Chapter 4. Source Models
The learning objectives for this chapter are to:
Explain why and how source models are used in risk assessment.
Describe simple source models used for liquids and gases: holes, pipe flow, and liquid pools.
Describe more complex source models: holes in tanks.
Describe more complex source models: evaporation and flashing or boiling liquids.
Distinguish between realistic and worst-case releases.
Perform conservative analysis.
Most incidents in chemical plants result in spills of toxic, flammable, or explosive materials. Source models are an important part of the consequence modeling procedure shown in Figure 4-1. Figure 4-1 also identifies the particular chapters in this book that are related to the topics shown. Additional information is provided elsewhere.1
1Center for Chemical Process Safety. Guidelines for Consequence Analysis of Chemical Releases (New York, NY: American Institute of Chemical Engineers, 1999).

Many incidents result in the loss of containment of material from the process. These materials may have hazardous properties, which could include toxic properties and energy content. Typical incidents might include the rupture or break of a pipeline, a hole in a tank or pipe, runaway reaction, or fire external to the vessel.
Once the incident is identified, source models are selected to describe how materials are discharged from the process. The source model provides a description of the rate of discharge,the total quantity discharged (or total time of discharge), and the state of the discharge (that is solid, liquid, vapor, or a combination). A dispersion model is subsequently used to describe how th material is transported downwind and dispersed to some concentration levels (discussed further in Chapter 5). For releases of flammable materials, fire and explosion models convert the source model information on the release into energy hazard potentials, such as thermal radiation and explosion overpressures (discussed further in Chapter 6). Effect models convert these incident-specific results into effects on people (injury or death) and structures, as described in Chapter 2. Environmental impacts could also be considered, but we do not do so here.
4-1 Introduction to Source Models
Source models are constructed from fundamental or empirical equations representing the physical and chemical processes occurring during the release of materials. For a reasonably complex plant, many source models are needed to describe the release. Some development and modification of the original models is usually required to fit the specific situation.
In many cases, the results are only estimates because the physical properties of the materials are not adequately characterized or because the physical processes themselves are not completely understood. If uncertainty exists, the parameters should be selected to maximize the release rate and quantity. This approach ensures that a design is conservative (discussed further in Sections 4-9 and 4-10).
Release mechanisms are classified into wide- or limited-aperture releases, where apertures are holes and cracks in tanks and pipes, leaks in flanges, valves, and pumps, and severed or ruptured pipes and tanks. In the limited-aperture case, material is released at a slow enough rate that upstream conditions are not immediately affected—the assumption of constant upstream pressure is frequently valid. In the wide-aperture case, a large hole develops in the process unit, releasing a substantial amount of material in a short time. An example is the overpressuring and rupture of a storage tank.
Limited-aperture releases are conceptualized in Figure 4-2. Relief valves, designed to prevent the overpressuring of tanks and process vessels, are also potential sources of released material.

Figure 4-3 shows how the physical state of the material affects the release mechanism. For gases or vapors stored in a tank, a leak results in a jet of gas or vapor. For liquids, a leak below the liquid level in the tank results in a stream of escaping liquid. If the liquid is stored under pressure above its atmospheric boiling point, a leak below the liquid level will result in a stream of liquid flashing partially into vapor. Small liquid droplets or aerosols might also form from the flashing stream, with the possibility of transport away from the leak by wind currents. A leak in the vapor space above the liquid can result in either a vapor stream or a two-phase stream composed of vapor and liquid.

Several basic source models are used repeatedly and will be developed in detail here:
Flow of liquid through a hole
Flow of liquid through a hole in a tank
Flow of liquids through pipes
Flow of gases or vapor through holes
Flow of gases or vapor through pipes
Flashing liquids
Liquid pool evaporation or boiling
Many other incidents—such as tank overflow, catastrophic failure, excessive heating, and so forth—can be described using source models. Other source models, specific to certain materials, are introduced in subsequent chapters.
4-2 Flow of Liquid through a Hole
A mechanical energy balance describes the various energy forms associated with flowing fluids:
∫dPρ+Δ(ˉu22αgc)+ggcΔz+F=−Ws˙m(4-1)
where
P is the pressure (force/area),
ρ is the fluid density (mass/volume),
ū is the average instantaneous velocity of the fluid (length/time2),
gc is the gravitational constant (length mass/force time2),
α is the unitless velocity profile correction factor with the following values:
α = 0.5 for laminar flow, α = 1.0 for plug flow, andα approaches 1.0 for turbulent flow,
g is the acceleration due to gravity (length/time2),
z is the height above datum (length),
F is the net frictional loss term (length force/mass),
Ws is the shaft work (force length/time), defined as the positive work done by the system on the surroundings, and
˙m is the mass flow rate (mass/time).
The Δ function represents the final minus the initial state.
For incompressible liquids, the density is constant, and
∫dPρ=ΔPρ(4-2)
Consider a process unit that develops a small hole, as shown in Figure 4-4. The pressure energy of the liquid contained within the process unit is converted to kinetic energy as the fluid escapes through the hole. Frictional forces between the moving liquid and the wall of the leak convert some of the kinetic energy of the liquid into thermal energy, resulting in a reduced velocity.

