Chapter 7. Concepts to Prevent Fires and Explosions
The learning objectives for this chapter are to:
Describe inerting and other methods to adjust the gas composition.
Discuss static electricity ignition hazards and their control.
Explain design concepts to prevent fires and explosions.
A threefold strategy is used to prevent or limit the damage due to fires and explosions: (1) prevent flammable mixtures, (2) eliminate and reduce ignition sources, and (3) mitigate the damage after a fire or explosion has occurred. In normal industrial practice, all three strategies must be addressed.
As the discussion in Chapter 6 made clear, the elimination of ignition sources alone is not enough to prevent fires and explosions: Ignition sources are too plentiful to use as the primary prevention mechanism. A more robust design is to prevent the presence of flammable mixtures as the primary control, followed by the elimination of ignition sources as a secondary control.
For any fire or explosion to occur, three conditions must be met (as shown in the fire triangle depicted in Figure 6-1). First, a fuel must be present. Second, oxygen or an oxidant must be present to support the combustion reaction. Finally, a source of ignition must be available to initiate the reaction. If any of these three conditions of the fire triangle is eliminated, the triangle is broken and it is not possible for a fire or explosion to occur.
Damage resulting from fires and explosions is minimized by stopping fires or explosions as quickly as possible as well as by designing the process equipment (and control centers) to withstand their effects. Industry accomplishes this by using the best engineering practices, known as Recognized and Generally Acceptable Good Engineering Practices (RAGAGEP). See Chapter 1 for additional information on RAGAGEP.
7-1 Inerting
The purpose of inerting is to adjust the gas composition in process equipment, using an inert gas, so as to prevent the existence of flammable atmospheres. The inert gas is usually nitrogen or carbon dioxide, although steam is sometimes used. The limiting oxygen concentration (LOC) is frequently used as a concentration target for inerting.
For many gases, the LOC is approximately 10%, and for many dusts, it is approximately 8%, although wide variability is expected. The commonly used control concentrations1 are shown in Table 7-1.
1NFPA 69, Standard on Explosion Prevention Systems (Quincy, MA: National Fire Protection Association, 2014).
Table 7-1 NFPA Inerting Control Concentrations
Condition |
Actual LOC |
NFPA recommended control concentration1 |
|---|---|---|
Oxygen concentration is continuously monitored |
Greater than or equal to 5% Less than 5% |
2% below the actual LOC Less than 60% of LOC |
Oxygen concentration is not continuously monitored |
Greater than or equal to 7.5% Less than 7.5% |
At least 4.5% below the actual LOC No more than 40% of LOC |
For an empty vessel to be charged with a flammable material, the vessel is first inerted before the flammable material is charged. An inerting system is required to maintain a safe atmosphere in the vapor space above the liquid. Ideally, this system should include an automatic inert gas addition feature to control the oxygen concentration below the LOC. This control system should have an analyzer to continuously monitor the oxygen concentration in relation to the LOC and a controlled inert gas feed system to add inert gas when the oxygen concentration approaches the control concentration. Often, however, the inerting system consists only of a regulator designed to maintain a fixed positive inert pressure in the vapor space; this ensures that inert gas is always flowing out of the vessel rather than air flowing in. The analyzer system, however, results in a significant savings in inert gas usage while improving safety.
A commonly used low set point is 1% below the recommended control concentration. If the actual LOC is less than or equal to 5% oxygen, then the recommended control concentration is 3% or 2% below the actual LOC. Nitrogen addition is started at 3% (the National Fire Protection Association’s [NFPA’s] recommendation), and nitrogen addition is stopped at 2% below the LOC.
Several purging methods are used to initially reduce the oxygen concentration to the low set point: vacuum purging, pressure purging, combined pressure–vacuum purging, vacuum and pressure purging with impure nitrogen, sweep-through purging, and siphon purging.
Vacuum Purging
Vacuum purging is the most commonly used inerting procedure for vessels. This procedure is not used for large storage vessels, however, because those containers are usually not rated for vacuum. Most can withstand only a pressure of a few inches of water gauge.
Many process vessels, including reactors, are often designed for full vacuum—that is, –760 mm Hg gauge or 0.0 mm Hg absolute. Consequently, vacuum purging is a common procedure employed with reactors. The steps in the vacuum purging process include (1) drawing a vacuum on the vessel until the target pressure is reached, (2) relieving the vacuum with an inert gas, and (3) repeating steps 1 and 2 until the target oxygen concentration is obtained.
The process for vacuum purging using the stepwise procedure is shown in Figure 7-1. A vessel of known size is vacuum-purged from an initial oxygen concentration yo to a final target oxygen concentration yj. The vessel is initially at pressure PH and is vacuum-purged using a vacuum at pressure PL. The objective is to determine the number of cycles required to achieve the target oxygen concentration. Note that the high and low pressures selected for the cycles shown in Figure 7-1 depend on the design pressure of the vessel and the low pressure capability of the facility’s vacuum system.

The oxygen concentration at the end of each vacuum cycle is the same as the concentration at the beginning of the vacuum cycle: Evacuation of the gas from the vessel does not change the concentration. For each pressurization cycle, if the inert does not contain any oxygen, then the number of moles of oxygen remains constant.
Assuming a high pressure PH and a low pressure or vacuum PL for each cycle and assuming ideal gas behavior, the total moles of gas at each pressure are
nH=PHVRgT(7-1)
nL=PLVRgT(7-2)
where nH and nL are the total moles at the high and low pressures, respectively.
The number of moles of oxidant for the low-pressure PL and high-pressure PH are computed using Dalton’s law:
(noxy)1L=y0nL(7-3)
(noxy)1H=y0nH(7-4)
where 1L and 1H are the first vacuum and first atmospheric states, respectively.
When the vacuum is relieved with pure inert gas, the moles of oxidant remains the same as in the vacuum state and the moles of nitrogen increases. The new (lower) oxidant concentration is
y1=(noxy)1LnH(7-5)
where y1 is the oxygen concentration after the first purge with nitrogen. Substituting Equation 7-3 into Equation 7-5 gives
y1=(noxy)1LnH=y0(nLnH)
If the vacuum and inert relief process is repeated, the concentration after the second purge is
y2=(noxy)2LnH=y1nLnH=y0(nLnH)2
This process is repeated as often as required to decrease the oxidant concentration to a desired level. The concentration after j purge cycles—that is, vacuum and relief—is given by the following general equation:
yj=y0(nLnH)j=y0(PLPH)j(7-6)
This equation assumes that the pressure limits PH and PL are identical for each cycle.
The total moles of inert gas added for each cycle is constant. For j cycles, it is given by
ΔnN2= j(PH−PL)VRgT(7-7)
Equations 7-1 through 7-7 assume the gas is well mixed in the vessel, the ideal gas law applies, and the temperature is constant.
Example 7-1
Use a vacuum purging technique to reduce the oxygen concentration within a 1000-gal vessel to 1 ppm. Determine the number of purges required and the total nitrogen used. The temperature is 75°F, and the vessel initially contains air at ambient conditions. A vacuum pump is used that reaches a pressure of 20 mm Hg absolute, and the vacuum is subsequently relieved with pure nitrogen until the pressure returns to 1 atm absolute.
Solution
The concentration of oxygen at the initial and final states is
y0=0.21 lb-mole O2/total molyf=1 ppm=1×10−6 1b-mole O2/total mol
The required number of cycles is computed using Equation 7-6:
yj=yo(PLPH)j ln(yjyo)=j ln(PLPH) j=ln(10−6/0.21)ln(20 mm Hg/760 mm Hg)=3.37
This is normally rounded up to four purge cycles.
The total nitrogen used is determined from Equation 7-7. The low-pressure PL is
PL=(20 mm Hg760 mm Hg)(14.7 psia)=0.387 psiaΔnN2=j(PH−PL)VRgT=4(14.7−0.387) psia(1000 gal)(1 ft3/ 7.48 gal)(10.73 psia ft3/1b-moleoR)(75+460)oR=1.33 lb-mole=37.2 1bm of nitrogen=16.9 kg of nitrogen
Pressure Purging
Vessels can be pressure-purged by adding inert gas under pressure. After the inert gas is added and mixes throughout the vessel, it is vented to the atmosphere, usually down to atmospheric pressure. More than one pressure cycle may be necessary to reduce the oxidant content to the desired concentration. For pressure purging, the high pressure is specified by the inert gas pressure and the pressure rating of the vessel.
The cycles required to reduce the oxygen concentration to a target value are shown in Figure 7-2. In this case, the vessel is initially at PL and is pressurized using a source of pure inert at PH. The objective is to determine the number of pressure purge cycles required to reach the desired concentration.

Because the vessel is pressurized with pure inert gas, the number of moles of oxygen remains constant during pressurization, and the mole fraction of oxygen decreases. During depressurization, the composition of the gas within the vessel remains constant, but the total number of moles is reduced. Thus, the oxygen mole fraction remains unchanged.
The equation used for this pressure purging is identical to Equation 7-6, where nL is now the total moles at atmospheric pressure (low pressure) and nH is the total moles under pressure (high pressure). In this case, however, the initial concentration of oxidant in the vessel (yo) is computed after the vessel is pressurized (the first pressurized state). The number of moles for this pressurized state is nH and the number of moles for the atmospheric case is nL.
One practical advantage of pressure purging versus vacuum purging is the potential for cycle time reductions. The pressurization process is much more rapid compared to the relatively slow process of pulling a vacuum. Also, the capacity of vacuum systems decreases significantly as the absolute vacuum is decreased. Pressure purging, however, uses more inert gas. Therefore, the best purging process is selected based on cost and performance.
Example 7-2
Use a pressure purging technique to reduce the oxygen concentration in the same vessel discussed in Example 7-1. Determine the number of purges required to reduce the oxygen concentration to 1 ppm using pure nitrogen at a pressure of 5 barg and at a temperature of 25°C. Also, determine the total nitrogen required. Compare the quantities of nitrogen required for the two purging processes.
Solution
The final oxygen concentration (yf) is specified to be 1 ppm or 10–6 lb-mole oxygen/total lb-mole. The number of cycles required is computed using Equation 7-6:
1 bar=0.987 atm=14.5 psi 1 atm=1.013 bar
yj=yo(PLPH)jj=ln(10−6/ 0.21)ln[1.013 bar /(5+1.013) bar]=6.88
The number of purge cycles, therefore, is 7. Thus, 7 pressure purges are required, compared to 4 for the vacuum purge process. The quantity of nitrogen used for this inerting operation is determined using Equation 7-7:
ΔnN2=j(PH−PL)VRgT=7(6.013−1.013) bar3.785 m3 (0.08314 bar m3/ kg−mole oK)(298oR)=5.34 kg-mole=149 kg of nitrogen=328 lb nitrogen
Pressure purging requires 7 purges and 149 kg of nitrogen, compared to 4 purges and 16.9 kg of nitrogen for vacuum purging. This result illustrates the need for a cost performance comparison to determine whether the time saved in pressure purging justifies the added cost for nitrogen.
Combined Pressure–Vacuum Purging
In some cases, both pressure and vacuum capabilities are available and are used simultaneously to inert a vessel. The computational procedure depends on whether the vessel is first evacuated or pressurized.
The purging cycles for a pressure-first purge are shown in Figure 7-3. In this case, the beginning of the cycle is defined as the end of the initial pressurization. If the initial oxygen mole fraction is 0.21, the oxygen mole fraction at the end of this initial pressurization is given by
y0=0.21(PoPH)(7-8)

At this point, the remaining cycles are identical to those for pressure purging and Equation 7-6 applies. However, the number of cycles j is the number of cycles after the initial pressurization.
The purging cycles for an evacuate-first purge are shown in Figure 7-4. In this case, the beginning of the cycle is defined as the end of the initial evacuation. The oxygen mole fraction at this point is the same as the initial mole fraction. Furthermore, the remaining cycles are identical to those in the vacuum purge operation and Equation 7-6 is directly applicable. However, the number of cycles j is the number of cycles after the initial evacuation.

