Chapter 2. Toxicology
The learning objectives for this chapter are:
Learn how toxicants enter and are eliminated from the body.
Understand effects of toxicants, including response to dose.
Use probit equations to determine response to dose.
Understand exposure limits, such as threshold limit value (TLV) and permissible exposure level (PEL).
Many years ago, toxicology was defined as the science of poisons. Unfortunately, the word poison could not be defined adequately. Paracelsus, an early investigator of toxicology during the 1500s, stated the problem: “All substances are poisons; there is none which is not a poison. The right dose differentiates a poison and a remedy.” Harmless substances, such as water, can become fatal if delivered to the biological organism in large enough doses. A fundamental principle of toxicology is there are no harmless substances, only harmless ways of using substances.
Today, toxicology is more adequately defined as the qualitative and quantitative study of the adverse effects of toxicants on biological organisms. A toxicant can be a chemical or physical agent, including dusts, fibers, noise, and radiation. A good example of a physical agent is asbestos fiber, a known cause of lung damage and cancer.
The toxicity of a chemical or physical agent is a property of the agent describing its effect on biological organisms. The term toxic hazard describes the likelihood of damage to biological organisms based on exposure resulting from transport and other physical factors of usage. The toxic hazard of a substance can be reduced by the application of appropriate industrial hygiene techniques (see Chapter 3). The toxicity, however, cannot be changed.
The AICHE Center for Chemical Process Safety (CCPS)1 has more detailed definitions of these two terms:
1AICHE Center for Chemical Process Safety (CCPS) online glossary, www.aiche.org/ccps/resources/glossary.
Toxicity: The quality, state, or degree to which a substance is poisonous and/or may chemically produce an injurious or deadly effect upon introduction into a living organism.
Toxic hazard: A measure of the danger posed to living organisms by a toxic agent, determined not only by the toxicity of the agent itself, but also by the means by which it may be introduced into the subject organisms under prevailing conditions.
2-1 How Toxicants Enter the Body
For higher-order organisms, the path of the chemical agent through the body is well defined. After the toxicant enters the organism, it moves into the bloodstream. From there, it is either eventually eliminated or transported to the target organ. The damage is exerted at the target organ. A common misconception is that damage occurs in the organ where the toxicant is most concentrated. Lead, for instance, is stored in humans mostly in the bone structure, but the damage occurs in many organs. For corrosive chemicals, the damage to the organism can occur without absorption or transport through the bloodstream.
Toxicants enter biological organisms by the following routes:
Ingestion: through the mouth into the stomach
Inhalation: through the mouth or nose into the lungs
Injection: through cuts in the skin
Dermal absorption: through the skin membrane
All these entry routes can be controlled by the application of proper industrial hygiene techniques, summarized in Table 2-1. These control techniques are discussed in more detail in Chapter 3 on industrial hygiene. Of the four routes of entry, the inhalation and dermal routes are the most significant to industrial facilities. Inhalation is the easiest to quantify by the direct measurement of airborne concentrations; the usual exposure is by vapor, but small solid and liquid particles can also contribute.
Table 2-1 Entry Routes for Toxicants and Methods for Control
Entry route |
Entry organ |
Method for control |
|---|---|---|
Ingestion |
Mouth or stomach |
Enforcement of rules on eating, drinking, and smoking |
Inhalation |
Mouth or nose |
Ventilation, respirators, hoods, and other personal protection equipment |
Injection |
Cuts in skin |
Proper protective clothing |
Dermal absorption |
Skin |
Proper protective clothing |
Injection, inhalation, and dermal absorption generally result in the toxicant entering the bloodstream unaltered. Toxicants entering through ingestion are frequently modified or excreted in bile. Toxicants that enter by injection and dermal absorption are difficult to measure and quantify. Some toxicants are absorbed rapidly through the skin.
Figure 2-1 shows the expected blood-level concentration as a function of time and route of entry. The blood-level concentration depends on a wide range of parameters, so large variations in this behavior are expected. Injection usually results in the highest blood-level concentration, followed by inhalation, ingestion, and absorption. The peak concentration generally occurs earliest with injection, followed by inhalation, ingestion, and absorption.

The gastrointestinal (GI) tract, the skin, and the respiratory system play significant roles in the various routes of entry.
Gastrointestinal Tract
The GI tract plays the most significant role in toxicants entering the body through ingestion. Food or drink is the usual mechanism of exposure. Airborne particles (either solid or liquid) can also lodge in the mucus of the upper respiratory tract and be swallowed.
The rate and selectivity of absorption by the GI tract are highly dependent on many conditions. The type of chemical, its molecular weight, molecule size and shape, acidity, susceptibility to attack by intestinal flora, rate of movement through the GI tract, and many other factors affect the rate of absorption.
Skin
The skin plays important roles in both the dermal absorption and injection routes of entry. Injection includes both entry by absorption through cuts and mechanical injection with hypodermic needles. Mechanical injection can occur as a result of improper hypodermic needle storage in a laboratory drawer.
