12
Resource Allocation Determines Alternate Cell Fate in Bistable Genetic Switch
Priya Chakraborty and Sayantari Ghosh⋆
Department of Physics, National Institute of Technology Durgapur, Durgapur, West Bengal, India
Abstract
Living cells need a constant availability of certain resources to have a sustained gene expression process. Limited availability of cellular resources for gene expression, like ribosomes and RNA Polymerase, significantly modifies the system dynamics. Factors like the variation in rate of binding or variation in efficiency of the recruited resource have the potential to affect crucial dynamic phenomena like cell fate determination. In this paper we have taken a very important motif, a bistable genetic toggle switch, and explored the effect of resource imbalance in this circuit in terms of the bifurcations taking place. We show that initial asymmetric biasing to resources via resource affinity or gene copy number significantly modifies the cell fate transition, both in pitchfork and saddle node type bifurcation. Our study establishes that in a limited resource environment, controlled resource allocation can be an important factor for robust functioning of the synthetic or cellular genetic switches.
Keywords: Gene regulation, resource allocation, cell fate decision, pitchfork bifurcation, saddle node bifurcation, genetic toggle switch
12.1 Introduction
Proteins that govern all functionalities in a living cell are produced by two major steps: transcription and translation, which indeed are a combination of several intermediate processes. In order to understand intriguing cellular processes like cellular decision making or to operate a synthetic circuit inside the cell, a detailed mathematical study of cellular or synthetic gene regulatory dynamics is of prime concern. Extreme non-linearity inside cellular systems and the nature of coupling of one ongoing process with another makes this understanding of the dynamics extremely difficult. However, some re-occurring genetic pattern, called a motif, can be found in living organisms performing some typical tasks for the cell. Scientists are focusing largely to explore repeatedly occurring motifs to understand the cell dynamics as well as cellular decision making from the last few decades. Genetic toggle is one of the most extensively studied biological motifs [7, 16, 18], where two genes (lacI and tetR) mutually repress each other’s expression. Dynamics of this genetic motif are usually studied from the perspective of saddle-node bifurcation. Here, in the case of saddle node bifurcation of toggle, the system undergoes a transition to a bistable state from a monostable one and after a specific range of parameters, again, the system becomes monostable. Thus, a specific region of bistability separates two otherwise monostable regions in the phase plane. The system can also undergo a pitchfork bifurcation for some specific symmetry, as studied in some recent works [2]. In case of pitchfork type bifurcation, a monostable region converts to a bistable one at a transition point and remains bistable for rest. Bistabilty introduces some irreversibility in biological systems, that once the system attains its steady state, it retains its same steady state even on application of some output perturbations on a small scale. Thus, in the case of cell fate differentiation and cellular decision making, the genetic toggle motif is taken as a canonical circuit for understanding [1].
From its early invention as a synthetic circuit by Gardner et al. [7], different approaches for robust controlling of genetic toggle are proposed. Some of them involve real time feedback control [12], auto regulation, noise [19], the addition of some diffusible molecules like isopropyl-β-D-thiogalacto-pyranoside (IPTG), and anhydrotetracycline (aTc) to control the promoter activity as well. The search for a novel control parameter is still going on and in some recent studies it was established that the limited availability of cellular ingredients serving in the process of protein production can act as a robust parameter in cell dynamics.
