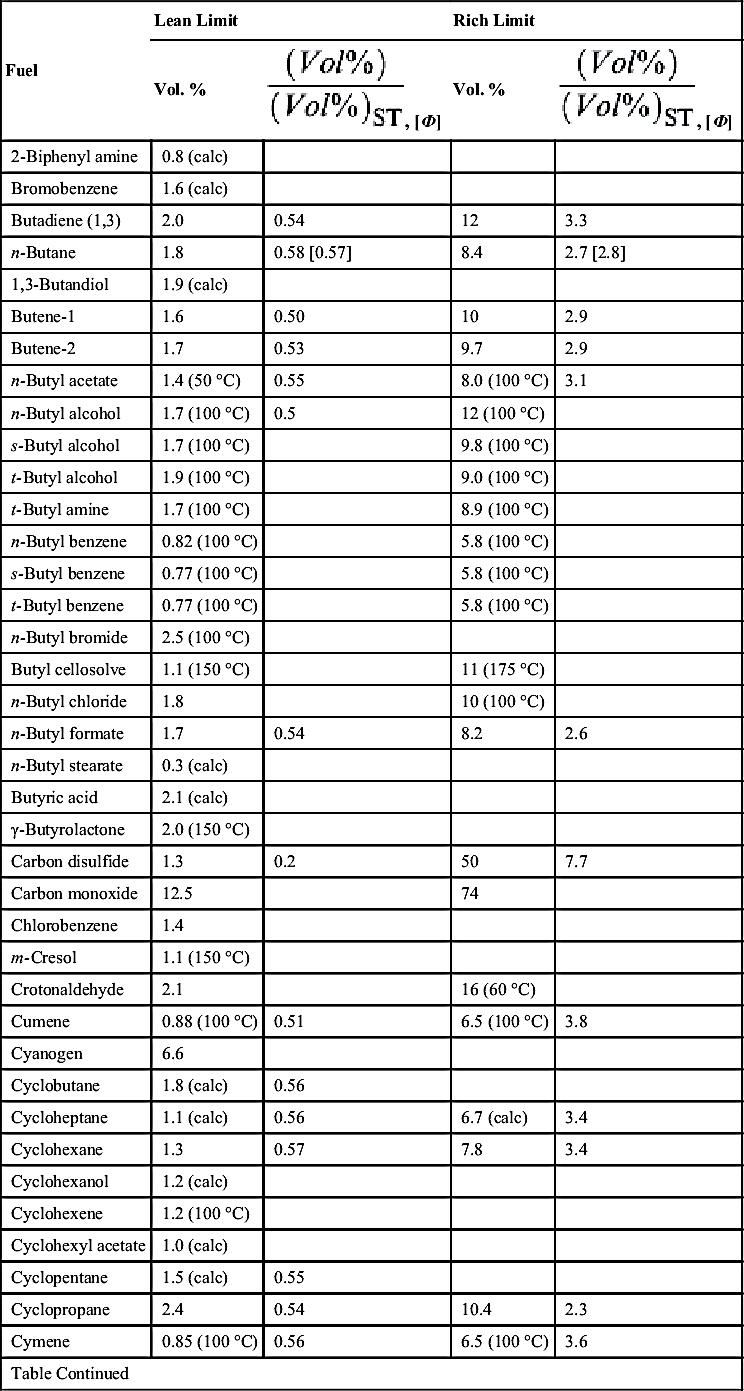
| Fuel | Lean Limit | Rich Limit | ||
| Vol. % | Vol. % | |||
| Decaborane | 0.2 (calc) | 0.11 | ||
| Decalin | 0.74 (100 °C) | 4.9 (100 °C) | ||
| n-Decane | 0.75 (53 °C) | 0.56 | 5.6 (86 °C) | 4.2 |
| Deuterium | 4.9 | 75 | ||
| Diborane | 0.8 | 0.12 | 88 | 13.5 |
| Diethyl amine | 1.8 | 10 | ||
| Diethyl aniline | 0.8 (calc) | |||
| 1,4-Diethyl benzene | 0.8 (100 °C) | |||
| Diethyl cyclohexane | 0.75 | |||
| Diethyl ether | 1.9 | 0.56 | 36 | 11 |
| 3,3-Diethyl pentane | 0.7 (100 °C) | |||
| Diethyl ketone | 1.6 (calc) | 0.55 | ||
| Diisobutyl carbinol | 0.82 (100 °C) | 6.1 (175 °C) | ||
| Diisobutyl ketone | 0.79 (100 °C) | 6.2 (100 °C) | ||
| Diisopropyl ether | 1.4 | 0.57 | 7.9 | 3.5 |
| Dimethyl amine | 2.8 | |||
| 2,2-Dimethyl butane | 1.2 | 7.0 | ||
| 2,3-Dimethyl butane | 1.2 | 7.0 | ||
| Dimethyl decalin | 0.69 (100 °C) | 5.3 (110 °C) | ||
| Dimethyl dichlorosilane | 3.4 | |||
| Dimethyl ether | 3.4 | 0.52 | 27 | 4.1 |
| N, N-Dimethyl formamide | 1.8 (100 °C) | 14 (100 °C) | ||
| 2,3-Dimethyl pentane | 1.1 | 6.8 | ||
| 2,2-Dimethyl propane | 1.4 | 7.5 | ||
| Dimethyl sulfide | 2.2 | 0.50 | 20 | 4.5 |
| Dioxane | 2.0 | 22 | ||
| Dipentene | 0.75 (150 °C) | 6.1 (150 °C) | ||
