Appendix F
Laminar flame speeds
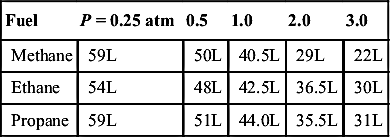
The compilation of laminar flame speed data given in Tables F1 and F2 is due to Gibbs and Calcote [J Chem Eng Data 4, 226 (1959)]. The reader is referred to the quoted paper for details on the chosen values. The data are for premixed fuel–air mixtures at 25 °C and 100 °C and 1 atm pressure. Additional data (obtained from a counter-flow, double-flame, burner configuration vs the Bunsen cone burner configuration) are included from Law [in Reduced Kinetic Mechanisms for Applications in Combustion Systems, N. Peters and B. Rogg, eds., Lecture Notes in Physics Monographs 15, 15–26 (1993). Springer-Verlag, NY] and Vagelopoulos, Egolfopoulos, and Law [Proceedings of the Combustion Institute 25, 1341–1347 (1994)]. The values of Law and coworkers are denoted by the letters L and L2 following the flame speed. Table F3 from Law reports flame speed data as a function of pressure.
Table F1
Burning Velocities of Various Fuels at 25 °C Air–fuel Temperature (0.31 mol% H2O in air). Burning Velocity S as a Function of Equivalence Ratio ϕ in cm/s
| Fuel | Φ = 0.7 | 0.8 | 0.9 | 1.0 | 1.1 | 1.2 | 1.3 | 1.4 | Smax | Φ at Smax |
| Saturated Hydrocarbons | ||||||||||
| Ethane | 30.6, | 36.0, | 40.6, | 44.5, | 47.3, | 47.3, | 44.4, | 37.4, | 47.6 | 1.14 |
| 22.0L | 29.0L | 36.5L | 42.5L | 43.0L | 42.5L | 40.0L | 27.5L | |||
| Propane | – | – | 42.3, | 45.6, | 46.2, | 42.4, | 34.3, | – | 46.4 | 1.06 |
| 24.0L, | 32.0L, | 39.5L, | 44.0L, | 45.0L, | 43.5L, | 37.0L, | 28.0L, | |||
| 23.0L2 | 30.0L2 | 37.0L2 | 39.0L2 | 41.0L2 | 40.5L2 | 33.5L2 | 25.0L2 | |||
| n-Butane | – | 38.0 | 42.6 | 44.8 | 44.2 | 41.2 | 34.4 | 25.0 | 44.9 | 1.03 |
| Methane | – | 30.0, | 38.3, | 43.4, | 44.7, | 39.8, | 31.2, | – | 44.8 | 1.08 |
| 20.5L, | 28.0L, | 36.0L, | 40.5L, | 42.0L, | 37.0L, | 27.0L, | 17.5L, | |||
| 17L2 | 25.0L2 | 33.0L2 | 38L2 | 38.5L2 | 34.0L2 | 24.0L2 | 13.5L2 | |||
| n-Pentane | – | 35.0 | 40.5 | 42.7 | 42.7 | 39.3 | 33.9 | – | 43.0 | 1.05 |