In the intermediate steps of protein production, majorly transcription and translation, the genes collect resources for successful completion of its expression from the cell. RNAP, transcription factor (TF), ribosome, degradation machinery, etc. are various resources that the cell supplies to the synthetic genetic circuit implemented in it or uses for its endogenous gene functionalities. It is experimentally verified that the cell does not contain these resources abundantly and depending upon the mode of operation, the availability of resources in different cells varies significantly. In the translation process, the ribosome is considered to be the most important resource the gene circuit collects from the cell. Though the presence of this essential cellular resource in all the prokaryotes and eukaryotes is a fact, the amount of free ribosome in different living beings is different. Even for different cells having different functionalities, they differ in availability of free ribosomes. Like the pancreatic cells, in eukaryotes, they are dedicated for most of the protein production over other cells and contain an unusually high number of ribosomes. On the contrary, the smooth endoplasmic reticulum (SER) does not have ribosomes on its surface and thus does not participate in protein production. This limited availability of the essential translational resource inside the cell significantly affects the ongoing dynamics. In a low protein activity or for a demand of low resource for the implemented synthetic construct, this limited availability may not affect things, but in higher protein activity or for a larger resource demand for the synthetic construct, unprecedented resource competition comes into the picture. Different experimental and theoretical studies establish that ribosome limitation, significantly modifying the circuit dynamics and at a larger scale the system chooses the favorable state. In a recent study, it is established that the protein production curve can be largely modified in terms of sensitivity and amplification by controlling ribosome availability and its distribution in the system [5]. Due to the nature of coupling of different ongoing processes, the competition in outer motifs, which seems unimportant in the study of motif interest, significantly modifies the dynamic behavior and can ruin the entire system, resulting in some emergent responses in output [4]. Not only limited to ribosomes, this competition can arise for RNAP, gene copy numbers, degradation machinery, and many more. Yuriy Mileiko and team in a recent study have shown that gene copy number variation brings a significant change in the dynamics of some well-known motifs [13]. The effect of decoy binding is also established by some recent papers [9, 11]. This trend of exploring the consequences of resource availability and distribution in cell dynamics is new and scientists are getting exciting novel mechanisms of system modelling from it.
Cellular decision-making, environmental sensing, and cell to cell communication are three key processes underlying pattern formation and development in microscopic and complex organisms. From a theoretical point of view, though the cellular decision making seems to be reversible, in practice most of the time this is irreversible due to some secondary effects arising from the process. The biologically programmed cell death (apoptis) or cell death in response to injury (lysis) is most the promising reason here. Several approaches in determining cell fate like feedback-controlled regulation, cell size, and growth rate dependency [10, 17] are established in the recent past. The effect of noise, especially when a group of cells is participating, statistically predicts the most probable result, as shown in [1]. Though the presence of these various efforts of determining cell fate transition, the effect of limited availability of resource or asymmetry in resource affinity inside the cell and in the cell fate dynamics is not explored much. This asymmetry serving as initial biasness has the capacity to pre-pattern the cell fate.
In this paper, we have taken a simple genetic toggle motif and considered, along with the mutual repression of each other, that both the genes are collecting resources (say ribosome here) from the same pool with different affinities. We also explore the condition for variability in gene copy numbers for the pitchfork type of bifurcation here. Some of the major findings of this paper are:
- Variability in resource affinity as well as gene copy number introduces asymmetry in pitchfork bifurcation and pre-patterns the cell fate. The greater the asymmetry, the more the system pre-patterns itself in determining an alternate cell fate.
- Asymmetry in resource affinity regulates the range of bistability and thus robustness of the switch in saddle node bifurcation.
- Total availability of resource regulates the point of bifurcation and region of interest in the system.
12.2 Model Formulation
Let us consider X and Y are the two proteins repressing each others promoter activity, forming a toggle switch, as shown in Figure 12.1. The hammerhead symbols represent the repression here. We also consider both these proteins are collecting resources from the same pool for their expression. We particularly focus on the pool of ribosome here, the essential resource for the translation process, for the transcriptional complex to be translated as a protein product.
Moving a step closer to cell dynamics, we consider that the ribosomes are distributed over small several cytoplasmic compartments inside the cell. Let T represent this local pool of resources available in the immediate vicinity of the toggle switch. Thus, our consideration of the two participants of the concerned toggle switch collects resource ribosomes from the pool T, which stands from its biological relevancy without any doubt.
The available mRNA pool for translation is represented by gx and gy for protein X and Y, respectively.