The skin’s outer layer—called the stratum corneum—consists of dead, dried cells that are resistant to permeation by toxicants. Absorption also occurs through the hair follicles and sweat glands, but this is normally negligible. The absorption properties of the skin vary as a function of location and the degree of hydration. The presence of water increases the skin hydration and results in increased permeability and absorption.
Most chemicals are not absorbed readily by the skin. A few chemicals, however, do show remarkable skin permeability. Phenol, for example, requires only a small area of skin for the body to absorb an adequate amount to result in death.
Skin absorption varies by skin location on the body. For males, if we define the foot skin as 1× absorption, then the palm and ankle are 5×, the back and forearm are 10×, and the forehead and scalp are 34×.
Respiratory System
The respiratory system plays a significant role in toxicants entering the body through inhalation. The main function of the respiratory system is to exchange oxygen and carbon dioxide between the blood and the inhaled air. Approximately 8 L of air is breathed per minute, but only a fraction of the total air within the lung is exchanged with each breath. These demands increase significantly with physical exertion.
The respiratory system is divided into two areas: the upper respiratory system and the lower respiratory system. The upper respiratory system is composed of the nose, sinuses, mouth, pharynx (the section between the mouth and the esophagus), larynx (the voice box), and the trachea (the windpipe). The lower respiratory system consists of the lungs and its smaller structures, including the bronchi and the alveoli. The bronchial tubes carry fresh air from the trachea through a series of branching tubes to the alveoli. The alveoli are small, blind air sacs where the gas exchange with the blood occurs. An estimated 700 million alveoli are found in a normal lung. These alveoli contribute a total surface area of approximately 75 m2. Small capillaries found in the walls of the alveoli transport the blood; an estimated 100 mL of blood is in the capillaries at any moment. More detailed information on the respiratory system is provided at the Gikipedia website.2
2Respiratory System, http://gikipedia.org/wiki/the+respiratory+system+facts.
The upper respiratory tract is responsible for filtering, heating, and humidifying the air. Fresh air brought in through the nose is completely saturated with water and regulated to the proper temperature by the time it reaches the larynx. The mucus lining in the upper respiratory tract assists in filtering out solid contaminants in the air.
The upper and lower respiratory tracts respond differently to the presence of toxicants. The upper respiratory tract is affected mostly by water-soluble toxicants. These materials either react or dissolve in the mucus to form acids and bases. Toxicants in the lower respiratory tract affect the alveoli by physically blocking the transfer of gases (as with insoluble dusts) or reacting with the wall of the alveoli to produce corrosive or toxic substances. Phosgene gas, for example, reacts with the water on the alveoli wall to produce hydrochloric acid (HCl) and carbon dioxide.
Upper respiratory toxicants include hydrogen halides (hydrogen chloride, hydrogen bromide), oxides (nitrogen oxides, sulfur oxides, sodium oxide), and hydroxides (ammonium hydroxide, sodium dusts, and potassium hydroxides). Lower respiratory toxicants include monomers (such as acrylonitrile), halides (fluorine, chlorine, bromine), and other miscellaneous substances such as hydrogen sulfide, phosgene, methyl cyanide, acrolein, asbestos dust, silica, and soot.
Dusts and other insoluble materials present a particular difficulty to the lungs. Particles that enter the alveoli are removed slowly. For dusts, the following simple rule usually applies: The smaller the dust particles, the farther they penetrate into the respiratory system. Particles greater than 5 μm in diameter are usually filtered by the upper respiratory system, whereas particles with diameters between 2 and 5 μm generally reach the bronchial system. Particles smaller than 0.2 μm settle out too slowly and are mostly exhaled with the air.
2-2 How Toxicants Are Eliminated from the Body
Toxicants are eliminated or rendered inactive by the following routes:
Excretion: through the kidneys, liver, lungs, or other organs
Detoxification: by changing the chemical into something less harmful by biotransformation
Storage: in the fatty tissue
The kidneys are the dominant means of excretion in the human body. They eliminate substances that enter the body by ingestion, inhalation, injection, and dermal absorption. The toxicants are extracted by the kidneys from the bloodstream and are excreted in the urine.
Toxicants that are ingested into the digestive tract are frequently excreted by the liver. In general, chemical compounds with molecular weights greater than about 300 are excreted by the liver into bile. Compounds with lower molecular weights enter the bloodstream and are excreted by the kidneys. The digestive tract tends to selectively detoxify certain agents, whereas substances that enter through inhalation, injection, or dermal absorption generally arrive in the bloodstream unchanged.
The lungs are also a means for elimination of substances, particularly those that are volatile. Chloroform and alcohol, for example, are excreted partially by this route.
Other routes of excretion are the skin (by means of sweat), hair, and nails. These routes usually play minor roles compared to the excretion processes of the kidneys, liver, and lungs.