| n-Heptane | – | 37.0 | 39.8 | 42.2 | 42.0 | 35.5 | 29.4 | – | 42.8 | 1.05 |
| 2,2,4-Trimethylpentane | – | 37.5 | 40.2 | 41.0 | 37.2 | 31.0 | 23.5 | – | 41.0 | 0.98 |
| 2,2,3-Trimethylpentane | – | 37.8 | 39.5 | 40.1 | 39.5 | 36.2 | – | – | 40.1 | 1.00 |
| 2,2-Dimethylbutane | – | 33.5 | 38.3 | 39.9 | 37.0 | 33.5 | – | – | 40.0 | 0.98 |
| Isopentane | – | 33.0 | 37.6 | 39.8 | 38.4 | 33.4 | 24.8 | – | 39.9 | 1.01 |
| 2,2-Dimethylpropane | – | – | 31.0 | 34.8 | 36.0 | 35.2 | 33.5 | 31.2 | 36.0 | 1.10 |
| Unsaturated Hydrocarbons | ||||||||||
| Acetylene | – | 107, | 130 | 144, | 151 | 154, | 154 | 152, | 155 | 1.25 |
| – | 107L | – | 136L | – | 151L | – | 155L | |||
| Ethylene | 37.0, | 50.0, | 60.0, | 68.0, | 73.0, | 72.0, | 66.5, | 60.0, | 73.5 | 1.13 |
| 37.0L | 48.0L | 60.0L | 66.0L | 70.0L | 72.0L | 71.0L | 65.0L | |||
| Propylene | – | 62.0 | 66.6 | 70.2 | 72.2 | 71.2 | 61.0 | – | 72.5 | 1.14 |
| Table Continued | ||||||||||

| Fuel | Φ = 0.7 | 0.8 | 0.9 | 1.0 | 1.1 | 1.2 | 1.3 | 1.4 | Smax | Φ at Smax |
| 1,3-Butadiene | – | – | 42.6 | 49.6 | 55.0 | 57.0 | 56.9 | 55.4 | 57.2 | 1.23 |
| n-1-Heptene | – | 46.8 | 50.7 | 52.3 | 50.9 | 47.4 | 41.6 | – | 52.3 | 1.00 |
| Propylene | – | – | 48.4 | 51.2 | 49.9 | 46.4 | 40.8 | – | 51.2 | 1.00 |
| n-2-Pentene | – | 35.1 | 42.6 | 47.8 | 46.9 | 42.6 | 34.9 | – | 48.0 | 1.03 |
| 2,2,4-Trimethyl-3-pentene | – | 34.6 | 41.3 | 42.2 | 37.4 | 33.0 | – | – | 42.5 | 0.98 |
| Substituted Alkyls | ||||||||||
| Methanol | 34.5, | 42.0, | 48.0, | 50.2, | 47.5, | 44.4, | 42.2, | 50.4 | 1.08 | |
| At 45 °C | 21.5L | 31.0L | 37.5L | 48.0L | 54.0L | 53.5L | 48.0L | 42.0L | ||
| Isopropyl alcohol | – | 34.4 | 39.2 | 41.3 | 40.6 | 38.2 | 36.0 | 34.2 | 41.4 | 1.04 |
| Triethylamine | – | 32.5 | 36.7 | 38.5 | 38.7 | 36.2 | 28.6 | – | 38.8 | 1.06 |
| n-Butyl chloride | 24.0 | 30.7 | 33.8 | 34.5 | 32.5 | 26.9 | 20.0 | – | 34.5 | 1.00 |
| Allyl chloride | 30.6 | 33.0 | 33.7 | 32.4 | 29.6 | – | – | – | 33.8 | 0.89 |
