Specific reaction rate constants
Table C1
H2/O2 Mechanisma
| A | n | E | ||
| H2–O2 Chain Reactions | ||||
| 1.1 | H + O2 ⇄ O + OH | 1.04 × 1014 | 0.00 | 64.06 |
| 1.2d | O + H2 ⇄ H + OH | 5.08 × 1012 | 0.00 | 33.26 |
| 8.79 × 1014 | 0.00 | 80.21 | ||
| 1.3 | OH + H2 ⇄ H + H2O | 2.16 × 108 | 1.51 | 14.35 |
| 1.4 | OH + OH ⇄ O + H2O | 3.34 × 104 | 2.42 | −80.76 |
| H2–O2 Dissociation/Recombination Reactions | ||||
| 1.5b | H2 + M ⇄ H + H + M | 4.58 × 1019 | −1.40 | 435.16 |
| H2 + Ar ⇄ H + H + Ar | 5.84 × 1018 | −1.10 | 435.16 | |
| 1.6b | O + O + M ⇄ O2 + M | 6.16 × 1015 | −0.50 | 0.00 |
| O + O + Ar ⇄ O2 + Ar | 1.89 × 1013 | 0.00 | −7.49 | |
| 1.7b | O + H + M ⇄ OH + M | 4.71 × 1018 | −1.00 | 0.00 |
| 1.8b | H2O + M ⇄ H + OH + M | 6.06 × 1027 | −3.32 | 505.45 |
| H2O + H2O ⇄ H + OH + H2O | 1.01 × 1026 | −2.44 | 502.94 | |
| HO2 Reactions | ||||
| 1.9c | H + O2 ⇄ HO2, k∞ | 4.65 × 1012 | 0.44 | 0.00 |
| H + O2 + M ⇄ HO2 + M, M = N2, k0 | 6.37 × 1020 | −1.72 | 2.18 | |
| α = 0.8, T∗∗∗ = 1.0 × 10−30, T∗ = 1.0 × 10+30 | ||||
| H + O2 + M ⇄ HO2 + M, M = Ar, k0 | 9.04 × 1019 | −1.50 | 2.06 | |
| α = 0.5, T∗∗∗ = 1.0 × 10−30, T∗ = 1.0 × 10+30 | ||||
| 1.10 | HO2 + H ⇄ H2 + O2 | 2.75 × 106 | 2.09 | −6.07 |
| 1.11 | HO2 + H ⇄ OH + OH | 7.08 × 1013 | 0.00 | 1.23 |
| 1.12 | HO2 + O ⇄ OH + O2 | 2.85 × 1010 | 1.00 | −3.03 |
| 1.13 | HO2 + OH ⇄ H2O + O2 | 2.89 × 1013 | 0.00 | −2.08 |
| Table Continued | ||||

| A | n | E | ||
| H2O2 Reactions | ||||
| 1.14d | HO2 + HO2 ⇄ H2O2 + O2 | 4.20 × 1014 | 0.00 | 50.21 |
| 1.30 × 1011 | 0.00 | −6.82 | ||