Vacuum and Pressure Purging with Impure Nitrogen
The equations developed for vacuum and pressure purging apply to processes using pure inert gases only. In reality, many of the nitrogen separation processes available today do not provide pure nitrogen; instead, they typically provide nitrogen in the 98% range, with the remainder of the gas being usually oxygen.
Assume that the nitrogen used as an inerting gas also contains oxygen at a constant mole fraction of yoxy. For a pressure purging procedure. the total moles of oxygen present at the end of the first pressurization is given by the moles initially present plus the moles included with the nitrogen. This amount is
noxy=y0(PLVRgT)+yoxy(PH−PL)VRgT(7-9)
The total moles in the vessel at the end of the first pressurization is given by Equation 7-1. Thus, the mole fraction of oxygen at the end of this cycle is
y1=noxyntot=y0(PLPH)+yoxy(1−PLPH)(7-10)
This result is generalized into a recursive equation (Equation 7-11) and a generalized equation (Equation 7-13), for the oxygen concentration at the end of the jth pressure cycle:
yj=yj−1(PLPH)+yoxy(1−PLPH)(7-11)
Rearrange Equation 7-11 to give:
yj−yoxy=(yj−1−yoxy)(PLPH)
Use the preceding equation to determine the concentration after one purge:
y1−yoxy=(y0−yoxy)(PLPH)(7-12)
Repeat this process for the second purge:
y2−yoxy=(y1−yoxy)(PLPH)
Replace (y1−yoxy)
y2−yoxy=(y0−yoxy)(PLPH)(PLPH)=(y0−yoxy)(PLPH)2
Repeat this process to give the following general equation:
yj−yoxy=(y0−yoxy)(PLPH)j(7-13)
Equation 7-13 is used in place of Equation 7-6 for both pressure and vacuum purging with impure inert gases.
Pressure purging is faster because the pressure differentials are greater but it uses more inert gas than vacuum purging. Vacuum purging uses less inert gas because the oxygen concentration is reduced primarily by vacuum on the very first cycle. When combining vacuum and pressure purging, less nitrogen is used to if the initial cycle is a vacuum cycle.
Sweep-Through Purging
The sweep-through purging process adds the purge gas into a vessel at one opening and withdraws the mixed gas from the vessel to the atmosphere (or scrubber) from another opening. This purging process is commonly used when the vessel or equipment is not rated for pressure or vacuum; the purge gas is added and withdrawn at a fixed pressure, normally atmospheric.
Purging results are defined by assuming perfect mixing within the vessel, constant temperature, and constant pressure. Under these conditions, the mass or volumetric flow rate for the exit stream is equal to the inlet stream. The material balance around the vessel is
VdCdt=C0Qv−CQv(7-14)
where
V is the vessel volume,
C is the concentration of oxidant within the vessel (mass or volumetric units),
C0 is the inlet oxidant concentration (mass or volumetric units),
Qv is the volumetric flow rate (volume/time), and
t is time.
The mass or volumetric flow rate of oxidant into the vessel is C0Qv, and the flow rate of oxidant exiting is CQv. Equation 7-14 is rearranged and integrated:
Qv∫t0dt=V∫C2C1dC(C0−C)(7-15)
The volumetric quantity of inert gas required to reduce the oxidant concentration from C1 to C2 is Qvt, and it is determined using the following equation:
Qvt=Vln(C1−C0C2−C0)(7-16)
If pure inert gas is used for the sweep purge, then C0 = 0.
Example 7-3
A storage vessel contains 100% air by volume and must be inerted with nitrogen until the oxygen concentration is less than 1.25% by volume. The vessel volume is 1000 ft3. Compute the volume of nitrogen required, assuming the nitrogen contains 0.01% oxygen.
Solution
The volume of nitrogen required, Qvt, is determined using Equation 7-15:
Qvt=Vln(C1−C0C2−C0)=(1000 ft3)ln(21.0−0.011.25−0.01)=2830 ft3
This is the quantity of impure nitrogen added (containing 0.01% oxygen). The quantity of pure nitrogen required to reduce the oxygen concentration to 1.25% is
Qvt=(1000 ft3)ln(21.01.25)=2821 ft3
Siphon Purging
The sweep-through process requires large quantities of nitrogen, as shown by Equation 7-16. This could be expensive when purging large storage vessels. In such a case, siphon purging is used to minimize this purging expense.
The siphon purging process starts by filling the vessel with liquid water or any liquid compatible with the material to be stored in the vessel. The purge gas is subsequently added to the vapor space of the vessel as the liquid is drained from the vessel. The volume of purge gas is equal to the volume of the vessel, and the rate of purging is equivalent to the volumetric rate of liquid discharge.
It is typically not possible to fill the vessel entirely with liquid. Thus, when using the siphon purging process, it may be desirable to first fill the vessel as much as possible with liquid and then use the sweep-through purge process to remove oxygen from the residual head space. This method decreases the oxygen concentration to low concentrations, but requires only a small extra expense for the additional sweep-through purging.
Using the Flammability Diagram to Avoid Flammable Atmospheres
The flammability diagram introduced in Chapter 6 is important for determining whether a flammable mixture exists and for providing target concentrations for inerting and purging procedures. The objective is to avoid flammable mixtures.
The procedure for taking a vessel out of service is illustrated in Figure 7-5. The vessel is initially at point A and contains pure fuel gas at atmospheric pressure. If air is used to purge the vessel, the composition follows line AR, which goes through the flammability zone. If nitrogen is first pumped into the vessel, the gas composition follows along line AS. One approach is to continue the nitrogen flow until the vessel contains pure nitrogen. However, this requires a large amount of nitrogen and is costly. A more efficient procedure is to inert with nitrogen until point S is reached. Then air can be introduced, and the gas composition follows along the line SR. In this case, the flammability zone is avoided and a safe vessel preparation procedure is ensured.
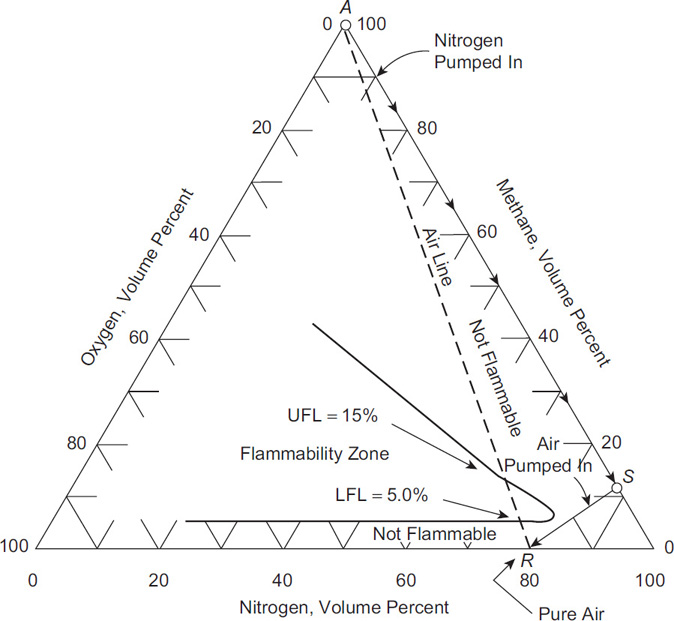
One might suggest an even more optimized procedure, which involves first pumping air into the vessel until a point is reached on the air line above the UFL. This is followed by pumping nitrogen into the vessel, followed by pumping in air. This approach avoids the nose of the flammability zone and minimizes the consumption of nitrogen. Unfortunately, the air forms a flammable mixture at the entry point as the pure air mixes with the fuel-rich gas mixture in the vessel. The flammability diagram reflects only the average gas composition within the vessel. Using nitrogen first avoids this problem.
When using the nitrogen purge process, one must determine the location of point S in Figure 7-5. The approach is shown in Figure 7-6. Point S is approximated by a line starting at the pure air point R and connecting through a point M at the intersection of the LFL with the stoichiometric combustion line. Because the gas compositions at points R and M are known, the composition at point S is determined graphically or by using the following equation:
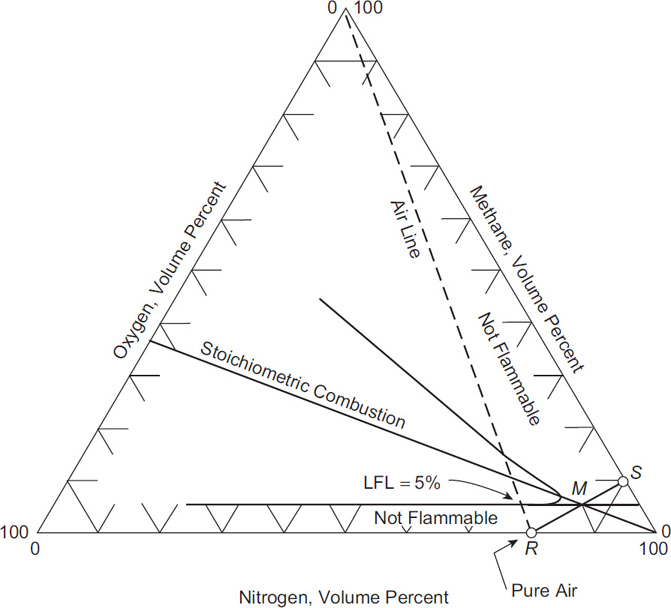
OSFC=LFL1−z(LFL21)(7-17)
where OSFC is the out-of-service fuel concentration—that is, the fuel concentration at point S in Figure 7-6. The LFL is the volume percent of fuel in air at the lower flammability limit, and z is the stoichiometric oxygen coefficient from the combustion reaction given in Chapter 6. The derivation of Equation 7-17 is provided on the website for this textbook.
Another approach is to estimate the fuel concentration at point S by extending the line from point R through the intersection of the minimum oxygen concentration (M ) and the stoichiometric combustion line. The analytical result is
OSFC=LOCz(1−LOC21)(7-18)
where
LOC is the limiting oxygen concentration (also called the minimum oxygen concentration) in volume percent oxygen,
z is the stoichiometric coefficient for oxygen given in Equation 6-9, and
LFL is the fuel concentration at the lower flammability limit, in volume percent of fuel in air.
The derivation of Equation 7-18 is provided on the website for this textbook.
Equations 7-17 and 7-18 are approximations of the fuel concentration at point S. Fortunately, these equations are mostly (but not always) conservative—that is, less than the experimentally determined OSFC value. For instance, for methane the LFL is = 5.3% and z is 2. Thus Equation 7-17 predicts an OSFC of 10.7% fuel. This is compared to an experimentally determined OSFC of 14.5% (from Table 7-2). By using an experimental LOC of 12%, an OSFC value of 14% is determined. This is closer to the experimental value but still conservative. For ethylene, 1,3-butadiene, and hydrogen, Equation 7-18 predicts a higher OSFC than the experimentally determined value. For all other species in Table 7-2, Equation 7-17 estimates an OSFC that is less than the experimental value.
Table 7-2 Experimental In-Service Oxygen Concentrations (ISOCs) and Out-Of-Service Fuel Concentrations (OSFCs)
Chemical |
OSFC (vol. % fuel) |
ISOC (vol. % oxygen) |
Chemical |
OSFC (vol. % fuel) |
ISOC (vol. % oxygen) |
|---|---|---|---|---|---|
Methane |
14.5 |
13 |
Cyclopropane |
7.0 |
12.0 |
Ethane |
7.0 |
11.7 |
Methyl alcohol |
15.0 |
10.8 |
Propane |
6.2 |
12.0 |
Ethyl alcohol |
9.5 |
11.0 |
Butane |
5.8 |
12.5 |
Dimethyl ether |
7.1 |
11.0 |
n-Pentane |
4.2 |
12.0 |
Diethyl ether |
3.8 |
11.0 |
n-Hexane |
3.8 |
12.2 |
Methyl formate |
12.5 |
11.0 |
Natural gas |
11.0 |
12.8 |
Isobutyl formate |
6.5 |
12.7 |
Ethylene |
6.0 |
10.5 |
Methyl acetate |
8.5 |
11.7 |
Propylene |
6.0 |
12.0 |
Acetone |
7.8 |
12.0 |
2-Methylpropene |
5.5 |
12.5 |
Methyl ethyl ketone |
5.3 |
11.5 |
1-Butene |
4.8 |
11.7 |
Carbon disulfide |
2.5 |
6.0 |
3-Methyl-1-butene |
4.0 |
11.5 |
Gasoline (115/145) |
3.8 |
12.0 |
1,3-Butadiene |
4.9 |
10.8 |
JP-4 |
3.5 |
11.7 |
Acetylene |
4.0 |
7.0 |
Hydrogen |
5.0 |
5.7 |
Benzene |
3.7 |
11.8 |
Carbon monoxide |
19.5 |
7.0 |
Source: C. V. Mashuga and D. A. Crowl. “Application of the Flammability Diagram for Evaluation of Fire and Explosion Hazards of Flammable Vapors.” Process Safety Progress 17, no. 3 (1998): 176.
Figure 7-7 shows the procedure for placing a vessel into service. The vessel begins with air, shown as point A. Nitrogen is pumped into the vessel until point S is reached. Then fuel is pumped in following line SR until point R is reached. The problem is to determine the oxygen (or nitrogen) concentration at point S. The in-service oxygen concentration (ISOC) represents the maximum oxygen concentration at point S in Figure 7-7 that just avoids the flammability zone, with a small margin of safety.