For this limited-aperture release, assume a constant gauge pressure Pg within the process unit. The external pressure is atmospheric, so ΔP = Pg. The shaft work is zero, and the velocity of the fluid within the process unit is assumed negligible. The change in elevation of the fluid during the discharge through the hole is also negligible, so Δz = 0. The frictional losses in the leak are approximated by a constant discharge coefficient Cl, defined as
−ΔPρ−F=C21(−ΔPρ)(4-3)
The modifications are substituted into the mechanical energy balance (Equation 4-1) to determine ū, the average discharge velocity from the leak:
ˉu=C1√α√2gcPgρ(4-4)
A new discharge coefficient C0 is defined as
Co=C1√α(4-5)
The resulting equation for the velocity of fluid exiting the leak is
ˉu=Co√2gcPgρ(4-6)
The mass flow rate Qm resulting from a hole of area A is given by
Qm=ρˉμA=ACo√2ρgcPg(4-7)
The total mass of liquid spilled depends on the total time that the leak is active.
The discharge coefficient Co is a complicated function of the Reynolds number of the fluid escaping through the leak and the diameter of the hole. The following guidelines are suggested:
For sharp-edged orifices and for Reynolds numbers greater than 30,000, Co approaches the value 0.61. Under these conditions, the exit velocity of the fluid is independent of the size of the hole.
For a well-rounded nozzle, the discharge coefficient approaches 1.
For short sections of pipe attached to a vessel (with a length/diameter ratio not less than 3), the discharge coefficient is approximately 0.81.
When the discharge coefficient is unknown or uncertain, use a value of 1.0 to maximize the computed flows.
More details on discharge coefficients for these types of liquid discharges are provided elsewhere.2
2Robert H. Perry and Don W. Green. Perry’s Chemical Engineers Handbook, 8th ed. (New York, NY: McGraw-Hill, 2008), pp. 8–59.
Example 4-1
At 1:00 pm, the plant operator notices a drop in pressure in a pipeline transporting benzene. The pressure is immediately restored to 7 barg. At 2:30 pm, a 2 cm-diameter leak is found in the pipeline and immediately repaired. Estimate the total amount of benzene spilled. The specific gravity of benzene is 0.8794.
Solution
The drop in pressure observed at 1:00 pm is indicative of a leak in the pipeline. The leak is assumed to be active between 1:00 pm and 2:30 pm, for a total of 90 minutes. The area of the hole is
A=πd24=(3.14)[(2cm)(1m/100cm)]24=3.14×10−4m2
The density of the benzene is
ρ=(0.8794)(1000 kg/m3)=879.4 kg/m3
The leak mass flow rate is given by Equation 4-7. A discharge coefficient of 0.61 is assumed for this orifice-type leak:
Qm=AC0√2ρgcPg=(3.14×10−4m2)(0.61)√(2)(879.4 kgm3)(1 kg m/s2N)(7 bar)(105 N/m21 bar)=6.72 kg/s
The total quantity of benzene spilled is
(6.72 kg/s)(90 min)(60 s/min)=3.63×104 kg
4-3 Flow of Liquid through a Hole in a Tank
A storage tank is shown in Figure 4-5. A hole develops at a height hL below the fluid level. The flow of liquid through this hole is represented by the mechanical energy balance (Equation 4-1) and the incompressible assumption (Equation 4-2).
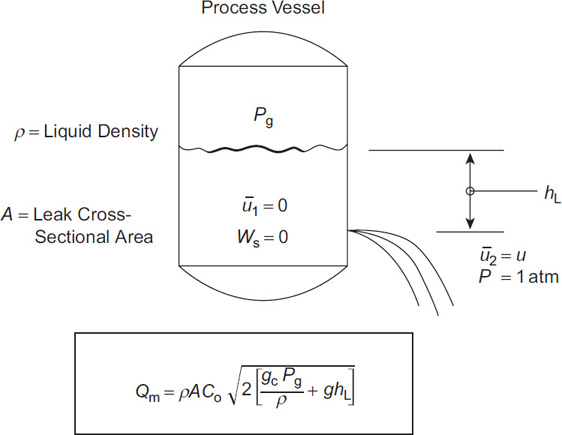
The gauge pressure on the tank is Pg, and the external gauge pressure is atmospheric, or 0. The shaft work Ws is zero, and the velocity of the fluid in the tank is zero.
A dimensionless discharge coefficient C1 is defined as
−ΔPρ−ggcΔz−F=C21(−ΔPρ−ggcΔz)(4-8)
The mechanical energy balance (Equation 4-1) is solved for ū, the average instantaneous discharge velocity from the leak:
ˉu = C1√α√2(gPcgρ+ghL)(4-9)
where hL is the liquid height above the leak. A new discharge coefficient Co is defined as
Co=C1√α(4-10)
The resulting equation for the instantaneous velocity of fluid exiting the leak is
ˉu =Co√2(gPcgρ + ghL)(4-11)
The instantaneous mass flow rate Qm resulting from a hole of area A is given by
Qm = ρˉuA = ρACo√2(gcPgρ + ghL)(4-12)
As the tank empties, the liquid height decreases and the velocity and mass flow rate decrease.
Assume that the gauge pressure Pg on the surface of the liquid is constant. This would occur if the vessel was padded with an inert gas to prevent explosion or was vented to the atmosphere. For a tank of constant cross-sectional area At, the total mass of liquid in the tank above the leak is
m=ρAthL(4-13)
The rate of change of mass within the tank is
dmdt=−Qm(4-14)
where Qm is given by Equation 4-12. By substituting Equations 4-12 and 4-13 into Equation 4-14, and by assuming a constant tank cross-section and liquid density, we can obtain a differential equation representing the change in the fluid height:
dhLdt = −CoAAt√2(gPcgρ+ ghL)(4-15)
Equation 4-15 can be rearranged and integrated from an initial height h0L to any height hL:
∫hLh0LdhL√2gcPgρ+2ghL=−CoAAt∫t0dt(4-16)
This equation is integrated to
1g√2gcPgρ+2ghL−1g√2gcPgρ+2ghoL=−CoAAtt(4-17)
Solving for hL, the liquid level height in the tank, yields
hL = hoL − CoAAt√2gPcgρ + 2ghoL t + g2(CoAAtt)2(4-18)
Equation 4-18 is substituted into Equation 4-12 to obtain the mass discharge rate at any time t:
Qm = ρCoA√2(gPcgρ + ghoL)−ρgC2oA2Att(4-19)
The first term on the right-hand side of Equation 4-19 is the initial mass discharge rate at hL= hoL.