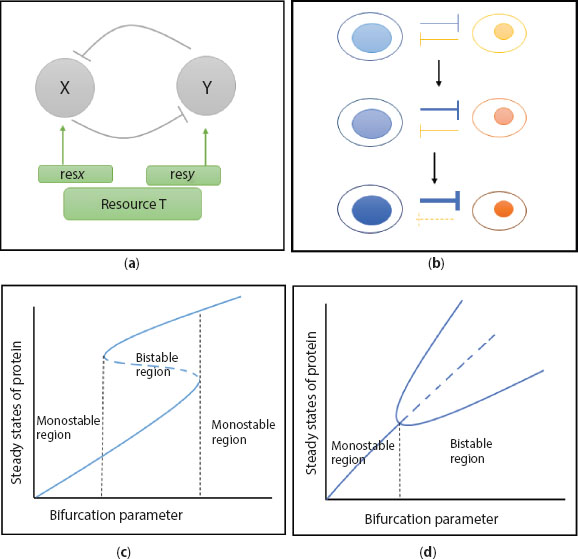
Figure 12.1 Model Motif. (a) Genetic toggle, two proteins X and Y mutually repress each others promoter activity. Hammerhead symbol represents the repression here. Also, both the participants of the toggle collect resources from the same pool T with the affinities resx and resy respectively. (b) Schematic diagram of inter cellular competition determining cell fate. Strength of repression drives the cell to a particular fate. (c) Saddle node bifurcation in genetic toggle. A bistable region separates two monostable regions in phase space. (d) Pitchfork bifurcation in genetic toggle. Monostable region switches to a bistable region at bifurcation point.
The available mRNA pool gx and gy, collecting ribosomes from the pool T, with affinities resx and resy, makes a ribosome bound complex cx and cy which will be translated to protein X and Y at a rate of ∈x and ∈y, respectively. This asymmetry in resource allocation is very insightful here. The polycistronic mRNA pool in most of the bacterial organisms contains multiple ribosome binding sites (RBS) [3] and the rate of translation depends on the rate of recruitment of ribosomes to this RBS, as well as on the rate of translation initiation. The rate of ribosome recruitment also depends upon many factors. Including all these rates, we generalize the resource allocation or the protein production, which can have different rates as well.
As mentioned, from the total pool T, the ribosome bound complexes are presented by cx and cy, thus further free pool of resource ribosome for translation is estimated by (T − cx − cy).
The mutual repression is captured by a Michaelis–Menten type term in our model and the hill function co-operativity n is taken as 2. The ODE representing the scenario is given by Equation 12.1 below.

In a steady state, all the rates of change are equal to 0 and we investigated the system.
12.3 Result Section
12.3.1 Pitchfork Bifurcation in Genetic Toggle
Pitchfork bifurcation occurs at specific equilibrium with perfect symmetry conditions of the toggle system. For one variable dynamic system, several studies are present, while for a two variable dynamic system, a few studies in the recent past [2] investigated this phenomena in brief. For a conventional pitchfork model, we take the production rates of the two proteins to be equal, thus here we take ∈x, the production rate of X from its complex cx, and, ∈y, the rate of production of Y from its complex cy is equal.
12.3.1.1 Resource Affinity Regulates the Symmetry of Pitchfork Bifurcation
Following the conventional way of pitchfork bifurcation in a toggle system, the protein production rates are equal and a detailed literature review strongly supports our consideration of taking different resource affinity values without any loss of generality. We find that the resource affinity value regulates the symmetry of pitchfork bifurcation significantly. We investigated the model for fixed values of n = 2, gx = gy = 5, T = 5, resy = 2, and changing ∈x so that for every point ∈x = ∈y for four different values of resx. When resx = resy, we find a beautiful symmetric pitchfork bifurcation in the output, while more interestingly asymmetry in resource affinity values destroys the symmetry in the output pitchfork with a significant impression. Starting with a lower resource affinity for X, resx < resy, there is a smooth transition of the pitchfork to a low state of the system, as shown in Figure 12.2a, while a higher state is only accessible for a very large perturbation in the system. While starting from a higher resource affinity, resx > resy for Figure 12.2d, the continuous accessible state is the higher production state, while the lower production states are only accessible for a large perturbation in the system. The same resource affinity of X and Y (resx = resy = 2 for Figure 12.2c) results in a symmetric pitchfork. Also, it is interesting to note that the larger the asymmetry, the higher the stability of the chosen transitioned state and the lower the chances for its transition to another steady state even when perturbation is present from the outside (comparing Figure 12.2a and Figure 12.2d with Figure 12.2c). So, the results indicate to the conclusion that an initial asymmetry in resource allocation pre-patterns the cells to a higher production regime or in a lower production regime and determines the cell fate.