If a detailed flammability diagram is lacking, then the ISOC is estimated. One approach is to use the intersection of the LFL with the stoichiometric combustion line. A line is drawn from the top apex of the triangle (R) through this intersection to the nitrogen axis, as shown in Figure 7-8. The composition at S is determined graphically or with

ISOC=zLFL1−(LFL100)(7-19)
Where
ISOC is the in-service oxygen concentration in volume percent oxygen,
z is the stoichiometric coefficient for oxygen given in Equation 6-9, and
LFL is the fuel concentration at the lower flammability limit, in volume percent of fuel in air.
Equation 7-19 is derived on the website for this textbook. The nitrogen concentration at point S is equal to 100 – ISOC.
An expression to estimate the ISOC using the intersection of the minimum oxygen concentration and the stoichiometric line is also found using a similar procedure. The analytical result is
ISOC=zLOCz−LOC100(7-20)
where LOC is the minimum oxygen concentration in volume percent oxygen.
A comparison of the estimates using Equations 7-19 and 7-20 with the experimental values in Table 7-2 shows that Equation 7-19 predicts a lower oxygen value than the experimental values for all species except for methyl formate. Equation 7-20 predicts a lower oxygen concentration than the experimental value for all species in Table 7-2 except for butane, methyl-1-butene, 1,3-butadiene, isobutyl formate, and acetone. The calculated values are deliberately not shown in Table 7-2. Obtaining direct and reliable experimental data under conditions as close as possible to process conditions is recommended.
7-2 Static Electricity
A common ignition source within chemical plants is sparks resulting from static charge buildup and sudden discharge. Static electricity is perhaps the most elusive of ignition sources. Despite considerable efforts to mitigate this risk, serious explosions and fires caused by static ignition continue to plague the chemical process industry.
The best design methods for preventing the emergence of this type of ignition source are developed by understanding the fundamentals relevant to static charge. These fundamentals are then used to design features within a plant to prevent the accumulation of static charge or to recognize situations where the buildup of static electricity is inevitable and unavoidable. For unavoidable static buildup, design features are added to continuously and reliably inert the atmosphere around the regions where static sparks are likely.
Fundamentals of Static Charge
Static charge buildup is a result of physically separating a poor conductor from a good conductor or another poor conductor. When different materials touch each other, the electrons move across the interface from one surface to the other. Upon separation, more of the electrons remain on one surface than on the other—that is, one material becomes positively charged and the other negatively charged. In such a case, the charges are equal but opposite in polarity.
If both the materials are good conductors, the charge buildup due to separation is small because the electrons are able to quickly scurry between the surfaces. Conversely, if one or both of the materials are insulators or poor conductors, electrons are not as mobile and may become trapped on one of the surfaces, resulting in charge accumulation.
Household examples that result in static charge accumulation include walking across a rug, placing different materials in a tumble dryer, removing a sweater, and combing hair. The clinging fabrics and sometimes audible sparks are the result of the buildup of static charge.
Common industrial examples include pumping a nonconductive liquid through a pipe, mixing immiscible liquids, pneumatically conveying solids, and leaking steam that contacts an ungrounded conductor. The static charges in these examples accumulate to develop large voltages. Subsequent grounding produces large and energetic sparks.
For industrial operations where flammable vapors may be present, any charge accumulation exceeding 0.1 mJ is considered dangerous. Static charges of this magnitude are easy to generate. For example, the static buildup created just by walking across a carpet averages about 20 mJ and exceeds several thousand volts.
Basic electrostatic relationships are used to understand and investigate these situations. These relationships may include field strengths produced by static charges, electrostatic potential, capacitance, relaxation times, currents, and potentials in flow systems.
An electrostatic discharge occurs when two materials at different potentials or polarities come close enough together to generate a charge transfer. In an explosive environment, this sudden transfer of charges may be energetic enough to act as an ignition source. To prevent ignitions created in this way, one must understand (1) how charges accumulate on objects, (2) how charges discharge by means of charge transfer, and (3) how to estimate the resulting energy discharged in relationship to the minimum ignition energy (MIE) of the material present.
Charge Accumulation
Four charge accumulation mechanisms are relevant to dangerous electrostatic discharges in a chemical plant:2
2J. A. Cross. Electrostatics: Principles, Problems, and Applications (Bristol, UK: Adam Higler, 1987).
Contact and frictional charging: When two materials, one or both of which are insulators, are brought into contact, a charge separation occurs at the interface. If the two objects are then separated, some of the charges remain separated, giving the two materials opposite but equal charges.
Double-layer charging: Charge separation occurs on a microscopic scale in a liquid at any interface (solid–liquid, gas–liquid, or liquid–liquid). As the liquid flows, it carries a charge and it leaves a charge of opposite sign on the other surface—for example, a pipe wall.
Induction charging: This phenomenon is applicable only to materials that are electrically conductive. A person with insulated shoes, for example, may approach an overhead vessel that is positively charged (previously filled with positively charged solids). Electrons in the person’s body (head, shoulders, and arms) migrate toward the positive charge of the vessel, thereby causing an equal quantity of positive charges to accumulate on the opposite side of the body. This leaves a part of the body positively charged by induction. When a metal object is touched, a transfer of the electrons occurs, creating a spark.
Charging by transport: When charged liquid droplets or solid particles settle on an isolated object, the object becomes charged. The transferred charge is a function of the object’s capacitance and of the conductivities of the particles.
Some nonconductive hydrocarbon liquids have such low conductivities that once they receive a charge, they retain it for a relatively long time. This can result in the potential hazard of a hydrocarbon liquid storage tank. This critical factor is described in a CSB video on static electricity.3
3Static Sparks Explosion in Kansas. https://www.csb.gov/videos/static-sparks-explosion-in-kansas/.
Electrostatic Discharges4
4T. B. Jones and J. L. King. Powder Handling and Electrostatics (Chelsea, MI: Lewis Publishers, 1991).
A charged object can be discharged to a ground or to an oppositely charged object when the field intensity exceeds 3 MV/m (breakdown voltage of air) (where 1 MV/m = 106 V/m = 1000 V/mm) or when the surface reaches a maximum charge density of 7 × 10-5 coulombs/m2. This discharge can occur by six methods: (1) spark, (2) propagating brush, (3) conical pile (sometimes known as Maurer discharge), (4) brush, (5) lightning-like, and (6) corona discharges.
A spark discharge (Figure 7-9) is a discharge between two metallic objects. Because both objects are conductive, the electrons move to exit at a single point of the charged object, and they enter the second object at a single point. This will result in an energetic spark that can ignite a flammable dust or gas.

A propagating brush discharge (Figures 7-9 and 7-10) is a discharge from a grounded conductor when it approaches a charged insulator that is backed by a conductor. These discharges are energetic and can ignite flammable gases and dusts. Data show that propagating brush discharges are not possible if the breakdown voltage of the insulator is 4 kV or less.5
5B. Maurer. “Discharges Due to Electrostatic Charging of Particles in Large Storage Silos.” German Chemical Engineering 3 (1979): 189–195.

A conical pile discharge (Figure 7-9) is a form of a brush-type discharge that occurs at the conical surface of a pile of powder.6 The necessary conditions for this discharge are (1) a powder with a high resistivity (greater than 1010 ohm m), (2) a powder with coarse particles 0.1 mm in diameter, (3) a powder with a high charge-to-mass ratio (for example, charged by pneumatic transport), and (4) a filling rate greater than approximately 0.5 kg/s. Conical pile discharges are relatively intense discharges, with energies up to several hundred millijoules, and can ignite flammable gases and dusts. To ignite dusts, the coarse particles need a fraction of fines to give an explosive atmosphere.
6M. Glor and B. Maurer. “Ignition Tests with Discharges from Bulked Polymeric Granules in Silos (Cone Discharge).” Journal of Electrostatics 30 (1993): 123–134.
A brush discharge (Figure 7-9) is a discharge between a relatively sharp-pointed conductor (radius of 0.1 to 100 mm) and either another conductor or a charged insulated surface. This discharge radiates from the conductor in a brush-like configuration. Such a discharge is less intense compared to a point-to-point spark discharge, and it is unlikely to ignite dusts. However, brush discharges can ignite flammable gases.
Lightning-like discharges (Figure 7-9) are discharges from a cloud in the air over the powder. Experiments have shown that lightning-like discharges do not occur in vessels with volumes less than 60 m3 or in silos with diameters less than 3 m.7 There is currently no physical evidence indicating that lightning-like discharges have resulted in industrial deflagrations.
7P. Boschung, W. Hilgner, G. Luttgens, B. Maurer, and A. Wider. “An Experimental Contribution to the Question of the Existence of Lightning-like Discharges in Dust Clouds.” Journal of Electrostatics 3 (1977): 303–310.
A corona discharge (Figure 7-11) is similar to a brush discharge. The electrode conductor has a sharp point. The discharge from such an electrode has sufficient energy to ignite only the most sensitive gases (for example, hydrogen).

