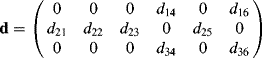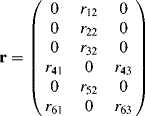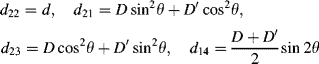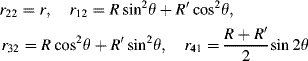Chapter 4
Ferroelectric Liquid Crystals for Nonlinear Optical Applications
4.1 Introduction
4.1.1 Overview of Nonlinear Optical Materials (NLO) and Electro-Optic (EO) Materials
NLO materials have become increasingly indispensable to today's EO technology. Electro-optics involve these two important technologies (electronics and photonics), and the interdisciplinary field requires expertise from many fields such as mathematics, physics, chemistry, material science, electrical, and optical engineering. Electrons perform information functions in electronic devices, while photons are capable of performing the same functions in photonic devices at much faster speed but without production of too much heat. EO devices provide a means of converting information from the electronic domain to the photonic domain or vice versa. They have been widely used in telecommunication systems, and are particularly attractive in emerging optical computing applications.
Since the discovery of second harmonic generation (SHG) in inorganic materials in 1961 [1], a variety of inorganic crystalline materials including lithium niobate (LiNbO3), potassium niobate (KNbO3), potassium titanyl phosphate (KTiOPO4), and potassium dihydrogen phosphate (KH2PO4), etc., have been successfully developed. Today's EO and NLO commercial markets are completely dominated by inorganic crystalline materials (mainly lithium niobate) because some of them have been found as superior NLO and EO materials for a variety of devices including modulators, parametric oscillators, optical switches, etc. Lithium niobate is today's benchmark in commercial EO technology because of its excellent performance in telecommunication applications. While inorganic crystals have the advantages of being well characterized, they have evident disadvantages such as shock sensitivity, high cost, and poor processability.
Compared with inorganic crystalline materials, organic materials show superior versatility, flexibility, durability, facile fabrication, and processability. It is possible to design organic materials using molecular engineering and thereby tailor the properties for a specific NLO and EO application because of the availability of a very wide range of chemical structural modifications in organic molecules. Moreover, organic materials show much smaller reduced half-wave voltages (νπ = λ/(n3reff), n is the refractive index) and much larger figures-of-merit (![]() for EO modulators and n3reff/ε for photorefractive applications) than inorganic materials because of their large effective EO coefficients, (reff) and small dielectric constants (ε). For example, the figure-of-merit (n3reff/ε) for a typical organic single crystal (optimized stilbene) is 5300 pm/V, while for inorganic lithium niobate it is only 11 pm/V [2]. There are also concerns for organic NLO materials, such as environmental stability, photochemical stability, mechanical strength, and performance at low and high temperatures.
for EO modulators and n3reff/ε for photorefractive applications) than inorganic materials because of their large effective EO coefficients, (reff) and small dielectric constants (ε). For example, the figure-of-merit (n3reff/ε) for a typical organic single crystal (optimized stilbene) is 5300 pm/V, while for inorganic lithium niobate it is only 11 pm/V [2]. There are also concerns for organic NLO materials, such as environmental stability, photochemical stability, mechanical strength, and performance at low and high temperatures.
Significant and dramatic advancement has been made for organic NLO materials in past decades. The field of organic NLO materials has been reviewed by many authors in numerous excellent review papers [3–15] and book chapters [16, 17]. Enormous data are available and meaningful insights have been gained through a comprehensive range of reviews on newly emerging organic NLO materials. On the basis of their nature, organic NLO materials are classified into six different categories: (i) single crystals [12, 14], (ii) metal-organic hybrids [5, 9, 12], (iii) Langmuir–Blodgett (LB) films [16, 17], (iv) self-assembled systems [9–12], (v) liquid crystals, and (vi) poled polymers [3, 4, 6, 7, 13, 15]. Each type of material has its own advantages and disadvantages.
Organic single crystals consisting of organic conjugated molecules carrying strong electron donors and electron acceptors exhibit very large NLO coefficients and excellent figures-of-merit, but they are mechanically soft, difficult to design and process with precision, and almost impossible to process into optical quality films. Growing large-size single crystals is usually difficult. LB films and self-assembled multilayers exhibit very large SHG activity and possess high degree of molecular orientation and precise control of film thicknesses and layer architectures. However, these NLO materials, particularly LB films, are soft and have very unstable structures. Although sometimes self-assembled multilayers are also included into metal-organic hybrids, herein metal-organic hybrids denote only organometallic complexes including compounds with covalent bonds between metal atoms and other elements or with coordination bonds between metal ions and organic ligands. These types of complexes include at least one metal atom or ion in their molecules and exhibit large molecular hyperpolarizabilities (β) owing to the existence of two special types of charge-transfer transitions, metal-to-ligand and ligand-to-metal. The incorporation of transition metals into organic molecules favors crystallization, thereby facilitating to grow large-size single crystals of organometallic compounds. Like other organic chromophore compounds, organometallics incorporating strong chromophores can be used in five other categories.
Poled polymers [3, 4, 6] are among the most important organic NLO materials, and their macroscopic nonlinearities are generally realized by the electric field poling technique. To maintain a stable polar alignment in the thermodynamically unstable poled state, it is essential to utilize either high glass-transition temperature (Tg) polymers with NLO chromophores as pendants or cross-linked polymers with NLO chromophores locked in the polymer network. They can be easily processed into thin films by spin-coating and be readily integrated with silicon very-large-scale integration (VLSI) technology. Over the past decade, considerable effort and progress have been made on these materials from the viewpoint of EO activity, with current values of EO coefficients up to approximately 500 pm/V and of optical losses under 2 dB/cm. Thermal and photochemical stability have been also greatly improved. However, how to make one material possess all of the aforementioned properties remains problematic, let alone the challenge of addressing other properties such as solubility, shrinkage, adhesion, and mechanical stress.
Liquid crystals (LCs), particularly ferroelectric liquid crystals (FLCs) [18] as a special type of organic NLO material, are very appealing for NLO applications. FLCs possess thermodynamically stable intrinsic polar order, and their polar direction can be controlled via ferroelectric (FE) switching under an external electric field, enabling fabrication of more complex devices. Furthermore, as in LC displays, FLC materials can be readily integrated with silicon VLSI technology, allowing for hybrid technologies utilizing the FLCs' NLO properties.
4.1.2 Scope of This Chapter
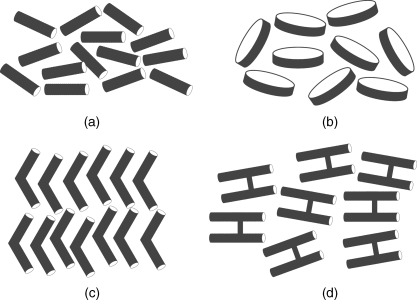
Although LCs have been known to scientists for over one hundred years, it was only in the late 1960s that LCs began to be used in display applications. Today LCs have proven extremely valuable in commercial display products such as TVs, automobile gauges, road signs, watches, and computer displays. It was not until 1980s that LCs started to be recognized as potential NLO materials. For many decades, predominant LC molecules have been variants on simple rod shapes (Figure 4.1a). Several new types of LC molecules have been developed in the past three decades, such as disc molecules (Figure 4.1b) [19], bent-core (BC) (banana-shaped) molecules (Figure 4.1c) [20], and H-shaped twin molecules (Figure 4.1d) [21], which exhibit novel and potentially valuable properties that conventional rod-shaped calamitic LCs cannot possess. For instance, banana liquid crystals and H-shaped dimesogens exhibit larger NLO coefficients than rod-shaped NLO FLCs, and discotic LCs have been widely used for organic photovoltaics and other organic semiconductor devices [22].
Figure 4.1 Different shaped liquid crystals: (a) conventional rod-shaped LCs, (b) disc-shaped LCs, (c) banana-shaped LCs, and (d) H-shaped twin LCs.
In this chapter we focus on the NLO applications of LCs, particularly FLCs. The early developments on rod-shaped FLCs and their SHG and Pockels effects were reviewed in several papers and book chapters [23–26]. Herein we focus on fascinating new developments on H-shaped dimesogens and bent-core mesogens. We will also discuss miscellaneous NLO LC materials and their ferroelectricity-related behaviors. Some important rod-shaped FLCs and their polymeric versions will be included in this chapter for comparison with other types of NLO FLC materials. The cross-linked polymeric versions of rod-shaped FLCs and their NLO applications will be highlighted since these pyroelectric polymers formed via photo-induced in situ polymerization are thermally and mechanically stable enough to be used for EO devices (e.g., waveguides). Cross-linked polymerization in these materials helps maintain the macroscopic nonlinearity via locking the chromophores into polymer networks. It should be pointed out that this chapter is by no means comprehensive and does not cover all the known FLC materials. Our idea is instead to give the readers a summary of this area and also an overview of the exciting new advances in NLO FLCs and their potential industrial applications by selectively choosing some targets. Owing to the limitation of the available literature and our knowledge, we apologize to all those who are not mentioned in this chapter, but have made contributions to the research and development of this subfield.
4.2 Fundamentals
4.2.1 Ferroelectricity in LCs
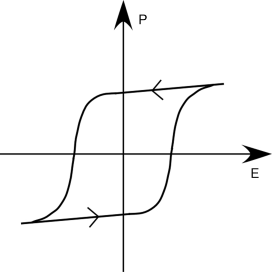
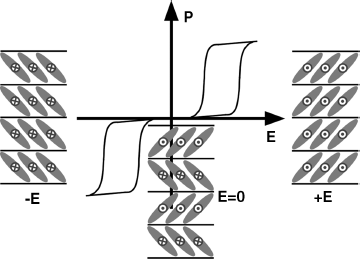
Ferroelectrics are a subgroup of pyroelectrics which show a non-vanishing spontaneous polarization at zero electric field. The distinguishing feature between a ferroelectric and a pyroelectric is that the direction of the spontaneous polarization (P) for a ferroelectric can be switched by an external electric field (E), yielding a typical single hysteresis loop (Figure 4.2) [27]. Both ferroelectrics and pyroe-lectrics are useful for NLO and other applications (e.g., ferroelectric RAM for computers [28]).
Figure 4.2 A typical single hysteresis loop for ferroelectrics.
Liquid crystals, as a state of matter between liquid and solid, uniquely combine both long range order (as in crystals) and mobility (as in liquids) in the same material. The order leads to the anisotropy of LC molecules on both molecular and supermolecular levels, and the mobility enables LC molecules to respond easily to external stimuli. LCs self-assemble into macroscopically ordered structures which can be classified into four major categories based on the orientational or positional orders of their molecules: nematic (N), smectic (Sm), columnar (Col), and cubic mesophases. Nematic mesophases have only long range orientational order, smectic mesophases exhibit one-dimensional (1D) positional order and form 2D layered structures, columnar mesophases (also called modulated layer mesophases) possess 2D positional order and form tube-like 1D structures, and cubic mesophases [29, 30] have 3D-ordered structural organization. Note that the shapes of the molecules are not exactly related to the types of mesophases formed. For example, linear rod-shaped (e.g., polycatenar mesogens [31]), disc-shaped molecules [32], and molecules without specific shape or with a spherical shape [33, 34] all can organize as nematic, smectic, columnar, and 3D-ordered mesophases. The most common smectic phases formed by rod-shaped LC molecules are the smectic A (SmA) phase with their molecular long axes (i.e., the director) parallel to the layer normal, and the smectic C (SmC) phase with the director tilted with respect to the layer normal.
The incorporation of chirality into LC systems leads to the formation of helical superstructures in nematic phases (e.g., cholesteric phases, N*) and tilted smectic phases (e.g., SmC* phases) and can also induce whole new classes of chiral phases such as blue phases (BP*), twist grain boundary phases (TGB*), and 3D-ordered mesophases [35]. The non-tilted chiral SmA* phase exhibits the electroclinic effect under an external electric field, while chirality induces polar order in the tilted chiral smectic LC phases (e.g., SmC* and SmCA* phases). The two tilted chiral phases possess spontaneous polarizations and show FE and antiferroelectric (AF) switching under surface-stabilized conditions upon the application of an external electric field. Hence, the SmC* phase is also called the ferroelectric phase, while the SmCA* phase is similarly called the antiferroelectric phase. There also exist some intermediate chiral subphases (denoted as SmC1/n*, n > 2) between ferro- and antiferroelectric phases [36], which exhibit ferrielectric switching behavior under surface-stabilized conditions with the applied electric field and are therefore called ferrielectric phases.
FLCs are a natural and compelling candidate for NLO and EO applications due to the lack of inversion symmetry. Although the helix causes the vanishment of the spontaneous polarization for bulk materials at zero field, the FLCs exhibit bistable FE switching under surface-stabilized conditions. Traditional FLCs are composed of chiral rod-shaped molecules that possess a net electric-dipole moment perpendicular to the director and parallel to the layer plane. Because of the way in which molecules pack together, the minimum energy state is one in which all the molecules point on average in the same direction locally. However, dipole–dipole interactions in bulk materials favor the escape of macroscopic polarity by the formation of helical supermolecular structures. Such bulk materials are not so interesting for NLO applications. The discovery of the surface-stabilized ferroelectric liquid crystal (SSFLC) by Clark and Lagerwall [37] triggered not only the development of FLC-based displays but also the research of FLCs as NLO materials. It should be noted that FLC materials can be generated either from the pure chiral materials (e.g., one chiral compound or a mixture of several chiral compounds) possessing the SmC* phase or by doping an achiral SmC host with a small percentage of one or more rod-shaped chiral compounds.
In a surface-stabilized cell with a bookshelf geometry, the FLC molecules self-assemble into parallel layers (Figure 4.3). Within each sheet the director is tilted away from the layer normal by the tilt angle θ that is characteristic of the particular liquid crystal mixture and which depends on temperature. The net molecular dipole moments are within the layers and perpendicular to the director. The FLC molecules can switch on a smectic cone between two stable states upon the application of an electric field, thereby yielding a single hysteresis loop for ferroelectricity (Figure 4.4) [38].
Figure 4.3 A bistable FLC cell with a bookshelf geometry showing that the director ![]() is tilted away from the layer normal
is tilted away from the layer normal ![]() by the tilt angle θ. The net molecular dipole moments P are within the layers and perpendicular to the director. d(T) is the layer spacing.
by the tilt angle θ. The net molecular dipole moments P are within the layers and perpendicular to the director. d(T) is the layer spacing.
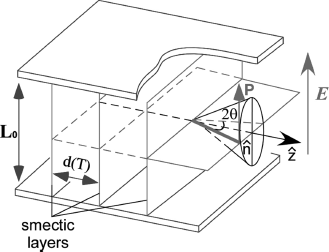
Figure 4.4 An ideal bistable FLC cell showing a typical single hysteresis loop (ferroelectricity) and two bistable FE states.