Figure 12.2 Resource Affinity Regulates Symmetry of Pitchfork Bifurcation. Concentration of protein X wrt the rate of X production ∈x plot. n = 2, resy = 2, gx = gy = 5, T = 5, for all the plots. Resource affinity for X production, i.e., resx =1 for (a), resx=1.8 for (b), resx = 2 for (c), and resx = 3 for (d).
12.3.1.2 Availability of Total mRNA Pool Regulates the Symmetry of Pitchfork Bifurcation
We find similar results with respect to the gene copy number available for translation for a particular protein. The number of copies of a particular gene present in the genotype is usually called the gene copy number. A symmetry in the presence of gene copy number with symmetry in other system parameters shows a perfect pitchfork in the output, while asymmetry in the initial condition of gene copy number pre-patterns the system to a higher or lower production state, as shown in Figure 12.3. It is interesting to note that the greater the asymmetry in the initial gene copy number, the greater the perturbation the system demands to transit from its continuous steady state to the other.
12.3.1.3 Total Resource Availability Regulates the Point of Bifurcation in the System
We find that total resource availability significantly regulates the bifurcation point of the system, as shown in Figure 12.4. We investigate the model for two fixed values of T, increasing the total resource available for translation and the position of bifurcation comes to a lower value of input signal. Also, the range of steady states drastically changes.
12.3.2 Saddle Node Bifurcation in Genetic Toggle
The genetic toggle, most conventionally known as the genetic toggle switch, is biologically most important for its on/off switch like behavior which plays a significant role in determining cell fate. From its early invention, researchers are deliberately searching for the ways of robust controlling of the toggle switch. We find beautiful control on the toggle switch by controlling resource distribution.

Figure 12.3 Availability of Total mRNA Pool Regulates Symmetry of Pitchfork Bifurcation and Cell Fate Transition. Concentration of protein X vs. rate of X production ∈x plot. resx = resy = 2, n = 2, T = 5, gy = 5 is fixed for all the plots and ∈x = ∈y. Gene copy numbers available for production of X, i.e., gx =3 for (a), gx = 4.5 for (b), gx = 5 for (c), and gx = 6 for (d).

Figure 12.4 Total Resource T Availability Regulates Point of Pitchfork Bifurcation in Genetic Toggle. Concentration of protein X vs. rate of X production ∈x plot. n = 2, gx = gy = 5, ∈x = ∈y, resx = resy = 2 for both the plots. T = 5 for (a) plot and T = 10 for (b) plot.
12.3.2.1 Resource Distribution Regulates the Point of Bifurcation in Toggle Switch
We find the resource distribution significantly regulates the point of bifurcation in the saddle node of genetic toggle as well, as shown in Figure 12.5. We investigate the system for 3 different values of resx, keeping all other parameters fixed at resy = 2, n = 2, gx = gy = 5, T = 5, and ∈y = 2 and plot the concentration of protein X with respect to the activator of X production ∈x. Considering the blue line (continuous and dashed) primarily, which shows the scenario when resx = resy = 2 and that resource allocation for X to Y is the same, we find a change in resx shifts the curve left to the green curve (or right to the red curve) for a higher affinity for resources to X than Y, resx = 3, > resy = 2 (for a lower affinity for resource to X than Y, resx = 1, < resy = 2).