Energy from Electrostatic Discharges
Figure 7-12 illustrates the energy generated in electrostatic discharges versus the minimum ignition energies of gases and vapors and dusts. In general, the results illustrate that most flammable gases and vapors can be ignited by spark, brush, conical pile, and propagating brush discharges, and that most flammable dusts can be ignited only by sparks, propagating brush, and conical pile discharges. The regions enclosed by the dotted lines in Figure 7-12 indicate regions of uncertainty.

Energy of Electrostatic Ignition Sources
A spark is generated between two conductors when the distance between the conductors is small compared to the diameter of the conductors and when the electric field intensity between the conductors is approximately 3 MV/m. A brush discharge is generated if the distance between the conductors is large compared to the radius of curvature of the conductor.
The energy of a spark discharge is a function of the accumulated charge (Q in coulombs) on the object, the capacitance of the object (C in farads), and the potential, or voltage (V, in volts), of the object. These three variables are related by the expression C = Q/V. The actual energy (expressed in joules) associated with the discharge process is given by
J=Q22C(7-21)
Equation 7-21 assumes a capacitance-type discharge (that is, a spark); however, capacitance and voltage are not defined in nonconductive systems. As a consequence, Equation 7-21 is valid only for capacitive sparks, but it is used qualitatively for the other discharges.
A criterion that is commonly used to estimate the potential hazard of a discharge is to compare the MIE of the fuel–air mixture to the equivalent energy of the discharge. A precise experimental determination of the MIE is often required under the specific conditions of the system. MIEs are shown in Table 6-4 for a number of flammable gases and in Table 6-7 for dusts.
The static discharge energy is a function of the accumulated charge. In an industrial setting, this accumulated charge is usually the result of either contact or friction charging for flowing solids and double-layer charging for flowing liquids. In each case, the charge (electrons) is transported with the material. The measure of this flow of electrons is a streaming current and is expressed in coulombs per second or amps.
Streaming Current
A streaming current IS is the flow of electricity produced by transferring electrons from one surface to another by a flowing fluid or solid. When a liquid or solid flows through a pipe (metal or glass), an electrostatic charge develops due to the streaming material. This current is analogous to a current in an electrical circuit. The relation between a liquid streaming current and the pipe diameter, pipe length, fluid velocity, and fluid properties is given by the following equation:8
8L. G. Britton. Avoiding Static Ignition Hazards in Chemical Operations (New York: American Institute of Chemical Engineers, 1999).
Is=[10×10−6amp(m / s)2(m)2](ud)2[1−exp(−Luτ)](7-22)
where
Is is the streaming current (amps),
u is the velocity (m/s),
d is the pipe diameter (m),
L is the pipe length (m), and
τ is the liquid relaxation time (seconds).
The relaxation time of the liquid is the time for the charge to fall by approximately 37% of its initial value, assuming no charges are added—that is, the time required for a charge to dissipate by leakage. It is determined using
τ=εrε0γc(7-23)
where
τ is the relaxation time (seconds),
εr is the relative dielectric constant (unitless),
ε0 is the permittivity constant, with a value of
8.85×10−12coulomb2Nm2=2.7×10−12coulombvolt ft=8.85×10−12coulombvolt m8.85×10−14coulombvolt cm=8.85×10−14sohm cm=8.85×10−14mho s cm, and
γc is the specific conductivity (mho/cm).
Specific conductivities and relative dielectric constants are listed in Table 7-3. Notice that resistance = 1/conductivity = 1/(mho/cm) = ohm cm.
Table 7-3 Properties for Electrostatic Calculations
Material |
Specific conductivitya(mho/cm) |
Dielectric constant |
|
|---|---|---|---|
Liquids |
|
|
|
|
Benzene |
7.6 × 10–8 to < 1 × 10–18 |
2.3 |
|
Toluene |
< 1 × 10–14 |
2.4 |
|
Xylene |
< 1 × 10–15 |
2.4 |
|
Heptane |
< 1 × 10–18 |
2.0 |
|
Hexane |
< 1 × 10–18 |
1.9 |
|
Methanol |
4.4 × 10–7 |
33.7 |
|
Ethanol |
1.5 × 10–7 |
25.7 |
|
Isopropanol |
3.5 × 10–6 |
25.0 |
|
Water |
5.5 × 10–6 |
80.4 |
Other materials and air |
|
|
|
|
Air |
— |
1.0 |
|
Cellulose |
1.0 × 10–9 |
3.9–7.5 |
|
Pyrex |
1.0 × 10–14 |
4.8 |
|
Paraffin |
10–16 to 0.2 × 10–18 |
1.9–2.3 |
|
Rubber |
0.33 × 10–13 |
3.0 |
|
Slate |
1.0 × 10–8 |
6.0–7.5 |
|
Teflon |
0.5 × 10–13 |
2.0 |
|
Wood |
10–10 to 10–13 |
3.0 |
aResistance = 1/conductivity = 1/(mho/cm) = ohm cm.
Source: J. H. Perry. Chemical Engineers’ Handbook, 3rd ed. (New York, NY: McGraw-Hill, 1950), p. 1734.
Charges also accumulate when solids are transported. The buildup results from the separation of solid particle surfaces. Because solid geometries are almost always ill defined, electrostatic calculations for solids are handled empirically.
The streaming current that is generated while transporting solids is a function of the solids processing method (see Table 7-4) and the flow rate, as shown by
Is=(coulombskg)(kgs)(7-24)
where
IS is coulombs/second or amps,
coulombs/kg is given in Table 7-4, and
kg/s is the solids flow rate.
Table 7-4 Charge Buildup for Various Operations
Process |
Charge (coulomb/kg) |
|---|---|
Sieving |
10–9 to 10–11 |
Pouring |
10–7 to 10–9 |
Grinding |
10–6 to 10–7 |
Micronizing |
10–4 to 10–7 |
Sliding down an incline |
10–5 to 10–7 |
Pneumatic transport of solids |
10–5 to 10–7 |
Source: R. A. Mancini. “The Use (and Misuse) of Bonding for Control of Static Ignition Hazards.” Plant/Operations Progress 7, no. 1 (1988): 24.
Some generally accepted guidelines for electrostatic calculations are shown in Table 7-5.
Table 7-5 Accepted Electrostatic Values for Calculations
Voltage to produce spark between needle points ½ in. apart |
14,000 V |
|
Voltage to produce spark between plates 0.01 mm apart |
350 V |
|
Maximum charge density before corona discharge |
2.65 × 10–9 coulomb/cm2 |
|
Minimum ignition energies (mJ) |
|
|
Vapors in air |
0.1 |
|
Mists in air |
1.0 |
|
Dusts in air |
10.0 |
|
Approximate capacitances C (micro-micro farads = 10–12 farads) |
|
|
Humans |
100–400 |
|
Automobiles |
500 |
|
Tank truck (2000 gal) |
1000 |
|
Tank (12-ft diameter with insulation) |
100,000 |
|
Capacitance between two 2-in. flanges (⅛-in. gap) |
20 |
|
Contact zeta potential |
0.01–0.1 V |
|
Source: F. G. Eichel. “Electrostatics.” Chemical Engineering (March 13, 1967): 163.
Electrostatic Voltage Drops
Figure 7-13 illustrates a tank with a feed line. Fluid flows through the feed line and drops into the tank. The streaming current builds up a charge and voltage in the feed line to the vessel and in the vessel itself. The voltage from the electrical ground in the metal line to the end of the glass pipe is calculated using
V=IsR(7-25)

The resistance R (in ohms) is computed using the conductivity of the fluid γc (in mho/cm), the length of the conductor L (in cm), and the area A of the conductor (in cm2):
R=LγcA(7-26)
This relationship shows that as the area of the conductor increases, the resistance decreases; if the conductor length increases, the resistance increases.
Energy of Charged Capacitors
A capacitor is used to store electrostatic energy, with generally two electrical conductors (plates) separated by a dielectric (i.e., insulator). The amount of work required to increase the charge on a capacitor from Q to Q + dQ is dJ = VdQ, where V is the potential difference and the charge is Q. Because V = Q/C, the integration gives Equation 7-21, and substitutions give
J=CV22(7-27)
J=QV2(7-28)
The units used in Equations 7-27 and 7-28 are usually C in farads, V in volts, Q in coulombs, and J in joules.
Capacitances of various materials used in the chemical industry are given in Table 7-6.
Table 7-6 Capacitance of Various Objects
Object |
Capacitance (farad) |
|---|---|
Small scoop, soda can, tools |
5 × 10–12 |
Buckets, small drums |
20 × 10–12 |
50–100-gal containers |
100 × 10–12 |
Person |
200 × 10–12 |
Automobile |
500 × 10–12 |
Tank truck |
1000 × 10–12 |
Source: R. A. Mancini. “The Use (and Misuse) of Bonding for Control of Static Ignition Hazards.” Plant/Operations Progress 7, no. 1 (1988): 24.
Charges can accumulate as a result of a streaming current dQ/dt = I. Assuming a constant streaming current,
Q=Is t(7-29)
where IS is in amps and t is in seconds. Equation 7-29 assumes that the system starts with no accumulation of charge, only one constant source of charge IS, and no current or charge loss terms.
Example 7-4
Determine the voltage developed between a charging nozzle and a grounded tank, as shown in Figure 7-14. Also, compute the energy stored in the nozzle and the energy accumulated in the liquid. Explain the potential hazards in this process for a flow rate of
1 gpm
150 gpm
The data are
Hose length: 20 ft
Hose diameter: 2 in.
Liquid conductivity: 10–8 mho/cm
Dielectric constant εr: 25.7
Density: 0.88 g/cm3