The liver is the dominant organ in the detoxification process. In this organ, detoxification occurs by biotransformation, in which the chemical agents are transformed by reaction into either harmless or less harmful substances. Biotransformation reactions can also occur in the blood, intestinal tract wall, skin, kidneys, and other organs.
The final mechanism for elimination is storage. This process involves depositing the chemical agent mostly in the fatty areas of the organism, but also in the bones, blood, liver, and kidney. Storage can create a future problem if the organism’s food supply is reduced and the fatty deposits are metabolized; the stored chemical agents will be released into the bloodstream, resulting in possible damage.
For massive exposures to chemical agents, damage can occur to the kidneys, liver, or lungs, significantly reducing the organism’s ability to excrete the substance.
2-3 Effects of Toxicants on the Body
Table 2-2 lists some of the effects or responses from toxic exposure. The challenge is to determine whether exposures have occurred before substantial symptoms appear. This is accomplished through a variety of medical tests. The results from these tests must be compared to a medical baseline study, performed before any exposure.
Table 2-2 Various Responses to Toxicants
Effects that are irreversible: Carcinogen—causes cancer Mutagen—causes chromosome damage Reproductive hazard—causes damage to reproductive system Teratogen—causes birth defects |
Effects that may or may not be reversible: Dermatotoxic—affects skin Hemotoxic—affects blood Hepatotoxic—affects liver Nephrotoxic—affects kidneys Neurotoxic—affects nervous system Pulmonotoxic—affects lungs |
Respiratory problems are diagnosed using a spirometer. The patient exhales as hard and as fast as possible into the device. The spirometer measures (1) the total volume exhaled, called the forced vital capacity (FVC), with units in liters; (2) the forced expired volume measured at 1 second (FEV1), with units in liters per second; (3) the forced expiratory flow in the middle range of the vital capacity (FEV 25–75%), measured in liters per second; and (4) the ratio of the observed FEV1 to FVC × 100 (FEV1/FVC%).
Reductions in expiration flow rate are indicative of bronchial disease, such as asthma or bronchitis. Reductions in FVC are due to reduction in the lung or chest volume, possibly as a result of fibrosis (an increase in the interstitial fibrous tissue in the lung). The air remaining in the lung after exhalation is called the residual volume (RV). An increase in the RV is indicative of deterioration of the alveoli, possibly because of emphysema. The RV measurement requires a specialized tracer test with helium.
Nervous system disorders are diagnosed by examining the patient’s mental status, cranial nerve function, motor system reflexes, and sensory systems. An electroencephalogram (EEG) tests higher brain and nervous system functions.
Changes in skin texture, pigmentation, vascularity, and hair and nail appearance are indicative of possible toxic exposures.
Blood counts are also used to determine toxic exposures. Measurements of the red and white blood cells, hemoglobin content, and platelet count are performed easily and inexpensively. However, blood counts are frequently insensitive to toxic exposure; marked changes are seen only after substantial exposure and damage.
Kidney function is determined through a variety of tests that measure the chemical content and quantity of urine. With early kidney damage, proteins or sugars are found in the urine.
Liver function is determined through a variety of chemical tests on the blood and urine.
2-4 Toxicological Studies
A major objective of a toxicological study is to quantify the effects of the suspect toxicant on a target organism. Animals are used in most toxicological studies, usually with the hope that the results can be extrapolated to humans. Once the effects of a suspect agent have been quantified, appropriate procedures are established to ensure that the agent is handled properly.
Before undertaking a toxicological study, the following items must be identified:
The toxicant
The target or test organism
The effect or response to be monitored
The dose range
The period of the test
The toxicant must be identified with respect to its chemical composition and its physical state. For example, benzene can exist in either liquid or vapor form. Each physical state preferentially enters the body by a different route and requires a different toxicological study.
The test organism can range from a simple single cell to higher animals. The selection of this organism depends on the effects considered and other factors such as the cost and availability of the test organism. For studies of genetic effects, single-cell organisms might be satisfactory. For studies determining the effects on specific organs such as the lungs, kidneys, or liver, higher organisms are a necessity.
The dose units depend on the method of delivery. For substances delivered directly into the organism (by ingestion or injection), the dose is measured in milligrams of agent per kilogram of body weight. This relationship enables researchers to apply the results obtained from small animals such as mice (fractions of a kilogram in body weight) to humans (about 70 kg for males and 60 kg for females). For gaseous airborne substances, the dose is measured in either parts per million (ppm) by volume or milligrams of agent per cubic meter of air (mg/m3). For airborne particulates, the dose is measured in milligrams of agent per cubic meter of air (mg/m3) or millions of particles per cubic foot (mppcf).
The period of the test depends on whether long-term or short-term effects are of interest. Acute toxicity is the effect of a single exposure or a series of exposures that occur close together in a short period of time. Chronic toxicity is the effect of multiple exposures occurring over a long period of time. Chronic toxicity studies are difficult to perform because of the time involved; most toxicological studies are based on acute exposures. Toxicological studies can sometimes be complicated by latency, an exposure that results in a delayed response.