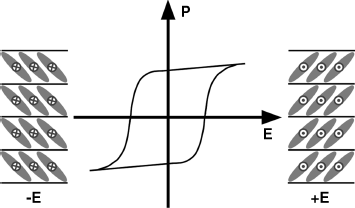
The antiferroelectric liquid crystals (AFLCs) are also important for NLO applications since the AF ground state can be switched to the two FE states, which show NLO activity, under an external electric field. The AFLC (SmCA*) phase is monostable and has three important states: one ground-state AF state and two field-induced FE states (Figure 4.5). Therefore, the field-induced switching among the three states exhibits a typical double hysteresis curve with a zero polarization at zero field.
Figure 4.5 An ideal surface-stabilized AFLC cell showing a double hysteresis curve (antiferroelectricity), and one ground-state AF state and two FE states.
The SmC1/3* ferrielectric phase in the non-helical model has four FE states, and the field-induced switching yields a triple hysteresis curve (Figure 4.6) with a non-zero spontaneous polarization at zero field. In the bulk materials, the SmC1/3* phase exhibits a vanishing spontaneous polarization at zero field due to the formation of the helical superstructure [39], which is also similarly found in the SmC* case. Hence, many bulk LC materials exhibiting the SmC*, SmC1/3* or in general SmC1/n* phases should be precisely called neither ferro- or ferrielectric nor pyroelectric but helielectric [40]. When the electric field is above the threshold for unwinding the helix (i.e., E > Eth), the spontaneous polarization reappears to enable FE switching on a smectic cone between the two complete FE states.
Figure 4.6 An ideal surface-stabilized ferrielectric cell showing a triple hysteresis curve (ferrielectricity) and four FE states.

The vanishing spontaneous polarization at zero field in the SmC* phase should be distinguished from that in the SmCA* phase. The former will exhibit non-zero spontaneous polarization (i.e., ferroelectricity) unless the helix forms at zero field, while the latter phase will exhibit zero spontaneous polarization (i.e., antiferroelectricity) whether the helix forms at zero field or not since the opposite dipoles between the adjacent layers cancel each other out. The threshold voltage for the switching of an AFLC is about one order of magnitude larger than that of a FLC. These indicate that ferroelectricity in the SmC* phase is strongly dependent on cell conditions. A bistable cell enables a SmC* material to behave as a proper and real FLC (Figure 4.4).
When an FLC, AFLC, or ferrielectric liquid crystal is polymerized in situ under their field-induced FE states, macroscopic polar order is locked into cross-linked polymer networks. The removal of the electric field may not influence the macroscopically polar structures of these polymers. However, the high viscosity of the polymers makes it impossible to reverse the spontaneous polarization by applying an external electric field. These polymers, therefore, are pyroelectrics rather than ferroelectrics. They should be precisely called pyroelectric liquid crystal polymers (PLCPs).
We have discussed the importance of chirality to ferroelectricity in LCs. The introduction of chirality, in fact, is not the only way to realize ferroelectricity in LCs. Takezoe and Watanabe stated that it is possible to induce ferroelectricity by reducing the symmetry to Cn or Cnv in a LC system [41]. Four different molecular systems were proposed: (i) polar uniaxial nematic LC with C∞v symmetry, (ii) polar biaxial nematic LC with C1v (Cs) symmetry, (iii) polar uniaxial SmA LC with C∞v symmetry, and (iv) polar biaxial smectic LC with C2v symmetry. The discovery that achiral bent-core (banana-shaped) molecules form macroscopic polarity by Niori et al. [42] in 1996 supports the feasibility of the fourth molecular system. Most bent-core molecules form AF phases (see Section 4.3.3 for details). Polar ordering was reported in aromatic polyesters exhibiting nematic phases [43], but the high viscosity of the polymers prevents field-driven FE switching. These materials are pyroelectrics rather than ferroelectrics. In contrast, strong SHG at zero field and the reversal of the polarization under a field were observed in the thermotropic or lyotropic cholesteric phase of a α-helical polypeptide [44], indicating the first proper ferroelectricity in nematic LCs (also see Section 4.3.4).
4.2.2 Microscopic to Macroscopic Nonlinearity
As in other organic materials, the nonlinearity in a bulk FLC material also results from that of individual molecules. The dipole polarization μ of an isolated organic molecule under an external electric field can be written as:
(4.1) ![]()
where μ0 is the permanent dipole moment of the molecule, αij is the linear molecular polarizability, and βijk and γijkl are the first and second hyperpolarizabilities, respectively. The subscripts i, j, and k refer to a molecule-based coordinate system, and E is the applied electric field. Symmetry requires that all terms of even order in the electric field vanish for a molecule with an inversion center.
The macroscopic polarization P of a bulk nonlinear material under an external electric field E can be expressed as:
(4.2) ![]()
where P0 is the spontaneous polarization, and χ(n) is the nonlinear optical susceptibility of nth order. IJK is the coordinate system of the bulk material. As in the molecular case, a bulk material with an inversion center possesses zero even-order susceptibilities. This also indicates that a centrosymmetric bulk material does not exhibit second-order susceptibility even if the molecules constituting it do possess second-order non-zero hyperpolarizability βijk. Therefore, enabling bulk materials to possess macroscopic second-order nonlinearities has an indispensable requisite that their non-centrosymmetric nonlinear molecules are arranged in such a manner that the bulk materials do not have a center of symmetry.
The NLO coefficient dIJK is an important parameter in the case of SHG. dIJK is related to χ(2) by:
(4.3) ![]()
Building a relationship between microscopic (β) and macroscopic (d) nonlinear properties helps not only understand the measured values for bulk organic materials but also design new types of molecules and bulk materials. According to the most simple oriented-gas model [45], all contributions to the optical nonlinearity due to intermolecular interactions are neglected except for local-field corrections, and therefore, only intramolecular interactions are considered. Thus a simple relation between the NLO coefficients dIJK for frequency doubling and molecular hyperpolarizabilities βijk is described as:
where θs are the angles between molecular charge-transfer axes ijk and the reference system IJK, respectively, and the f factors are local-field corrections at different frequencies. Note that this equation assumes that there is only one symmetry equivalent position in the unit cell.
The f factors at different frequencies are often calculated within the simple approximation of the Lorentz model:
The principal indices nI are for light with frequencies 2ω and ω.
According to the orientated gas model [45], the EO coefficients rIJK for the Pockels effect are similarly related to the molecular hyperpolarizabilities βijk through:
The f factors are similarly calculated by:
where εI is the dielectric constant along the principal I axis. Here the β terms are calculated for the EO process instead of SHG.
Note that the hyperpolarizabilities in Eqs. (4.4) and (4.6) are different (![]() in Eq. (4.4) and
in Eq. (4.4) and ![]() in Eq. (4.6)), though both are related to each other. In the simplest approach (two-level model) [45], the hyperpolarizabilites for SHG and EO effect are given by:
in Eq. (4.6)), though both are related to each other. In the simplest approach (two-level model) [45], the hyperpolarizabilites for SHG and EO effect are given by:
where
(4.10) ![]()
Here, μeg is the element of matrix of the electric-dipole operator between the excited and ground state, λ0 is the charge-transfer transition wavelength, Δμ is the difference between the excited and ground-state dipole moments, and ε0 is the vacuum permittivity. Note that β0 for similar chromophores can be readily computed by using Eq. (4.8) and the published data of ![]() , measured by Cheng et al. [46] at the fundamental wavelength of λ = 1907 nm using electric field induced second harmonic (EFISH) technique.
, measured by Cheng et al. [46] at the fundamental wavelength of λ = 1907 nm using electric field induced second harmonic (EFISH) technique.
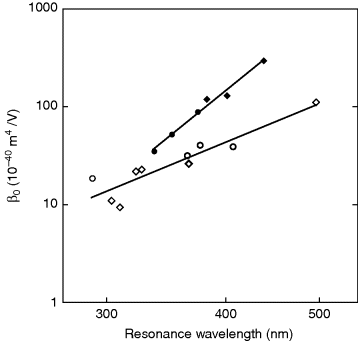
These authors [46] reported the hyperpolarizabilities of more than one hundred compounds that were carefully measured in solution using the EFISH technique at a fundamental wavelength of 1907 nm. They found a remarkable dependence of the experimental β values and the charge-transfer transition wavelength λ0. For a given type of molecule (e.g., disubstituted benzene or stilbene derivatives), β0 depends mainly on λ0 without any further reference to the details of the donor and acceptor. Figure 4.7 shows β0 as a function of the resonance wavelength (λ0) in solution for some benzenes and stilbenes. These two families are the prototypical NLO chromophores with one and two aromatic rings and have already been incorporated into many LC molecules.
Figure 4.7 Log–log plot of the dependence of the zero-frequency hyperpolarizability on the resonance wavelength in solution for disubstituted benzenes (open circles) and stilbenes (closed circles). The straight lines are empirical relations between β0 and λ0, with ![]() for the benzenes and
for the benzenes and ![]() for the stilbenes. β0 is expressed in 10−40 m4/V and λ0 in nm. Note that the β0 data are adopted from reference [46].
for the stilbenes. β0 is expressed in 10−40 m4/V and λ0 in nm. Note that the β0 data are adopted from reference [46].
4.2.3 SHG and Pockels Effect in LCs
4.2.3.1 SHG Measurements
SHG is a NLO process in which photons in a laser beam interacting with a material generate new photons with twice the frequency. SHG is, therefore, called frequency doubling. In addition to its use in high-resolution imaging in biological and medical science, another important application in industry is to generate a green laser with the wavelength of 532 nm using the readily available Nd:YAG laser with the wavelength of 1064 nm.
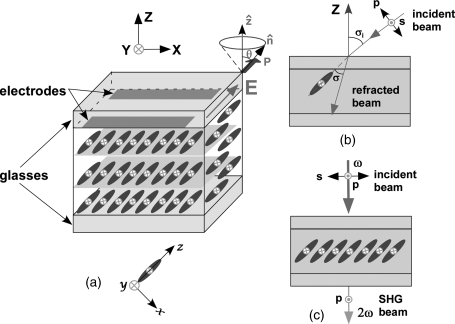
For a FLC material with a C2 symmetry axis along the polar Y-axis direction (Figure 4.8a), the only non-zero components of the contracted susceptibility d tensor in the XYZ coordinate system are given by
Figure 4.8 Schematic illustration of (a) a homeotropically aligned FLC cell with the spontaneous polarization P parallel to the polar axis which is the Y-axis in the XYZ coordinate system and the y-axis in the xyz molecular coordinate system, (b) SHG experiments using phase matching method, and (c) SHG experiments using Maker fringe method at normal incidence. In (b), the phase matching is achieved by rotating the cell around the polar axis, θ is the tilt angle, σi is the incident angle, and σ is the angle between the optical propagation direction and the FLC molecule director ![]() . (See the color version of this figure in Color Plates section.)
. (See the color version of this figure in Color Plates section.)
The number of independent dij coefficients is reduced to four (d22, d21, d23, and d14) with d21 = d16, d23 = d34, and d14 = d25 = d36 according to Kleinman symmetry. For a material with a C2v point group, only three independent coefficients (d22, d21 = d16, d23 = d34, and d14 = d25 = d36 = 0) remain (see the SmCaPF structure with a C2v axis symmetry in Section 4.3.3) upon the application of Kleinman symmetry.
To determine the dij coefficients, SHG is measured for different combinations of polarizations of the fundamental and second harmonic lights. Measurements are generally performed in homeotropic cells. A horizontal electric field, parallel to the cell surface, is usually applied to align this type of cell (Figure 4.8a). Different interaction lengths are employed because the SHG signal also depends on the phase mismatch between the lights at 2ω and ω [47]. To change the interaction length, the cell can be measured at different angles of incidence (Figure 4.8b), or different cells with various thicknesses can be used at normal incidence (Figure 4.8c). Very often FLCs present the favorable condition of phase matching, when the SHG intensity is the largest [48]. Typically this happens for an ee-o process (i.e., generation of a 2ω photon with ordinary (o) polarization from two ω photons with extraordinary (e) polarization). The effective susceptibility deff for this process is expressed as
(4.12) ![]()
where σ is the angle between the FLC director and the refracted beam (Figure 4.8b) at which the phase matching condition is achieved.
For a FLC material possessing strong absorption, the data process is cumbersome, and the approach proposed by Herman and Hayden for absorbing materials is followed [49].
In addition to the above measurements in homeotropic cells, the dij coefficients can be also measured in planar aligned cells with quasi-bookshelf (QBS) geometry, where the smectic layers are essentially perpendicular to the glass surface [50].
4.2.3.2 Pockels Effect Measurement
The Pockels effect, also called Pockels EO effect, consists in the change of the refractive indices of a given material upon the application of an electric field. The Pockels effect depends on the material's macroscopic susceptibility χ(2). The induced index change varies linearly with the applied electric field. Pockels cells are widely used in a variety of scientific and technological areas, particularly in fiber-optic communications.
For a FLC material with a C2 symmetry axis along the polar Y-axis direction (Figure 4.8a), the only non-zero components of the r tensor in the XYZ coordinate system are given by
The number of independent rij coefficients is reduced to four (r22, r12, r32, and r41) with r12 = r61, r32 = r43, and r41 = r52 = r63 if Kleinman symmetry is fulfilled. For a material with a C2v point group, only three independent coefficients (r22, r12 = r61, r32 = r43, and r41 = r52 = r63 = 0) are left upon the assumption of Kleinman symmetry.
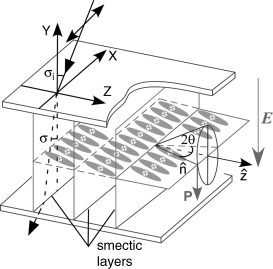
Although the Pockels effect has been extensively investigated in polymeric materials, and many methods are developed to measure rij coefficients for poled polymeric films [2], reports on FLC materials including PLCPs are relatively limited. Hult and co-workers adopted a crossed-polarizer method to measure the EO coefficient (r22 − r12) of a PLCP in a surface-stabilized FLC cell with a bookshelf geometry (Figure 4.9) [51].
Figure 4.9 A surface-stabilized FLC cell with a bookshelf structure utilized for EO measurements. θ is the tilt angle, σi is the incident angle, σ is the angle of refraction, the Z-axis is parallel to the layer normal, the incident polarized light propagates in the XY plane, and the polar direction is parallel to the electric field with both of them perpendicular to the cell surface.