Solution
Because the hose and nozzle are not grounded, the voltage generated at the nozzle tip is V = IR. The resistance is computed using Equation 7-25 for the conducting fluid with a resistance length equivalent to the hose length (from the ground near the pump to the nozzle) and a resistance area equivalent to the cross-sectional area of the conducting fluid:
L=(20 ft)(12 in/ft)(2.54 cm/in)=610 cmA=πr2=(3.14)(1 in)2=3.14 m2=20.3 cm2
LA=(20 ft)(12 in/ft)(2.54 cm/in)=610 cm=πr2=(3.14)(1 in)2=3.14 m2=20.3 cm2 Using Equation 7-26, we obtain
R=(1γc)(LA)=(108ohm cm)(610 cm20.3 cm2)=3.00×109ohm
R=(1γc)(LA)=(108ohm cm)(610 cm20.3 cm2)=3.00×109ohm The streaming current is a function of the velocity and the pipe diameter. The average velocity in the pipe is
u=(1 gal/min3.14 in2)(ft37.48 gal)(144 in2ft2)(1 min60 s)=0.102 ft/s=3.1×10−2 m/s
u=(1 gal/min3.14 in2)(ft37.48 gal)(144 in2ft2)(1 min60 s)=0.102 ft/s=3.1×10−2 m/s The relaxation time is estimated using Equation 7-23:
τ=εrε0γc=(25.7)(8.85×10−14mho scm)10−8 mho / cm=22.7×10−5 s
τ=εrε0γc=(25.7)(8.85×10−14mho scm)10−8 mho / cm=22.7×10−5 s The streaming current is now determined using Equation 7-22:
Is=[10×10−6 amp(m/s)2(m)2](ud)2[1−exp(−Luτ)]=[10×10−6 amp(m/s)2(m)2][(3.1×10−2 m/s)(2 in12 in/ft×3.28 ft/m)]2[1−exp(−20 ft0.102 ft/s×2.27×10−4s)]=[10×10−6 amp(m/s)2(m)2](1.57×10−3 m2/s)2(1−0)=2.48×10−11 amp
Is=[10×10−6 amp(m/s)2(m)2](ud)2[1−exp(−Luτ)]=[10×10−6 amp(m/s)2(m)2][(3.1×10−2 m/s)(2 in12 in/ft×3.28 ft/m)]2[1−exp(−20 ft0.102 ft/s×2.27×10−4s)]=[10×10−6 amp(m/s)2(m)2](1.57×10−3 m2/s)2(1−0)=2.48×10−11 amp Method 1: Compute the energy accumulated in the capacitor formed between the flanges at the nozzle. A spark between the flanges may be an ignition source. The voltage drop down the 20-ft line is the same as the voltage drop from the hose flange to the nozzle flange, assuming that the nozzle is grounded. The voltage is therefore
V=IR=(2.48×10−11 amp)(3.0×109 ohm)=0.074 volt
V=IR=(2.48×10−11 amp)(3.0×109 ohm)=0.074 volt The capacitance between the two 2-in flanges is given in Table 7-5:
C=20×10−12 farads=20×10−12 coulomb/volt
C=20×10−12 farads=20×10−12 coulomb/volt The energy is determined using Equation 7-27:
J=CV22=[20×10−12 coulomb/volt (0.074 volt)22]=5.49×10−14 J
J=CV22=[20×10−12 coulomb/volt (0.074 volt)22]=5.49×10−14 J This is significantly lower than the energy required to ignite a flammable gas (0.1 mJ). An electrostatic hazard at the nozzle is not expected.
Method 2: Compute the energy accumulated in the capacitor formed by the tank of liquid. A brush discharge can jump from this liquid to a metal component, such as a grounded thermocouple. The accumulated charge is computed using Equation 7-29:
Q=Ist
Q=Ist with the time equal to the filling time of the vessel:
t=(300 gal / 1 gpm)(60 s / min)=18,000 s
t=(300 gal / 1 gpm)(60 s / min)=18,000 s Substitution into Equation 7-29 gives
Q=Ist=(2.48×10−11 amp)(18,000 s) =4.46×10−7 coulomb
Q=Ist=(2.48×10−11 amp)(18,000 s) =4.46×10−7 coulomb The capacitance of the liquid is estimated to be one-tenth of the capacitance of a 2000-gal vessel, shown in Table 7-5; therefore
C=100×10−12 farads=100×10−12 coulomb/volt
C=100×10−12 farads=100×10−12 coulomb/volt and the accumulated energy is determined using Equation 7-21:
J=Q22C=(4.46×10−7 coulomb)22(100×10−12 farads)=9.9×10−4 J=0.99 mJ
J=Q22C=(4.46×10−7 coulomb)22(100×10−12 farads)=9.9×10−4 J=0.99 mJ This exceeds the energy required to ignite a flammable gas (0.1 mJ). In this situation the vessel should be purged with nitrogen to maintain the concentration of the flammable vapor below the LOC.
This case is identical to case a except that the flow rate is higher, 150 gpm versus 1 gpm for case (a):
u=(0.102 ft/s)(150 gpm1 gpm) =4.66 m/s
u=(0.102 ft/s)(150 gpm1 gpm) =4.66 m/s The resistance is the same as for case (a)—that is, 3 × 109 ohm:
τ=22.7×10−5 s
τ=22.7×10−5 s The streaming current is, from Equation 7-21,
Is=[10×10−6 amp(m / s)2(m)2][(4.66 m / s)(0.051 m)]2[1−exp(−20 ft15.3 ft / s×2.27×10−4 s)]=(5.65×10−7 amp)(1−0)=5.65×10−7 amp
Is=[10×10−6 amp(m / s)2(m)2][(4.66 m / s)(0.051 m)]2[1−exp(−20 ft15.3 ft / s×2.27×10−4 s)]=(5.65×10−7 amp)(1−0)=5.65×10−7 amp Method 1: Compute the energy accumulated in the capacitor formed between the flanges at the nozzle:
V=IR=(5.65×10−7 amp)(3×109 ohm)=1695 volts
V=IR=(5.65×10−7 amp)(3×109 ohm)=1695 volts The accumulated energy is again computed using Equation 7-26:
J=CV22=(20×10−12 coulomb)(1695 volts)22=0.000029 J=0.029 mJ
J=CV22=(20×10−12 coulomb)(1695 volts)22=0.000029 J=0.029 mJ This is less than the energy required to ignite a flammable gas (0.1 mJ).
Method 2: Compute the energy accumulated in the capacitor formed by the tank of fluid:
t=300 gal150 gpm(60 smin)=160 sQ=ISt=(5.65×10−7 amp)(160 s)=9×10−5 coulombsJ=Q22C=(9×10−5 coulombs)22(100×10−12 coulomb/volt)=40 J >> 0.1 mJ
This energy exceeds 0.1 mJ. This problem illustrates the importance of inerting. It is relatively easy to build up energies that exceed 0.1 mJ.
Capacitance of a Body
The buildup of a charge on one surface relative to another surface produces a capacitor. In the chemical industry, the properties of the developed capacitor are estimated by assuming parallel flat plate or spherical geometries. For example, the capacitance of a tank or a person is estimated by assuming spherical geometries, and the capacitance of a person’s shoe sole or of a noncorrosive tank lining is estimated assuming parallel flat plates. Several examples are shown in Figure 7-15.

The capacitance C of a body is Q/V. For a sphere with radius r, the voltage developed when a charge Q is accumulated is determined using the following equation from physics:
V=14πε0Qεrr(7-30)
Because C = Q/V, the capacitance for a spherical body is
C=4πεrε0r(7-31)
where
εr is the relative dielectric constant (unitless),
ε0 is the permittivity (8.85 × 10–12 coulomb2/Nm2 = 2.7× 10–12 coulomb/volt ft),
r is the sphere radius, and
C is the capacitance.
For two parallel plates,
V=QLεrε0A(7-32)
Therefore, the capacitance between parallel plates is
C=εrε0AL(7-33)
where
A is the area of the surface and
L is the thickness of the dielectric.
Example 7-5
Estimate the capacitance of a person (1.9 m tall) standing on a dry wooden floor.
Solution
This person’s capacitance is estimated assuming that the person’s shape is spherical and that the “sphere” is surrounded by air (er is 1.0 for air). Using Equation 7-31 for a sphere, we have
C=4πεrε0r=4(3.14)(1.0)(8.85×10−12coulombvolt m)(1.9 m2)=1.05×10−10coulombvolt=1.05×10−10 farad
The calculated capacitance is close to the value listed for a person in Table 7-6.
Example 7-6
Estimate the capacitance of a person standing on a conductive floor. Assume that the person’s shoe soles separate the person from the floor; that is, the shoe sole is the dielectric of the capacitor. Given:
Shoe sole area (ft2) = 2 shoes (0.4 ft2 each)
Shoe sole thickness = 0.2 in.
Dielectric constant of shoe soles = 3.5
Solution
Use Equation 7-32, which for flat parallel plates is
C=εrε0AL=(3.5)(2.7×10−12coulombvolt ft)(0.8 ft2)(0.2 in12 in/ft)=4.54×10−10 farads
Example 7-7
Estimate the charge buildup, and accumulated energy, as a result of a person (insulated from the floor) charging 13.6 kg of a dry powder, using a scoop, into a 75-L insulated drum. Assume that the person’s capacitance is 200 × 10–12 farad.
Solution
This operation is a sliding-contact type operation. From Table 7-4, this operation gives a charge of 10–5 coulomb/kg. Therefore, the charge buildup is
Q=(10−5coulombskg)(13.6 kg)=1.36×10−4 coulombs
The accumulated energy, using Equation 7-21, is
J=Q22C=(1.36×10−4coulombs)22(200×10−12 farad)=46.2 Joules=46,200 mJ
These results illustrate that the energy exceeds the requirement for generating a spark capable of igniting a flammable gas. This spark would be discharged if the person approached a ground with a hand or with the scoop.
An equal and opposite charge is also accumulated in the powder in the insulated drum. Therefore, the charged powder is another ignition source. For example, if a grounded object of any kind is placed close to the solids, an energetic brush discharge could be generated.
Sparks resulting from static charge and discharge continue to cause major fires and explosions within the chemical industry. The fundamentals and examples developed in these sections were designed to emphasize the importance of this subject. The emphasis on the fundamentals is intended to assist with understanding to prevent incidents with static electricity.
7-3 Controlling Static Electricity
Charge buildup, resulting sparks, and the ignition of flammable materials are inevitable events if control methods are not appropriately used. In practice, design engineers recognize this problem and install special features to prevent (1) sparks by eliminating the buildup and accumulation of static charge and (2) ignition by inerting.
Inerting (Section 7-1) is the most effective and reliable method for preventing ignition. It is always used when working with flammable liquids that are 5°C (or less) below the flash point (closed cup). Methods for preventing charge buildup are described in the following paragraphs.
General Design Methods to Prevent Electrostatic Ignitions
The design objective is to prevent the buildup of charges on a product (liquid or powder) as well as on surrounding objects (equipment or personnel). Also remember that for every charged object, there exists its oppositely charged counterpart. Four methods are used to achieve this objective:
Prevent charges from accumulating to dangerous levels by reducing the rate of charge generation and increasing the rate of charge relaxation. This method is generally used when handling liquids.
Prevent charges from accumulating to dangerous levels by designing the system to include charge reduction by means of low-energy discharges. This method is generally used when handling powders.
When dangerous discharges cannot be eliminated, prevent the possibility of an ignition by maintaining oxidant levels below the combustible levels (inerting) or by maintaining fuel levels below a target concentration, such as the LOC, LFL, or above the UFL.
Measures to mitigate the consequences of an explosion are also options for consideration (for example, deflagration venting and explosion suppression).
The special design features for preventing specific electrostatic ignitions are described in the following paragraphs.
Sparks are prevented by grounding and bonding. This procedure prevents two metallic objects (close to each other) from having different potentials. Grounding and bonding are used to avoid the possibility of isolated metal parts or objects. Isolated objects are notorious for building up large potentials and energetic sparks when they are approached by another conductor at a lower potential.
Propagating brush discharges are prevented by keeping the nonconductive surfaces or coatings thin enough or conductive enough to have a breakdown voltage less than 4 kV. These discharges are also prevented by keeping the metallic backings grounded, so as to eliminate the accumulation of a high-density charge on the metallic interface and a countercharge on the nonconductor surface.
Conical pile discharges are prevented by increasing the conductivity (add additives), by decreasing the charge rate to less than 0.5 kg/s, or by using containers with a volume less than 1 m3. The most effective way of preventing ignitions from conical pile discharges is inerting.
Brush discharges are prevented by keeping the nonconductive surfaces thin enough or conductive enough to have a breakdown voltage (ud) of 4 kV. Nonconductive coatings with a thickness greater than 2 mm, however, are capable of brush discharges even with a ud less than 4 kV. To prevent brush discharges, a thickness of less than 2 mm is necessary. This fixes the charges accumulated on the nonconductor, and fixed charges cannot be transferred in a brush discharge. Brush discharges from nonconductive liquids are prevented by increasing the conductivity using conductive additives. The most effective way of preventing ignitions from brush discharges is inerting.
Lightning-like discharges are prevented by keeping the vessel volume to less than 60 m3 or the vessel diameter to less than 3 m. If this condition is not met, then the system needs to be inerted.
Relaxation
When pumping fluids into a vessel through a pipe on top of the vessel, the separation process produces a streaming current Is, which causes charge buildup. It is possible to substantially reduce this electrostatic hazard by adding an enlarged section of pipe just before entering the tank. This decreases the flow rate and increases the hold time for charge relaxation. The residence time in this relaxation section of pipe should be approximately twice the relaxation time determined from Equation 7-23.
In actual practice, it was found that a hold time equal to or greater than one-half the calculated relaxation time is sufficient to eliminate charge buildup.9 The “twice the relaxation time” rule, therefore, provides a safety factor of 4. The American Petroleum Institute recommends a ud, from Equation 7-22, of less than 0.5 m2/s for road tanker filling and 0.8 m2/s for railcar filling.10
9F. G. Eichel. “Electrostatics.” Chemical Engineering (March 13, 1967): 153.
10API RP 2003, Protection Against Ignitions Arising Out of Static, Lightning, and Stray Currents, 8th ed. (Washington, DC: American Petroleum Institute, 2015).
Bonding and Grounding
The voltage difference between two conductive materials is reduced to zero by bonding the two materials—that is, by bonding one end of a conducting wire to one of the materials and bonding the other end to the second material. When comparing two sets of bonded materials they may have different voltages, however. The voltage difference between these two sets is reduced to zero by bonding each set to ground—that is, by grounding.
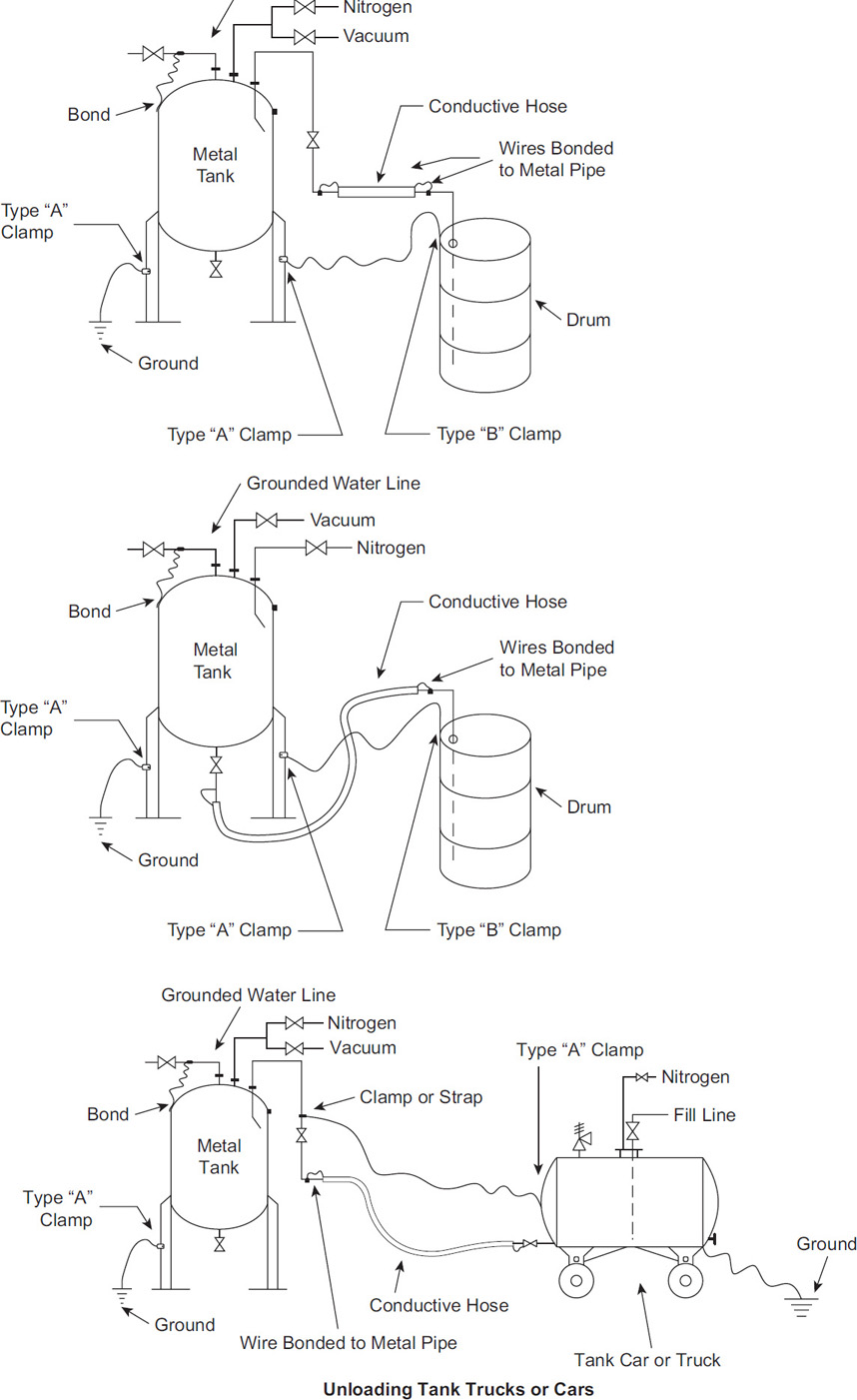
Bonding and grounding reduces the voltage of an entire system to ground level or zero voltage. It also eliminates the charge buildup between various parts of a system, eliminating the potential for static sparks. Examples of grounding and bonding are illustrated in Figures 7-16 and 7-17.