2-5 Dose versus Response
Biological organisms respond differently to the same dose of a toxicant. These differences are a result of age, sex, weight, diet, general health, and other factors. For example, consider the effects of an irritant vapor on human eyes. Given the same dose of vapors, some individuals will barely notice any irritation (weak or low response), whereas other individuals will be severely irritated (high response).
Consider a toxicological test run on a large number of individuals. Each individual is exposed to the same dose and the response is recorded. A plot of the type shown in Figure 2-2 is prepared with the data. The fraction or percentage of individuals experiencing a specific response is plotted. Curves of the form shown in Figure 2-2 are frequently represented by a normal or Gaussian distribution, given by the equation

where
f (x) is the probability (or fraction) of individuals experiencing a specific response,
x is the response,
σ is the standard deviation, and
μ is the mean.
The standard deviation and mean characterize the shape and the location of the normal distribution curve, respectively. They are computed from the original data f (xi) using the equations
where n is the number of data points. The quantity σ 2 is called the variance.
The mean determines the location of the curve with respect to the x axis, and the standard deviation determines the shape. Figure 2-3 shows the effect of the standard deviation on the shape. As the standard deviation decreases, the distribution curve becomes more pronounced around the mean value.

The area under the curve of Figure 2-2 represents the percentage of individuals affected for a specified response interval. In particular, the response interval within 1 standard deviation of the mean represents 68.2% of the individuals, as shown in Figure 2-4a. A response interval of 2 standard deviations represents 95.5% of the total individuals (Figure 2-4b). The area under the entire curve represents 100% of the individuals.

Example 2-1
Seventy-five people are tested for skin irritation due to of a specific dose of a substance. The responses are recorded on a scale from 0 to 10, with 0 indicating no response and 10 indicating a high response. The number of individuals exhibiting a specific response is given in the following table:
Response |
Number of individuals affected |
|---|---|
0 |
0 |
1 |
5 |
2 |
10 |
3 |
13 |
4 |
13 |
5 |
11 |
6 |
9 |
7 |
6 |
8 |
3 |
9 |
3 |
10 |
2 |
Total 75 |
Plot a histogram of the number of individuals affected versus the response.
Determine the mean and the standard deviation.
Plot the normal distribution on the histogram of the original data.
Solution
The histogram is shown in Figure 2-5. The number of individuals affected is plotted versus the response. An alternative method is to plot the percentage of individuals versus the response.
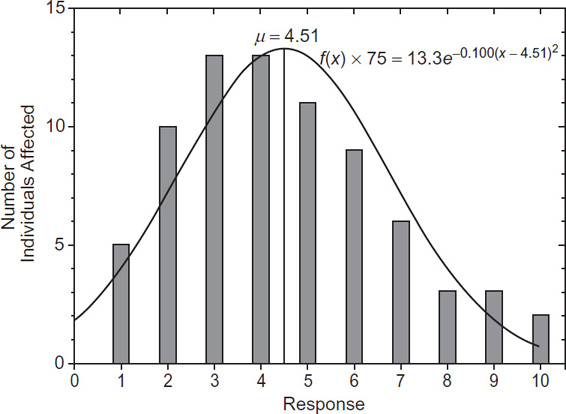
Figure 2-5 Number of individuals affected based on response. The mean is computed using Equation 2-2:
The standard deviation is computed using Equation 2-3:
The normal distribution is determined using Equation 2-1. Substituting the mean and standard deviations, we find
The distribution is converted to a function representing the number of individuals affected by multiplying by the total number of individuals, in this case 75. The corresponding values are shown in Table 2-3 and Figure 2-5.
Table 2-3 Theoretical Frequency and Number of People Affected for Each Response for Example 2-1
X |
f(X) |
75f(X) |
|---|---|---|
0 1 2 3 4 4.51 5 6 7 8 9 10 |
0.0232 0.0519 0.0948 0.1417 0.173 0.178 0.174 0.143 0.096 0.0527 0.0237 0.00874 |
1.74 3.89 7.11 10.6 13.0 13.3 13.0 10.7 7.18 3.95 1.78 0.655 |
The toxicological experiment is repeated for a number of different doses, and normal curves similar to Figure 2-5 are drawn. The standard deviation and mean response are determined from the data for each dose. A complete dose–response curve is produced by plotting the cumulative mean response at each dose. Error bars are drawn at ±σ around the mean. A typical result is shown in Figure 2-6.

For convenience, the response is plotted versus the logarithm of the dose, as shown in Figure 2-7. This form provides a much straighter line in the middle of the response curve than the simple response versus dose form of Figure 2-6.