4.3 NLO and EO LC Materials
4.3.1 Rod-Shaped NLO LCs
4.3.1.1 LC Monomers
The discovery that all chiral tilted smectic phases exhibit a spontaneous polarization by R.B. Meyer and co-workers [52], followed by a ground-breaking work on SSFLCs [37], led to extensive investigations of SmC* materials. The first NLO work in FLCs was reported in 1981 by Vtyurin et al. [53] who performed the SHG measurement of a Schiff-base FLC, DOBAMBC. Phase matched SHG work on DOBAMBC was reported in 1985 by Shtykov et al. [54], giving the effective coefficient of deff = 0.0008 pm/V (Table 4.1). Six years later Ozaki et al. [55] confirmed the above measurement and also found that a commercial FLC mixture including phenylpyrimidine-based components, ZLI3654, exhibited a deff value of 0.005 pm/V, almost one order of magnitude larger than that of DOBAMBC. Although Liu et al. [56] reported that another commercial FLC mixture having phenyl benzoates, SCE9, exhibited a similar deff value of 0.0037 pm/V, it was the first report to measure all four individual dij coefficients of a FLC. An interesting FLC with a chiral chloro group, 3M2CPOOB, was found to have similar NLO coefficients to SCE9 [57]. Since the aforementioned FLC materials contain neither an electron donor nor an electron acceptor along the FLC polar axis in their molecules, small NLO coefficients are expected.
Table 4.1 Representative rod-shaped NLO FLC monomersa and their properties.
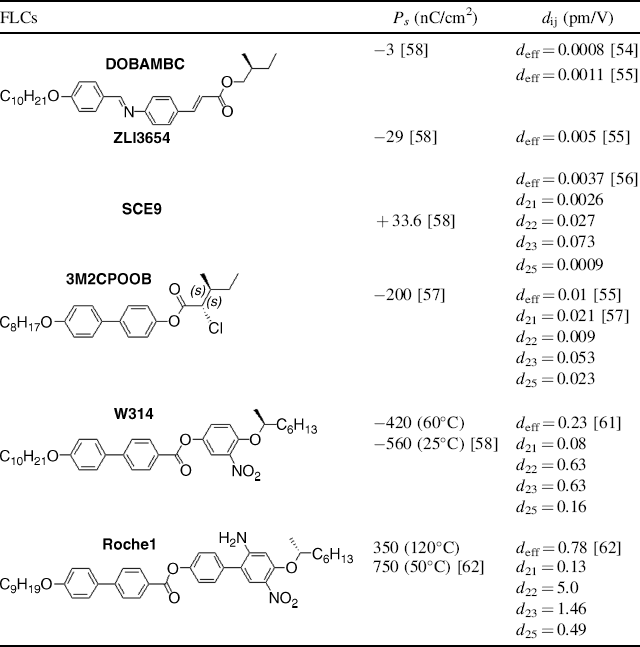
a. The chirality (R or S configuration in the chiral centers) of the FLC molecules determines the sign of polarizations and is ambiguous in many papers, and we give the chrality of these molecules unambiguously according to the original literature.
According to the oriented-gas model (see Eqs. (4.4) and (4.6)), the NLO and EO coefficients of materials are closely related to the molecular hyperpolarizabilities. The increase of the molecular hyperpolarizability will definitely enhance the NLO and EO coefficients if materials have similar polar order parameters. The factors influencing the molecular hyperpolarizability have been well established for organic chromophore materials: (i) charge separation between the donor and acceptor of a molecular chromophore, and (ii) the distance between the charge-separated species. To enhance the molecular hyperpolarizability of a FLC, incorporation of a strong chromophore, which carries both a strong donor and a strong acceptor and has two of them bridged by a highly π-conjugated linkage, into the FLC molecules along the polar direction instead of the director orientation is necessary. Such structural modifications without killing the SmC* phase, particularly in rod-shaped molecules, are a big challenge.
One of the most important NLO FLCs, (o-nitroalkoxy)phenyl 4′-alkoxylbiphenyl-4-carboxylate (W314), was successfully synthesized in 1991 by the Walba's group [58], on the basis of careful analysis of the Boulder Model for the polar order in FLCs and elegant design of molecules [59, 60]. W314 possesses a spontaneous polarization (−560 nC/cm2 at room temperature) [58], and SHG measurements exhibited the deff value of 0.23 pm/V with the largest coefficients of d22 = d23 = 0.63 pm/V [61]. This is over a 40-fold increase over the deff coefficient of ZLI3654.
It should be mentioned that W314 does not contain any laterally substituted donor along the polar direction, which may weaken charge separation induced by the strong electron acceptor (NO2). Realizing that the 4-nitroaniline unit might be a promising NLO functional group possibly incorporated into an FLC molecule along its polar direction, Schmitt et al. designed and synthesized a four-ring compound (Roche1) including both a laterally substituted amino group and a nitro group to create a strong NLO chromophore perpendicular to the molecular long axis. Roche1 exhibited a phase sequence of Cr-SmC*-SmA*-Iso and the largest spontaneous polarization (350 nC/cm2 at 120°C and 750 nC/cm2 at 50°C) ever reported for rod-shaped NLO FLCs [62]. SHG measurements of Roche1 exhibited the largest coefficient of d22 = 5.0 pm/V reported for conventional rod-shaped FLCs. These values indicate that Roche1 molecules show a perfect combination of chirality, NLO activity, and SmC* mesogenicity. Other reports on the SHG of rod-shaped FLCs existed [63–66] but the measured NLO coefficients were relatively smaller than those in W314 and Roche1.
As shown in Table 4.1, the NLO coefficient from DOBAMBC to Roche1 increased by about five thousand times because the 4-nitroaniline unit was incorporated into the molecules of Roche1 along its polar direction. In contrast, although DOBAMBC possesses a highly conjugated chromophore along the molecular long axis, its NLO coefficient was extremely small. This clearly indicates that the incorporation of a chromophore along the polar direction is essential to achieve strong NLO strength. Note that an FLC with a large spontaneous polarization does not always show large NLO coefficients (SCE9 vs. 3M2CPOOB), because the spontaneous polarization is related to the molecular dipole, while the NLO coefficients depend on the molecular hyperpolarizability. For FLCs with small NLO coefficients, the d22 coefficients along the polar direction are smaller than the d23 coefficients, indicating the absence of functional groups with strong hyperpolarizabilities along their polar direction. This further supports the importance of a strong chromophore along the polar direction in FLC molecules.
4.3.1.2 LC Polymers
One way to improve the thermal and mechanical properties of FLC monomers is to build polymers from them. The improvement in the thermal and mechanical stability can be useful for NLO applications.
FLC polymers can be classified into three categories: side-chain polymers, main-chain polymers, and cross-linked polymers. Zentel et al. [67] reported the first side-chain FLC polymers with a methylsiloxane backbone (Table 4.2), which exhibited a spontaneous polarization of 430 nC/cm2. Based on the modified version of W314 with double bonds incorporated into the two chain termini of W314, Walba et al. [68] synthesized a main-chain FLC polymer using a tetramethyldisiloxane unit as a linkage, which showed a spontaneous polarization of ~600 nC/cm2 at room temperature. Later they used the same monomer to synthesize a series of novel main-chain FLC oligomers [69] by acyclic diene metathesis (ADMET) polymerization using a Grubbs' catalyst [70]. It is interesting to note that these oligomers exhibited enantiotropic SmC* phases with very broad phase ranges. The spontaneous polarization and tilt angle for one of them were measured to be 639 nC/cm2 and 34° (Table 4.2), respectively, at 70°C. Unfortunately, neither SHG nor Pockels effect was measured for these compounds. However, on the basis of their monomeric analogue W314, the d22 and d23 coefficients of these compounds were expected to be more than 0.6 pm/V.
Table 4.2 Representative LC polymers and their properties.
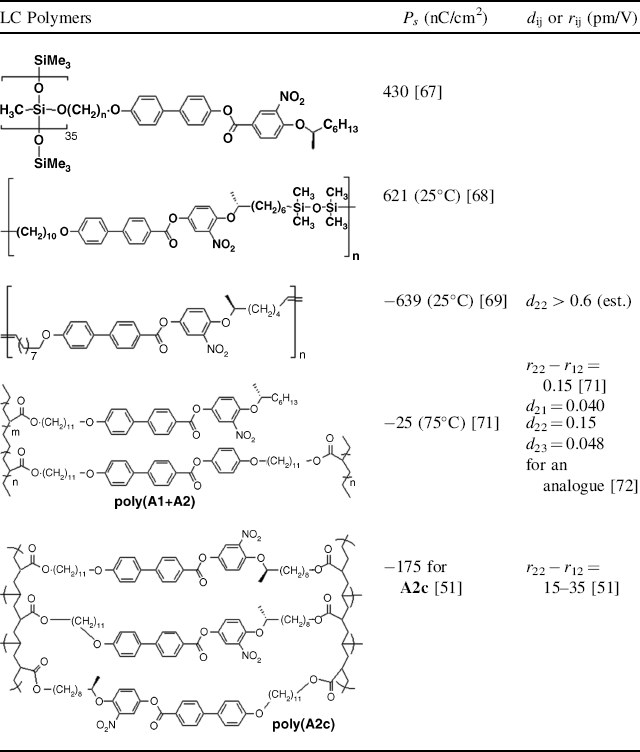
By incorporating the acrylate moiety into the chain termini of W314, the Hult group successfully developed a series of the mono- and bi-functional acrylate FLC monomers [50, 51, 71]. In situ photo-induced polymerization of these monomers alone, or co-polymerization of their respective mixtures or their mixtures with bi-functional cross-linking agents in cells generated a variety of completely or partially cross-linked pyroelectric LC polymeric films (e.g., poly(A1 + A2) and poly(A2c)) which possess good NLO and EO properties (Table 4.2). This technique has an obvious advantage that pyroelectric polymers possessing a uniform optic axis, and thermal and mechanical stability, are efficiently produced in a relatively easy way. A series of papers on these types of polymeric materials have been published, which cover the range of their syntheses [51, 71, 72], SHG and EO measurements [50, 51, 71–73], and waveguide devices [74–76]. The waveguide loss in these devices was reported to be 1.07 ± 0.95 dB/cm, which meets the requirement for NLO devices. Although the partially cross-linked polymer (poly(A1 + A2)) and its analogues exhibited smaller EO and NLO coefficients [71, 72], a completely cross-linked polymer (poly(A2c)) was shown to give a relatively large EO coefficient (r22 − r12 = 15–35 pm/V) [51]. This value makes the new material interesting for NLO and EO devices.
4.3.2 H-Shaped Ferroelectric Dimesogens
The incorporation of a strong chromophore into a FLC molecule along its polar axis, i.e., perpendicular to the molecular long axis, is essential to achieve strong NLO strength. Although a wide range of NLO chromophores [77] are available for molecular tailoring via organic synthesis, the simple rod-shape of conventional FLC molecules limits our ability to incorporate correctly oriented strong NLO chromophores while retaining the SmC* phase. Large laterally substituted functional groups including donors, acceptors, and π-conjugated linkages on the central core, will influence the molecular packing, thereby inhibiting the formation of the smectic layers. A new approach to making strong NLO FLCs was pioneered by the Walba's group [21], based on a novel molecular structure. Instead of using a conventional rod-shaped molecule, a pair of rod-shaped molecules are connected in a side-by-side “H” dimer configuration by a π-conjugated azo linkage. This configuration forces the strong chromophore to orient along the polar direction of the FLC material, producing larger NLO and EO coefficients. Another advantage of this structure is that a wider range of chromophores can be accommodated along the polar direction without significantly influencing the molecular packing to form the smectic layers.
The first H-shaped twin mesogens (also called fused twins) were reported by Malthete and co-workers [78] in 1975, which include a 1,4,5,8-tetrasubstituted naphthalene central core and exhibited a nematic phase. Later, several types of H-shaped twin mesogens containing a methylene group [79], sulfur groups (e.g., S, SO, and SO2) (Table 4.3) [80], or substituted and unsubstituted 1,4-phenylene segments [81] as lateral bridges, were reported by different groups. Although some of these compounds formed the SmC phase, these are unsuitable for NLO applications since these bridges are not conjugated linkages allowing for charge separation between a donor and an acceptor. It was the Walba's group who first incorporated a strong disperse red-1 chromophore, DR-1, into an H-shaped twin molecule (Table 4.3) [21]. However, the first reported two azo-bridged molecules exhibited the SmA* or SmB* phases instead of the desired SmC* phase [21]. The SmC* phase was generated by doping WD1 into an achiral SmC host in a range of 20–60 wt% of WD1.
Table 4.3 Representative H-Shaped Dimesogens and Their Properties.
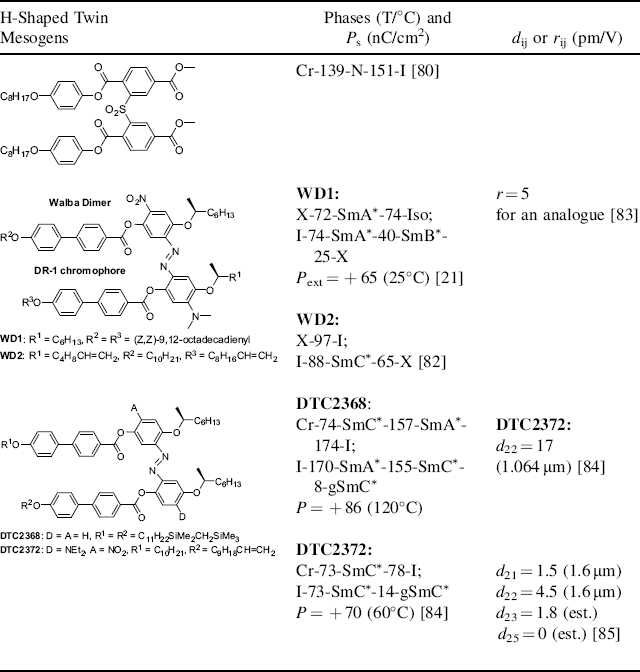
The extrapolated polarization was measured using these SmC* mixtures. The introduction of terminous double bonds into the carbon chain of the azo-bridged dimer molecule induced a thermodynamically unstable monotropic SmC* phase (WD2 in Table 4.3). ADMET polymerization of this dimer monomer using a Grubbs' catalyst produced a main-chain FLC polymer possessing an enantiotropic SmC* phase [82]. The spontaneous polarization was not measured due to the high viscosity of the polymer. Note that neither of the aforementioned H-shaped twin mesogens were investigated by SHG or Pockels effect experiments. Very recently, an analogue possessing an opposite DR-1 chromophore dipole orientation and a SmA* phase was reported to give an extrapolated coefficient of r ≈ 5 pm/V [83].