| 1.15c | H2O2 ⇄ OH + OH, k∞ | 2.00 × 1012 | 0.90 | 204.00 |
| H2O2 + M ⇄ OH + OH + M, k0 | 2.49 × 1024 | −2.30 | 204.00 | |
| α = 0.42, T∗∗∗ = 1.0 × 10−30, T∗ = 1.0 × 10+30 | ||||
| 1.16 | H2O2 + H ⇄ H2O + OH | 2.41 × 1013 | 0.00 | 16.61 |
| 1.17 | H2O2 + H ⇄ H2 + HO2 | 4.82 × 1013 | 0.00 | 33.26 |
| 1.18 | H2O2 + O ⇄ OH + HO2 | 9.55 × 106 | 2.00 | 16.61 |
| 1.19d | H2O2 + OH ⇄ H2O + HO2 | 1.74 × 1012 | 0.00 | 1.33 |
| 7.59 × 1013 | 0.00 | 30.42 | ||
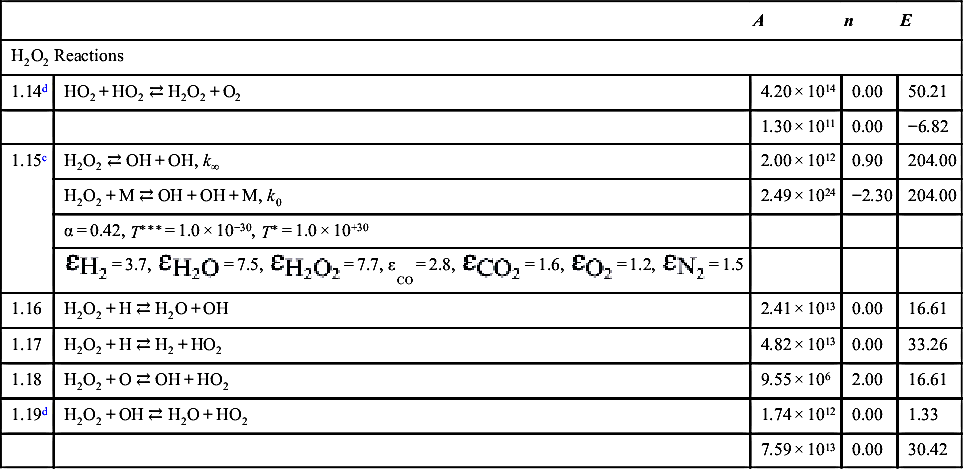
Table C2
CO/H2/O2 Mechanisma
| A | n | E | ||
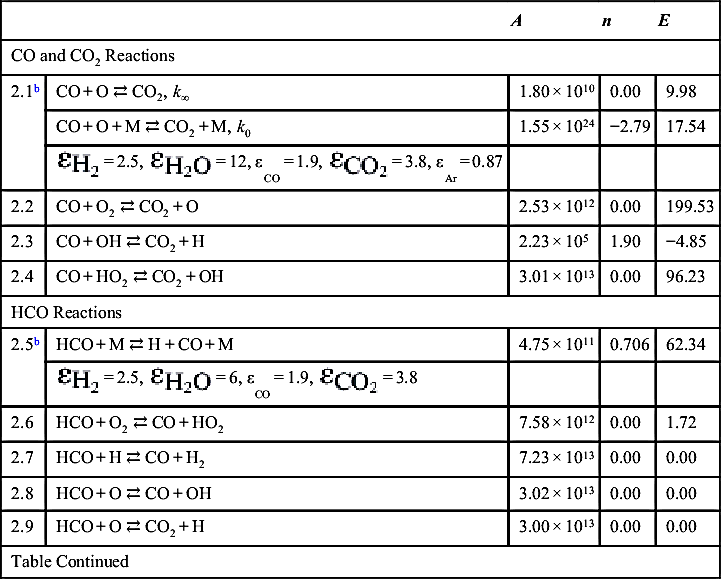
| CO and CO2 Reactions | ||||
| 2.1b | CO + O ⇄ CO2, k∞ | 1.80 × 1010 | 0.00 | 9.98 |
| CO + O + M ⇄ CO2 + M, k0 | 1.55 × 1024 | −2.79 | 17.54 | |
| 2.2 | CO + O2 ⇄ CO2 + O | 2.53 × 1012 | 0.00 | 199.53 |
| 2.3 | CO + OH ⇄ CO2 + H | 2.23 × 105 | 1.90 | −4.85 |
| 2.4 | CO + HO2 ⇄ CO2 + OH | 3.01 × 1013 | 0.00 | 96.23 |
| HCO Reactions | ||||
| 2.5b | HCO + M ⇄ H + CO + M | 4.75 × 1011 | 0.706 | 62.34 |
| 2.6 | HCO + O2 ⇄ CO + HO2 | 7.58 × 1012 | 0.00 | 1.72 |
| 2.7 | HCO + H ⇄ CO + H2 | 7.23 × 1013 | 0.00 | 0.00 |
| 2.8 | HCO + O ⇄ CO + OH | 3.02 × 1013 | 0.00 | 0.00 |
| 2.9 | HCO + O ⇄ CO2 + H | 3.00 × 1013 | 0.00 | 0.00 |
| Table Continued | ||||
| A | n | E | ||
| 2.10 | HCO + OH ⇄ CO + H2O | 3.02 × 1013 | 0.00 | 0.00 |
| 2.11 | HCO + HO2 ⇄ CO2 + OH + H | 3.00 × 1013 | 0.00 | 0.00 |
| 2.12 | HCO + HCO ⇄ H2 + CO + CO | 3.00 × 1012 | 0.00 | 0.00 |

Li J, Zhao Z, Kazakov A, Chaos M, Dryer FL, Scire Jr JJ. A comprehensive kinetic mechanism for CO, CH2O, and CH3OH combustion. Int J Chem Kinet 2007;39:109–36.