| Isopropyl mercaptan | – | 30.0 | 33.5 | 33.0 | 26.6 | – | – | – | 33.8 | 0.94 |
| Ethylamine | – | 28.7 | 31.4 | 32.4 | 31.8 | 29.4 | 25.3 | – | 32.4 | 1.00 |
| Isopropylamine | – | 27.0 | 29.5 | 30.6 | 29.8 | 27.7 | – | – | 30.6 | 1.01 |
| n-Propyl chloride | – | 24.7 | 28.3 | 27.5 | 24.1 | – | – | – | 28.5 | 0.93 |
| Isopropyl chloride | – | 24.8 | 27.0 | 27.4 | 25.3 | – | – | – | 27.6 | 0.97 |
| n-Propyl bromide | No ignition | |||||||||
| Silanes | ||||||||||
| Tetramethylsilane | 39.5 | 49.5 | 57.3 | 58.2 | 57.7 | 54.5 | 47.5 | – | 58.2 | 1.01 |
| Trimethylethoxysilane | 34.7 | 41.0 | 47.4 | 50.3 | 46.5 | 41.0 | 35.0 | – | 50.3 | 1.00 |
| Aldehydes | ||||||||||
| Acrolein | 47.0 | 58.0 | 66.6 | 65.9 | 56.5 | – | – | – | 67.2 | 0.95 |
| Propionaldehyde | – | 37.5 | 44.3 | 49.0 | 49.5 | 46.0 | 41.6 | 37.2 | 50.0 | 1.06 |
| Acetaldehyde | – | 26.6 | 35.0 | 41.4 | 41.4 | 36.0 | 30.0 | – | 42.2 | 1.05 |
| Table Continued | ||||||||||

| Fuel | Φ = 0.7 | 0.8 | 0.9 | 1.0 | 1.1 | 1.2 | 1.3 | 1.4 | Smax | Φ at Smax |
| Ketones | ||||||||||
| Acetone | – | 40.4 | 44.2 | 42.6 | 38.2 | – | – | – | 44.4 | 0.93 |
| Methyl ethyl ketone | – | 36.0 | 42.0 | 43.3 | 41.5 | 37.7 | 33.2 | – | 43.4 | 0.99 |
| Esters | ||||||||||
| Vinyl acetate | 29.0 | 36.6 | 39.8 | 41.4 | 42.1 | 41.6 | 35.2 | – | 42.2 | 1.13 |
| Ethyl acetate | – | 30.7 | 35.2 | 37.0 | 35.6 | 30.0 | – | – | 37.0 | 1.00 |
| Ethers | ||||||||||
| Dimethyl ether | – | 44.8 | 47.6 | 48.4 | 47.5 | 45.4 | 42.6 | – | 48.6 | 0.99 |
| Diethyl ether | 30.6 | 37.0 | 43.4 | 48.0 | 47.6 | 40.4 | 32.0 | – | 48.2 | 1.05 |
| Dimethoxymethane | 32.5 | 38.2 | 43.2 | 46.6 | 48.0 | 46.6 | 43.3 | – | 48.0 | 1.10 |
| Diisopropyl ether | – | 30.7 | 35.5 | 38.3 | 38.6 | 36.0 | 31.2 | – | 38.9 | 1.06 |
| Thio Ethers | ||||||||||
| Dimethyl sulfide | – | 29.9 | 31.9 | 33.0 | 30.1 | 24.8 | – | – | 33.0 | 1.00 |
| Peroxides | ||||||||||
| Di-tert-butyl peroxide | – | 41.0 | 46.8 | 50.0 | 49.6 | 46.5 | 42.0 | 35.5 | 50.4 | 1.04 |
| Aromatic Compounds | ||||||||||