Zhang et al. found that the replacement of the dimethylamino group in the Walba dimer with a diethylamino group induced the SmC* phase (Table 4.3). A series of dimers were reported to exhibit the thermodynamically stable enantiotropic SmC* phase in a broad phase range [84]. A single polarization peak was observed in surface-stabilized cells upon the application of a triangular wave voltage, suggesting FE switching. SHG measurements of a dimer using Maker fringe method gave the extrapolated d22 coefficient of 17 pm/V at 1064 nm, more than three times as large as that for the best calamitic NLO FLC, Roche1 [62]. The d22 value is obviously resonance-enhanced due to the strong absorption of DR-1 chromophore at the second harmonic wavelength of 532 nm. SHG measurements at the longer incident wavelength of 1600 nm (i.e., much less resonance-enhanced) gave the values of d22 = 4.5 pm/V and d21 = 1.5 pm/V [85]. It is interesting to note that the d22 coefficients for the mixtures of DTC2372 in the host DTC2368 are linearly related to the mole fractions of DTC2372.
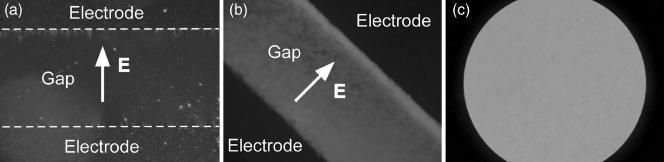
H-shaped dimesogens not only possess larger NLO coefficients than rod-shaped materials, but also achieve good alignment in both homeotropic cells with coplanar electrodes (Figure 4.10a and b) and planar cells (Figure 4.10c) with buffed polyimide (PI) or nylon alignment layers. These are interesting characteristics for NLO and EO applications.
Figure 4.10 Textures of compound DTC2372 between crossed polarizers (a and b) in a homeotropically aligned cell (2 µm thickness, 70°C, DC voltage of 1200 V across the gap of 100 µm) and (c) a planarly aligned cell (70°C) with PI alignment layers at both sides. (a) Polarizers along the indicatrix axes, and (b) the cell in (a) was rotated by about 45°.
4.3.3 BC LCs
4.3.3.1 Introduction
Since bent-core mesogens are a very special type of mesogen, and possess quite distinct properties from other types of mesogens previously reported, herein we give them a more detailed introduction and discussion. In 1996 Niori et al. presented a paper [42] where the existence of polar liquid crystal phases in materials formed by achiral molecules was proven. Until then, structural polarity and molecular chirality had been unequivocally linked to each other. The studied compounds contain a bent-shape molecular core. For that reason, those materials were called BC LCs or banana-shaped mesogens.
A typical mesogenic BC molecule is shown in Figure 4.11 [86]. From the structural point of view, the molecule is conformed by a bent central unit, two rod-like wings, and linking groups. The bending angle is normally close to 120°. Terminal chains are usually alkyl or alkoxy flexible chains, as in normal calamitic molecules. This modification of the molecular geometry, apparently ingenuous, of a rod-like molecule brings about, apart from conventional phases such as N, SmA or SmC, completely new mesogenic structures. The new mesophases were initially named as B followed by a number. This nomenclature has been presently abandoned, at least in part, and has been substituted by another less ambiguous and more descriptive classification.
Figure 4.11 The general molecular structure of a bent-core molecule. The different constituents are: CU central unit, RC1 and RC2 linear rigid cores which can be the same or different, X and Y linking groups, Z lateral substituents, and R1 and R2 terminal chains.
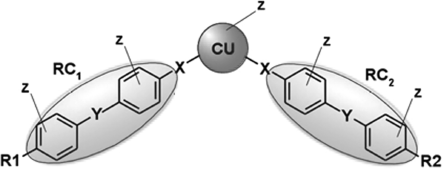
Figure 4.12 is a diagram where the common way to represent BC molecules depending on the point of view is shown. In a rigid model, the molecule is conveniently described as a bow with a long axis (the string n) and a short axis (the arrow b). Furthermore, a tilted smectic layer of achiral BC molecules is a chiral structure, and the same happens with some columnar stackings [87, 88]. This fact is represented with diagrams with different colors (red (−) and blue (+)) for opposite chirality.
Figure 4.12 Schematic representation of an isolated bent-core molecule (left). When the molecule is placed in a tilted smectic layer, the structure becomes chiral, and opposite chirality is represented by different colors (right).

There are lots of molecular arrangements characteristic of BC LCs: smectic [88], undulated [89], and columnar phases of rather different types [90, 91]. In addition, there are biaxial nematic organizations [92] (though microscopically they seem to be SmC cybotactic nuclei [93, 94]), isotropic-like dark conglomerate phases [95, 96], and more specific structures such as B4 [97, 98], B5 [99] or B6 [100, 101]. In this chapter we will focus our attention only on the B2 (or SmCP) phases. For the reader interested in more details we refer to reviews on the subject of BC LCs. In references [102, 103] an enlightening description of the banana phases can be found. Another extensive and well-documented review [20] gives special attention to structure–property relationship and chirality-related phenomena. Some functionalities of these materials are also well surveyed [104].
4.3.3.2 SmCP Phases
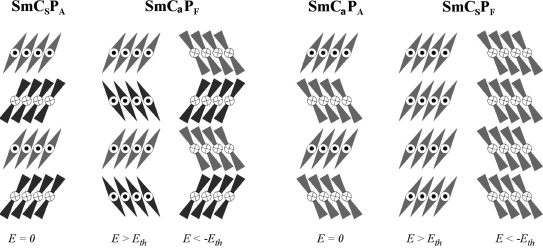
The most peculiar characteristics of BC LCs derive precisely from their anomalous molecular shape. The molecules are forced to adopt a compact packing arrangement that restricts their rotational freedom around the main molecular director (the string of the bow n) and, to minimize the excluded volume, molecules tend to stack in with parallel bent directions (see the banana bunch in Figure 4.13). In the SmCP phases, molecules are arranged in layers with a non-null tilt angle and each layer has identical polar order. Depending on the orientation of the tilt and molecular dipole directions in adjacent layers, there are four basic SmCP phases [88] (see Figure 4.14). The layer chirality alters from layer to layer in the SmCsPA (synclinic antiferroelectric) and SmCaPF (anticlinic ferroelectric) phases, giving rise to racemic structures. The other two variants SmCaPA (anticlinic antiferroelectric) and SmCsPF (synclinic ferroelectric) are homochiral. If we extend the correlation to more than two layers there are more possible tilt-polarization combinations. They can also be described as a repetition of stacks of the four different basic structures as described before, including information on the interface between the stacks. These suprastructures are designated as [SmCsPF]aPs or [SmCsPF]aPA [105]. The multi-layer stacking structure [SmCsPF]aPs was induced from dark conglomerate phases by an external electric field and was also used to reasonably explain FE switching in a columnar phase [106].
Figure 4.13 In a bunch of bananas, the packing is more compact if the bent directions are parallel to each other.

Figure 4.14 From left to right: SmCsPA, SmCaPF, SmCaPA, and SmCsPF states. The first two are racemic and consist of layers with opposite chirality, and the last two are homogeneously chiral structures. As indicated, an electric field above a threshold value Eth can transfer SmCsPA to SmCaPF, and SmCaPA to SmCsPF via switching on a smectic cone.
An electric field parallel to the layers can induce a polarization reorientation. The switching can be either around the smectic cone or along the long molecular axis (Figure 4.15). The kind of switching depends on factors such as the field frequencies, field intensity, or material viscosity. In most cases of practical interest, the first kind of switching (right) takes place and the chirality of the layers is kept in the process [88, 107]. In this situation a SmCsPA structure is changed to SmCaPF and SmCaPA is switched to SmCsPF. Recently, a new type of switching mode involving both switching modes (Figure 4.15) in the same molecule was reported in the columnar (i.e., modulated smectic) phase formed by the BC molecule with a bulky carbosilane terminous [108].
Figure 4.15 Switching by molecular rotation around the long axis (left) changes both chirality and polarity, while switching on the tilt cone (more common, right) only changes polarity with retention of chirality. (See the color version of this figure in Color Plates section.)

The ground-state FE phases (i.e., SmCaPF and SmCsPF) for bent-core molecules are rare and also difficult to identify [109]. A chiral BC compound P8OPIMB6* was unambiguously confirmed to be a ground state ferroelectric (SmCaPF) by both EO switching and zero-field SHG experiments [110]. It was later found that the appearance of ferroelectricity or antiferroelectricity for these type of homologues shows even–odd behavior [111]. Most BC compounds show ground-state AF phases (i.e., SmCaPA and SmCsPA). It has been suggested [112] that the ground-state AF behavior takes place because the end chains in SmCPA structures are essentially parallel, permitting out of layer fluctuations. This supplies a favorable entropic contribution, which is absent in SmCPF variants, to the free energy. From the viewpoint of NLO, we will be especially interested in the FE states. In the following we will concentrate on the two FE structures (SmCaPF and SmCsPF), no matter how they are generated, spontaneously or induced by an electric field.
4.3.3.3 Qualitative Aspects of NLO Properties of BC SmCP Phases
Soon after its discovery, SmCP structures were pointed out as candidates with potentialities in the field of electronic nonlinear optics [113–115]. Among the NLO properties, we include both SHG and electronic EO (Pockels) effect. Intuitively these capabilities can be understood in terms of two reasons:
(a) the packing conditions derived from the molecular shape, and (b) the molecular geometry itself, which allows for a significant electronic response at molecular level along the polar axis and results in a relatively high molecular hyperpolarizability.SmCP structures are known to exhibit high spontaneous polarization Ps. In fact, the degree of polar stereocontrol achievable in these phases is much better than that in poled polymers or calamitic SmC* phases. The Ps values (in the range of 500–1000 nC/cm2) in these materials imply that, typically, more than one half of the molecular dipoles are oriented along the polar axis. In contrast, only a few percent of chromophores are aligned in poled polymers, or ~20% in conventional FLCs.
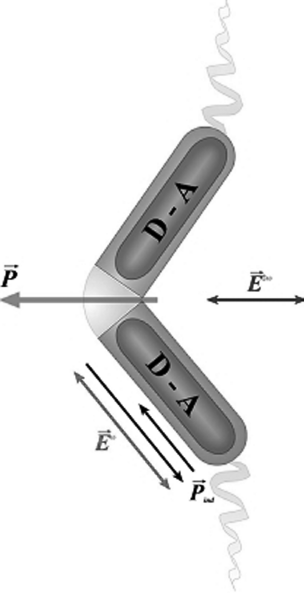
The second point is illustrated in Figure 4.16, where a simplified model for a NLO BC molecule is presented. It is assumed that the NLO activity arises from two one-dimensional β-units integrated in the wings of the molecule. The β-units are simple donor–acceptor groups linked by a conjugated electronic system. As can be seen, there is always a molecular β response along the short axis (b), which macroscopically implies the existence of an important net component of NLO activity along the polarization direction. On the other hand, it is well known that to attain a high NLO response, the distance that allows for valid interaction between donors and acceptors must be as long as possible. In contrast to rod-like molecules, BC molecules can incorporate into their wings large β functional arrays, involving strong donor and acceptor groups with two or more conjugated aromatic rings between, without critically damaging its mesogenic character. In summary, due to their outstanding molecular shape, BC molecules are allowed simultaneously to have an inherently or field-induced FE character and large components of β along the macroscopic polar axis.
Figure 4.16 In BC molecules donor–acceptor (D-A) groups can be large since they can be integrated into the molecules without spoiling their mesogenic character. Moreover, there is always a nonlinear response along the polarization direction (red arrow).
4.3.3.4 The Second-Order Susceptibility and EO Tensors
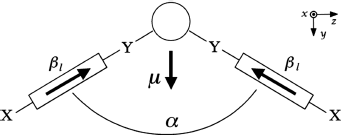
The above ideas will now be elaborated in a more quantitative manner. If the NLO activity is assumed to be mainly driven by the longitudinal hyperpolarizability βl along the lateral structures in Figure 4.17, the molecular hyperpolarizability tensor ![]() is given in reduced notation by [116],
is given in reduced notation by [116],
Figure 4.17 Schematic representation of the chemical structure of an average BC molecule. X and Y represent donor and acceptor groups, respectively, which are connected to each other through a conjugated spacer. βyzz and βyyy are assumed to be the dominant hyperpolarizability coefficients, with the rest being negligible. The whole tensor can be considered to arise from a unique one-dimensional hyperpolarizability βl directed longitudinally along the wings of the molecule.
(4.14) 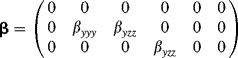
where
(4.15) ![]()
Here βl is the longitudinal hyperpolarizability for frequency doubling, i.e., ![]() .
.
The macroscopic second-order susceptibility tensor will be obtained using Eqs. (4.4) and (4.5). In the SmCsPF structure, the four independent dij coefficients (see Eq. (4.11) in Section 4.2.3.1) can be expressed in the XYZ frame of Figure 4.8a as:
where θ is the tilt angle, and the rest of the symbols are given by
Here the same local-field factor f has been assumed for ω and 2ω lights, and Ψ is the angle between the macroscopic polarization and the molecular short axis (b). The introduction of this angle is a simple way to incorporate into our model some molecular disorder, which is modeled as rotation around the director (n). In terms of Ψ, Ps is given by
μb is the molecular dipole moment component along the b direction.
For the SmCaPF structure, Eq. (4.16) remains valid with the exception of the coefficients d14 = d25 = d36 = 0.
If the perfect molecular order is assumed (Ψ = 0 and the thermal averages are equal to 1 or 0), further simplification is achieved. In this context, D′ = 0, and D and d are related to each other (see Eqs. (4.16) and (4.17)) via the bending angle, ![]() . If α = 120° then D/d = 3.
. If α = 120° then D/d = 3.
The above equations allow an idea of the d coefficients if some rather general assumptions are made and the microscopic parameters (βl and μb) are known. We will return to this point in Section 4.3.3.6.
The electronic contribution to the r tensor will similarly be obtained using Eqs. (4.6) and (4.7). In the SmCsPF structure, the four independent dij coefficients (see Eq. (4.13) in Section 4.2.3.2) can be similarly given by:
where R, R′ and r are proportional to D, D′ and d, respectively, with the same proportionality constant, i.e., R/D = R′/D′ = r/d = K, with
Here ω and ω′ are the frequencies of the light which are modulated and doubled in the EO and SHG processes, respectively.