| Diphenylamine | 0.7 (calc) | |||
| Diphenyl ether | 0.8 (calc) | |||
| Diphenyl methane | 0.7 (calc) | |||
| Divinyl ether | 1.7 | 0.42 | 27 | 6.7 |
| n-Dodecane | 0.60 (calc) | 0.54 | ||
| Ethane | 3.0 | 0.53 [0.52] | 12.4 | 2.2 [2.4] |
| Ethyl acetate | 2.2 | 0.55 | 11 | 2.7 |
| Ethyl alcohol | 3.3 | 0.5 | 19 (60 °C) | 2.9 |
| Ethyl amine | 3.5 | |||
| Ethyl benzene | 1.0 (100 °C) | 0.51 | 6.7 (100 °C) | 3.4 |
| Table Continued | ||||

| Fuel | Lean Limit | Rich Limit | ||
| Vol. % | Vol. % | |||
| Ethyl chloride | 3.8 | |||
| Ethyl cyclobutane | 1.2 | 0.53 | 7.7 | 3.4 |
| Ethyl cyclohexane | 0.95 (130 °C) | 0.56 | 6.6 (130 °C) | 3.9 |
| Ethyl cyclopentane | 1.1 | 0.56 | 6.7 (0.5 atm) | 3.4 |
| Ethyl formate | 2.8 | 0.50 | 16 | 2.8 |
| Ethyl lactate | 1.5 | |||
| Ethyl mercaptan | 2.8 | 0.63 | 18 | 4.4 |
| Ethyl nitrate | 4.0 | |||
| Ethyl nitrite | 3.0 | 50 | ||
| Ethyl propionate | 1.8 | 0.58 | 11 | 3.5 |
| Ethyl propyl ether | 1.7 | 0.62 | 9 | 3.3 |
| Ethylene | 2.7 | 0.41 [0.40] | 36 | 5.5 [8.0] |
| Ethyleneimine | 3.6 | 46 | ||
| Ethylene glycol | 3.5 (calc) | |||
| Ethylene oxide | 3.6 | 100 | ||
| Furfural alcohol | 1.8 (72 °C) | 16 (117 °C) | ||
| Gasoline (100/130) | 1.3 | 7.1 | ||
| Gasoline (115/145) | 1.2 | 7.1 | ||