Table C3
CH2O/CO/H2/O2 Mechanisma
| A | n | E | ||
| CH2O Reactions | ||||
| 3.1b | CH2O + M ⇄ HCO + H + M | 3.30 × 1039 | −6.30 | 418.00 |
| 3.2b | CH2O + M ⇄ CO + H2 + M | 3.10 × 1045 | −8.00 | 408.00 |
| 3.3 | CH2O + H ⇄ HCO + H2 | 5.74 × 107 | 1.90 | 11.50 |
| 3.4 | CH2O + O ⇄ HCO + OH | 1.81 × 1013 | 0.00 | 12.89 |
| 3.5 | CH2O + OH ⇄ HCO + H2O | 3.43 × 109 | 1.20 | −1.88 |
| 3.6 | CH2O + O2 ⇄ HCO + HO2 | 1.23 × 106 | 3.00 | 217.60 |
| 3.7 | CH2O + HO2 ⇄ HCO + H2O2 | 4.11 × 104 | 2.50 | 42.72 |
| 3.8 | HCO + HCO ⇄ CH2O + CO | 3.00 × 1013 | 0.00 | 0.00 |

Table C4
CH3OH/CH2O/CO/H2/O2 Mechanisma,e
| A | n | E | ||
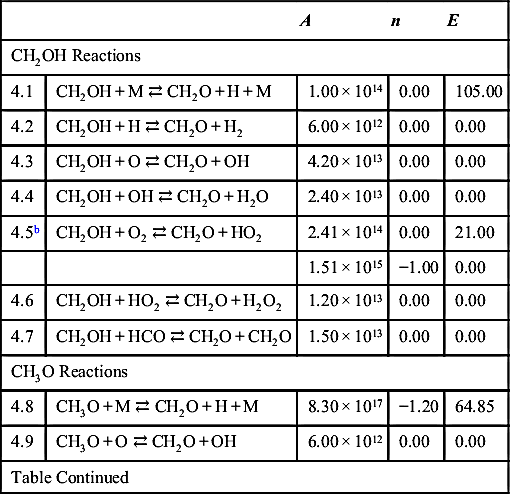
| CH2OH Reactions | ||||
| 4.1 | CH2OH + M ⇄ CH2O + H + M | 1.00 × 1014 | 0.00 | 105.00 |
| 4.2 | CH2OH + H ⇄ CH2O + H2 | 6.00 × 1012 | 0.00 | 0.00 |
| 4.3 | CH2OH + O ⇄ CH2O + OH | 4.20 × 1013 | 0.00 | 0.00 |
| 4.4 | CH2OH + OH ⇄ CH2O + H2O | 2.40 × 1013 | 0.00 | 0.00 |
| 4.5b | CH2OH + O2 ⇄ CH2O + HO2 | 2.41 × 1014 | 0.00 | 21.00 |
| 1.51 × 1015 | −1.00 | 0.00 | ||
| 4.6 | CH2OH + HO2 ⇄ CH2O + H2O2 | 1.20 × 1013 | 0.00 | 0.00 |
| 4.7 | CH2OH + HCO ⇄ CH2O + CH2O | 1.50 × 1013 | 0.00 | 0.00 |
| CH3O Reactions | ||||
| 4.8 | CH3O + M ⇄ CH2O + H + M | 8.30 × 1017 | −1.20 | 64.85 |
| 4.9 | CH3O + O ⇄ CH2O + OH | 6.00 × 1012 | 0.00 | 0.00 |
| Table Continued | ||||
| A | n | E | ||
| 4.10 | CH3O + OH ⇄ CH2O + H2O | 1.80 × 1013 | 0.00 | 0.00 |
| 4.11b | CH3O + O2 ⇄ CH2O + HO2 | 9.03 × 1013 | 0.00 | 50.12 |
| 2.20 × 1010 | 0.00 | 7.31 | ||