12.3.2.2 Region of Interest in Toggle Switch is Significantly Regulated by Resource Allocation
For a saddle node bifurcation, the most interesting region is the range of input signal for which the output protein concentration attains two drastically different concentrations depending upon the mode of forward or backward operation. When investigated in a bifurcation diagram, a set of stable equilibrium points is separated by unstable equilibrium points, the system cannot achieve physically. From Figure 12.5, we also get that the range of interest increases (or decreases) for X with a lower resource allocation resx = 1 < resy = 2 (for X getting higher resource than Y, resx = 3 > resy =2). It is interesting to note that lower resource availability to X than Y stabilises the bifurcation curve for larger fluctuation. This is not only giving us an opportunity for robust controlling of the system, but also signifies that initial biasing of the system towards the resource significantly modifies cell fate in terms of stability.
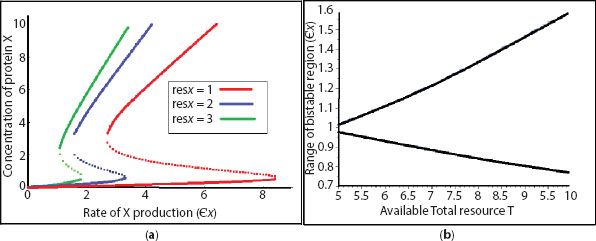
Figure 12.5 (a). Rate of X production ∈x vs. concentration of protein X plot in case of saddle node bifurcation in genetic toggle. resy = 2, gx = gy = 5, T = 5, n = 2, ∈y = 2. (b). Phase plot for range of bistable region ∈x, the rate of X production from its complex vs. total resource availability T. resx = resy = 1, n = 2, gx = gy = 5, ∈y = 1.
12.3.2.3 Total Resource Availability T Regulates Saddle Node Bifurcation Curve
Along with the variation in resource affinity, the availability of the total nutrients here significantly regulates the saddle node bifurcation curve, as shown in Figure 12.5b. The regulation is quite positive here though. Greater resources stabilize the system for a larger range of bistability and greater switch robustness, while with less availability of resources the switch response is not stable and the steady states can alter even for low fluctuation in the system.
12.4 Conclusion
Cellular decision making is a fundamental biological phenomenon by which a cell opts the different states prior to environmental conditions, leading to asymmetric cell differentiation. The underlying reason behind this is still not entirely explored. We take a simple genetic motif, genetic toggle here, and show that resource affinity asymmetry of the toggle participants, both for the saddle node and in pitchfork bifurcation, significantly biases the cell fate. This mutually repressing motif is very common in nature [6, 14], where in output the system shows patterning by choosing one cell fate over another. The availability of total resource pool significantly regulates the bifurcation point in the motif. In the case of a synthetic circuit, this resource limitation is very true because the gene circuit implemented in the host entirely depends upon the host’s resource for its expression and in the case of a cell, the limitation in cellular resource ribosome is a major factor, indicating our findings to be true also. We also investigated the effect of gene copy number in the case of pitchfork bifurcation, indicating an initial asymmetry that biases the cell fate to lower or higher production states accordingly.
Here, it is important to note that our entire consideration is valid for a low growth state of the system. The effect of growth rate on cell dynamics is well established [17]. Overexpression of endogenous genes, or adding some synthetic construct in the cell, destabilizes the resource distribution and makes the growth rate smaller, while growth causing dilution enhances protein degradation. So, some researchers point out that growth is a significantly regulatory parameter in every cellular phenomenon, but some experimental results also pointed out that these effects only depend upon experimental conditions, causing some momentary changes in dynamics. Our study mostly follows the experimental situation [8, 15] when the growth rate is low and competition effect is significant.
A perfect noise free environment in the cell is impracticable. For a single cell, though the consideration does not violate the reality, working with a group of cells, the predictability can vary significantly. The addition of noise in existing dynamics will give a result close to reality. Also, the limitation of other cellular nutrients in the way of gene expression can regulate the alternate protein production, stabilizing one state over other, regulating cell fate. In the future, we would like to extend our work for a complete scenario including transcriptional, translational, and degradation machinery competition in a noisy cell environment.