If the response of interest is death or lethality, the response versus log dose curve of Figure 2-7 is called a lethal dose (LD) curve. The dose that results in the death of 50% of the subjects is called the LD50 dose (lethal dose for 50% of the subjects). Other values such as LD10 or LD90 are sometimes also reported. If the LD is used for oral or dermal exposures, the concentration units are expressed as mg/kg of body weight.
The lethal concentration (LC) is used for inhalation exposures of gas, vapor, or dust/mist expressed as ppm or mg/L. With this measure, LC50 is the lethal concentration for 50% of the exposed subjects. Values for inhalation toxicity are based on 4-hour tests with laboratory animals.
If the response to the chemical or agent is minor and reversible (such as minor eye irritation), the response–log dose curve is called the effective dose (ED) curve. Values for ED50, ED10, and so forth are also used.
Finally, if the response to the agent is toxic (an undesirable response that is not lethal but is irreversible, such as liver or lung damage), the response–log dose curve is called the toxic dose (TD) curve.
The relationships among the various types of response–log dose curves are shown in Figure 2-8.

Most often, response–dose curves are developed using acute toxicity data. Chronic toxicity data are usually considerably different. Furthermore, the data are complicated by differences in group age, sex, and method of delivery. If several chemicals are involved, the toxicants might interact additively (the combined effect is the sum of the individual effects), synergistically (the combined effect is more than the individual effects), as potentiators (the presence of one chemical increases the effect of the other), or antagonistically (both chemicals counteract each other).
2-6 Dose and Response Using Probit Equation
Response versus dose curves can be drawn for a wide variety of exposures, including exposure to heat, pressure, radiation, impact, and sound. For computational purposes, the response versus dose curve is not convenient; instead, an analytical equation is preferred.
Many methods exist for representing the response–dose curve.3 For single exposures, the probit (probability unit) method is particularly well suited, providing a straight-line equivalent to the response–dose curve. The probit variable Y is related to the probability P by the following equation:4
3Phillip L. Williams, Robert C. James, and Stephen M. Roberts, eds. The Principles of Toxicology: Environmental and Industrial Applications, 2nd ed. (New York, NY: John Wiley & Sons, 2000).
4D. J. Finney. Probit Analysis (Cambridge, UK: Cambridge University Press, 1971), p. 23.
Equation 2-4 provides a relationship between the probability P and the probit variable Y. This relationship is plotted in Figure 2-9 and tabulated in Table 2-4.

Table 2-4 Transformation from Percentages to Probits. Computed from % = 5 + NORM.S.INV(%)
% |
0 |
10 |
20 |
30 |
40 |
50 |
60 |
70 |
80 |
90 |
|---|---|---|---|---|---|---|---|---|---|---|
0 |
– |
3.72 |
4.16 |
4.48 |
4.75 |
5.00 |
5.25 |
5.52 |
5.84 |
6.28 |
1 |
2.67 |
3.77 |
4.19 |
4.50 |
4.77 |
5.03 |
5.28 |
5.55 |
5.88 |
6.34 |
2 |
2.95 |
3.83 |
4.23 |
4.53 |
4.80 |
5.05 |
5.31 |
5.58 |
5.92 |
6.41 |
3 |
3.12 |
3.87 |
4.26 |
4.56 |
4.82 |
5.08 |
5.33 |
5.61 |
5.95 |
6.48 |
4 |
3.25 |
3.92 |
4.29 |
4.59 |
4.85 |
5.10 |
5.36 |
5.64 |
5.99 |
6.55 |
5 |
3.36 |
3.96 |
4.33 |
4.61 |
4.87 |
5.13 |
5.39 |
5.67 |
6.04 |
6.64 |
6 |
3.45 |
4.01 |
4.36 |
4.64 |
4.90 |
5.15 |
5.41 |
5.71 |
6.08 |
6.75 |
7 |
3.52 |
4.05 |
4.39 |
4.67 |
4.92 |
5.18 |
5.44 |
5.74 |
6.13 |
6.88 |
8 |
3.59 |
4.08 |
4.42 |
4.69 |
4.95 |
5.20 |
5.47 |
5.77 |
6.17 |
7.05 |
9 |
3.66 |
4.12 |
4.45 |
4.72 |
4.97 |
5.23 |
5.50 |
5.81 |
6.23 |
7.33 |
|
|
|
|
|
|
|
|
|
|
|
% |
99.1 |
99.2 |
99.3 |
99.4 |
99.5 |
99.6 |
99.7 |
99.8 |
99.9 |
|
|
7.37 |
7.41 |
7.46 |
7.51 |
7.58 |
7.65 |
7.75 |
7.88 |
8.09 |
|
|
|
|
|
|
|
|
|
|
|
|
% |
0.1 |
0.2 |
0.3 |
0.4 |
0.5 |
0.6 |
0.7 |
0.8 |
0.9 |
|
|
1.91 |
2.12 |
2.25 |
2.35 |
2.42 |
2.49 |
2.54 |
2.59 |
2.63 |
|
The probit relationship of Equation 2-4 transforms the sigmoid shape of the normal response versus dose curve into a straight line when plotted using a linear probit scale, as shown in Figure 2-10. Standard curve-fitting techniques are used to determine the best-fitting straight line.