The time te for the vessel to empty to the level of the leak is found by solving Equation 4-18 for t after setting hL = 0:
te = 1Cog(AtA)[√2(gPcgρ+ghoL)−√2gPcgρ](4-20)
If the vessel is at atmospheric pressure, Pg = 0 and Equation 4-20 reduces to
te = 1Cog(AtA)√2ghoL(4-21)
Example 4-2
A cylindrical tank 20 ft high and 8 ft in diameter is used to store benzene. The tank is padded with nitrogen to a constant regulated pressure of 1 atm gauge to prevent the formation of a flammable mixture. The liquid level within the tank is presently at 17 ft. A 1-in.-diameter puncture hole occurs in the tank 5 ft off the ground because of the careless driving of a forklift truck. Estimate (a) the gallons of benzene spilled, (b) the time required for the benzene to leak out, and (c) the maximum mass flow rate of benzene through the leak. The specific gravity of benzene at these conditions is 0.8794.
Solution
The density of the benzene is
ρ=(0.8794)(62.4 lbm/ ft3)=54.9 lbm/ ft3
The area of the tank is
At=πd24=(3.14)(8 ft)24=50.2 ft2
The area of the leak is
A=(3.14)(1 in)2(1 ft2/ 144 in2)4=5.45×10−3 ft2
The gauge pressure is
Pg=(1 atm)(14.7 lbf/ in2)(144 in2/ ft2)=2.12×103 lbf/ ft2
The volume of benzene above the leak is
V=Ath0L=(50.2 ft2)(17 ft−5 ft)(7.48 gal/ft3)=4506 gal
This is the total amount of benzene that will leak out.
The length of time for the benzene to leak out is given by Equation 4-20:
te=1Cog(AtA)[√2(gcPgρ+ghoL)−√2gcPgρ]=1(0.61)(32.17 ft/s2)(50.2 ft25.45×10−3 ft2) ×{[(2)(32.17 ft-lbm/lbf-s2)54.9 lbm/ft3+(2)(32.17 ft/s2)(12 ft)]1/2− √2484 ft2/s2}=(469 s2/ft)(7.22 ft/s)=3386 s=56.4 min
This appears to be more than adequate time to stop the leak or to invoke an emergency procedure to reduce the impact of the leak. However, the maximum discharge occurs when the hole first occurs.
The maximum discharge occurs at t = 0 at a liquid level of 17.0 ft. Equation 4-19 is used with t = 0 to compute the mass flow rate:
Qm=ρACo√2(gcPgρ+ghoL)=(54.9 1bm/ft3)(5.45×10−3 ft2)(0.61)√3.26×103 ft2/s2=10.4 1bm/s
A general equation to represent the draining time for any vessel of any geometry is developed as follows. Assume that the head space above the liquid is at atmospheric pressure. Then, by combining Equations 4-12 and 4-14, we get
dmdt=ρdVdt=−ρACo√2ghL(4-22)
By rearranging and integrating, we obtain
−1ACo√2g∫v2v1dV√hL=∫t0dt(4-23)
which results in the general equation for the draining time for any vessel:
t=1ACo√2g∫V2V1dV√hL(4-24)
Equation 4-24 does not assume that the hole is at the bottom of the vessel.
For a vessel with the shape of a vertical cylinder, we have
dV=πd24dhL(4-25)
By substituting into Equation 4-24, we obtain
t=πD24ACo√2g∫dhL√hL(4-26)
If the hole is at the bottom of the vessel, then Equation 4-26 is integrated from h = 0 to h=h0L.
Equation 4-26 then provides the emptying time for the vessel:
te=πD2/4ACo√2hoLg=1Cog(πD2/4A)√2gh0L(4-27)
which is the same result as Equation 4-21.
4-4 Flow of Liquids through Pipes
A pipe transporting liquid is shown in Figure 4-6. A pressure gradient across the pipe is the driving force for the movement of liquid. If the pipe diameter is constant, then the velocity is constant along the entire length of the pipe. Frictional forces between the liquid and the wall of the pipe convert kinetic energy into thermal energy, which in turn results in a decrease in the liquid pressure.

Flow of incompressible liquids through pipes is described by the mechanical energy balance (Equation 4-1) combined with the incompressible fluid assumption (Equation 4-2). The net result is
ΔPρ+Δˉu22αgc+ggcΔz+F=−Wsm(4-28)
The frictional loss term F in Equation 4-28 represents the loss of mechanical energy resulting from friction. It includes losses resulting from flow through lengths of pipe; fittings such as valves, elbows, orifices; and pipe entrances and exits. For each frictional device, a loss term of the following form is used:
F=Kf(u22gc)(4-29)
where
Kf is the excess head loss due to the pipe or pipe fitting (dimensionless) and u is the fluid velocity (length/time).