| n-Heptane | 1.05 | 0.56 | 6.7 | 3.6 |
| n-Hexadecane | 0.43 (calc) | 0.51 | ||
| n-Hexane | 1.2 | 0.56 | 7.4 | 3.4 |
| n-Hexyl alcohol | 1.2 (100°C) | 0.53 | ||
| n-Hexyl ether | 0.6 (calc) | |||
| Hydrazine | 4.7 | 0.27 | 100 | 5.8 |
| Hydrogen | 4.0 | 0.14 [0.10] | 75 | 2.54 [7.14] |
| Hydrogen cyanide | 5.6 | 40 | ||
| Hydrogen sulfide | 4.0 | 0.33 | 44 | 3.6 |
| Isoamyl acetate | 1.1 (100 °C) | 7.0 (100 °C) | ||
| Isoamyl alcohol | 1.4 (100 °C) | 9.0 (100 °C) | ||
| Isobutane | 1.8 | 8.4 | ||
| Isobutyl alcohol | 1.7 (100 °C) | 11 (100 °C) | ||
| Isobutyl benzene | 0.82 (100 °C) | 6.0 (175 °C) | ||
| Isobutyl formate | 2.0 | 8.9 | ||
| Isobutylene | 1.8 | 0.53 | 9.6 | 2.8 |
| Isopentane | 1.4 | |||
| Isophorone | 0.84 | |||
| Isopropylacetate | 1.7 (calc) | |||
| Isopropyl alcohol | 2.2 | |||
| Isopropyl biphenyl | 0.6 (calc) | |||
| Table Continued | ||||
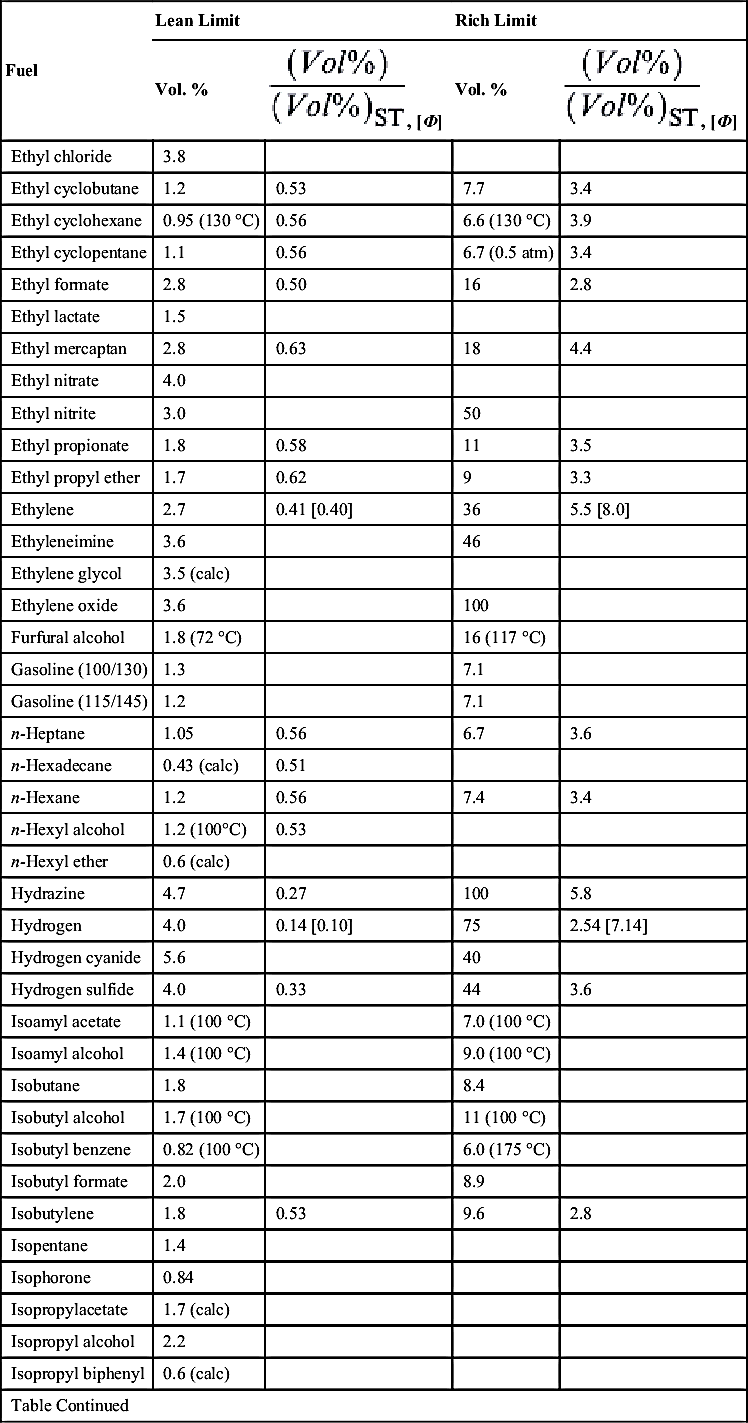
| Fuel | Lean Limit | Rich Limit | ||
| Vol. % | Vol. % | |||
| Jet fuel (JP-4) | 1.3 | 8 | ||
| Methane | 5.0 | 0.53 [0.50] | 15.0 | 1.6 [1.7] |
| Methyl acetate | 3.2 | 0.57 | 16 | 2.8 |
| Methyl acetylene | 1.7 | |||
| Methyl alcohol | 6.7 | 0.55 [0.51] | 36 (60 °C) | 2.9 [4.0] |
| Methylamine | 4.2 (calc) | |||
| Methyl bromide | 10 | 15 | ||