Table 2-5 lists a variety of probit equations for several different types of exposures. The causative factor represents the dose V. The probit variable Y is computed from
Table 2-5 Probit Correlations for a Variety of Exposures (The causative variable is representative of the magnitude of the exposure)
|
|
|
Probit parameters |
|
|---|---|---|---|---|
Type of injury or damage |
Causative variable |
k1 |
k2 |
|
Firea |
|
|
|
|
|
Burn death from flash fire |
teIe4/3/104 |
–14.9 |
2.56 |
|
Burn death from pool burning |
tI4/3/104 |
–14.9 |
2.56 |
Explosiona |
|
|
|
|
|
Death from lung hemorrhage |
po |
–77.1 |
6.91 |
|
Eardrum rupture |
po |
–15.6 |
1.93 |
|
Death from impact |
J |
–46.1 |
4.82 |
|
Injury from impact |
J |
–39.1 |
4.45 |
|
Injury from flying fragments |
J |
–27.1 |
4.26 |
|
Structural damage |
po |
–23.8 |
2.92 |
|
Glass breakage |
po |
–18.1 |
2.79 |
Toxic releaseb |
|
|
|
|
|
Ammonia death |
Σ C 2.0T |
–35.9 |
1.85 |
|
Carbon monoxide death |
Σ C 1.0T |
–37.98 |
3.7 |
|
Chlorine death |
Σ C 2.0T |
–8.29 |
0.92 |
|
Ethylene oxide deathc |
Σ C 1.0T |
–6.19 |
1.0 |
|
Hydrogen chloride death |
Σ C 1.0T |
–16.85 |
2.0 |
|
Nitrogen dioxide death |
Σ C 2.0T |
–13.79 |
1.4 |
|
Phosgene death |
Σ C 1.0T |
–19.27 |
3.69 |
|
Propylene oxide death |
Σ C 2.0T |
–7.42 |
0.51 |
|
Sulfur dioxide death |
Σ C 1.0T |
–15.67 |
1.0 |
|
Toluene death |
Σ C 2.5T |
–6.79 |
0.41 |
te: effective time duration (s)
Ie: effective radiation intensity (W/m2)
t: time duration of pool burning (s)
I: radiation intensity from pool burning (W/m2)
po: peak overpressure (N/m2)
J: impulse (N s/m2)
C: concentration (ppm)
T: time interval (min)
aSelected from Frank P. Lees. Loss Prevention in the Process Industries (London, UK: Butterworths, 1986), p. 208.
bCenter for Chemical Process Safety (CCPS). Guidelines for Consequence Analysis of Chemical Releases (New York, NY: American Institute of Chemical Engineers, 2000), p. 254.
cRichard W. Prugh. “Quantitative Evaluation of Inhalation Toxicity Hazards,” in Proceedings of the 29th Loss Prevention Symposium (American Institute of Chemical Engineers, July 31, 1995).
Note that the results computed using the probit equations in Table 2-5 are highly sensitive to the exponent in the causative variable: A small change in the exponent will change the results dramatically.
For spreadsheet computations, a more useful expression for performing the conversion from probits to percentage is given by
where erf is the error function. Using Microsoft Excel, the probit function is given by P = 5 + NORM.S.INV(%) and the inverse from the probit to probability is given by 100*NORM.S.Dist(Y-5, True).
Example 2-2
Determine the percentage of people who will die as a result of burns from pool burning if the probit variable Y is 4.39. Compare results from Table 2-4 and Equation 2-6.
Solution
The percentage from Table 2-4 is 27%. The same percentage can be computed using Equation 2-6, as follows:
where the error function is a mathematical function found in spreadsheets and software programs.
Example 2-3
Eisenberg5 reported the following data on the effect of explosion peak overpressures on eardrum rupture in humans:
5N. A. Eisenberg. Vulnerability Model: A Simulation System for Assessing Damage Resulting from -Marine Spills, NTIS Report AD-A015-245 (Springfield, VA: National Technical Information Service, 1975).
Percentage affected |
Peak overpressure (N/m2) |
|---|---|
1 |
16,500 |
10 |
19,300 |
50 |
43,500 |
90 |
84,300 |
Confirm the probit correlation for this type of exposure, as shown in Table 2-5.
Solution
The percentage is converted to a probit variable using Table 2-4. The results are:
Percentage |
Probit |
|---|---|
1 |
2.67 |
10 |
3.72 |
50 |
5.00 |
90 |
6.28 |
Figure 2-11 is a plot of the percentage affected versus the natural logarithm of the peak overpressure. This demonstrates the classical sigmoid shape of the response versus log dose curve. Figure 2-12 is a plot of the probit variable (with a linear probit scale) versus the natural logarithm of the peak overpressure. The straight line verifies the values reported in Table 2-5. The sigmoid curve of Figure 2-11 is drawn after converting the probit correlation back to percentages.