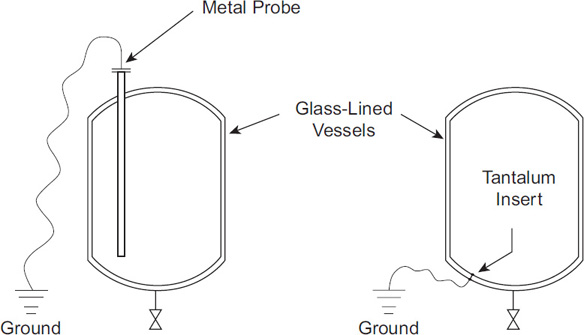
Materials inside glass or plastic-lined vessels are grounded using tantalum inserts or metal probes, as illustrated in Figure 7-18. This technique, however, is not effective when handling liquids with low conductivity. In this case, the fill line should extend to the bottom of the vessel (see Figure 7-19), to help eliminate the charge generation (and accumulation) resulting from separation during the filling operation. Also, the inlet velocities should be low enough to minimize the charge generated by streaming current IS.

Dip Pipes
An extended line, sometimes called a dip leg or dip pipe, reduces the electrical charge that accumulates when liquid is allowed to free fall. However, when using dip pipes, care must be taken to prevent siphoning back when the inlet flow is stopped. One method commonly employed to prevent siphoning is to place a hole in the dip pipe near the top of the vessel. Another technique is to use an angle iron instead of a pipe and to let the liquid flow down the angle iron (see Figure 7-19). These methods are also used when filling drums.
Increasing Conductivity with Additives
The conductivity of nonconducting organic materials can sometimes be increased by using antistatic additives. Examples of antistatic additives include water and polar solvents, such as alcohols. These additives are effective only when they are soluble in the offending liquid, because an insoluble phase gives an additional source of separation and charge buildup.
Handling Solids without Flammable Vapors
Charging solids with a ungrounded and conductive chute can result in a buildup of a charge on the chute. This charge can accumulate and ultimately produce a spark that may ignite a dispersed and flammable dust. Solids are transferred safely by bonding and grounding all conductive parts and/or by using nonconductive parts (drum and chute), as shown in Figure 7-20.

Handling Solids with Flammable Vapors
A safe design for handling solids that contain flammable vapors includes handling of the solids and liquids in a closed and inerted atmosphere (see Figure 7-21). For solvent-free solids, the use of nonconductive containers is permitted. For solids containing flammable solvents, only conductive and grounded containers are recommended.11
11Expert Commission for Safety in the Swiss Chemical Industry. “Static Electricity: Rules for Plant Safety,” Plant/Operations Progress 7, no. 1 (1988): 1.