| 4.12 | CH3O + HO2 ⇄ CH2O + H2O2 | 3.00 × 1011 | 0.00 | 0.00 |
| CH3OH Reactions | ||||
| 4.13c,d | CH2OH + H ⇄ CH3OH, k∞ | 1.06 × 1012 | 0.50 | 0.36 |
| CH2OH + H + M ⇄ CH3OH + M, k0 | 4.36 × 1031 | −4.65 | 21.26 | |
| α = 0.6, T∗∗∗ = 1.0 × 102, T∗ = 9.0 × 104, T∗∗ = 1 × 104 | ||||
| 4.14c,d | CH3O + H ⇄ CH3OH, k∞ | 2.43 × 1012 | 0.50 | 0.21 |
| CH3O + H + M ⇄ CH3OH + M, k0 | 4.66 × 1041 | −7.44 | 58.91 | |
| α = 0.7, T∗∗∗ = 1.0 × 102, T∗ = 9.0 × 104, T∗∗ = 1 × 104 | ||||
| 4.15 | CH3OH + H ⇄ CH2OH + H2 | 3.20 × 1013 | 0.00 | 25.50 |
| 4.16 | CH3OH + H ⇄ CH3O + H2 | 8.00 × 1012 | 0.00 | 25.50 |
| 4.17 | CH3OH + O ⇄ CH2OH + OH | 3.88 × 105 | 2.50 | 12.89 |
| 4.18 | CH3OH + OH ⇄ CH3O + H2O | 1.00 × 106 | 2.10 | 2.08 |
| 4.19 | CH3OH + OH ⇄ CH2OH + H2O | 7.10 × 106 | 1.80 | −2.49 |
| 4.20 | CH3OH + O2 ⇄ CH2OH + HO2 | 2.05 × 1013 | 0.00 | 187.86 |
| 4.21 | CH3OH + HO2 ⇄ CH2OH + H2O2 | 3.98 × 1013 | 0.00 | 81.17 |
| 4.22 | CH2OH + HCO ⇄ CH3OH + CO | 1.00 × 1013 | 0.00 | 0.00 |
| 4.23 | CH3O + HCO ⇄ CH3OH + CO | 9.00 × 1013 | 0.00 | 0.00 |
| 4.24 | CH3OH + HCO ⇄ CH2OH + CH2O | 9.64 × 103 | 2.90 | 54.85 |
| 4.25 | CH2OH + CH2OH ⇄ CH3OH + CH2O | 3.00 × 1012 | 0.00 | 0.00 |
| 4.26 | CH3O + CH2OH ⇄ CH3OH + CH2O | 2.40 × 1013 | 0.00 | 0.00 |
| 4.27 | CH3O + CH3O ⇄ CH3OH + CH2O | 6.00 × 1013 | 0.00 | 0.00 |
| 4.28 | CH3OH + CH3O ⇄ CH3OH + CH2OH | 3.00 × 1011 | 0.00 | 16.99 |

Li J, Zhao Z, Kazakov A, Chaos M, Dryer FL, Scire Jr JJ. A comprehensive kinetic mechanism for CO, CH2O, and CH3OH combustion. Int J Chem Kinet 2007;39:109–36.