Acknowledgement
PC and SG acknowledge the support by DST-INSPIRE, India, vide sanction Letter No. DST/INSPIRE/04/2017/002765 dated-13.03.2019.
References
- 1. G. Balázsi, A. van Oudenaarden, and J. J. Collins. Cellular decision making and biological noise: from microbes to mammals. Cell, 144(6):910–925, 2011.
- 2. I. Bose and S. Ghosh. Bifurcation and criticality. Journal of Statistical Mechanics: Theory and Experiment, 2019(4):043403, 2019.
- 3. D. H. Burkhardt, S. Rouskin, Y. Zhang, G.-W. Li, J. S. Weissman, and C. A. Gross. Operon mrnas are organized into orf-centric structures that predict translation efficiency. Elife, 6:e22037, 2017.
- 4. P. Chakraborty and S. Ghosh. Emergent correlations in gene expression dynamics as foot-prints of resource competition. The European Physical Journal E, 44(10):1–12, 2021.
- 5. P. Chakraborty and S. Ghosh. Emergent regulatory response and shift of half induction point under resource competition in genetic circuits. arXiv preprint arXiv:2112.04985, 2021.
- 6. E. Clark. Dynamic patterning by the drosophila pair-rule network reconciles long-germ and short-germ segmentation. PLoS biology, 15(9):e2002439, 2017.
- 7. T. S. Gardner, C. R. Cantor, and J. J. Collins. Construction of a genetic toggle switch in escherichia coli. Nature, 403(6767):339–342, 2000.
- 8. A. Gyorgy, J. I. Jiménez, J. Yazbek, H.-H. Huang, H. Chung, R. Weiss, and D. Del Vecchio. Isocost lines describe the cellular economy of genetic circuits. Biophysical journal, 109(3):639–646, 2015.
- 9. S. Jayanthi, K. S. Nilgiriwala, and D. Del Vecchio. Retroactivity controls the temporal dynamics of gene transcription. ACS synthetic biology, 2(8):431–441, 2013.
- 10. S. Klumpp, Z. Zhang, and T. Hwa. Growth rate-dependent global effects on gene expression in bacteria. Cell, 139(7):1366–1375, 2009.
- 11. T.-H. Lee and N. Maheshri. A regulatory role for repeated decoy transcription factor binding sites in target gene expression. Molecular systems biology, 8(1):576, 2012.
- 12. J.-B. Lugagne, S. S. Carrillo, M. Kirch, A. Köhler, G. Batt, and P. Hersen. Balancing a genetic toggle switch by real-time feedback control and periodic forcing. Nature communications, 8(1):1–8, 2017.
- 13. Y. Mileyko, R. I. Joh, and J. S. Weitz. Small-scale copy number variation and large-scale changes in gene expression. Proceedings of the National Academy of Sciences, 105(43):16659–16664, 2008.
- 14. Y. Saka and J. C. Smith. A mechanism for the sharp transition of morphogen gradient interpretation in xenopus. BMC developmental biology, 7(1):1–9, 2007.
- 15. I. Shachrai, A. Zaslaver, U. Alon, and E. Dekel. Cost of unneeded proteins in e. coli is reduced after several generations in exponential growth. Molecular cell, 38(5):758–767, 2010.
- 16. M. Strasser, F. J. Theis, and C. Marr. Stability and multiattractor dynamics of a toggle switch based on a two-stage model of stochastic gene expression. Biophysical journal, 102(1):19–29, 2012.
- 17. C. Tan, P. Marguet, and L. You. Emergent bistability by a growth-modulating positive feedback circuit. Nature chemical biology, 5(11):842–848, 2009.
- 18. T. Tian and K. Burrage. Bistability and switching in the lysis/lysogeny genetic regulatory network of bacteriophage λ. Journal of Theoretical Biology, 227(2):229–237, 2004.
- 19. J. Wang, J. Zhang, Z. Yuan, and T. Zhou. Noise-induced switches in network systems of the genetic toggle switch. BMC systems biology, 1(1):1–14, 2007.
Note
- ⋆ Corresponding author: [email protected]