| 3-Methyl-1-butene | 1.5 | 0.55 | 9.1 | 3.3 |
| Methyl butyl ketone | 1.2 (50 °C) | 0.58 | 8.0 (100 °C) | 3.3 |
| Methyl cellosolve | 2.5 (125 °C) | 20 (140 °C) | ||
| Methyl cellosolve acetate | 1.7 (150 °C) | |||
| Methyl ethyl ether | 2.2 (calc) | |||
| Methyl chloride | 7 (calc) | |||
| Methyl cyclohexane | 1.1 | 0.56 | 6.7 (calc) | 3.4 |
| Methyl cyclopentadiene | 1.3 (100 °C) | 7.6 (100 °C) | ||
| Methyl ethyl ketone | 1.9 | 0.52 | 10 | 2.7 |
| Methyl formate | 5.0 | 0.53 | 23 | 2.4 |
| Methyl cycolhexanol | 1.0 (calc) | |||
| Methyl isobutyl carbinol | 1.2 (calc) | |||
| Methyl isopropenyl ketone | 1.8 (50 °C) | 9.0 (50 °C) | ||
| Methyl lactate | 2.2 (100 °C) | |||
| Methyl mercaptan | 3.9 | 0.60 | 22 | 3.4 |
| 1-Methyl naphthalene | 0.8 (calc) | |||
| 2-Methyl pentane | 1.2 (calc) | |||
| Methyl propionate | 2.4 | 0.60 | 13 | 3.2 |
| Methyl propyl ketone | 1.6 | 0.55 | 8.2 | 2.8 |
| Methyl styrene | 1.0 (calc) | |||
| Methyl vinyl ether | 2.6 | 39 | ||
| Monoisopropyl bicyclohexyl | 0.52 | 4.1 (200 °C) | ||
| 2-Monoisopropyl biphenyl | 0.53 (175 °C) | 3.2 (200 °C) | ||
| Monomethylhydrazine | 4 | 0.52 | ||
| Naphthalene | 0.88 (78 °C) | 5.9 (122 °C) | ||
| Nicotine | 0.75 (calc) | |||
| Nitroethane | 3.4 | |||