For fluids flowing through pipes, the excess head loss term Kf is given by
Kf=4fLd(4-30)
where
f is the Fanning friction factor (unitless),
L is the flow path length (length), and
d is the flow path diameter (length).
The Fanning friction factor f is a function of the Reynolds number Re and the roughness of the pipe ε. Table 4-1 provides values of ε for various types of clean pipe. Figure 4-7 is a plot of the Fanning friction factor versus Reynolds number with the pipe roughness, ε/d, as a parameter.

Table 4-1 Roughness Factor ε for Pipesa
Pipe material |
Condition |
Typical ε |
|
|---|---|---|---|
mm |
inch |
||
Drawn brass, copper, stainless |
New |
0.002 |
0.00008 |
Commercial steel |
New |
0.046 |
0.0018 |
|
Light rust |
0.3 |
0.015 |
|
General rust |
2.0 |
0.08 |
Iron |
Wrought, new |
0.045 |
0.0018 |
|
Cast, new |
0.30 |
0.025 |
|
Galvanized |
0.15 |
0.006 |
Concrete |
Very smooth |
0.04 |
0.0016 |
|
Wood floated, brushed |
0.3 |
0.012 |
|
Rough, visible form marks |
2.0 |
0.08 |
Glass or plastic |
Drawn tubing |
0.002c |
0.0008c |
Rubber |
Smooth tubing |
0.01 |
0.004 |
|
Wire reinforced |
1.0 |
0.04 |
Fiberglassb |
|
0.005 |
0.0002 |
aRon Darby. “Fluid Flow.” Albright’s Chemical Engineering Handbook, Lyle F. Albright, ed. (Boca Raton, FL: CRC Press, 2009), p. 421.
bWilliam D. Stringfellow, ed. Fiberglass Pipe Handbook (Washington, DC: Society of the Plastics Industry, 1989).
cGenerally considered smooth pipe with ε = 0.
For laminar flow, the Fanning friction factor is given by
f=16Re(4-31)
For turbulent flow, the data shown in Figure 4-7 are represented by the Colebrook equation:
1√f=−4log(13.7εd+1.255Re√f)(4-32)
An alternative form of Equation 4-32, useful for determining the Reynolds number from the friction factor f, is
1Re = √f1.255(10−0.25/√f−13.7εd)(4-33)
For fully developed turbulent flow in rough pipes, f is independent of the Reynolds number, as shown by the nearly constant friction factors at high Reynolds numbers in Figure 4-7. For this case, Equation 4-33 is simplified to
1√f=4log(3.7dε)(4-34)
For smooth pipes, ε = 0 and Equation 4-32 reduces to
1√f=4log(Re√f1.255)(4-35)
For smooth pipes with a Reynolds number less than 100,000, the following Blasius approximation to Equation 4-35 is useful:
f=0.079Re−1/4(4-36)
A single equation has been proposed by Chen3 to provide the friction factor f over the entire range of Reynolds numbers shown in Figure 4-7. This equation is
3N. H. Chen. An Explicit Equation for Friction Factor in Pipe, Industrial Engineering and Chemistry Fundamentals 18 (1979): 296.
1√f=− 4log(ε/d3.7065−5.0452 logARe)(4-37)
where
A=[(ε/d)1.10982.8257+5.8506Re0.8981]
2-K Method
For pipe fittings, valves, and other flow obstructions, the traditional method has been to use an equivalent pipe length Lequiv in Equation 4-30. The problem with this method is that the specified length is coupled to the friction factor. An improved approach is to use the 2-K method,4, 5 which uses the actual flow path length in Equation 4-30—equivalent lengths are not used—and provides a more detailed approach for pipe fittings, inlets, and outlets. Darby6 suggests a 3-K method that fits the data over an even wider range of flow conditions.
4W. B. Hooper. The Two-K Method Predicts Head Losses in Pipe Fittings, Chemical Engineering (August 24, 1981): 96–100.
5W. B. Hooper. Calculate Head Loss Caused by Change in Pipe Size, Chemical Engineering (November 7, 1988): 89–92.
6R. Darby and R. P. Chhabra. Chemical Engineering Fluid Mechanics, 3rd ed. (Boca Raton, FL: CRC Press, 2017).
The 2-K method defines the excess head loss in terms of two constants, the Reynolds number and the pipe internal diameter:
Kf=K1Re+K∞(1+1IDinches)(4-38)
where
Kf is the excess head loss (dimensionless),
Kl and K∞ are constants (dimensionless),
Re is the Reynolds number (dimensionless), and
IDinches is the internal diameter of the flow path (inches).
Table 4-2 contains a list of K values for use in Equation 4-38 for various types of fittings and valves.