4.3.3.5 SHG and EO Properties on Presently Known BC LCs
So far most experimental tests have been focused on the determination of the d coefficients through measurements of SHG. The first results found in materials of the P-n-O-PIMB family were very promising, reporting d coefficients in the range of 10–50 pm/V [115–117], almost within the range needed for applications. However, subsequent measurements on these and other materials [118–121], did not confirm these data, the new d values being about one order of magnitude lower. The reasons for the disparities are still unclear. In this regard it is interesting to point out that the experiments are not easy to perform, and problems with the sample alignment or the quantitative analysis of the raw data must be in the origin of the discrepancies.
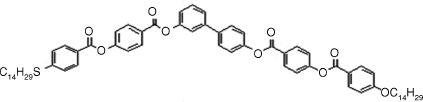
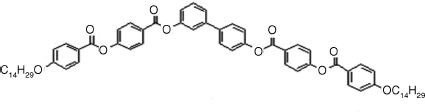
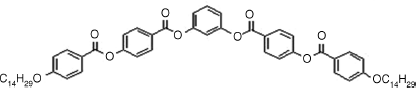
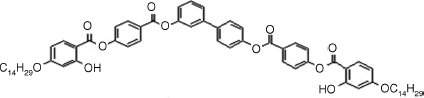
Table 4.4 D and d Coefficients (pm/V) for SHG in SmCPF Compoundsa
| Bent-core Mesogen | D or d (pm/V) |
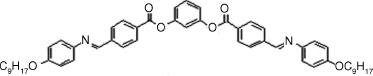 |
1: D = 6, d = 3.8 |
| SmCaPF [119] | |
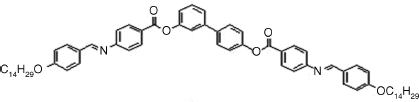 |
2: D = 8, d = 4 |
| SmCaPF [121] | |
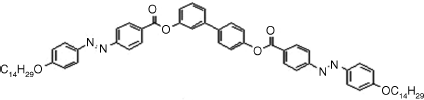 |
3: D = 6.1, d = 3.3 |
| SmCsPF [121] | |
 |
4: D = 2, d = 1.8 |
| SmCsPF [119] | |
 |
5: D = 2, d = 1.2 |
| SmCsPF [121] | |
 |
6: D = 3.1, d = 1.8b |
| SmCsPF | |
 |
7: D = 1.9, d = 1 |
| SmCsPF [119] | |
 |
8: D = 17, d = 8c |
| SmCsPF [122] | |
| a. Measurements were performed at the fundamental light wavelength of 1064 nm except for compound 8. SmCaPF and SmCsPF denote field-induced FE structures in spite of the ground-state AF structures. | |
| b. Unpublished results. | |
| c. Extrapolated from a 50% mixture at 1600 nm. | |
As shown in Table 4.4, all materials have AF ground states and were transformed into the SmCPF phases under field. The tilt angles are about 45°. The form of the d full tensor can be obtained from Eqs. (4.11) and (4.16) for the SmCsPF case. In all cases the coefficient D′ was found to be small, with D′ ≈ 0.1D. For the SmCaPF variant the same equation applies with d14 = d25 = d36 = 0.
It should be noted that all compounds, perhaps with the exception of compound 8, exhibit SHG efficiencies not large enough for NLO applications. The explanation for this fact as well as for the slight differences among these compounds can be understood in terms of the molecular structures. For example, the azo and imine groups (compounds 1–3) allow for a more delocalized charge distribution between donors and acceptors. In contrast, compounds 4–7 only contain ester groups, which significantly cut the electronic conjugation and, consequently, their efficiencies are lower.
Compound 8 is different [122]. In one of the wings of this molecule there are a strong acceptor, α-cyano cinnamate (ߝCH=CH(CN)COOߝ), and a piperazine moiety (donor), connected via an azo (ߝN=Nߝ) bridge, which preserves the conjugation. This material is the first BC liquid crystal specifically designed for NLO applications. There is significant enhancement in the SHG response which proves the basic efficacy of the synthesis design.
In comparison to SHG, electronic EO effects have been investigated comparatively less in these materials. These studies are very interesting because they directly prove the performance of the compounds for the construction of ultra-fast EO modulators. However, the experiments are difficult because it is necessary to handle high-frequency electric fields for the samples. The magnitude of the effect is measured through the EO r tensor. Up to now, the highest EO coefficients found are in the range of 10 pm/V achieved for a 40 MHz driving voltage in the material P8OPIMB6* [123].
Now the question is to find out to what extent these d or r values can still be improved. We will try to answer this question in the next section where we analyze what we can possibly expect about the optimum NLO properties of these materials.
4.3.3.6 Limits of the SHG and EO Responses in BC LCs
To find an estimate of the maximum NLO coefficients of any material we have to consider the limits of the molecular hyperpolarizabilities for a given molecular shape, some important structural parameters such as the degree of polar order or the density of molecules, and the refractive indices. The first parameter has the strongest influence on the NLO performance and depends on the nature of the donor and acceptor groups. We will restrict our disccusion to this point since for BC LCs there are no clear possibilities of influencing the rest of the factors by means of chemical design.
As has been pointed out before, in a one-dimensional donor–π-electron–acceptor system, the β values depend on the strength of the donor and acceptor and on the extension of the conjugation length. However, at least for aromatic-ring systems, the highest β per unit length is attained for two or three connecting rings. On the other hand, it is dubious to be able to maintain the mesogenic character of a BC molecule by attaching NLO moieties with arbitrary length to it. Trying to be realistic, we will consider the possibility of integrating NLO groups with only one or two aromatic rings into the BC LC molecules.
We have shown how to obtain the hyperpolarizability values for benzene- and stilbene-based chromophores (see Section 4.2.2 for details). Here we will show how to transfer these values to LCs. A similar study has been carried out by two groups [29, 37] for several organic solids. Upon transferring the data, however, it should be noted that in the bulk structure a red shift Δλ in λ0 is normally observed, i.e., λ0(LC) = λ0 + Δλ. Empirically, it is found Δλ ≈ 50 nm. Using this correction, the SHG and EO hyperpolarizabilities can be computed from Eqs. (4.8) and (4.9) at the desired wavelengths. Finally, from the knowledge of some structural parameters the full tensors d and r can be obtained using Eqs. (4.17) and (4.20), respectively.
To reach the limits of the NLO responses it is interesting to take advantage of the resonance enhancement. In accordance with this idea, the wavelength for operation should be near (but not just at) the absorption wavelength. Empirically, a good compromise is a wavelength about 100 nm away from the absorption peak, i.e., λc = λ0 + Δλ + 100 nm. The d and r values obtained in this way are considered to be optimized for SHG and EO effect respectively.
The SHG and EO hyperpolarizabilities are obtained from Eqs. (4.8) and (4.9) at λ = λc, and the rest of the parameters needed in the calculations (see Eqs. (4.16)–(4.20)) will be typical values of BC LCs. Specifically, we have used for all compounds N = 9 × 1026 molecules/m3 (compound 1 in Table 4.4), θ = 45°, nω = n2ω = 1.52, and ε = 5. The degree of molecular order is estimated as ![]() from typical Ps and μb data (see Eq. (4.18)), and
from typical Ps and μb data (see Eq. (4.18)), and ![]() (assuming a Gaussian distribution for Ψ). The results for the maximum D and R values are shown in Figures 4.18 and 4.19, respectively. The non-zero individual coefficients of the tensors are somewhat smaller. They are: d22 = d ≈ 0.24D (r22 ≈ 0.24R), and d21 ≈ 0.55D (r12 ≈ 0.55R).
(assuming a Gaussian distribution for Ψ). The results for the maximum D and R values are shown in Figures 4.18 and 4.19, respectively. The non-zero individual coefficients of the tensors are somewhat smaller. They are: d22 = d ≈ 0.24D (r22 ≈ 0.24R), and d21 ≈ 0.55D (r12 ≈ 0.55R).
Figure 4.18 Log–log plot of the dependence of the D coefficient for SHG on the operating wavelength for the second-harmonic light λc = λ0 + 150 nm. Open circles represent BC molecules with disubstituted benzenes as NLO active groups. Closed circles are the same for the stilbene case. The straight lines are empirical relations between D and λc, ![]() for the benzenes and
for the benzenes and ![]() for the stilbenes. D is expressed in pm/V and λc in nm.
for the stilbenes. D is expressed in pm/V and λc in nm.
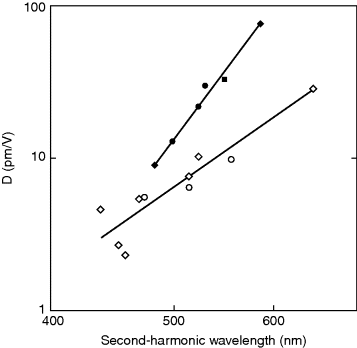
Figure 4.19 Log–log plot of the dependence of the R coefficient for the EO effect on the operating wavelength of the modulated light λc = λ0 + 150 nm. Open circles represent BC molecules with disubstituted benzenes as active groups. Closed circles are the same for the stilbene case. The straight lines are empirical relations between R and λc, ![]() for the benzenes and
for the benzenes and ![]() for the stilbenes. R is expressed in pm/V and λc in nm.
for the stilbenes. R is expressed in pm/V and λc in nm.
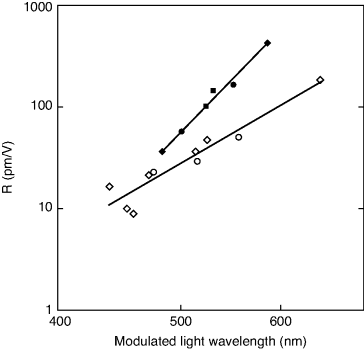
The predicted SHG efficiencies are moderate but not huge. Clearly an inevitable dilution effect of the NLO response takes place upon integrating relatively small NLO moieties into large BC LC molecules. More specifically, benzene chromophores are less likely to generate bent-core materials with stronger SHG strength, since the d coefficients are not better than those of some commercially available inorganic crystals such as LiNbO3. Stilbene derivatives are better, though, obviously, more difficult to achieve in the sense that it may be problematic to maintain the mesogenic character of a molecule with two relatively long chromophores. If only one active group is introduced into one of the molecular wings, the coefficients shown in Figure 4.18 should be halved. In the case of stilbene derivatives, the SHG efficiencies are still good. Regarding the EO effect, the materials seem even more promising. The optimized r values are rather high (Figure 4.19), and there is still a large margin for improvement starting from the currently available materials. Coefficients about r = 50 pm/V for modulation of visible or near IR light seem feasible.
Clearly these values should be enough for applications. However, to determine whether these compounds can be used in practice, other issues must also be considered. For example, one of the key points is the ease of processability of the materials to fabricate different devices. In this sense, BC molecules present some problems to align, and certainly they are more difficult to handle than classical LCs. In this respect, some aligning methods have been applied with some success, such as the application of an AC electric field in a direction parallel to the plane of the (uncoated) substrates [1 1 0] or simply shearing the sample [1 2 4]. However, the alignment of BC molecules is still at the research stage. Another problem to solve is the temperature range of the mesophases. In this regard, some progress has been made, and more and more structures are being reported to exhibit the SmCP phase transition close to room temperature. On the other hand, irrespective of the mesophase temperature ranges, the mesogenic structures can be “frozen” at room temperature using the technique of in situ photopolymerization [125–127], if the BC molecules are incorporated into photo-induced polymerizable groups such as acrylate or methacrylate. This approach offers the additional advantage of improving the mechanical properties and locking the FE order in the pyroelectric polymer without the necessity of any external electric field. In this respect, stable SHG has been demonstrated at room temperature for a homeotropically aligned SmCPF film prepared under these conditions [128]. Alternatively, some materials show a high tendency to freeze the SmCPF layer structures with polar order into the glassy ordered states at room temperature [19, 20, 129]. Likewise, other macromolecular designs such as polymers [130–134], Langmuir–Blodgett films [135–137], or sol–gel materials [138] can also be explored for BC mesomorphic structures.
In summary, we have shown that the bent molecular shape is appropriate for the correct design of NLO materials. Structures are possible with large efficiencies both in SHG and EO processes. Some improvements have been achieved, but more design and synthetic work are still required to approach the theoretical potentialities of these materials. Moreover, applications require other important points to be addressed. Many strategies are at hand but the problems to be solved are far from being trivial. Research in this area is a challenging task for the near future.
4.3.4 Miscellaneous LC NLO Materials
In addition to rod-shaped chiral FLCs, H-shaped chiral ferroelectric dimesogens, and BC achiral polar mesogens, there are other interesting LC materials which show polar order and NLO activities (Table 4.5). The incorporation of chirality into disc-shaped molecules led to ferroelectricity in the tilted columnar phases as was realized by Helfrich [139, 140] and Scherowsky [141, 142]. It was reported that the polar order is perpendicular to the columns and the tilted molecules can be switched by the applied electric field. Hennrich et al. [143] recently reported that an achiral octopolar C3-symmetric liquid crystal exhibited SHG activity at zero field, which was as strong as found in poled-polymer systems. This work indicated that this compound spontaneously assembles into a non-centrosymmetric supramolecular polar structure in the LC phases. An octopolar-based SiO2 solid-state hybrid composite was later found to show quadratic NLO activity in a non-resonant SHG experiment [144].
Table 4.5 Special Types of NLO LC Materials and Their Properties.
| Polar Mesogens | Phases (T/°C), Ps (nC/cm2), and d (pm/V) |
 |
Ps = 180 [140] |
 |
Cr-68-Col-74-Iso; |
| Iso-66-Col-~45-Cr | |
| Ps = 54 [142] | |
 |
Cr1-40-Cr2-59.5-Colh- |
| 68.5-ND-112-Iso [143] | |
 |
Cr-80-X-82-Colr-139-Colh- |
| 168-Iso | |
| Iso-164-Colh-137-Colr-74-Cr | |
| Ps = ~250 [145, 146] | |
 |
Cr-58-ColX-90-ColhPA- |
| 123-Colh-174-Iso [147, 148] | |
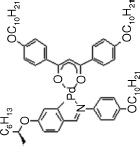 |
Cr-114.4-SmA*-118.4-Iso; |
| Iso-118-SmA*-111-SmC*-89-Cr [150] | |
| Ps = −29 | |
| deff = 0.047 [151] |
Switchable polar columnar phases were reported to be formed by two types of achiral molecules: BC polycatenar molecules [145, 146] and urea derivatives linearly linked by hydrogen bonding [147, 148]. The former umbrella-like polycatenar compound exhibited three columnar phases, Colh, ColhPA, and ColX phases. The Colh phase is paraelectric, and the ColhPA phase is axially polar and switchable under an external electric field with a spontaneous polarization of ~250 nC/cm2. The Colh–ColhPA phase transition involves the transformation from flat discs to cones for the shape of the columnar building blocks. The urea derivatives exhibited two columnar phases, Colh and Colr. Although polar switching was observed in both the systems, the absence of SHG signal at zero field indicated that neither of them is truly FE. The former has a ground-state AF organization in the ColhPA phase, while for the latter, the polar structure along the column was readily induced by cooperative interaction between the polarization and the applied electric field. The single hysteresis loop observed in both systems is due to the slow relaxation of the field-induced FE states to the ground-state apolar supramolecular structures. Takezoe et al. recently have reviewed different types of switchable polar columnar phases in a highlight paper [149].