| Nitromethane | 7.3 | |||
| Table Continued | ||||
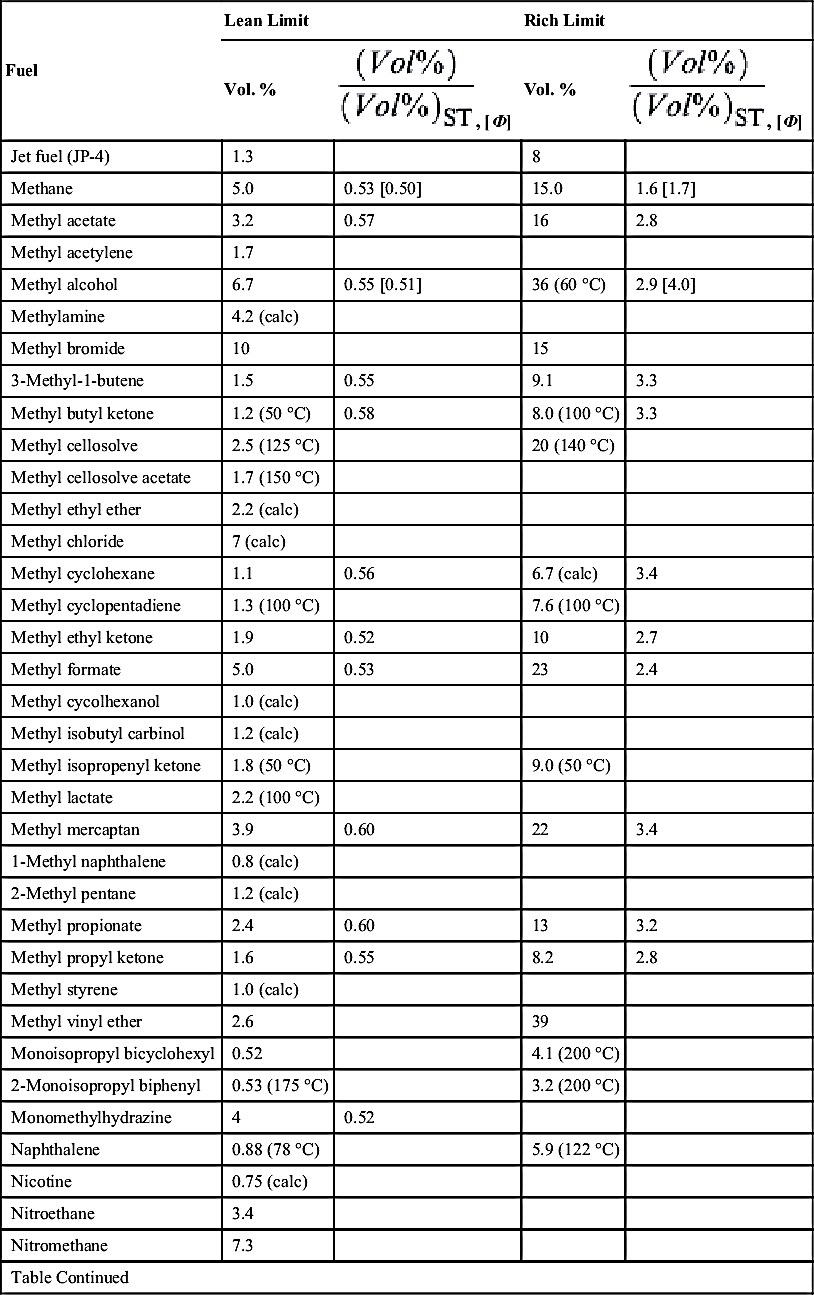
| Fuel | Lean Limit | Rich Limit | ||
| Vol. % | Vol. % | |||
| 1-Nitropropane | 2.2 | |||
| 2-Nitropropane | 2.5 | |||
| n-Nonane | 0.85 (43 °C) | 0.58 | ||
| n-Octane | 0.95 | 0.58 | ||
| Paraldehyde | 1.3 | 0.48 | ||
| Pentaborane | 0.42 | 0.12 | ||
| n-Pentadecane | 0.50 (calc) | 0.52 | ||
| n-Pentane | 1.4 | 0.55 | 7.8 | 3.1 |
| Phthalic anhydride | 1.2 (140 °C) | 9.2 (195 °C) | ||
| 3-Picoline | 1.4 (calc) | |||
| Pinane | 0.74 (160 °C) | 7.2 (160 °C) | ||
| Propadiene | 2.16 | |||
| Propane | 2.1 | 0.57 [0.56] | 9.5 | 2.5 [2.7] |
| 1,2-Propanediol | 2.5 (calc) | |||
| β-Propiolactone | 2.9 (75 °C) | |||
| Propionaldehyde | 2.9 | 0.59 | 17 | |
| n-Propyl acetate | 1.8 | 0.58 | 8 (90 °C) | 2.6 |
| n-Propyl alcohol | 2.2 (53 °C) | 0.49 | 14 (100 °C) | 3.2 |
| Propylamine | 2.0 | |||
| Propyl chloride | 2.4 (calc) | |||
| n-Propyl nitrate | 1.8 (125 °C) | 100 (125 °C) | ||
| Propylene | 2.4 | 0.54 [0.53] | 11 | 2.5 [2.7] |
| Propylene dichloride | 3.1 (calc) | |||
| Propylene glycol | 2.6 (96 °C) | |||
| Propylene oxide | 2.8 | 37 | ||
| Pyridine | 1.8 (60 °C) | 12 (70 °C) | ||
| Propargyl alcohol | 2.4 (50 °C) | |||
| Quinoline | 1.0 (calc) | |||
