4.7. Stirred Reactor Theory
In the discussion of premixed turbulent flames, the case of infinitely fast mixing of reactants and products was introduced. Generally this concept is referred to as a stirred reactor. Many investigators have employed stirred reactor theory not only to describe turbulent flame phenomena, but also to determine overall reaction kinetic rates [23] and to understand stabilization in high-velocity streams [65]. Stirred reactor theory is also important from a practical point of view because it predicts the maximum energy release rate possible in a fixed volume at a particular pressure.
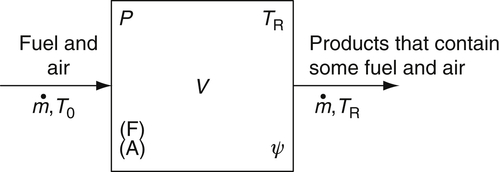
Consider a fixed volume V into which fuel and air are injected at a fixed total mass flow rate 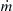 and temperature T0. The fuel and air react in the volume, and the injection of reactants and outflow of products (also equal to
and temperature T0. The fuel and air react in the volume, and the injection of reactants and outflow of products (also equal to  ) are so oriented that within the volume there is instantaneous mixing of the unburned gases and the reaction products (burned gases). The reactor volume attains some steady temperature TR and pressure P. The temperature of the gases leaving the reactor is, thus, TR as well. The pressure differential between the reactor and the exit is generally considered to be small. The mass leaving the reactor contains the same concentrations as those within the reactor and thus contains products as well as fuel and air. Within the reactor there exists a certain concentration of fuel (F) and air (A) and also a fixed unburned mass fraction, ψ. Throughout the reactor volume, TR, P, (F), (A), and ψ are constant and fixed; that is, the reactor is so completely stirred that all elements are uniform everywhere. Figure 4.49 depicts the stirred reactor concept in a generalized manner.
) are so oriented that within the volume there is instantaneous mixing of the unburned gases and the reaction products (burned gases). The reactor volume attains some steady temperature TR and pressure P. The temperature of the gases leaving the reactor is, thus, TR as well. The pressure differential between the reactor and the exit is generally considered to be small. The mass leaving the reactor contains the same concentrations as those within the reactor and thus contains products as well as fuel and air. Within the reactor there exists a certain concentration of fuel (F) and air (A) and also a fixed unburned mass fraction, ψ. Throughout the reactor volume, TR, P, (F), (A), and ψ are constant and fixed; that is, the reactor is so completely stirred that all elements are uniform everywhere. Figure 4.49 depicts the stirred reactor concept in a generalized manner.
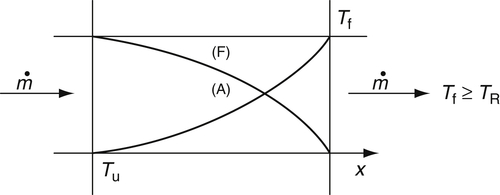
The stirred reactor may be compared to a plug flow reactor in which premixed fuel–air mixtures flow through the reaction tube. In this case, the unburned gases enter at temperature T0 and leave the reactor at the flame temperature Tf. The system is assumed to be adiabatic. Only completely burned products leave the reactor. This reactor is depicted in Figure 4.50.
The volume required to convert all the reactants to products for the plug flow reactor is greater than that for the stirred reactor. The final temperature is, of course, higher than the stirred reactor temperature.
It is relatively straightforward to develop the controlling parameters of a stirred reactor process. If ψ is defined as the unburned mass fraction, it must follow that the fuel–air mass rate of burning  is
is
![]()
and the rate of heat evolution 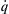 is
is
![]()
where q is the heat of reaction per unit mass of reactants for the given fuel-air ratio. Assuming that the specific heat of the gases in the stirred reactor can be represented by some average quantity 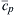 , one can write an energy balance as
, one can write an energy balance as
![]()
For the plug flow reactor or any similar adiabatic system, it is also possible to define an average specific heat that takes its explicit definition from
![]()
To a very good approximation these two average specific heats can be assumed equal. Thus, it follows that
![]()
The mass burning rate is determined from the ordinary expression for chemical kinetic rates; that is, the fuel consumption rate is given by
![]()
where (A/F) represents the air–fuel ratio. The concentration of the fuel can be written in terms of the total density and unburned mass fraction
![]()
which permits the rate expression to be written as

Now the great simplicity in stirred reactor theory is realizable. Since (F), (A), and TR are constant in the reactor, the rate of conversion is constant. It is now possible to represent the mass rate of burning in terms of the preceding chemical kinetic expression:
![]()
or
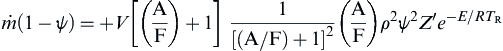
From the equation of state, by defining
![]()
and substituting for (1 − ψ) in the last rate expression, one obtains

By dividing through by P2, one observes that
![]()
or
![]()
which states that the heat release is also a function of TR.