Baena et al. [150] reported the first metal-containing FLCs (i.e., ferroelectric metallogens) with the molecules consisting of a planar central core and four tails, namely three achiral ones and one chiral one. This complex can be also regarded as an H-shaped dimesogen with the palladium atom as the lateral bridging group. SHG measurements [151] of this complex exhibited a small effective coefficient of deff = 0.047 pm/V probably due to the lack of an electron acceptor along the direction normal to the molecular long axes.
Despite usually found in the smectic or columnar phases, polar order can also appear in the nematic phase. The Watanabe's group [43, 152] reported that a commercially available aromatic copolyester (also called Vectra), consisting of 4-hydroxybenzoic acid (p-HBA) and 6-hydroxy-2-naphthoic acid (HNA) in a molar ratio of 73/27, exhibited strong SHG at zero field in its nematic phase. It was shown that dipole–dipole interaction exists in this biaxial polymer with Cs symmetry. However, owing to the high viscosity of this material, the macroscopic polarity cannot be reversed even with a strong electric field, suggesting that Vectra is pyroelectric rather than ferroelectric. Another interesting polar nematic material is α-helical polypeptide. This chiral polymer has been found to form macroscopically polar order along the helical axis in the thermotropic and lyotropic cholesteric phases [44, 153, 154]. The polarization in this peptide can be reversed with the applied electric field, indicating that the material is truly a proper ferroelectric. To the best of our knowledge, this polymer is the first known nematic ferroelectric.
4.4 Conclusions and Future Prospects
We have given an overview of FLC NLO materials and their efficiencies in SHG and the Pockels effect. Like poled polymers, FLCs are a promising alternative to inorganic crystals since their polar direction can be easily controlled with the applied electric field, and the LC film technology can be readily integrated with silicon VLSI technology on a large area and volume. As we discussed, real ferroelectricity is very difficult to achieve for LCs owing to strong dipole–dipole interaction in macroscopically polar superstructural organizations. So far there are only three types of materials: surfaced-stabilized bistable chiral FLCs, ground-state chiral bent-core SmCaPF materials, and a nematic α-helical polypeptide, which can be properly called ferroelectrics. However, as long as non-centrosymmetric superstructures can be achieved for LC materials, they are still interesting for NLO and EO applications. Hence, in this chapter we have discussed the helielectric SmC* materials, cross-linked pyroelectric LC polymers, polar nematic materials, field-induced polar banana materials with ground-state AF organization (e.g., SmCsPA and SmCaPA) and ground-state dark conglomerate phases (e.g., BC materials with silane termini), octopolar LC materials, field-induced polar discotic materials, and field-induced polar polycatenar materials with hexagonal columnar phases. Most of these materials do not possess SHG activity at zero field, but strong SHG and even polarization switching in some cases were observed upon field application.
Although relatively good alignment can be readily achieved for rod-shaped NLO FLCs, their NLO optical susceptibilities (χ(2)) are too small for commercial applications. BC materials are much better, but their alignment is poor, particularly in planar cells. H-shaped twin mesogens can achieve good alignment in both homeotropic and planar cells, and larger NLO activity should be obtained by incorporating stronger chromophores along the polar direction without influencing the SmC* phase. Thus, they could be promising candidates to penetrate commercial NLO and EO markets. Certainly the development of new types of NLO LC materials with special properties is also necessary.
Large nonlinear optical susceptibilities (χ(2)) are not the only requirement for applications in devices. Nalwa et al. in a book chapter [155] have listed 10 possible characteristics for an ideal NLO material: (1) large nonlinear figure-of-merit for frequency conversion, (2) high laser damage threshold, (3) fast optical response time, (4) wide phase matchable angle, (5) architectural flexibility for molecular design and morphology, (6) ability to process into crystals or thin films, (7) optical transparency, (8) ease of fabrication, (9) nontoxicity and good environmental stability, and (10) high mechanical strength and thermal stability. None of the single LC (or other types) materials could possess all of the above properties. Hence, the choice of materials for specific applications will depend on the particular properties required, and some trade-offs are certainly necessary. During the past decades, substantial progress has been made to enhance the EO activity of poled polymeric materials, reduce their EO losses, and improve their thermal and photochemical stability. In comparison to poled polymers, the NLO efficiency of LCs is relatively small. Incorporating strong chromophores into H-shaped twin molecules or bent-core molecules seems to be a good way to enhance the NLO activity in LC materials. To improve thermal and photochemical stability, cross-linked polymers should be used, and photochemically sensitive functional groups should be avoided as much as possible. EO loss will be a less critical issue since a normal pyroelectric LC polymer already has losses under 2 dB/cm [76]. Integration of these properties together into single materials, devices, or systems is a challenging task which will definitely lead to a bright future for NLO LC materials.
Acknowledgments
YQZ would like to thank Micron Technology Inc. for its support and approval to write this chapter. JE would like to thank the generous support from MICINN-FEDER of Spain-UE (Project MAT 2009-14636-C03) and the Basque Country Government (Project GIC10/45). The authors would like to sincerely thank Veronica Cepak and Chris Walker for taking on the burden of carefully reading the manuscript, as well as Mike O'Callaghan for his comments on parts of the manuscript.
1. P. A. Franken, A. E. Hill, C. W. Peters, and G. Weinreich. Generation of optical harmonics. Phys. Rev. Lett. 1961, 7, 118–119.
2. C. Bosshard and P. Günter. Electro-optical effects in molecular crystals and polymers. In: H. S. Nalwa and S. Miyata, Eds., Nonlinear Optics of Organic Molecules and Polymers, CRC Press, Inc., Boca Raton, FL, 1997.
3. L. R. Dalton, P. A. Sullivan, and D. H. Bale. Electric field poled organic electro-optic materials: state of the art and future prospects. Chem. Rev. 2010, 110, 25–55.
4. S. R. Marder. Organic nonlinear optical materials: where we have been and where we are going. Chem. Commun. 2006, 131–134.
5. B. J. Coe. Switchable nonlinear optical metallochromophores with pyridinium electron acceptor groups. Acc. Chem. Res. 2006, 39, 383–393.
6. D. M. Burland, R. D. Miller, and C. A. Walsh. Second-order nonlinearity in poled-polymer systems. Chem. Rev. 1994, 94, 31–75.
7. P. A. Sullivan and L. R. Dalton. Theory-inspired development of organic electro-optic materials. Acc. Chem. Res. 2010, 43, 10–18.
8. O. Ostroverkhova and W. E. Moerner. Organic photorefractives: mechanisms, materials, and applications. Chem. Rev. 2004, 104, 3267–3314.
9. P. Innocenzi and B. Lebeau. Organic–inorganic hybrid materials for non-linear optics. J. Mater. Chem. 2005, 15, 3821–3831.
10. S. Yitzchaik and T. J. Marks. Chromophoric self-assembled superlattices. Acc. Chem. Res. 1996, 29, 197–202.
11. O. R. Evans and W. Lin. Crystal engineering self-organized porphyrinic materials ring of NLO materials based on metal-organic coordination networks. Acc. Chem. Res. 2002, 35, 511–522.
12. C. M. Drain, A. Varotto, and I. Radivojevic. Self-organized porphyrinic materials. Chem. Rev. 2009, 109, 1630–1658.
13. J. Luo, X.-H. Zhou, and A. K.-Y. Jen. Rational molecular design and supramolecular assembly of highly efficient organic electro-optic materials. J. Mater. Chem. 2009, 19, 7410–7424.
14. T. P. Radhakrishnan. Molecular structure, symmetry, and shape as design elements in the fabrication of molecular crystals for second harmonic generation and the role of molecules-in-materials. Acc. Chem. Res. 2008, 41, 367–376.
15. L. R. Dalton, W. H. Steier, B. H. Robinson, C. Zhang, A. Ren, S. Garner, A. Chen, T. Londergan, L. Irwin, B. Carlson, L. Fifield, G. Phelan, C. Kincaid, J. Amend, and A. Jen. From molecules to opto-chips: organic electro-optic materials. J. Mater. Chem. 1999, 9, 1905–1920.
16. J.-M. Lehn. From molecular to supramolecular nonlinear optical properties, In: Materials for Nonlinear Optics. ACS Symposium Series, vol. 455, 1991 (Chapter 28).
17. J. M. Hoover, R. A. Henry, G. A. Lindsay, M. P. Nadler, S. F. Nee, M. D. Seltzer, and J. D. Stenger-Smith. Langmuir–Blodgett multilayers of fluorinated, main-chain chromophoric, optically nonlinear polymers. In: Macromolecular Assemblies in Polymeric Systems. ACS Symposium Series, vol. 493, 1992 (Chapter 9).
18. S. T. Lagerwall. Ferroelectric and Antiferroelectric Liquid Crystals, Wiley-VCH, Germany, 1999.
19. S. Chandrasekhar, B. K. Sadashiva, and K. A. Suresh. Liquid crystals of disc-like molecules. Pramana 1977, 9, 471–480.
20. R. A. Reddy and C. Tschierske. Bent-core liquid crystals: polar order, superstructural chirality and spontaneous desymmetrisation in soft matter systems. J. Mater. Chem. 2006, 169, 907–961.
21. D. M. Walba, D. J. Dyer, T. Sierra, P. L. Cobben, R. Shao, and N. A. Clark. Ferroelectric liquid crystals for nonlinear optics: orientation of the disperse red 1 chromophore along the ferroelectric liquid crystal polar axis. J. Am. Chem. Soc. 1996, 118, 1211–1212.
22. S. Sergeyev, W. Pisula, and Y. H. Geerts. Discotic liquid crystals: a new generation of organic semiconductors. Chem. Soc. Rev. 2007, 36, 1902–1929.
23. M. Trollsås, F. Sahlén, P. Busson, J. Örtegren, U. W. Gedde, A. Hult, M. Lindgren, D. Hermann, P. Rudquist, L. Komitov, B. Stebler, and S. T. Lagerwall. The influence of the molecular structure on the second-order nonlinear optical properties of pyroelectric liquid crystalline polymers. In: Organic Thin Films. ACS Symposium Series, vol. 695, 1998 (Chapter 23).
24. D. M. Walba, M. B. Ros, N. A. Clark, R. Shao, K. M. Johnson, M. G. Robinson, J. Y. Liu, and D. Doroski. Ferroelectric liquid crystals designed for electronic nonlinear optical applications. In: Materials for Nonlinear Optics. ACS Symposium Series, vol. 455, 1991 (Chapter 32).
25. J.-C. Dubois, P. L. Barny, P. Robin, V. Lemoine, and H. Rajbenbach. Properties of side chain liquid crystal and amorphous polymers. Applications to non-linear optics. Liq. Cryst. 1993, 14, 197–213.
26. M. Schadt. Linear and non-linear liquid crystal materials, electro-optical effects and surface interactions. Their application in present and future devices. Liq. Cryst. 1993, 14, 73–104.
27. W. Känzig. Ferroelectrics and antiferroelectrics. In: F. Seitz, T. P. Das, D. Turnbull, and E. L. Hahn,Eds., Solid State Physics, vol. 4, Academic Press, 1957.
28. Jj. F. Scott. Ferroelectric Memories, Springer, New York, 2000.
29. T. Kato, N. Mizoshita, and K. Kishimoto. Functional liquid-crystalline assemblies: self-organized soft materials. Angew. Chem. Int. Ed. 2006, 45, 38–68.
30. C. Tschierske. Micro-segregation, molecular shape and molecular topology – partners for the design of liquid crystalline materials with complex mesophase morphologies. J. Mater. Chem. 2001, 11, 2647–2671.
31. H.-T. Nguyen, C. Destrade, and J. Malthécte. Phasmids and polycatenar mesogens. Adv. Mater. 1997, 9, 375–378.
32. S. Kumar. Chemistry of Discotic Liquid Crystals: From Monomers to Polymers, CRC Press, Taylor & Francis Group, Boca Raton, FL, 2011.
33. C. Tschierske. Liquid crystal engineering – new complex mesophase structures and their relations to polymer morphologies, nanoscale patterning and crystal engineering. Chem. Soc. Rev. 2007, 36, 1930–1970.
34. X. H. Cheng, S. Diele, and C. Tschierske. Molecular design of liquid-crystalline block molecules: semifluorinated pentaerythritol tetrabenzoates exhibiting lamellar, columnar, and cubic mesophases. Angew. Chem. Int. Ed. 2000, 39, 592–595.
35. B. Pansu. Are surfaces pertinent for describing some thermotropic liquid crystal phases? Mod. Phys. Lett. B 1999, 13, 769–782.
36. H.-T. Nguyen, J. C. Rouillon, P. Cluzeau, G. Sigaud, C. Destrade, and N. Isaert. New chiral thiobenzoate series with antiferroelectric mesophases. Liq. Cryst. 1994, 17, 571–583.
37. N. A. Clark and S. T. Lagerwall. Submicrosecond bistable electro-optic switching in liquid crystals. Appl. Phys. Lett. 1980, 36, 899–901.
38. T. Ikeda, T. Sasaki, and K. Ichimura. Photochemical switching of polarization in ferroelectric liquid-crystal films. Nature 1993, 361, 428–430.
39. I. Dierking. Textures of Liquid Crystals, Wiley-VCH, Germany, 2003.
40. A. G. Duranda and P. Martinot-Lagardea. Physical properties of ferroelectric liquid crystals. Ferroelectrics 1980, 24, 89–97.
41. H. Takezoe and J. Watanabe. Ferroelectricity in liquid crystals, Mol. Cryst. Liq. Cryst. 1999, 328, 325–332.
42. T. Niori, T. Sekine, J. Watanabe, T. Furukawa, and H. Takezoe. Distinct ferroelectric smectic liquid crystals consisting of banana shaped achiral molecules. J. Mater. Chem. 1996, 6, 1231–1233.
43. T. Watanabe, S. Miyata, T. Furakawa, H. Takezoe, T. Nishi, A. Migita, M. Sone, and J. Watanabe. Nematic liquid crystals with polar ordering formed from simple aromatic polyester. Jpn. J. Appl. Phys. 1996, 35, L505–L507.
44. B. Park, Y. Kinoshita, H. Takezoe, and J. Watanab. Ferroelectricity in the lyotropic cholesteric phase of poly l-glutamate. Jpn. J. Appl. Phys. 1998, 37, L136–L138.