| Furan | 48.0 | 55.0 | 60.0 | 62.5 | 62.4 | 60.0 | – | – | 62.9 | 1.05 |
| Benzene | – | 39.4 | 45.6 | 47.6 | 44.8 | 40.2 | 35.6 | – | 47.6 | 1.00 |
| Thiophene | 33.8 | 37.4 | 40.6 | 43.0 | 42.2 | 37.2 | 24.6 | – | 43.2 | 1.03 |
| Table Continued | ||||||||||

| Fuel | Φ = 0.7 | 0.8 | 0.9 | 1.0 | 1.1 | 1.2 | 1.3 | 1.4 | Smax | Φ at Smax |
| Cyclic Compounds | ||||||||||
| Ethylene oxide | 57.2 | 70.7 | 83.0 | 88.8 | 89.5 | 87.2 | 81.0 | 73.0 | 89.5 | 1.07 |
| Butadiene monoxide | – | 6.6 | 47.4 | 57.8 | 64.0 | 66.9 | 66.8 | 64.5 | 67.1 | 1.24 |
| Propylene oxide | 41.6 | 53.3 | 62.6 | 66.5 | 66.4 | 62.5 | 53.8 | – | 67.0 | 1.05 |
| Dihydropyran | 39.0 | 45.7 | 51.0 | 54.5 | 55.6 | 52.6 | 44.3 | 32.0 | 55.7 | 1.08 |
| Cyclopropane | – | 40.6 | 49.0 | 54.2 | 55.6 | 53.5 | 44.0 | – | 55.6 | 1.10 |
| Tetrahydropyran | 44.8 | 51.0 | 53.6 | 51.5 | 42.3 | – | – | – | 53.7 | 0.93 |
| Tetrahydrofuran | – | – | 43.2 | 48.0 | 50.8 | 51.6 | 49.2 | 44.0 | 51.6 | 1.19 |
| Cyclopentadiene | 36.0 | 41.8 | 45.7 | 47.2 | 45.5 | 40.6 | 32.0 | – | 47.2 | 1.00 |
| Ethylenimine | – | 37.6 | 43.4 | 46.0 | 45.8 | 43.4 | 38.9 | – | 46.4 | 1.04 |
| Cyclopentane | 31.0 | 38.4 | 43.2 | 45.3 | 44.6 | 41.0 | 34.0 | – | 45.4 | 1.03 |
| Cyclohexane | – | – | 41.3 | 43.5 | 43.9 | 38.0 | — | – | 44.0 | 1.08 |
| Inorganic Compounds | ||||||||||
| Hydrogen | 102, | 120, | 145, | 170, | 204, | 245, | 213 | 290 | 325 | 1.80 |
| 124L | 150L | 187L | 210L | 230L | 245L | – | – | |||
| Carbon disulfide | 50.6 | 58.0 | 59.4 | 58.8 | 57.0 | 55.0 | 52.8 | 51.6 | 59.4 | 0.91 |
| Carbon monoxide | – | – | – | – | 28.5 | 32.0 | 34.8 | 38.0 | 52.0 | 2.05 |
| Hydrogen sulfide | 34.8 | 39.2 | 40.9 | 39.1 | 32.3 | – | – | – | 40.9 | 0.90 |

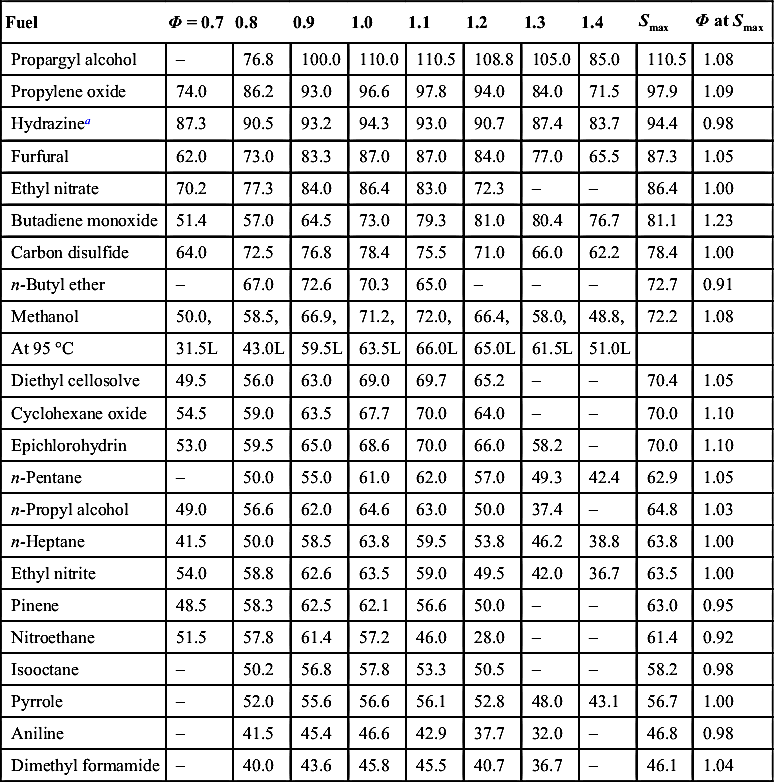
Table F2
Burning Velocities of Various Fuels at 100 °C Air–fuel Temperature (0.31 mol% H2O in air). Burning Velocity S as a Function of Equivalence Ratio ϕ in cm/s
| Fuel | Φ = 0.7 | 0.8 | 0.9 | 1.0 | 1.1 | 1.2 | 1.3 | 1.4 | Smax | Φ at Smax |