Table 4-2 2-K Constants for Loss Coefficients in Fittings and Valves
Fittings |
Description of fitting |
K1 |
K∞ |
|---|---|---|---|
Elbows |
Standard (r/D = 1), threaded |
800 |
0.40 |
90° |
Standard (r/D = 1), flanged/welded |
800 |
0.25 |
|
Long radius (r/D = 1.5), all types |
800 |
0.20 |
|
Mitered (r/D = 1.5): 1 weld (90°) |
1000 |
1.15 |
|
2 welds (45°) |
800 |
0.35 |
|
3 welds (30°) |
800 |
0.30 |
|
4 welds (22.5°) |
800 |
0.27 |
|
5 welds (18°) |
800 |
0.25 |
45° |
Standard (r/D = 1), all types |
500 |
0.20 |
|
Long radius (r/D = 1.5) |
500 |
0.15 |
|
Mitered, 1 weld (45°) |
500 |
0.25 |
|
Mitered, 2 welds (22.5°) |
500 |
0.15 |
180° |
Standard (r/D = 1), threaded |
1000 |
0.60 |
|
Standard (r/D = 1), flanged/welded |
1000 |
0.35 |
|
Long radius (r/D = 1.5), all types |
1000 |
0.30 |
Tees |
|
|
|
Used as elbows |
Standard, threaded |
500 |
0.70 |
|
Long radius, threaded |
800 |
0.40 |
|
Standard, flanged/welded |
800 |
0.80 |
|
Stub-in branch |
1000 |
1.00 |
Run-through |
Threaded |
200 |
0.10 |
|
Flanged/welded |
150 |
0.50 |
|
Stub-in branch |
100 |
0.00 |
Valves |
|
|
|
Gate, ball or plug |
Full line size, β = 1.0 |
300 |
0.10 |
|
Reduced trim,β = 0.9 |
500 |
0.15 |
|
Reduced trim,β = 0.8 |
1000 |
0.25 |
Globe |
Standard |
1500 |
4.00 |
|
Angle or Y-type |
1000 |
2.00 |
Diaphragm |
Dam type |
1000 |
2.00 |
Butterfly |
|
800 |
0.25 |
Check |
Lift |
2000 |
10.0 |
|
Swing |
1500 |
1.50 |
|
Tilting disk |
1000 |
0.50 |
Source: William B. Hooper. The Two-K Method Predicts Head Losses in Pipe Fittings, Chemical Engineering (August 24, 1981): 97.
For pipe entrances and exits, Equation 4-38 is modified to account for the change in kinetic energy:
Kf=K1Re+K∞(4-39)
For pipe entrances, K1 = 160 and K∞ = 0.50 for a normal entrance. For a Borda-type pipe connection to a tank where the pipe sticks up into the bottom of the tank for a short distance, K∞ = 1.0. For pipe exits, K1 = 0 and K∞ = 1.0. The K factors for the entrance and exit effects account for the changes in kinetic energy through these piping changes, so no additional kinetic energy terms in the mechanical energy balance must be considered. For high Reynolds numbers (that is, Re > 10,000), the first term in Equation 4-39 is negligible and Kf = K∞. For low Reynolds numbers (that is, Re < 50), the first term dominates and Kf = K1/Re.
Equations are also available for orifices7 and for changes in pipe sizes.8
7W. B. Hooper. The Two-K Method Predicts Head Losses in Pipe Fittings, Chemical Engineering (August 24, 1981): 96–100.
8W. B. Hooper. Calculate Head Loss Caused by Change in Pipe Size, Chemical Engineering (November 7, 1988): 89–92.
The 2-K method also represents liquid discharge through holes. From the 2-K method, an expression for the discharge coefficient for liquid discharge through a hole can be determined. The result is
Co=1√1+∑Kf(4-40)
where ∑Kf is the sum of all excess head loss terms, including entrances, exits, pipe lengths, and fittings, provided by Equations 4-30, 4-38, and 4-39. For a simple hole in a tank with no pipe connections or fittings, the friction is caused only by the entrance and exit effects of the hole. For Reynolds numbers greater than 10,000, Kf = 0.5 for the entrance and Kf = 1.0 for the exit. Thus ∑ Kf = 1.5, and from Equation 4-40, Co = 0.63, which nearly matches the suggested value of 0.61.
The general solution procedure to determine the mass flow rate of discharged material from a piping system is as follows:
Given the length, diameter, and type of pipe; pressures and elevation changes across the piping system; work input or output to the fluid resulting from pumps, turbines, and other sources; the number and type of fittings in the pipe; and properties of the fluid, including density and viscosity.
Specify the initial point (point 1) and the final point (point 2). This must be done carefully, because the individual terms in Equation 4-28 are highly dependent on this specification.
Determine the pressures and elevations at points 1 and 2. Determine the initial fluid velocity at point 1.
Guess a value for the velocity at point 2. If fully developed turbulent flow is expected, then this is not required.
Determine the friction factor for the pipe using Equations 4-31 through 4-37.
Determine the excess head loss terms for the pipe (using Equation 4-30), for the fittings (using Equation 4-38), and for any entrance and exit effects (using Equation 4-39). Sum the head loss terms, and compute the net frictional loss term using Equation 4-29. Use the velocity at point 2.
Compute values for all the terms in Equation 4-28, and substitute into the equation. If the sum of all the terms in Equation 4-28 is zero, then the computation is completed. If not, go back to step 4 and repeat the calculation.
Determine the mass flow rate using the equation ˙m=ρˉuA..
If fully developed turbulent flow is expected, the solution is direct. Substitute the known terms into Equation 4-28, leaving the velocity at point 2 as a variable. Solve for the velocity directly.
Example 4-3
Water contaminated with small amounts of hazardous waste is gravity-drained out of a large storage tank through a straight, new commercial steel pipe, 100 mm ID (actual internal diameter). The pipe is 100 m long, with a gate valve near the tank. The entire pipe assembly is horizontal. If the liquid level in the tank is 5.8 m above the pipe outlet, and the pipe is severed 33 m from the tank, compute the flow rate of material escaping from the pipe.
Solution
The draining operation is shown in Figure 4-8. Assuming negligible kinetic energy changes, no pressure changes, and no shaft work, the mechanical energy balance (Equation 4-28) applied between points 1 and 2 reduces to

ggcΔz+F=0
For water,
μ=1.0 × 10−3 kg/m sρ=1000 kg/m3
The K factors for the entrance and exit effects are determined using Equation 4-39. The K factor for the gate valve is found in Table 4-2, and the K factor for the pipe length is given by Equation 4-30.