45. C. Bosshard, K. Sutter, P. Petre, J. Hulliger, M. Flörsheimer, P. Kaatz, and P. Günter. Organic Nonlinear Optical Materials, Gordon and Breach, Basel, Switzerland, 1995.
46. L. T. Cheng, W. Tam, S. H. Stevenson, G. R. Meredith, G. Rikken, and S. R. Marder. Experimental investigations of organic molecular nonlinear optical polarizabilities. 1. Methods and results on benzene and stilbene derivatives. J. Phys. Chem. 1991, 95, 10631–10643.
47. J. Jerphagnon and S. K. Kurtz. Maker fringes: a detailed comparison of theory and experiment for isotropic and uniaxial crystals. J. Appl. Phys. 1970, 41, 1667–1681.
48. F. Zernike and J. E. Midwinter. Applied Nonlinear Optics, Wiley, New York, 1973.
49. W. N. Herman and L. M. Hayden. Maker fringes revisited: second-harmonic generation from birefringent or absorbing materials. J. Opt. Soc. Am. B 1995, 12, 416–427.
50. D. S. Hermann, P. Rudquist, S. T. Lagerwall, L. Komitov, B. Stebler, M. Lindgren, M. Trollsås, F. Sahlen, A. Hult, U. W. Gedde, C. Orrenius, and T. Norin. Second-harmonic generation in a novel crosslinked pyroelectric liquid crystal polymer (PLCP). Liq. Cryst. 1998, 24, 295–310.
51. M. Trollsås, C. Orrenius, F. Sahlén, U. W. Gedde, T. Norin, A. Hult, D. Hermann, P. Rudquist, L. Komitov, S. T. Lagerwall, and J. Lindström. Preparation of a novel cross-linked polymer for second-order nonlinear optics. J. Am. Chem. Soc. 1996, 118, 8542–8548.
52. R. B. Meyer, L. Liebert, L. Strzelecki, and P. Keller. Ferroelectric liquid crystals. J. Phys. Lett. 1975, 36, 69–71.
53. A. N. Vtyurin, V. P. Ermaeov, B. I. Ostrovskii, and V. F. Shabanov. Study of optical second harmonic generation in ferroelectric liquid crystal. Phys. Status Solidi B 1981, 107, 397–402.
54. N. M. Shtykov, M. I. Barnik, L. A. Beresnev, and L. M. Blinov. A study of a ferroelectric liquid crystal using second optical harmonic generation. Mol. Cryst. Liq. Cryst. 1985, 124, 379–390.
55. M. Ozaki, M. Utsumi, T. Gotou, Y. Morit, K. Daido, Y. Sadohara, and K. Yoshino. Static and dynamic properties of optical second harmonic generation in ferroelectric liquid crystal. Ferroelectrics 1991, 121, 259–274.
56. J. Y. Liu, M. G. Robinson, K. M. Johnson, and D. Doroski. Second-harmonic generation in ferroelectric liquid crystals. Opt. Lett. 1990, 15, 267–269.
57. K. Yoshino, M. Utsumi, Y. Morita, Y. Sadohara, and M. Ozaki. Second harmonic generation in ferroelectric liquid crystals and their mixtures. Liq. Cryst. 1993, 14, 1021–1032.
58. D. M. Walba, M. B. Ros, N. A. Clark, R. Shao, M. G. Robinson, J. Y. Liu, K. M. Johnson, and D. Doroski. Design and synthesis of new ferroelectric liquid crystals. 14. An approach to the stereocontrolled synthesis of polar organic thin films for nonlinear optical applications. J. Am. Chem. Soc. 1991, 113, 5471–5474.
59. D. M. Walba, M. B. Ros, N. A. Clark, R. Shao, K. M. Johnson, M. G. Robinson, J. Y. Liu, and D. Doroski. An approach to the design of ferroelectric liquid crystals with large second order electronic nonlinear optical susceptibility. Mol. Cryst. Liq. Cryst. 1991, 198, 51–60.
60. D. M. Walba, H. A. Razavi, A. Horiuchi, K. F. Eidman, B. Otterholm, R. C. Haltiwanger, N. A. Clark, R. Shao, D. S. Parmar, M. D. Wand, and R. T. Vohra. Evolution of the Boulder model for the molecular origins of the polarization in ferroelectric liquid crystals. Ferroelectrics 1991, 113, 21–36.
61. J. Y. Liu, M. G. Robinson, K. M. Johnson, D. M. Walba, M. B. Ros, N. A. Clark, R. Shao, and D. Doroski. The measurement of second-harmonic generation in novel ferroelectric liquid crystal materials. J. Appl. Phys. 1991, 70, 3426–3430.
62. K. Schmitt, R.-P. Herr, M. Schadt, J. Fünfschilling, R. Buchecker, X. H. Chen, and C. Benecke. Strongly non-linear optical ferroelectric liquid crystals for frequency doubling. Liq. Cryst. 1993, 14, 1735–1752.
63. A. Taguchi, Y. Ouchi, H. Takezoe, and A. Fukuda. Angle phase matching in second harmonic generation from a ferroelectric liquid crystal. Jpn. J. Appl. Phys. 1989, 37, L997–L999.
64. K. Kajikawa, T. Isozaki, H. Takezoe, and A. Fukuda. Mirrorless microcavity spontaneously formed in ferroelectric liquid crystals. Jpn. J. Appl. Phys. 1992, 31, L679–L681.
65. B. Park, M. Lim, J.-H. Lee, J.-H. Kim, and S.-D. Lee. Nonlinear optical anisotropy associated with polar ordering in ferroelectric liquid crystals. Ferroelectrics 1996, 179, 231–240.
66. M. Stanley, S. E. Day, D. A. Dunmur, and M. Grayson. Comparison of modelling and experimental values of non-linear optical coefficients of SCE13(*). Ferroelectrics 1996, 179, 249–256.
67. H. Kapitza, R. Zentel, R. J. Twieg, C. Nguyen, S. U. Vallerien, F. Kremer, and C. G. Willson. Ferroelectric liquid crystalline polysiloxanes with high spontaneous polarization and possible applications in nonlinear optics. Adv. Mater. 1990, 2, 539–543.
68. P. Keller, R. Shao, D. M. Walba, and M. Brunet. The first high polarization ferroelectric main chain liquid crystalline polymers. Liq. Cryst. 1995, 18, 915–918.
69. D. M. Walba, P. Keller, R. Shao, N. A. Clark, M. Hillmyer, and R. H. Grubbs. Main-chain ferroelectric liquid crystal oligomers by acyclic diene metathesis polymerization. J. Am. Chem. Soc. 1996, 118, 2740–2741.
70. R. H. Grubbs, S. J. Miller, and G. C. Fu. Ring-closing metathesis and related processes in organic synthesis. Acc. Chem. Res. 1995, 28, 446–452.
71. M. Trollsås, F. Sahlén, U. W. Gedde, A. Hult, D. Hermann, P. Rudquist, L. Komitov, S. T. Lagerwall, B. Stebler, J. Lindström, and O. Rydlund. Novel thermally stable polymer materials for second-order nonlinear optics. Macromolecules 1996, 29, 2590–2598.
72. C. Artal, M. B. Ros, J. L. Serrano, N. Pereda, J. Etxebarría, C. L. Folcia, and J. Ortega. SHG characterization of different polar materials obtained by in situ photopolymerization. Macromolecules 2001, 34, 4244–4255.
73. M. Lindgren, D. S. Hermann, J. Örtegren, P.-O. Arntzen, U. W. Gedde, A. Hult, L. Komitov, S. T. Lagerwall, P. Rudquist, B. Stebler, F. Sahlén, and M. Trollsås. Second-harmonic light generation in pyroelectric liquid-crystal polymers. J. Opt. Soc. Am. B 1998, 15, 914–922.
74. V. S. U. Fazio, S. T. Lagerwall, V. Zauls, S. Schrader, P. Busson, A. Hult, and H. Motschmann. Nonlinear optical properties of a channel waveguide produced with crosslinkable ferroelectric liquid crystals. Eur. Phys. J. E 2000, 3, 245–251.
75. V. S. U. Fazio, S. T. Lagerwall, P. Busson, A. Hult, and H. Motschmann. Phase-matched second-harmonic generation in a ferroelectric liquid crystal waveguide. Appl. Phys. Lett. 2000, 77, 319–321.
76. V. S. U. Fazio, V. Zaulsc, S. Schraderc, P. Busson, A. Hult, S. T. Lagerwall, and H. Motschmann. Enhanced phase-matched second-harmonic generation in a ferroelectric liquid crystal waveguide. Mol. Cryst. Liq. Cryst. 2001, 368, 293–302.
77. T. Verbiest, S. Houbrechts, M. Kauranen, K. Clays, and A. Persoons. Second-order nonlinear optical materials: recent advances in chromophore design. J. Mater. Chem. 1997, 7, 2175–2189.
78. J. Malthete, J. Billard, and J. Jacques. Tetrabenzoates of mesomorphic naphthalenetetrol. C. R. Acad. Sci., Paris C 1975, 281, 333–335.
79. A. C. Griffin, S. F. Thames, and M. S. Bonner. Effect of molecular structure on mesomorphism. Two series of novel methylene-bridged liquid crystal. Mol. Cryst. Liq. Cryst. 1977, 34, 135–139.
80. H. Dehne, A. Roger, D. Demus, S. Diele, H. Kresse, G. Pelzl, W. Wedler, and W. Weissflog. Sulphur ligated siamese twin mesogens. Liq. Cryst. 1989, 6, 47–62.
81. W. Weissflog, D. Demus, S. Diele, P. Nitschke, and W. Wedler. From laterally branched mesogens to novel twin molecules. Liq. Cryst. 1989, 5, 111–122.
82. D. M. Walba, L. Xiao, K. Keller, R. Shao, D. Link, and N. A. Clark. Ferroelectric liquid crystals for second order nonlinear optics. Pure Appl. Chem. 1999, 71, 2117–2123.
83. D. M. Walba, E. Garcia, J. Niessink-Trotter, M. Richard, R. Shao, M. Nakata, and N. A. Clark. ILCC2008, Jeju, Korea, abstracts I, p. 228.
84. Y. Zhang, J. Martinez-Perdiguero, U. Baumeister, C. Walker, J. Etxebarria, M. Prehm, J. Ortega, C. Tschierske, M. J. O'Callaghan, A. Harant, and M. Handschy. Laterally azo-bridged H-shaped ferroelectric dimesogens for second-order nonlinear optics: ferroelectricity and second harmonic generation. J. Am. Chem. Soc. 2009, 131, 18386–18392.
85. J. Martinez-Perdiguero, Y. Zhang, C. Walker, J. Etxebarria, C. L. Folcia, J. Ortega, M. J. O'Callaghan, and U. Baumeister. Second harmonic generation in laterally azo-bridged H-shaped ferroelectric dimesogens. J. Mater. Chem. 2010, 20, 4905–4909.
86. C. Y. Yelamaggad, M. Mathews, S. A. Nagamani, D. S. S. Rao, S. K. Prasad, S. Findesein, and W. Weissflog. A novel family of salicylaldimine-based five-ring symmetric and non-symmetric banana-shaped mesogens derived from laterally substituted resorcinol: synthesis and characterization. J. Mater. Chem. 2007, 17, 284–298.
87. G. Heppke and D. Moro. Liquid crystals – chiral order from achiral molecules. Science 1998, 279, 1872–1873.
88. D. R. Link, G. Natale, R. Shao, J. E. Maclennan, N. A. Clark, E. Körblova, and D. M. Walba. Spontaneous formation of macroscopic chiral domains in a fluid smectic phase of achiral molecules. Science 1997, 278, 1924–1927.
89. D. A. Coleman, J. Fernsler, N. Chattham, M. Nakata, Y. Takanishi, E. Körblova, D. R. Link, R. Shao, W. G. Jang, J. E. Maclennan, O. Mondainn-Monval, C. Boyer, W. Weissflog, G. Pelzl, L. C. Chien, J. Zasadzinski, J. Watanabe, D. M. Walba, H. Takezoe, and N. A. Clark. Polarization-modulated smectic liquid crystal phases. Science 2003, 301, 1204–1211.
90. J. Watanabe, T. Niori, T. Sekine, and H. Takezoe. Frustrated structure induced on ferroelectric smectic phases in banana-shaped molecular system. Jpn. J. Appl. Phys. 1998, 37, L139–L142.
91. J. Szydlowska, J. Mieckowski, D. Matraszek, D. W. Bruce, E. Gorecka, D. Pociecha, and D. Guillon. Bent-core liquid crystals forming two- and three-dimensional modulated structures. Phys. Rev. E 2003, 67, 031702.
92. B. R. Acharya, A. Primak, and S. Kumar. Biaxial nematic phase in bent-core thermotropic mesogens. Phys. Rev. Lett. 2004, 92, 145506.
93. C. Keith, A. Lehmann, U. Baumeister, M. Prehm, and C. Tschierske. Nematic phases of bent-core mesogens. Soft Matter 2010, 6, 1704–1721.
94. A. G. Vanakaras and D. J. Photinos. Thermotropic biaxial nematic liquid crystals: spontaneous or field stabilized? J. Chem. Phys. 2008, 128, 154512.
95. J. Ortega, C. L. Folcia, J. Etxebarria, N. Gimeno, and M. B. Ros. Interpretation of unusual textures in the B2 phase of a liquid crystal composed of bent-core molecules. Phys. Rev. E 2003, 68, 011707.
96. L. E. Hough, M. Spannuth, M. Nakata, D. A. Colemanm, C. D. Jones, G. Dantlgraber, C. Tschierske, J. Watanabe, E. Körblova, D. M. Walba, J. E. Maclennan, M. A. Glaser, and N. A. Clark. Chiral isotropic liquids from achiral molecules. Science 2009, 325, 452–456.
97. J. Thisayukta, J. Watanabe, and H. Takezoe. Study on helical structure of the B-4 phase formed from achiral banana-shaped molecule. Jpn. J. Appl. Phys. 2001, 40, 3277–3287.
98. L. E. Hough, H. T. Jung, D. Krüerke, M. S. Heberling, M. Nakata, C. D. Jones, D. Chen, D. R. Link, J. Zasadzinski, G. Heppke, J. P. Rabe, W. Stocker, E. Körblova, D. M. Walba, M. A. Glaser, and N. A. Clark. Helical nanofilament phases. Science 2009, 325, 456–460.
99. J. Svoboda, V. Novotna, V. Kozmin, M. Glogarova, W. Weissflog, S. Diele, and G. Pelzl. A novel type of banana liquid crystals based on 1-substituted naphthalene-2,7-diol cores. J. Mater. Chem. 2003, 13, 2104–2110.