2-7 Relative Toxicity
Toxicants are compared for relative toxicity based on the LD, ED, or TD curve. If the response–dose curve for chemical A is to the left of the response–dose curve for chemical B, then chemical A is more toxic. Care must be taken when comparing two response–dose curves when partial data are available. If the slopes of the curves differ substantially, the situation shown in Figure 2-13 might occur. If only a single data point is available in the upper part of the curves, it might appear that chemical A is always more toxic than chemical B. The complete data, however, show that chemical B is more toxic at lower doses and chemical A is more toxic at higher doses.

2-8 Threshold Limit Values
The lowest value on the response versus dose curve is called the threshold dose. Below this dose, the body is able to detoxify and eliminate the agent without any detectable effects. In reality, the response is only identical to zero when the dose is zero, but for small doses the response is not detectable.
The American Conference of Governmental Industrial Hygienists (ACGIH) has established threshold doses, called threshold limit values (TLVs), for a large number of chemical agents. The TLV refers to airborne concentration that corresponds to the conditions under which no adverse effects are normally expected during a worker’s lifetime. The exposure occurs only during normal working hours, eight hours per day and five days per week. The TLV was formerly called the maximum allowable concentration (MAC).
Three types of TLVs (TLV-TWA, TLV-STEL, and TLV-C) are distinguished, as defined in Table 2-6. More TLV-TWA data are available compared to TWA-STEL or TLV-C data.
Table 2-6 Definitions for Threshold Limit Values (TLVs)
TLV type |
Definition |
|---|---|
TLV-TWA |
Threshold limit value—time-weighted average |
|
The concentration for a conventional 8-hour workday and a 40-hour workweek, to which it is believed that nearly all workers may be repeatedly exposed, day after day, for a working lifetime without adverse effect. |
TLV-STEL |
Threshold limit value—short-term exposure limit |
|
A 15-minute TWA exposure that should not be exceeded at any time during a workday, even if the 8-hour TWA is within the TLV-TWA. The TLV-STEL is the concentration to which it is believed that workers can be exposed continuously for a short period of time without suffering (1) irritation, (2) chronic or irreversible tissue damage, (3) dose-rate–dependent toxic effects, or (4) narcosis of sufficient degree to increase the likelihood of accidental injury, impaired self-rescue, or materially reduced work efficiency. Exposures above the TLV-TWA up to the TLV-STEL should be less than 15 minutes, should occur no more than four times per day, and there should be at least 60 minutes between successive exposures in this range. |
TLV-C |
Threshold limit value—ceiling |
|
The concentration that should not be exceeded during any part of the working exposure. |
Source: American Conference of Governmental Industrial Hygienists. 2018 TLVs and BEIs (Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 2018).
The TLVs are “intended for use only as guidelines or recommendations to assist in the evaluation and control of potential workplace health hazards and for no other use (e.g., neither for evaluating or controlling community air pollution; nor for estimating the toxic potential of continuous, uninterrupted exposures or other extended work periods; nor for proving or disproving an existing disease or physical condition in an individual).”6 Further, these values are not fine lines between safe and dangerous conditions.
6American Conference of Governmental Industrial Hygienists., 2018 TLVs and BEIs (Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 2018).
The U.S. Occupational Safety and Health Administration (OSHA) has defined its own threshold dose, called a permissible exposure level (PEL). However, the PEL values are not as numerous and are not updated as frequently as the TLVs. PELs have legal authority and may result in fines if exceeded. TLVs are promulgated by a professional society and do not have legal authority.
For some toxicants (particularly carcinogens), exposures at any level are not permitted. These toxicants have zero thresholds.
Another quantity frequently reported is the amount that is “immediately dangerous to life and health” (IDLH). Exposures to this quantity and greater should be avoided under any circumstances.
TLVs are reported using ppm (parts per million by volume), mg/m3 (milligrams of vapor per cubic meter of air), or, for dusts, mg/m3 or mppcf (millions of particles per cubic foot of air). For vapors, mg/m3 is converted to ppm using the equation
where
T is the temperature in degrees K,
P is the absolute pressure in atm, and
M is the molecular weight in g/g-mole.
TLV and PEL values for a variety of toxicants are provided in Appendix E. Note that even though the PELs are legal limits and the TLVs are guidelines, every effort should be made to reduce the workplace exposure concentrations as much as possible.
Online Resources
American Conference of Governmental Industrial Hygienists (ACGIH), www.acgih.org.
NIOSH Pocket Guide to Chemical Hazards. This is a free database of chemical hazards. www.cdc.gov/niosh/npg/.
Society of Toxicology. This is a professional organization of scientists from academic institutions, government, and industry representing toxicologists. www.toxicology.org.