For the pipe entrance,
Kf=160Re+0.50
For the gate valve,
Kf=300Re+0.10
For the pipe exit,
Kf=1.0
For the pipe length,
Kf=4fLd=4f(33 m)0.10 m=1320f
Summing the K factors gives
∑Kf=460Re+1320f+1.60
For Re > 10,000, the first term in the equation is small. Thus
∑Kf=1320f+1.60
and it follows that
F=∑Kf(ˉu22gc)=(660f+0.80)(ˉu2gc)
The gravitational term in the mechanical energy equation is given by
ggcΔz=9.8 m/s21 kg m/s2/N(0-5.8 m)=−56.8 Nm/kg=−56.8 J/kg
Because there is no pressure change and no pump or shaft work, the mechanical energy balance (Equation 4-28) reduces to
ˉu222gc+ggcΔz+F=0.
Solving for the exit velocity and substituting for the height change gives
ˉu22=−2gc(ggcΔz+F)=−2gc(−56.8+F)
The Reynolds number is given by
Re=dˉuρμ=(0.1 m)(ˉu)(1000 kg/m3)1.0×10−3 kg/m s=1.0×105 ˉu
For new commercial steel pipe, from Table 4-1, ε = 0.046 mm and
εd=0.046 mm100 mm=0.00046
Because the friction factor f and the frictional loss term F are functions of the Reynolds number and velocity, the solution is found by trial and error. The trial-and-error solution is shown in the following table:
Guessed |
Re |
f |
F |
Calculated |
|---|---|---|---|---|
3.00 |
300,000 |
0.00451 |
34.09 |
6.75 |
3.50 |
350,000 |
0.00446 |
46.00 |
4.66 |
3.66 |
366,000 |
0.00444 |
50.18 |
3.66 |
Thus, the velocity of the liquid discharging from the pipe is 3.66 m/s. The table also shows that the friction factor f changes little with the Reynolds number. Thus, we can approximate it using Equation 4-34 for fully developed turbulent flow in rough pipes. Equation 4-34 produces a friction factor value of 0.0041. Then
F=(660f+0.80)ˉu22=3.51ˉu22
By substituting and solving, we obtain
ˉu22=−2gc(−56.8+3.51ˉu22) = 113.6−7.02ˉu22ˉu2 =3.76 m/s
This result is close to the more exact trial-and-error solution.
The cross-sectional area of the pipe is
A=πd24=(3.14)(0.1 m2)4=0.00785 m2
The mass flow rate is given by
Qm=ρˉuA=(1000 kg/m3)(3.66 m/s)(0.00785 m2)=28.8 kg/s
This represents a significant flow rate. If the plant personnel are able to recognize the leak and respond in 15 min to stop the release, a total of 26,000 kg of hazardous waste will be spilled. In addition to the material released by the flow, the liquid contained within the pipe between the valve and the rupture will spill. An alternative system must be designed to limit the release. This could include a reduction in the emergency response period, replacement of the pipe by a pipe with a smaller diameter, or modification of the piping system to include additional remotely activated control valves to stop the flow.
4-5 Flow of Gases or Vapors through Holes
For flowing liquids, the kinetic energy changes are frequently negligible and the physical properties (particularly the density) are constant. For flowing gases and vapors, these assumptions are valid only for small pressure changes (P1/P2 < 2) and low velocities (less than 0.3 times the speed of sound in gas). Energy contained within the gas or vapor as a result of its pressure is converted into kinetic energy as the gas or vapor escapes and expands through the hole. The density, pressure, and temperature change as the gas or vapor exits through the leak.
Gas and vapor discharges are classified as either throttling or free expansion releases. In throttling releases, the gas issues through a small crack with large frictional losses; little of the energy inherent to the gas pressure is converted to kinetic energy. In free expansion releases, most of the pressure energy is converted to kinetic energy, so that the assumption of isentropic behavior is usually valid.
Source models for throttling releases require detailed information on the physical structure of the leak; they are not considered here. Free expansion release source models require only the diameter of the leak.
A free expansion leak is shown in Figure 4-9. The mechanical energy balance (Equation 4-1) describes the flow of compressible gases and vapors. Assuming negligible potential energy changes and no shaft work results in a reduced form of the mechanical energy balance describing compressible flow through holes:

∫dPρ+Δ(ˉu22αgc)+F=0(4-41)
A discharge coefficient Cl is defined in a similar fashion to the coefficient defined in Section 4-2:
−∫dPρ − F = C21(−∫dPρ)(4-42)
Equation 4-42 is combined with Equation 4-41 and integrated between any two convenient points. An initial point (denoted by subscript “o”) is selected where the velocity is zero and the pressure is Po. The integration is completed to any arbitrary final point (denoted without a subscript). The result is
C21∫PPodPρ+ˉu22αgc=0(4-43)
For any ideal gas undergoing an isentropic expansion,
Pvγ = Pργ = constant(4-44)
where γ is the ratio of the heat capacities, γ = Cp/Cv. Substituting Equation 4-44 into Equation 4-43, defining a new discharge coefficient Co identical to that in Equation 4-5, and integrating results in an equation representing the velocity of the fluid at any point during the isentropic expansion:
ˉu2=2gcC2oγγ−1Poρ0[1−(PPo)(γ−1)/γ]=2gcC2oRgToMγγ−1[1−(PPo)(γ−1)/γ](4-45)
The second form incorporates the ideal gas law for the initial density ρo. Rg is the ideal gas constant, and To is the temperature of the source. Using the continuity equation
Qm=ρˉuA(4-46)
and the ideal gas law for isentropic expansions in the form
ρ=ρo(PPo)1/γ(4-47)
results in the following expression for the mass flow rate:
Qm=CoAPo√2gcMRgTo γγ−1[(PPo)2/γ−(PPo)(γ+1)/γ](4-48)
Equation 4-48 describes the mass flow rate at any point during the isentropic expansion.