This derivation was made as if the overall order of the air–fuel reaction were 2. In reality, this order is found to be closer to 1.8. The development could have been carried out for arbitrary overall order n, which would give the result
![]()
A plot of 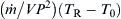 versus TR reveals a multivalued graph that exhibits a maximum, as shown in Figure 4.51. The part of the curve in Figure 4.51 that approaches the value Tx asymptotically cannot exist physically since the mixture could not be ignited at temperatures this low. In fact, the major part of the curve, which is to the left of Topt, has no physical meaning. At fixed volume and pressure it is not possible for both the mass flow rate and temperature of the reactor to rise. The only stable region exists between Topt to Tf. Since it is not possible to mix some unburned gases with the product mixture and still obtain the adiabatic flame temperature, the reactor parameter must go to zero when TR = Tf.
versus TR reveals a multivalued graph that exhibits a maximum, as shown in Figure 4.51. The part of the curve in Figure 4.51 that approaches the value Tx asymptotically cannot exist physically since the mixture could not be ignited at temperatures this low. In fact, the major part of the curve, which is to the left of Topt, has no physical meaning. At fixed volume and pressure it is not possible for both the mass flow rate and temperature of the reactor to rise. The only stable region exists between Topt to Tf. Since it is not possible to mix some unburned gases with the product mixture and still obtain the adiabatic flame temperature, the reactor parameter must go to zero when TR = Tf.
The value of TR, which gives the maximum value of the heat release, is obtained by maximizing the last equation. The result is
![]()
For hydrocarbons, the activation energy falls within a range of 120–160 kJ/mol and the flame temperature in a range of 2000–3000 K. Thus,
![]()
Stirred reactor theory reveals a fixed maximum mass loading rate for a fixed reactor volume and pressure. Any attempts to overload the system will quench the reaction. It is also worth noting that stirred reactor analysis for both non-dilute and dilute systems gives the maximum overall energy release rate possible for a given fuel–oxidizer mixture in a fixed volume at a given pressure. Attempts have been made to determine chemical kinetic parameters from stirred reactor measurements. The usefulness of such measurements at high temperatures and for non-dilute fuel–oxidizer mixtures is limited. Such analyses are based on the assumption of complete instantaneous mixing, which is impossible to achieve experimentally. However, for dilute mixtures at low and intermediate temperatures where the Damkohler number (Da = τm/τc) is small, studies have been performed to investigate the behaviors of reaction mechanisms [66].
Using the notation of Chapter 2, Section 2.8.3, the stirred reactor species and energy equations may be written in terms of the individual species equations and the energy equation. The species equations are given by
![]() (4.78)
(4.78)
where mj is the mass of the jth species in the reactor (and reactor outlet), V is the volume of the reactor,  is the chemical production rate of species j, MWj is the molecular weight of the jth species,
is the chemical production rate of species j, MWj is the molecular weight of the jth species, 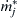 is the mass flow rate of species j in the reactor inlet, and
is the mass flow rate of species j in the reactor inlet, and  is the mass flow rate of species j in the reactor outlet.
is the mass flow rate of species j in the reactor outlet.
For a constant mass flow rate and mass in the reactor, Eqn (4.78) may be written in terms of species mass fractions, Yj, as
![]() (4.79)
(4.79)
At steady-state (dYj/dt = 0), and therefore
![]() (4.80)
(4.80)
The energy equation for an adiabatic system at steady-state is simply
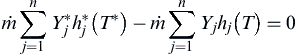
where
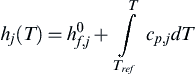
Equation (4.80) is a set of nonlinear algebraic equations and may be solved using various techniques [67]. Often the nonlinear differential Eqn (4.79) are solved to the steady-state condition in place of the algebraic equations using the stiff ordinary differential equation solvers described in Chapter 2 [68]. See Appendix I for more information on available numerical codes.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.