Table C5
CH4/CH3OH/CH2O/CO/H2/O2 Mechanisma
| A | n | E | ||
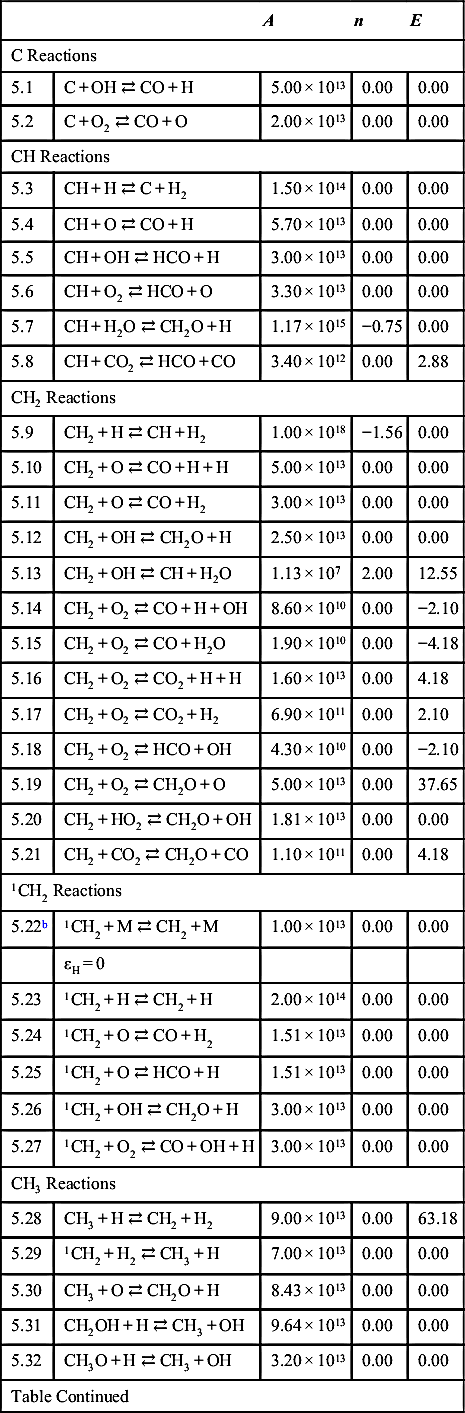
| C Reactions | ||||
| 5.1 | C + OH ⇄ CO + H | 5.00 × 1013 | 0.00 | 0.00 |
| 5.2 | C + O2 ⇄ CO + O | 2.00 × 1013 | 0.00 | 0.00 |
| CH Reactions | ||||
| 5.3 | CH + H ⇄ C + H2 | 1.50 × 1014 | 0.00 | 0.00 |
| 5.4 | CH + O ⇄ CO + H | 5.70 × 1013 | 0.00 | 0.00 |
| 5.5 | CH + OH ⇄ HCO + H | 3.00 × 1013 | 0.00 | 0.00 |
| 5.6 | CH + O2 ⇄ HCO + O | 3.30 × 1013 | 0.00 | 0.00 |
| 5.7 | CH + H2O ⇄ CH2O + H | 1.17 × 1015 | −0.75 | 0.00 |
| 5.8 | CH + CO2 ⇄ HCO + CO | 3.40 × 1012 | 0.00 | 2.88 |
| CH2 Reactions | ||||
| 5.9 | CH2 + H ⇄ CH + H2 | 1.00 × 1018 | −1.56 | 0.00 |
| 5.10 | CH2 + O ⇄ CO + H + H | 5.00 × 1013 | 0.00 | 0.00 |
| 5.11 | CH2 + O ⇄ CO + H2 | 3.00 × 1013 | 0.00 | 0.00 |
| 5.12 | CH2 + OH ⇄ CH2O + H | 2.50 × 1013 | 0.00 | 0.00 |
| 5.13 | CH2 + OH ⇄ CH + H2O | 1.13 × 107 | 2.00 | 12.55 |
| 5.14 | CH2 + O2 ⇄ CO + H + OH | 8.60 × 1010 | 0.00 | −2.10 |
| 5.15 | CH2 + O2 ⇄ CO + H2O | 1.90 × 1010 | 0.00 | −4.18 |
| 5.16 | CH2 + O2 ⇄ CO2 + H + H | 1.60 × 1013 | 0.00 | 4.18 |
| 5.17 | CH2 + O2 ⇄ CO2 + H2 | 6.90 × 1011 | 0.00 | 2.10 |
| 5.18 | CH2 + O2 ⇄ HCO + OH | 4.30 × 1010 | 0.00 | −2.10 |
| 5.19 | CH2 + O2 ⇄ CH2O + O | 5.00 × 1013 | 0.00 | 37.65 |
| 5.20 | CH2 + HO2 ⇄ CH2O + OH | 1.81 × 1013 | 0.00 | 0.00 |
| 5.21 | CH2 + CO2 ⇄ CH2O + CO | 1.10 × 1011 | 0.00 | 4.18 |
| 1CH2 Reactions | ||||
| 5.22b | 1CH2 + M ⇄ CH2 + M | 1.00 × 1013 | 0.00 | 0.00 |
| εH = 0 | ||||
| 5.23 | 1CH2 + H ⇄ CH2 + H | 2.00 × 1014 | 0.00 | 0.00 |
| 5.24 | 1CH2 + O ⇄ CO + H2 | 1.51 × 1013 | 0.00 | 0.00 |