For many safety studies, the maximum flow rate of vapor through the hole is required. This is determined by differentiating Equation 4-48 with respect to P/Po and setting the derivative equal to zero. The result is solved for the pressure ratio resulting in the maximum flow:
PchokedPo=(2γ+1)γ/(γ−1)(4-49)
The choked pressure Pchoked is the maximum downstream pressure resulting in maximum flow through the hole or pipe. For downstream pressures less than Pchoked, the following statements are valid: (1) The velocity of the fluid at the throat of the leak is the velocity of sound at the prevailing conditions, and (2) the velocity and mass flow rate cannot be increased further by reducing the downstream pressure—they are independent of the downstream conditions. This type of flow is called choked, critical, or sonic flow and is illustrated in Figure 4-10.

An interesting feature of Equation 4-49 is that for ideal gases, the choked pressure is a function only of the heat capacity ratio γ. Thus:
Gas |
γ |
Pchoked |
|---|---|---|
Monatomic |
≅ 1.67 |
0.487Po |
Diatomic and air |
≅ 1.40 |
0.528Po |
Triatomic |
≅ 1.32 |
0.542Po |
For an air leak to atmospheric conditions (Pchoked = 14.7 psia), if the upstream pressure is greater than 14.7/0.528 = 27.8 psia, or 13.1 psig, the flow will be choked and maximized through the leak. Conditions leading to choked flow are common in the process industries.
The maximum flow is determined by substituting Equation 4-49 into Equation 4-48:
(Qm)choked=CoAPo√γgcMRgTo(2γ+1)(γ+1)/(γ−1)(4-50)
where
M is the molecular weight of the escaping vapor or gas,
To is the temperature of the source, and
Rg is the ideal gas constant.
For sharp-edged orifices with Reynolds numbers greater than 30,000 (and not choked), a constant discharge coefficient Co of 0.61 is indicated. However, for choked flows the discharge coefficient increases as the downstream pressure decreases.9 For these flows and for situations where Co is uncertain, a conservative value of 1.0 is recommended.
9Robert H. Perry and Cecil H. Chilton. Chemical Engineers Handbook, 7th ed. (New York, NY: McGraw-Hill, 1997), pp. 10–16.
Values for the heat capacity ratio γ for a variety of gases are provided in Table 4-3.
Table 4-3 Heat Capacity Ratios γ for Selected Gases
Gas |
Chemical formula or symbol |
Approximate molecular weight (M) |
Heat capacity ratio |
|---|---|---|---|
Acetylene |
C2H2 |
26.0 |
1.30 |
Air |
– |
29.0 |
1.40 |
Ammonia |
NH3 |
17.0 |
1.32 |
Argon |
Ar |
39.9 |
1.67 |
Butane |
C4H10 |
58.1 |
1.11 |
Carbon dioxide |
CO2 |
44.0 |
1.30 |
Carbon monoxide |
CO |
28.0 |
1.40 |
Chlorine |
Cl2 |
70.9 |
1.33 |
Ethane |
C2H6 |
30.0 |
1.22 |
Ethylene |
C2H4 |
28.0 |
1.22 |
Helium |
He |
4.0 |
1.66 |
Hydrogen |
H2 |
2.0 |
1.41 |
Hydrogen chloride |
HCl |
36.5 |
1.41 |
Hydrogen sulfide |
H2S |
34.1 |
1.30 |
Methane |
CH4 |
16.0 |
1.32 |
Methyl chloride |
CH3Cl |
50.5 |
1.20 |
Natural gas |
– |
19.5 |
1.27 |
Nitric oxide |
NO |
30.0 |
1.40 |
Nitrogen |
N2 |
28.0 |
1.41 |
Nitrous oxide |
N2O |
44.0 |
1.31 |
Oxygen |
O2 |
32.0 |
1.40 |
Propane |
C3H8 |
44.1 |
1.15 |
Propene (propylene) |
C3H6 |
42.1 |
1.14 |
Sulfur dioxide |
SO2 |
64.1 |
1.26 |
Source: Crane Co. Flow of Fluids through Valves, Fittings, and Pipes, Technical Paper 410 (New York, NY: Crane Co., 2009). www.flowoffluids.com.
Example 4-4
A 0.2-cm hole forms in a tank containing nitrogen at 14 bar gauge and 25°C. Determine the mass flow rate through this leak. The external pressure is 1 atm.
Solution
From Table 4-3, for nitrogen γ = 1.41. Then, from Equation 4-49,
PchokedPo = (2γ+1)γ/(γ−1)= (22.41)1.41/0.41=0.527
The absolute pressure in the tank is 14 bar + 1.013 barg = 15.01 bara.