TOXNET, Toxicology Data Network provided by the U.S. National Library of Medicine. This includes free databases on toxicology, hazardous chemicals, environmental health, and toxic releases. www.toxnet.nlm.nih.gov.
U.S. Department of Labor, Occupational Safety and Health Administration. This includes all regulations and PEL values. www.osha.gov.
Suggested Reading
American Conference of Governmental Industrial Hygienists. 2018 TLVs and BEIs (Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 2018).
American Conference of Governmental Industrial Hygienists. Documentation of the Threshold Limit Values and Biological Exposure Indices, 7th ed. (Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 2018).
Eula Bingham, Barbara Cohrssen, and Charles H. Powell, eds. Patty’s Toxicology, 6th ed. (New York, NY: John Wiley & Sons, 2012).
D. J. Finney. Probit Analysis (Cambridge, UK: Cambridge University Press, 1971).
Curtis D. Klassen, ed. Casarett and Doull’s Toxicology: The Basic Science of Poisons, 8th ed. (New York, NY: McGraw-Hill, 2013).
Richard J. Lewis, Sr., ed. Sax’s Dangerous Properties of Industrial Materials, 12th ed. (New York, NY: Wiley Interscience, 2012).
Sam Mannan, ed., Lees’ Loss Prevention in the Process Industries, 4th ed. (Amsterdam: Elsevier, 2012), Chapter 18.
Problems
2-1. Using the data provided in Example 2-1, determine (a) the mean and variance, (b) the frequency as a function of the response, and (c) the number affected as a function of the response. Also, (d) show graphs for the frequency and number affected.
2-2. Using the data provided in Example 2-1, determine the accumulated frequency (a) between minus infinity and infinity (using Equation 2-1), (b) between the mean and infinity (using Equation 2-10), and (c) between the mean plus the two standard deviations and infinity (Equation 2-1). State any conclusions.
2-3. Using Equation 2-6, determine the probability for probits of 4.39, 5.25, and 6.23.
2-4. A blast produces a peak overpressure of 47,000 N/m2.
What fraction of structures will be damaged by exposure to this overpressure?
What fraction of people exposed will die as a result of lung hemorrhage?
What fraction of people exposed will have their eardrums ruptured?
What conclusions about the effects of this blast can be drawn?
Repeat this problem with 30,000, 80,000, and 100,000 N/m2.
2-5. A volatile substance evaporates from an open container into a room of volume 28.3 m3. The evaporation rate is 100 mg/min. If the air in the room is assumed to be well mixed, how many m3/min of fresh air must be supplied to ensure that the concentration of the volatile is maintained below its TLV of 100 ppm? The temperature is 25°C, and the pressure is 1 atm. The volatile molecular weight is 100 g/g-mole. Under most circumstances, the air in a room cannot be assumed to be well mixed. How would poor mixing affect the quantity of air required?
2-6. If 500 workers in a plant are exposed to the following concentrations of ammonia for the given number of hours, how many deaths will be expected?
1000 ppm for 1 hour
2000 ppm for 2 hours
300 ppm for 3 hours
150 ppm for 2 hours
Repeat this problem with the concentrations given in parts (a) through (d), but assume the times are 2, 4, 6, and 2 hours, respectively.
2-7. Using TLV data from Appendix E, convert TLV in ppm to mg/m3 for the following compounds. Assume a temperature of 25°C and a pressure of 1 atm.
benzene
chlorine
cyclohexanol
ethylene oxide
2-8. Estimate four exposure concentrations in ppm that will result in fatalities for 80% of the exposed individuals if they are exposed to chlorine for 2, 4, 6, and 8 minutes.
2-9. Determine the deaths resulting from the following exposures to chlorine:
200 ppm for 150 min
100 ppm for 50 min
50 ppm for 20 min
2-10. The peak overpressure expected as a result of the explosion of a tank in a plant facility is approximated by the equation:
where P is the overpressure in N/m2 and r is the distance from the blast in meters. The plant employs 500 people who work in an area from 3 to 150 m from the potential blast site. Estimate the number of fatalities due to lung hemorrhage as a result of this blast. Assume there are 5 shells around the center and the people are evenly distributed through the area.
2-11. Use Appendix E to determine the hazardous properties of ammonia—that is, TLV-TWA, TLV-STEL, TLV-C, and PEL.
2-12. At what overpressure (Pa and psi) would 50% of structures be damaged?
2-13. For methane in air at 1 atm and 298 K, how many ppm is 100 mg/m3?
2-14. Humans breathe about 500 mL of air per breath and take about 12 breaths per minute during normal activities. If a person is exposed to an atmosphere containing benzene at a concentration of 10 ppm (by volume), how many grams of benzene will be deposited in the lungs during an 8-hour shift if all the benzene that enters remains in the lungs? How many drops of liquid is this? A drop of liquid contains about 0.05 cm3. The specific gravity of benzene is 0.879. If you were the worker, would this exposure level be acceptable?
Additional homework problems are available in the Pearson Instructor Resource Center.