100. D. Shen, S. Diele, G. Pelzl, I. Wirth, and C. Tschierske. Designing banana-shaped liquid crystals without Schiff's base units: m-terphenyls, 2,6-diphenylpyridines and V-shaped tolane derivatives. J. Mater. Chem. 1999, 9, 661–672.
101. S. Kang, M. Tokita, Y. Takanishi, H. Takezoe, and J. Watanabe. Structure of a B6-like phase formed from bent-core liquid crystals determined by microbeam X-ray diffraction. Phys. Rev E 2007, 76, 042701.
102. G. Pelzl, S. Diele, and W. Weissflog. Banana-shaped compounds – a new field of liquid crystals. Adv. Mater. 1999, 11, 707–724.
103. H. Takezoe and Y. Takanishi. Bent-core liquid crystals: their mysterious and attractive world. Jpn. J. Appl. Phys. 2006, 45, 597–625.
104. J. Etxebarria and M. B. Ros. Bent-core liquid crystals in the route to functional materials. J. Mater. Chem. 2008, 18, 2919–2926.
105. C. Keith, G. Dantlgraber, R. A. Reddy, U. Baumeister, and C. Tschierske. Ferroelectric and antiferroelectric smectic and columnar liquid crystalline phases formed by silylated and non-silylated molecules with fluorinated bent cores. Chem. Mater. 2009, 19, 694–710.
106. Y. Zhang, U. Baumeister, C. Tschierske, M. J. O'Callaghan, and C. Walker. Achiral bent-core molecules with a series of linear or branched carbosilane termini: dark conglomerate phases, supramolecular chirality and macroscopic polar order. Chem. Mater. 2010, 22, 2869–2884.
107. M. Nakata, R. Shao, J. E. Maclennan, W. Weissflog, and N. A. Clark. Electric-field-induced chirality flipping in smectic liquid crystals: the role of anisotropic viscosity. Phys. Rev. Lett. 2006, 96, 067802.
108. Y. Zhang, M. J. O'Callaghan, U. Baumeister, and C. Tschierske. Bent-core mesogens with branched carbosilane termini: flipping suprastructural chirality without reversing polarity. Angew. Chem. Int. Ed. 2008, 47, 6892–6896.
109. D. M. Walba, E. Korblova, R. Shao, J. E. Maclennan, D. R. Link, M. A. Glaser, and N. A. Clark. A ferroelectric liquid crystal conglomerate composed of racemic molecules. Science 2000, 288, 2181–2184.
110. M. Nakata, D. R. Link, F. Araoka, J. Thisayukta, Y. Takanishi, K. Ishikawa, J. Watanabe, and H. Takezoe. A racemic layer structure in a chiral bent-core ferroelectric liquid crystal. Liq. Cryst. 2001, 28, 1301–1308.
111. S. K. Lee, S. Heo, J. G. Lee, K.-T. Kang, K. Kumazawa, K. Nishida, Y. Shimbo, Y. Takanishi, J. Watanabe, T. Doi, T. Takahashi, and H. Takezoe. Odd-even behavior of ferroelectricity and antiferroelectricity in two homologous series of bent-core mesogens. J. Am. Chem. Soc. 2005, 127, 11085–11091.
112. C. Keith, R. A. Reddy, M. Prehm, U. Baumeister, H. Kresse, J. L. Chao, H. Hahn, H. Lang, and C. Tschierske. Layer frustration, polar order and chirality in liquid crystalline phases of silyl-terminated achiral bent-core molecules. Chem. Eur. J. 2007, 13, 2556–2577.
113. F. Kentischerk, R. Macdonald, P. Warnik, and G. Heppke. Second harmonic generation (SHG) investigations of different phases of banana shaped molecules. Liq. Cryst. 1998, 25, 341–347.
114. S. W. Choi, Y. Kinoshita, B. Park, H. Takezoe, T. Niori, and J. Watanabe. Second-harmonic generation in achiral bent-shaped liquid crystals. Jpn. J. Appl. Phys. 1998, 37, 3408–3411.
115. R. Macdonald, F. Kentischer, P. Warnick, and G. Heppke. Antiferroelectricity and chiral order in new liquid crystals of nonchiral molecules studied by optical second harmonic generation. Phys. Rev. Lett. 1998, 81, 4408–4411.
116. F. Araoka, B. Park, Y. Kinoshita, K. Ishikawa, H. Takezoe, J. Thisayukta, and J. Watanabe. Evaluation of the first-order hyperpolarizability tensor of bent molecules by means of hyper-Rayleigh scattering method. Jpn. J. Appl. Phys. 1999, 38, 3526–3529.
117. F. Araoka, J. Thisayukta, K. Ishikawa, J. Watanabe, and H. Takezoe. Polar structure in a ferroelectric bent-core mesogen as studied by second-harmonic generation. Phys. Rev. E 2002, 66, 021705.
118. J. A. Gallastegui, C. L. Folcia, J. Etxebarria, J. Ortega, I. De Francisco, and M. B. Ros. Determination of the second order susceptibility tensor in banana-shaped liquid crystals. Liq. Cryst. 2002, 29, 1329–1333.
119. J. Ortega, J. A. Gallastegui, C. L. Folcia, J. Etxebarria, N. Gimeno, and M. B. Ros. Second harmonic generation measurements in aligned samples of liquid crystals composed of bent-core molecules. Liq. Cryst. 2004, 31, 579–584.
120. C. L. Folcia, I. Alonso, J. Ortega, J. Etxebarria, I. Pintre, and M. B. Ros. Achiral bent-core liquid crystals with azo and azoxy linkages: structural and nonlinear optical properties and photoisomerization. Chem. Mater. 2006, 18, 4617–4626.
121. I. C. Pintre, N. Gimeno, J. L. Serrano, M. B. Ros, I. Alonso, C. L. Folcia, J. Ortega, and J. Etxebarria. Liquid crystalline and nonlinear optical properties of bent-shaped compounds derived from 3,4′-biphenylene. J. Mater. Chem. 2007, 17, 2219–2227.
122. I. C. Pintre, J. L. Serrano, M. B. Ros, J. Martinez-Perdiguero, I. Alonso, J. Ortega, C. L. Folcia, J. Etxebarria, R. Alicante, and B. Villacampa. Bent-core liquid crystals in a route to efficient organic nonlinear optical materials. J. Mater. Chem. 2010, 20, 2965–2971.
123. M. Rickard, M. Nakata, H. Takezoe, J. Watanabe, and N. A. Clark. Electronic electrooptic phase modulation using bent-core liquid crystals. Appl. Phys. Lett. 2005, 87, 261115.
124. A. Jakli, S. Rauch, D. Lotzsch, and G. Heppke. Uniform textures of smectic liquid-crystal phase formed by bent-core molecules. Phys. Rev. E 1998, 57, 6737–6740.
125. C. D. Keum, A. Kanazawa, and T. Ikeda. Novel cross-linked organic materials with pronounced spontaneous polarization based on bis-dipolar molecules. Adv. Mater. 2001, 13, 321–323.
126. A. C. Sentman and D. L. Gin. Polymerizable bent-core mesogens: switchable precursors to ordered, polar polymer materials. Angew. Chem. Int. Ed. 2003, 42, 1815–1819.
127. J. Barbera, N. Gimeno, L. Monreal, R. Piñol, M. B. Ros, and J. L. Serrano. SmCP-networked films obtained by in situ photopolymerization of neat reactive banana-shaped liquid crystals. J. Am. Chem. Soc. 2004, 126, 7190–7191.
128. J. A. Gallastegui, C. L. Folcia, J. Etxebarria, J. Ortega, N. Gimeno, and M. B. Ros. Fabrication and nonlinear optical properties of monodomain polymers derived from bent-core mesogens. J. Appl. Phys. 2005, 98, 083501.
129. S. Rauch, C. Selbmann, P. Baukt, H. Sawade, G. Heppke, O. Morales-Saavedra, M. Y. Young, and A. Jakli. Glass forming banana-shaped compounds: vitrified liquid crystal states. Phys. Rev. E 2004, 69, 021707.
130. C. Keith, R. A. Reddy, and C. Tschierske. The first example of a liquid crystalline side-chain polymer with bent-core mesogenic units: ferroelectric switching and spontaneous achiral symmetry breaking in an achiral polymer. Chem. Commun. 2005, 871–873.
131. X. Chen, K. K. Tenneti, C. Y. Li, Y. Bai, X. Wan, X. Fan, Q. F. Zhou, L. Rong, and B. S. Hsiao. Side-chain liquid crystalline poly(meth)acrylates with bent-core mesogens. Macromolecules 2007, 40, 840–848.
132. C. Keith, G. Dantlgraber, R. A. Reddy, U. Baumeister, M. Prehm, H. Hahn, H. Lang, and C. Tschierske. The influence of shape and size of silyl units on the properties of bent-core liquid crystals – from dimers via oligomers and dendrimers to polymers. J. Mater. Chem. 2007, 17, 3796–3805.
133. S. Demel, C. Slugovc, F. Stelzer, K. Fodor-Csorba, and G. Galli. Alternating diene metathesis polycondensation (ALTMET) – a versatile tool for the preparation of perfectly alternating AB copolymers. Macromol. Rapid Commun. 2003, 24, 636–641.
134. E. J. Choi, J. C. Ahn, L. C. Chien, C. K. Lee, W. C. Zin, D. C. Kim, and S. T. Shin. Main chain polymers containing banana-shaped mesogens: synthesis and mesomorphic properties. Macromolecules 2004, 37, 71–78.
135. L. Zou, J. Wang, V. J. Beleva, E. E. Kooijman, S. V. Primak, J. Risse, W. Weissflog, A. Jakli, and E. K. Mann. Langmuir monolayers of bent-core molecules. Langmuir 2004, 20, 2772–2780.
136. J. Wang, L. Zou, W. Weissflog, A. Jakli, and E. K. Mann. Anisotropy in Langmuir layers of a bent-core liquid crystal. Langmuir 2006, 22, 3198–3206.
137. A. R. Geivandov, S. P. Palto, S. G. Yudin, L. M. Blinov, G. Pelzl, and W. Weissflog. Ferro- and antiferroelectric properties of Langmuir–Blodgett films composed of mesogenic bent-core molecules. Ferroelectrics 2006, 344, 247–254.
138. O. G. Morales-Saavedra, E. Rivera, J. O. Flores-Flores, R. Castañeda, J. G. Bañuelos, and J. M. Saniger. Preparation and optical characterization of catalyst free SiO2 sonogel hybrid materials. J. Sol-Gel Sci. Technol. 2007, 41, 277–289.
139. H. Bock and W. Helfrich. Ferroelectrically switchable columnar liquid crystal. Liq. Cryst. 1992, 12, 697–703.
140. H. Bock and W. Helfrich. Two ferroelectric phases of a columnar dibenzopyrene. Liq. Cryst. 1995, 18, 387–399.
141. G. Scherowsky and X. H. Chen. New switchable columnar liquid crystals. Liq. Cryst. 1994, 17, 803–810.
142. G. Scherowsky and X. H. Chen. Ferroelectric switching in columnar phases of novel chiral discotic liquid crystals. J. Mater. Chem. 1995, 5, 417–421.
143. G. Hennrich, A. Omenat, I. Asselberghs, S. Foerier, K. Clays, T. Verbiest, and J. L. Serrano. Liquid crystals from C3-symmetric mesogens for second-order nonlinear optics. Angew. Chem. Int. Ed. 2006, 45, 4203–4206.
144. V. Torres-Zúñiga, O. G. Morales-Saavedra, G. Hennrich, J. O. Flores-Flores, and R. Ortega-Martínez. Morphology, linear and nonlinear optical response of octopolar chromophores embedded in a silica sonogel matrix. Mol. Cryst. Liq. Cryst. 2010, 527, 1–11.
145. E. Gorecka, D. Pociecha, J. Mieczkowski, J. Matraszek, D. Guillon, and B. Donnio. Axially polar columnar phase made of polycatenar bent-shaped molecules. J. Am. Chem. Soc. 2004, 126, 15946–15947.
146. E. Gorecka, D. Pociecha, J. Matraszek, J. Mieczkowski, Y. Shimbo, Y. Takanishi, and H. Takezoe. Polar order in columnar phase made of polycatenar bent-core molecules. Phys. Rev. E 2006, 73, 031704.
147. K. Kishikawa, S. Nakahara, Y. Nishikawa, S. Kohmoto, and M. Yamamoto. A ferroelectrically switchable columnar liquid crystal phase with achiral molecules: superstructures and properties of liquid crystalline ureas. J. Am. Chem. Soc. 2005, 127, 2565–2571.
148. Y. Okada, S. Matsumoto, Y. Takanishi, K. Ishikawa, S. Nakahara, K. Kishikawa, and H. Takezoe. Polarization switching in a columnar liquid crystalline urea as studied by optical second-harmonic generation interferometry. Phys. Rev. E 2005, 72, 020701(R).
149. H. Takezoe, K. Kishikawa, and E. Gorecka. Switchable columnar phases. J. Mater. Chem. 2006, 16, 2412–2416.
150. M. J. Baena, P. Espinet, M. B. Ros, J. L. Serrano, and A. Ezcurra. Improved properties of ferroelectric liquid crystals from palladium β-diketone complexes. Angew. Chem. Int. Ed. 1993, 32, 1203–1205.
151. P. Espinet, J. Etxebarría, C. L. Folcia, J. Ortega, M. B. Ros, and J. Serrano. Second harmonic generation with metal-containing ferroelectric liquid crystals. Adv. Mater. 1996, 8, 745–748.
152. M. Koike, C.-C. Yen, Y. Liu, H. Tsuchiya, M. Tokita, S. Kawauchi, H. Takezoe, and J. Watanabe. Unusual nematic liquid crystal with polar Cs symmetry formed from aromatic polyesters with head−tail character. Macromolecules 2007, 40, 2524–2531.
153. J. Watanabe, Y. Hirose, M. Tokita, T. Watanabe, and S. Miyata. Polar structure in polypeptide cholesteric liquid crystals evidenced from observation of second-harmonic generation due to the helicoidal cavity effect. Macromolecules 1998, 31, 5937–5939.
154. C.-C. Yen, M. Tokita, B. Park, H. Takezoe, and J. Watanabe. Spontaneous organization of helical polypeptide molecules into polar packing structure. Macromolecules 2006, 39, 1313–1315.
155. H. S. Nalwa, T. Watanabe, and S. Miyata. Organic materials for second-order nonlinear optics. In: H. S. Nalwa and S. Miyata, Eds., Nonlinear Optics of Organic Molecules and Polymers, CRC Press, Boca Raton, FL, 1997.