7-4 Explosion-Proof Equipment and Instruments
All electrical devices are potential ignition sources; in turn, special design features are required to prevent the ignition of flammable vapors and dusts when such devices are used. The fire and explosion hazards are directly proportional to the number and type of electrically powered devices in a process area.12
12Expert Commission for Safety in the Swiss Chemical Industry. “Static Electricity: Rules for Plant Safety,” Plant/Operations Progress 7, no. 1 (1988):1.
Most safety practices for electrical installations are based on the National Electric Code (NEC).13 States, municipalities, and insurance companies may have their own installation requirements, but they are usually based on the NEC.
13NFPA 70, The National Electrical Code (Quincy, MA: National Fire Protection Association, 2008).
Process areas are divided into two major types of environments: XP and non-XP. XP, which stands for “explosion-proof,” means flammable materials might be present at certain times. Non-XP means that flammable materials are not present, even under abnormal conditions. In non-XP designated areas, open flames, heated elements, and other sources of ignition may be present.
Explosion-Proof Housings
In an XP area, the electrical equipment and some instrumentation must have special explosion-proof housings. These housings are not intended to prevent flammable vapors and gases from entering, but rather are designed to withstand an internal explosion and prevent the combustion from spreading beyond the inside of the enclosure. A motor starter, for example, is enclosed in a heavy cast walled box with the strength needed to withstand explosive pressures.
The explosion-proof design includes the use of conduit with special sealed connections around all junction boxes. Figure 7-22 shows features of electrical equipment rated for an XP area. The on/off switch housing is especially large and bulky. The thickness of the metal is approximately ¾ inch. The flanges are large so that if flammable vapors enter the box and are ignited, the burning gas is quenched as it flows out through the flange faces. This bulkiness is necessary to remove the heat of a burning gas and to extinguish the flame before it exits into a potentially flammable environment.
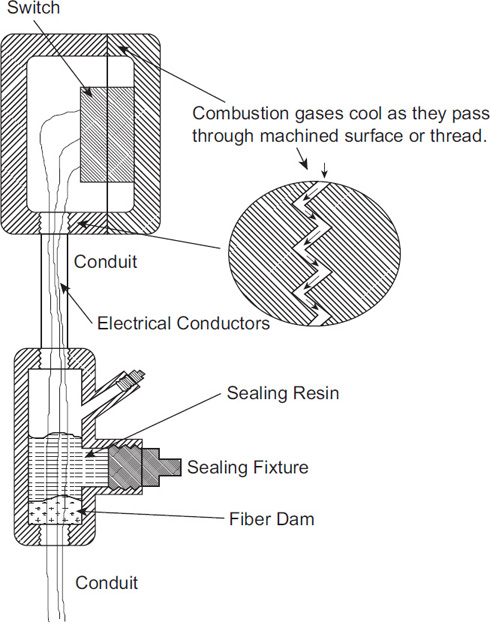
The sealing pipe fitting below the switch in Figure 7-22 is called an EYS. It is approximately 2½ inches long and 1½ inches in diameter. The procedure to seal this fitting is as follows:
The wires are separated within the fitting.
A fiber dam is created at the lower part of the fixture.
An epoxy liquid is poured into the fixture, completing the sealing of the fixture once the epoxy hardens.
A reasonably complex chemical plant may include hundreds of these fixtures, all of which must be sealed properly. Also, any scratches or dirt on the flanges will defeat the gas quenching and result in the combustion propagating to the external environment.
Area and Material Classification
The design of electrical equipment and instrumentation is based on the nature of the process hazards or specific process classifications. The classification method is defined in the NEC; it is a function of the nature and degree of the process hazards within a particular area. The rating method includes Classes I, II, and III; Groups A–G; and Divisions 1 and 2.
The classes are related to the nature of the flammable material:
Class I: Locations where flammable gases or vapors are present.
Class II: Same for combustible dusts.
Class III: Hazard locations where combustible fibers or dusts are present but not likely to be in suspension.
The groups designate the presence of specific chemical types. Chemicals that are grouped have equivalent hazards:
Group A: acetylene
Group B: hydrogen, ethylene
Group C: carbon monoxide, hydrogen sulfide
Group D: butane, ethane, ethyl alcohol
Group E: aluminum dust
Group F: carbon black
Group G: flour
Division designations are categorized in relationship to the probability of the material being within the flammable or explosive regions:
Division 1: Probability of ignition is high; that is, flammable concentrations are normally present.
Division 2: Hazardous only under abnormal or emergency conditions. Flammable materials are normally contained in closed containers or systems.
Design of an XP Rated Area
When designing an XP rated area, all electrical equipment and instrumentation must be specified for the class, group, and division. All equipment and instrumentation within an area must be appropriately specified and installed. The overall classification of an area is only as good as the piece of equipment with the lowest classification.
7-5 Ventilation
Proper ventilation is another method used to prevent fires and explosions. Ventilation is intended to dilute the explosive vapors with air to prevent explosions and to confine the hazardous flammable mixtures.
Open-Air Plants
Open-air plants are recommended because the average wind velocities are high enough to safely dilute volatile chemical leaks that may exist within a plant. Although safety precautions are always practiced to minimize leaks, accidental releases from pump seals and other potential release points may occur.
Example 7-8
A plant handling substantial quantities of flammable toluene is located 1000 ft from a residential area. There is some concern that a sizable leak of flammable vapors will form a flammable cloud, with subsequent ignition occurring in the residential area. Determine the minimum mass flow rate of toluene leakage required to produce a vapor cloud in the residential area with a concentration equal to the LFL. Assume a wind speed of 5 miles/hr (mph) and D atmospheric stability.
Solution
Assume a continuous leak at ground level. The plume concentration directly downwind along the cloud centerline is given by Equation 5-17:
〈C〉(x,0,0)=Qmπσyσzu
Solving for Qm, the mass flow rate from the leak, we obtain
Qm=〈C〉(x,0,0)πσyσzu
The LFL for toluene is 1.2% in air (from Appendix B). Converting the units, we obtain
(0.012m3 toluenem3 air)(1 g-mole toluene22.4×10−3 m3 toluene)(92 g toluene1 g-mole toluene)=49.3 g / m3
The wind speed is 5 mph = 2.23 m/s. The distance downwind is 1000 ft = 304 m. From Figure 5-10,
σy =22 m and σz =12 m
Substituting, we obtain
Qm=(49.3 g / m3)(3.14)(22 m)(12 m)(2.23 m / s)=9.11×104 g / s=91.1 kg/s=201 lbm/ s
Any leak with a flow rate greater than 201 lbm/s is capable of producing a flammable cloud in the residential area. Of course, the toxic effects of this cloud must also be considered. The LFL of 1.2% = 12,000 ppm is greater than toluene’s TLV-TWA of 20 ppm.
Plants Inside Buildings
Frequently, processes cannot be constructed outside. In this case, local and dilution ventilation systems are required. These ventilation systems were discussed in detail in Chapter 3, Section 3-3.
Local ventilation is the most effective method of controlling flammable gas releases. Dilution ventilation, however, is also used because the potential points of release are usually numerous and it may be mechanically or economically impossible to cover every potential release point with only local ventilation.
Empirically determined design criteria for ventilation systems for flammable materials inside storage and process areas are identified in Table 7-7. The NFPA ventilation requirements and classification of flammables are given in Table 7-7. There are more details regarding ventilation and classifications in the referenced NFPA document.
Table 7-7 NFPA Ventilation Requirements and Classifications of Flammable Materials
Ventilation area |
Rate |
Conditions |
|---|---|---|
Inside storage and process |
1 ft3/(min ft2) or 0.305 m3/(min m2) |
Designed to maintain concentration at or below 25% of LFL. |
|
|
Exhaust discharge shall be in a safe location outside the building. |
|
|
Recirculation of exhaust air is permitted only when it is monitored continuously and designed to automatically sound an alarm and stop recirculation providing full exhaust outside when the concentration is over 25% of LFL. |
|
|
Ventilation should cover all areas within the specified area. |
Classification of Flammables |
Conditions |
|
Class IA |
Flash point (FP) < 73°F and boiling point (BP) < 100°F or FP < 22.8°C and BP < 37.8°C |
|
Class IB |
FP < 73°F and BP ≥ 100°F or FP < 22.8°C and BP ≥ 37.8°C |
|
Class IC |
FP > 73°F but BP < 100°F or FP > 22.8°C but BP < 37.8°C |
|
Class II |
FP > 100°F and BP < 140°F or FP > 37.8°C and BP < 60°C |
|
Class III |
FP > 140°F or FP > 60°C |
|
Source: Data taken from National Fire Protection Association, Flammables and Combustible Liquids Code (Quincy, MA: National Fire Protection Association, 2018).
The effectiveness of a ventilation system is determined using material balance equations, described in Chapter 2 and illustrated in Example 7-9.
Example 7-9
Determine the concentration of toluene over a diked area (9.5 m2) that contains toluene as a result of a spill. Assume that the process area (230 m2) is designed to handle Class I flammable materials and that the liquid and air temperature is 18.3°C. The vapor pressure of toluene at 18.3°C is 20 mm Hg. The LEL is 1.2% by volume.
Solution
The source models for spills are described in Chapter 3, Equations 3-14 and 3-18. The concentration of volatiles in a ventilated area resulting from the evaporation from a pool is given by Equation 3-14:
Cppm=KAPsatkQvP×106
where
K is the mass transfer coefficient, determined using Equation 3-18,
A is the area of the pool,
Psat is the saturation vapor pressure of the liquid,
k is the nonideal mixing factor,
Qv is the volumetric ventilation rate, and
P is the pressure.
The ventilation capacity for this process area is based on the design criterion of 1 ft3/min ft2 = 0.30 m3/min m2 (Table 7-7); therefore
Qv=(0.305 m3min m2) (230 m2)=70.15m3min
Also,
M=92Psat=20 mm HgA=9.5 m2
The mass transfer coefficient is computed using Equation 3-18
with M0, and K0 for water—that is, 18 and 0.83 cm/s, respectively:
K=K0(M0M)1/3=0.83(1892)1/3=0.482cm / s=0.289 m/min
The nonideal mixing factor k ranges between 0.1 and 0.5. Because no information is given about the ventilation, k is used as a parameter. Substituting into Equation 3-14, we obtain
kCppm=KAPsat×106QvP=(0.289 m / min)(9.5 m2)(20 / 760)atm×106(70.15 m3/min)(1atm)=1030 ppm
The concentration range is estimated to be
Cppm=2060 ppm=0.206% by volume, for k=0.5Cppm=10,300 ppm=1.03% by volume, for k=0.1
These concentrations are considerably below the LFL of 1.2% by volume, which illustrates that the specified ventilation rate for Class I liquids is satisfactory for handling relatively large spills of flammable materials. The concentrations do, however, exceed the TLV for this substance.
7-6 Sprinkler Systems
Sprinkler systems contain fires in a specific area. Such a system consists of an array of sprinkler heads connected to a water supply. The heads are mounted in a high location (usually at or near the ceiling) and disperse a fine spray of water over an area when activated. Sprinkler systems require maintenance to ensure they operate and have an adequate and uninterrupted water supply.
The sprinkler heads may be activated by a variety of methods. For example, the heads may be activated individually by the melting of a fusible link holding a plug in the head assembly. Once turned on, the sprinklers cannot be turned off unless the main water supply is stopped. These kinds of wet pipe systems are used for storage areas, laboratories, control rooms, and small pilot areas.
Another approach uses open sprinkler heads and activates the entire sprinkler array from a common control point. The control point is connected to an array of heat and/or smoke detectors that trigger the sprinklers when an abnormal condition is detected. If a fire is detected, the entire sprinkler array within an area is activated, possibly in areas not even affected by the fire. This kind of deluge system is used for plant process areas and larger pilot plants.
Sprinkler systems can cause considerable water damage when activated, depending on the contents of the building or process structure. From a statistical perspective, the amount of water damage caused by the sprinklers is lower than from damage due to the fire without sprinklers.
There are various classes of fire hazards require different sprinkler designs. The detailed descriptions of these classes and sprinkler specifications are given in NFPA 13.14 An average chemical plant is classified as an ordinary hazard (Group 3) area. Various sprinkler specifications for this type of area are given in Table 7-8.
14NFPA 13, Standard for the Installation of Sprinkler Systems (Quincy, MA: National Fire Protection Association, 2016).
Table 7-8 Fire Protection for Chemical Plants
Sprinkler System Types (see NFPA 13 for details) Antifreeze sprinkler system: a wet pipe system that contains an antifreeze solution and that is connected to a water supply. Deluge sprinkler system: open sprinklers and an empty line that is connected to a water supply line through a valve that is opened upon detection of heat or a flammable material. Dry pipe sprinkler system: a system filled with nitrogen or air under pressure. When the sprinkler is opened by heat, the system is depressurized, allowing water to flow into the system and out the open sprinklers. Wet pipe sprinkler system: a system containing water that discharges through the opened sprinklers via heat. Design Densities (see NFPA 15 for details) Source of fire: not less than 0.50 gpm/ft2 of floor area. Pumps and related equipment: 0.50 gpm/ft2 of projected area. Vessels: 0.25 gpm/ft2 of exposed surface, including top and bottom. Vertical distance of nozzle should not exceed 12 ft. Horizontal structural steel: 0.10 gpm/ft2 of surface area. This may not be necessary if the steel is insulated. Vertical structural steel: 0.25 gpm/ft2 of surface area. This may not be necessary if the steel is insulated. Metal pipe, tubing, and conduit: not less than 0.15 gpm/ft2 of surface area. Cable trays: not less than 0.3 gpm/ft2 of projected plane area (horizontal or vertical). Nozzle Discharge Rate and Requited Pressure for Ultra-High-Speed Water Spray System (see NFPA 15 for details) Flow: 25 gpm/nozzle (flow should be equal to or greater than 25 gpm) Pressure at nozzle: 50 psig (pressure should be equal to or greater than 50 psig) |