| Styrene | 1.1 (29 °C) | |||
| Sulfur | 2.0 (247 °C) | |||
| p-Terphenyl | 0.96 (calc) | |||
| Tetraborane | 0.4 (calc) | 0.11 | ||
| n-Tetradecane | 0.5 (calc) | 0.52 | ||
| Tetrahydrofurane | 2.0 | |||
| Tetralin | 0.84 (100 °C) | 5.0 (150 °C) | ||
| 2,2,3,3-Tetramethyl pentane | 0.8 | |||
| Toluene | 1.2 (100 °C) | 0.53 | 7.1 (100 °C) | 3.1 |
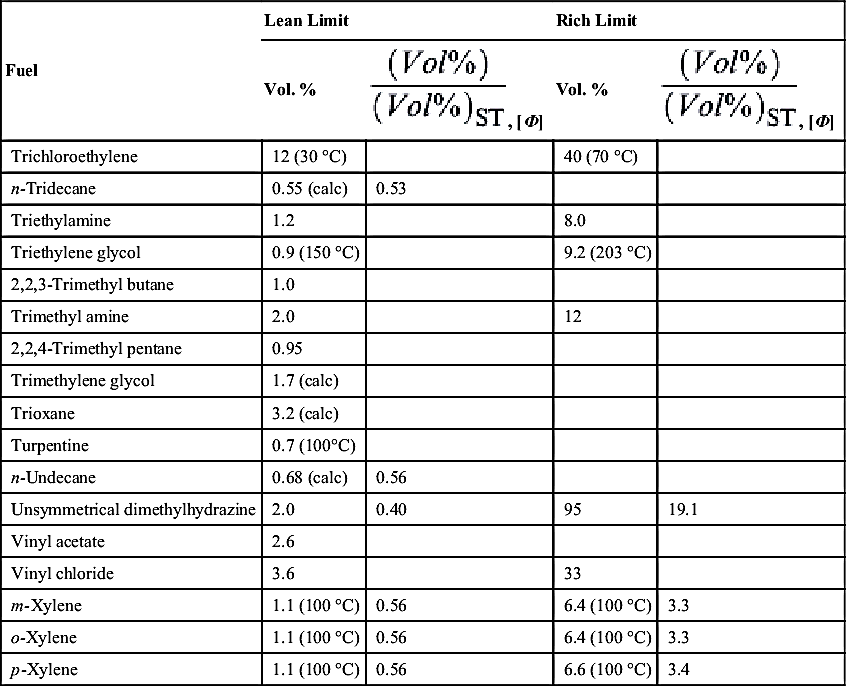
| Table Continued | ||||

| Fuel | Lean Limit | Rich Limit | ||
| Vol. % | Vol. % | |||
| Trichloroethylene | 12 (30 °C) | 40 (70 °C) | ||
| n-Tridecane | 0.55 (calc) | 0.53 | ||
| Triethylamine | 1.2 | 8.0 | ||
| Triethylene glycol | 0.9 (150 °C) | 9.2 (203 °C) | ||
| 2,2,3-Trimethyl butane | 1.0 | |||
| Trimethyl amine | 2.0 | 12 | ||
| 2,2,4-Trimethyl pentane | 0.95 | |||
| Trimethylene glycol | 1.7 (calc) | |||
| Trioxane | 3.2 (calc) | |||
| Turpentine | 0.7 (100°C) | |||
| n-Undecane | 0.68 (calc) | 0.56 | ||
| Unsymmetrical dimethylhydrazine | 2.0 | 0.40 | 95 | 19.1 |
| Vinyl acetate | 2.6 | |||
| Vinyl chloride | 3.6 | 33 | ||
| m-Xylene | 1.1 (100 °C) | 0.56 | 6.4 (100 °C) | 3.3 |
| o-Xylene | 1.1 (100 °C) | 0.56 | 6.4 (100 °C) | 3.3 |
| p-Xylene | 1.1 (100 °C) | 0.56 | 6.6 (100 °C) | 3.4 |
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.