| 5.25 | 1CH2 + O ⇄ HCO + H | 1.51 × 1013 | 0.00 | 0.00 |
| 5.26 | 1CH2 + OH ⇄ CH2O + H | 3.00 × 1013 | 0.00 | 0.00 |
| 5.27 | 1CH2 + O2 ⇄ CO + OH + H | 3.00 × 1013 | 0.00 | 0.00 |
| CH3 Reactions | ||||
| 5.28 | CH3 + H ⇄ CH2 + H2 | 9.00 × 1013 | 0.00 | 63.18 |
| 5.29 | 1CH2 + H2 ⇄ CH3 + H | 7.00 × 1013 | 0.00 | 0.00 |
| 5.30 | CH3 + O ⇄ CH2O + H | 8.43 × 1013 | 0.00 | 0.00 |
| 5.31 | CH2OH + H ⇄ CH3 + OH | 9.64 × 1013 | 0.00 | 0.00 |
| 5.32 | CH3O + H ⇄ CH3 + OH | 3.20 × 1013 | 0.00 | 0.00 |
| Table Continued | ||||
| A | n | E | ||
| 5.33b,c | CH3 + OH ⇄ CH3OH, k∞ | 2.79 × 1018 | −1.40 | 5.56 |
| CH3 + OH + M ⇄ CH3OH + M, k0 | 4.00 × 1036 | −5.92 | 13.14 | |
| α = 0.412, T∗∗∗ = 1.95 × 102, T∗ = 5.9 × 103, T∗∗ = 6.39 × 103 | ||||
| 5.34 | CH3 + OH ⇄ CH2 + H2O | 7.50 × 106 | 2.00 | 20.92 |
| 5.35 | CH3 + OH ⇄ 1CH2 + H2O | 8.90 × 1019 | −1.80 | 33.75 |
| 5.36 | CH3 + O2 ⇄ CH3O + O | 1.99 × 1018 | −1.57 | 122.30 |
| 5.37 | CH3 + O2 ⇄ CH2O + OH | 3.74 × 1011 | 0.00 | 61.26 |
| 5.38 | CH3 + HO2 ⇄ CH3O + OH | 2.41 × 1010 | 0.76 | −9.73 |
| 5.39 | CH3O + CO ⇄ CH3 + CO2 | 1.60 × 1013 | 0.00 | 49.37 |
| CH4 Reactions | ||||
| 5.40b,c | CH3 + H ⇄ CH4, k∞ | 1.27 × 1016 | −0.63 | 1.60 |
| CH3 + H + M ⇄ CH4 + M, k0 | 2.48 × 1033 | −4.76 | 10.21 | |
| α = 0.412, T∗∗∗ = 1.95 × 102, T∗ = 5.9 × 103, T∗∗ = 6.39 × 103 | ||||
| 5.41 | CH4 + H ⇄ CH3 + H2 | 5.47 × 107 | 1.97 | 46.90 |
| 5.42 | CH4 + O ⇄ CH3 + OH | 3.15 × 1012 | 0.50 | 43.06 |
| 5.43 | CH4 + OH ⇄ CH3 + H2O | 5.72 × 106 | 1.96 | 11.04 |
| 5.44 | CH3 + HO2 ⇄ CH4 + O2 | 3.16 × 1012 | 0.00 | 0.00 |
| 5.45 | CH4 + HO2 ⇄ CH3 + H2O2 | 1.81 × 1011 | 0.00 | 77.74 |