Source: Data taken from NFPA 13, Standard for the Installation of Sprinkler Systems (Quincy, MA: National Fire Protection Association, 2016); and NFPA 15, Standards for Water Spray Fixed Systems for Fire Protection (Quincy, MA: National Fire Protection Association, 2017).
Sometimes vessels need special water protection to keep the vessel walls cool during fires. High surface temperatures can result in metal failure at pressures far below the vessel’s maximum allowable working pressure (MAWP) with potentially disastrous consequences. In hydrocarbon spill fires, unprotected vessels (those with no insulation or water spray) can fail within minutes.
A water spray protection system around vessels is recommended to prevent this type of failure. Such systems, which are commonly called deluge systems, are designed to keep the vessel cool, flush away potentially hazardous spills, and help to knock down gas clouds.15 Deluge systems can also provide enough time to transfer material out of a storage tank into another (safe) area.
15NFPA 1, Fire Code (Quincy, MA: National Fire Protection Association, 2009).
Vessel deluge systems are usually designed as open-head systems, which are activated when a fire is detected and/or a flammable gas mixture is detected. The deluge system is usually opened when the flammable gas concentration is a fraction of the LFL (approximately 25%) or when a fire is detected by heat. Table 7-8 provides descriptions and design specifications for these systems.
Monitors are fixed water hydrants with an attached discharge gun. They are also installed in process areas and storage tank areas. Fire hydrants and monitors are spaced 150 to 250 ft apart around process units, located so that all areas of the plant can be covered by two streams. The monitor is usually located 50 ft from the equipment being protected.16 Fire monitors discharge water at a rate of 500 to 2000 gpm.
16D. C. Kirby and J. L. De Roo. “Water Spray Protection for a Chemical Processing Unit: One Company’s View.” Plant/Operations Progress 13, no. 4 (1984): 254.
Example 7-10
Determine the sprinkler requirements for a chemical process area within a building; this 100 ft by 30 ft area handles reactive solvents. Determine the number of sprinkler spray nozzles and pump specifications. Assume a 10-psig frictional loss within the system; and a 15-ft elevation of the sprinkler system above the pump.
Solution
Data for designing this system are found in Table 7-8.
Total water requirement=(0.50 gpm / ft2)(100 ft)(30 ft)=1500 gpmNumber of sprinkler nozzles=1500 gpm(25 gpm / nozzle)=60
If not a whole number it is rounded up.
The pressure required at the pump is the sum of the minimum pressure at the nozzle (specified as 50 psi), the pressure loss resulting from friction (10 psi), and the pressure resulting from the pipe elevation over the pump (15 ft of water or 6.5 psi). Therefore, the total pressure is 66.5 psi, which is rounded up to 67 psi. The pump power is now determined:
ft-lbfs=(67 lbfin2)(144 in2ft2)(1500 galmin)(1 min60 s)(ft37.48 gal)=32,250Horsepower=(32,250 ft-lbf/s)(1 HP550ft-lbfs)=58.6HP
Therefore, this sprinkler requires a pump with a capacity of 1500 gpm and a 58.6-HP motor, assuming an efficiency of 100%.
Actually, fire pumps are usually designed with discharge pressures of 100–125 psig so that the hose and monitor nozzle streams will have an effective reach. In addition, the size of the monitor is governed by requirements in the fire codes.17
17NFPA 1, Fire Code (Quincy, MA: National Fire Protection Association, 2009).
7-7 Industry’s Fire and Explosion Protection Strategy
The successful prevention of fires and explosions in chemical plants requires a combination of multiple design techniques. A complete description of these techniques is far beyond the scope of this text. A partial list, shown in Table 7-9, is given to illustrate that safety technology is relatively complex (the appropriate application requires significant knowledge and experience) and to serve as a checklist for engineers when identifying features for preventing fires and explosions.
Table 7-9 Responsibilities for Preventing and Mitigating Fires and Explosions
Strategies |
Responsibilities |
|---|---|
Standards and practices |
Regulations (PSM) |
|
Codes and practices (NFPA, API, and CCPS) |
|
Risk-based process safety (RBPS by CCPS) |
Passive and active |
Maintenance programs (e.g., mechanical integrity inspections) |
|
Design (e.g., drainage, containment, and accessibility) |
|
Fireproofing |
|
Deluge systems |
|
Controls (e.g., isolation valves and flame arrestors) |
|
Formation and training of emergency response teams |
Plant infrastructure |
Plant equipment layout (e.g., accessibility) |
|
Control rooms (e.g., air supplied and away from process) |
|
Water supply and distribution (e.g., hydrants and redundancy) |
|
Utilities (all available during emergencies) |
Source: J. A. Davenport. “Prevent Vapor Cloud Explosions.” Hydrocarbon Processing (March 1977): 205–214 and Orville M. Syle. “Loss Prevention Fundamentals for the Process Industry.” AICHE Loss Prevention Symposium, March 6–10, 1988.
A fire protection strategy is a systematic approach to identify, reduce, and manage fire hazards. The strategy used by industry to prevent fires and explosions is shown in Figure 7-23. As can be seen in the figure, the strategy is a combination of engineering practices, passive and active systems, and plant infrastructure.
Practices
Many incidents are caused by the failure to use RAGAGEP. This acronym incorporates five practices: (1) adherence to government regulations; (2) adherence to American Petroleum Institute (API) standards; (3) adherence to AICHE’s Center for Chemical Process Safety (CCPS) guidelines; (4) adherence to National Fire Protection Association (NFPA) fire codes and standards; and (5) industrial experience acquired by sharing information within industry and at professional meetings.
Passive and Active Systems
Passive and active safety systems include:
Elimination or reduction of fire hazards
Detection and control to minimize effects after a fire has started
Mitigation or reduction of the severity of fire consequences
Emergency response
Passive systems are responses to incidents without manual or control actions. They include (1) fireproofing (coating structures with fire-resistant cements); (2) drainage (slope surfaces away from process units to drain the flammable material to a safer location); (3) containment (increase the strength of vessels to contain fires and explosions); (4) separation (spacing between equipment); and (5) fire walls to minimize the spread of the fire.
Active systems are responses that require manual or control actions. They include (1) sprinklers to extinguish fires activated by heat detection; (2) deluge systems to spray a large quantity of water on process equipment to extinguish fires and prevent BLEVEs; (3) foam application to suppress the magnitude of the fire or release; and (4) fire brigade emergency responders using both plant and local fire departments.
Plant Fire Protection Infrastructure
The plant layout must be designed to ensure that equipment is accessible to maintenance workers as well as to emergency response teams and vehicles. The plant infrastructure also requires water for sprinklers and fire hydrants. A well-designed system includes a reliable source of water such as a municipal supply, well, lake, river, or water tower. Also, the design should include a good distribution system including pumps and backup pumps. The distribution and pump systems need redundancy to ensure that a common cause of failures—such as lightning or power failure—does not interrupt the source of water.
Documentation of Fire and Explosion Protection Strategy
The plant’s fire protection strategy must be documented and audited frequently. The purpose of the audit is to identify changes in equipment, procedures, or technology.
Suggested Reading
John Bond. Sources of Ignition, Flammability Characteristics of Chemicals and Products (Oxford, UK: Butterworth-Heinemann, 1991).
L. G. Britton. Avoiding Static Ignition Hazards in Chemical Operations (New York, NY: American Institute of Chemical Engineers, 1999).
D. A. Crowl. Understanding Explosions (New York, NY: American Institute of Chemical Engineers, 2003).
H. Deichelmann. The Electrostatic Charge of Glass-Lined Vessels and Piping. Pfaudler PWAG Report, 26th ed.
M. Glor. Electrostatic Hazards in Powder Handling (New York, NY: Wiley, 1988).
Thomas B. Jones and Jack L. King. Powder Handling and Electrostatics (Chelsea, MI: Lewis Publishers, 1991).
T. A. Kletz. What Went Wrong? (London, UK: Butterworth Heinemann, 2009).
J. F. Louvar, B. Maurer, and G. W. Boicourt. “Tame Static Electricity.” Chemical Engineering Progress (November 1994): 75–81.
G. Luttgens and M. Glor. Understanding and Controlling Static Electricity (Goethestrasse, Germany: Expert Verlag, 1989).
National Fire Protection Association. Fire Protection Handbook, 20th ed. (Boston, MA: National Fire Protection Association, 2008).
National Fire Protection Association. NFPA 77, Static Electricity (Boston, MA: National Fire Protection Association, 2014).
T. H. Pratt. Electrostatic Ignitions of Fires and Explosions (Marietta, GA: Burgoyne, 1997).
Problems
7-1. Use a vacuum inerting procedure with nitrogen to reduce the oxygen concentration to 1 ppm. The vessel has a volume of 3.78 m3 and initially contains air, the vacuum system can deliver 20 mm Hg absolute, the temperature is 24°C, and the highest pressure is 1 atm. Determine the number of purges and the total amount of nitrogen used (in kg). Repeat for a vessel with a volume of 37 m3 and a vacuum of 30 mm Hg.
7-2. Use a pressure inerting procedure with nitrogen to reduce the oxygen concentration to 1 ppm. The vessel has a volume of 3.78 m3 and is initially contains air, the nitrogen supply pressure is 4,136 mm Hg absolute, the temperature is 24°C, and the lowest pressure is 1 atm. Determine the number of purges and the total amount of nitrogen used (in kg). Repeat for a vessel with a volume of 37 m3 and a supply pressure of 3000 mm Hg.
7-3. Use a sweep through purge to reduce the oxygen concentration from 100% air (21% oxygen) to 1.25% oxygen. The vessel has a volume of 7500 gallon. Determine the ft3 of nitrogen required if the nitrogen contains 0.01% oxygen. Repeat this problem with nitrogen containing 0.02% oxygen and 0.05% oxygen.1
7-4. A 2000-m3 vessel containing liquid methanol must be prepared for entry. After draining, it must first be purged with nitrogen (98% nitrogen and 2% oxygen from a membrane unit) and then purged with air to reach a fuel composition of 0.1% of the TLV-TWA. Use sweep through purging, assuming a pressure of 1 atm, a temperature of 25°C, and purging rates (for nitrogen and air) of 10 m3/min.
Determine the composition of the vapor in the tank after draining.
Determine the target fuel composition for purging with nitrogen (with 2% oxygen).
Determine the amount of nitrogen (in kg) required.
Determine the quantity of sweep through air required.
7-5. Use Figure 7-14 to determine (a) the voltage and accumulated charge between the nozzle and the tank, and (b) the energy accumulated in the liquid. Do this for three different charge rates: 0.003785, 0.568, and 2 m3/min. Given: Hose length of 6.1 m, hose diameter of 5.1 cm, liquid conductivity of 1.0 × 10–8 mho/cm, dielectric constant of 25.7, vessel volume of 1.1 m3, and liquid density of 0.88.
7-6. Determine the capacitance of vessel volumes of 250, 500, 750, and 920 gallons.
7-7. Calculate the charge developed on a vessel being filled with carbon black. Consider two filling methods: (a) pouring from an open vessel and (b) pneumatic transport. Assume the vessel has a volume of 1.89 m3 and carbon black feed rate is 5 kg/s. Determine the accumulated charge and the energy. Does the energy exceed the MIE for dusts?
7-8. Calculate the charge developed on a vessel being filled with carbon black by pouring from another open vessel. Assume the vessel has a volume of 1.89 m3 and the feed rate is 0.1 kg/s. Identify the accumulated charge and the energy, and then determine whether the energy exceeds the MIE of dusts. Does the lower charge rate, compared to Problem 7-7, lower the potential hazard?
7-9. Determine the concentration of toluene over a diked area, where the diked area is 100 ft2, and the dike is in a process area 2500 ft2. The temperature is 65°F.
7-10. Repeat Problem 7-9 with a diked area of 2.32 m3 and a process area of 46.5 m2. The temperature is 18°C.
7-11. Repeat Problem 7-10 with a diked area of 2.32 m2 and a process area of 46.5 m2, but with a drainage pit that has a drain area of 0.018 m2. The temperature is 18°C.
Additional homework problems are available in the Pearson Instructor Resource Center.