| Propargyl alcohol | – | 76.8 | 100.0 | 110.0 | 110.5 | 108.8 | 105.0 | 85.0 | 110.5 | 1.08 |
| Propylene oxide | 74.0 | 86.2 | 93.0 | 96.6 | 97.8 | 94.0 | 84.0 | 71.5 | 97.9 | 1.09 |
| Hydrazinea | 87.3 | 90.5 | 93.2 | 94.3 | 93.0 | 90.7 | 87.4 | 83.7 | 94.4 | 0.98 |
| Furfural | 62.0 | 73.0 | 83.3 | 87.0 | 87.0 | 84.0 | 77.0 | 65.5 | 87.3 | 1.05 |
| Ethyl nitrate | 70.2 | 77.3 | 84.0 | 86.4 | 83.0 | 72.3 | – | – | 86.4 | 1.00 |
| Butadiene monoxide | 51.4 | 57.0 | 64.5 | 73.0 | 79.3 | 81.0 | 80.4 | 76.7 | 81.1 | 1.23 |
| Carbon disulfide | 64.0 | 72.5 | 76.8 | 78.4 | 75.5 | 71.0 | 66.0 | 62.2 | 78.4 | 1.00 |
| n-Butyl ether | – | 67.0 | 72.6 | 70.3 | 65.0 | – | – | – | 72.7 | 0.91 |
| Methanol | 50.0, | 58.5, | 66.9, | 71.2, | 72.0, | 66.4, | 58.0, | 48.8, | 72.2 | 1.08 |
| At 95 °C | 31.5L | 43.0L | 59.5L | 63.5L | 66.0L | 65.0L | 61.5L | 51.0L | ||
| Diethyl cellosolve | 49.5 | 56.0 | 63.0 | 69.0 | 69.7 | 65.2 | – | – | 70.4 | 1.05 |
| Cyclohexane oxide | 54.5 | 59.0 | 63.5 | 67.7 | 70.0 | 64.0 | – | – | 70.0 | 1.10 |
| Epichlorohydrin | 53.0 | 59.5 | 65.0 | 68.6 | 70.0 | 66.0 | 58.2 | – | 70.0 | 1.10 |
| n-Pentane | – | 50.0 | 55.0 | 61.0 | 62.0 | 57.0 | 49.3 | 42.4 | 62.9 | 1.05 |
| n-Propyl alcohol | 49.0 | 56.6 | 62.0 | 64.6 | 63.0 | 50.0 | 37.4 | – | 64.8 | 1.03 |
| n-Heptane | 41.5 | 50.0 | 58.5 | 63.8 | 59.5 | 53.8 | 46.2 | 38.8 | 63.8 | 1.00 |
| Ethyl nitrite | 54.0 | 58.8 | 62.6 | 63.5 | 59.0 | 49.5 | 42.0 | 36.7 | 63.5 | 1.00 |
| Pinene | 48.5 | 58.3 | 62.5 | 62.1 | 56.6 | 50.0 | – | – | 63.0 | 0.95 |
| Nitroethane | 51.5 | 57.8 | 61.4 | 57.2 | 46.0 | 28.0 | – | – | 61.4 | 0.92 |
| Isooctane | – | 50.2 | 56.8 | 57.8 | 53.3 | 50.5 | – | – | 58.2 | 0.98 |
| Pyrrole | – | 52.0 | 55.6 | 56.6 | 56.1 | 52.8 | 48.0 | 43.1 | 56.7 | 1.00 |
| Aniline | – | 41.5 | 45.4 | 46.6 | 42.9 | 37.7 | 32.0 | – | 46.8 | 0.98 |
| Dimethyl formamide | – | 40.0 | 43.6 | 45.8 | 45.5 | 40.7 | 36.7 | – | 46.1 | 1.04 |
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.