| 5.46 | CH3 + HCO ⇄ CH4 + CO | 1.20 × 1014 | 0.00 | 0.00 |
| 5.47 | CH3 + CH2O ⇄ CH4 + HCO | 3.64 × 10−6 | 5.42 | 4.18 |
| 5.48 | CH3 + CH3O ⇄ CH4 + CH2O | 2.41 × 1013 | 0.00 | 0.00 |
| 5.49 | CH3 + CH3OH ⇄ CH4 + CH2OH | 3.19 × 101 | 3.17 | 30.01 |
| 5.50 | CH4 + 1CH2 ⇄ CH3 + CH3 | 4.00 × 1014 | 0.00 | 0.00 |

Miller JA, Bowman CT. Mechanism and modeling of nitrogen chemistry in combustion. Prog Energy Combust Sci 1989;15:287–338. Li J, Zhao Z, Kazakov A, Chaos M, Dryer FL, Scire Jr JJ. A comprehensive kinetic mechanism for CO, CH2O, and CH3OH combustion. Int J Chem Kinet 2007;39:109–36. Held T. The oxidation of methanol, isobutene, and methyl tertiary-butyl ether, No. 1978-T, PhD dissertation, Princeton University, Princeton, NJ 08544, 1993. Burgess Jr DRF, Zachariah MR, Tsang W, Westmoreland PR. Thermochemical and chemical kinetic data for fluorinated hydrocarbons, NIST technical note 1412, NIST, Gaithersburg, MD, 1995. The CH4 and C2H6 (Table C6) mechanisms are based on the high temperature Miller–Bowman mechanism updated and extended to intermediate temperatures with kinetic data from the other listed sources. The reaction sets provided here have not been evaluated against experimental data. For validated CH4 mechanisms, the reader is referred to those given in the text of this appendix. For example, the GRI-Mech (Bowman CT, Frenklach M, Gardiner W, Golden D, Lissianski V, Smith G, Wang H. Gas Research Institute Report, 1995) mechanisms are optimized mechanisms for describing the combustion kinetics of methane/air mixtures with nitric oxide chemistry.
Table C6
C2H6/CH4/CH3OH/CH2O/CO/H2/O2 Mechanisma