Thus,
Pchoked=0.527(15.01 bara)=7.91 bara
Any external pressure less than 7.91 bara will result in choked flow through the leak. Because the external pressure is atmospheric in this case (1.013 bara), choked flow is expected and Equation 4-50 applies. The area of the hole is
A=πd24=(3.14)(0.2 cm)2(1 m2/104 cm2)4=3.14×10−6 m2
The discharge coefficient C0 is assumed to be 1.0. Also,
To=25+273=298 K(2γ+1)(γ+1)/(γ−1)=(22.41)2.41/0.41 =0.8305.87= 0.335
Then, using Equation 4-50,
(Qm)choked=CoAPo√γgcMRgTo(2γ+1)(γ+1)(γ−1)=(1.0)(3.14×10−6 m2)(15.01 bara)(105 N/m21 bar)×√(1.41)(1kg m/s2N)(28 kg/kg-mole)(8.314×103 N m/kg-mole K)(298 K)(0.335)=4.71 N√5.34×10−6 kg2/N2S2(Qm)choked=1.09×10−2 kg/s
4-6 Flow of Gases or Vapors through Pipes
Gas flow through pipes is modeled using two special cases: adiabatic and isothermal behavior. The adiabatic case corresponds to rapid gas flow through an insulated pipe. The isothermal case corresponds to flow through an uninsulated pipe maintained at a constant temperature; an underwater pipeline is an example. Real vapor flows behave somewhere between the adiabatic and isothermal cases. Unfortunately, the real case must be modeled numerically and no generalized and useful equations are available.
For both the isothermal and adiabatic cases, it is convenient to define a dimensionless Mach (Ma) number as the ratio of the gas velocity to the velocity of sound in the gas at the prevailing conditions:
Ma=ˉua(4-51)
where a is the velocity of sound. The velocity of sound is determined using the thermodynamic relationship
a=√gc(∂P∂ρ)S(4-52)
which for an ideal gas is equivalent to
a=√γgcRgT/M(4-53)
This demonstrates that for ideal gases, the sonic velocity is a function of temperature only. For air at 20°C, the velocity of sound is 344 m/s (1129 ft/s).
Adiabatic Flows
An adiabatic pipe containing a flowing gas is shown in Figure 4-11. For this particular case, the outlet velocity is less than the sonic velocity. The flow is driven by a pressure gradient across the pipe. As the gas flows through the pipe, it expands because of a decrease in pressure. This expansion leads to an increase in velocity and an increase in the kinetic energy of the gas. The kinetic energy is extracted from the thermal energy of the gas; thus, a decrease in temperature occurs. However, frictional forces are present between the gas and the pipe wall. These frictional forces increase the temperature of the gas. Depending on the magnitude of the kinetic and frictional energy terms, either an increase or a decrease in the gas temperature is possible.

The mechanical energy balance (Equation 4-1) also applies to adiabatic flows. For this case, it is more conveniently written in the form
dPρ+ˉudˉuαgc+ggcdz+dF=−δWsm(4-54)
The following assumptions are valid for this case:
ggc dz ≈ 0
Assuming a straight pipe without any valves or fittings, Equations 4-29 and 4-30 can be combined and then differentiated to result in
dF = 2fˉu2dLgcd
Because no mechanical linkages are present,
δWS = 0
An important part of the frictional loss term is the assumption of a constant Fanning friction factor f across the length of the pipe. This assumption is valid only at high Reynolds numbers.
A total energy balance is useful for describing the temperature changes within the flowing gas. For this open steady-flow process, the total energy balance is given by
dh + ˉud ˉuαgc + ggcdz = δq − δWsm(4-55)
where h is the enthalpy of the gas and q is the heat. The following assumptions are invoked:
dh = Cp dT for an ideal gas,
g/gc dz ≈ 0 is valid for gases,
δq = 0 because the pipe is adiabatic, and
δWs = 0 because no mechanical linkages are present.
These assumptions are applied to Equations 4-55 and 4-54. The equations are combined, integrated between the initial point denoted by subscript “o” and any arbitrary final point, and manipulated to yield, after considerable effort,10
10Octave Levenspiel. Engineering Flow and Heat Exchange, 2nd ed. (New York, NY: Springer, 1998), p. 43.
T2T1 = Y1Y2 where Yi = 1 + γ−12Ma2i(4-56)
P2P1 = Ma1Ma2√Y1Y2(4-57)
ρ2ρ1 = Ma1Ma2√Y2Y1(4-58)
G=ρˉu=Ma1P1√γgcMRgT1=Ma2P2√γgcMRgT2(4-59)
where G is the mass flux with units of mass/(area-time) and
γ+12 ln(Ma22Y1Ma21Y2) −(1Ma21−1Ma22) +γ (4fLd) = 0Kinetic EnergyCompressibilityPipe Friction (4-60)
Equation 4-60 relates the Mach numbers to the frictional losses in the pipe. The various energy contributions are identified. The compressibility term accounts for the change in velocity resulting from the expansion of the gas.
Equations 4-59 and 4-60 are converted to a more convenient and useful form by replacing the Mach numbers with temperatures and pressures, using Equations 4-56 through 4-58:
γ + 1γlnP1T2P2T1 − γ − 12γ(P21T22−P22T21T2−T1) (1P21T2−1P22T1) + 4fLd = 0(4-61)
G = √2gcMRg γγ−1 T2−T1(T1/P1)2 − (T2/P2)2(4-62)
For most problems, the pipe length (L), inside diameter (d), upstream temperature (T1) and pressure (P1), and downstream pressure (P2) are known. To compute the mass flux G, the procedure is as follows:
Determine pipe roughness ε from Table 4-1. Compute ε/d.
Determine the Fanning friction factor f from Equation 4-34. This assumes fully developed turbulent flow at high Reynolds numbers. This assumption can be checked later but is normally valid.
Determine T2 from Equation 4-61.
Compute the total mass flux G from Equation 4-62.
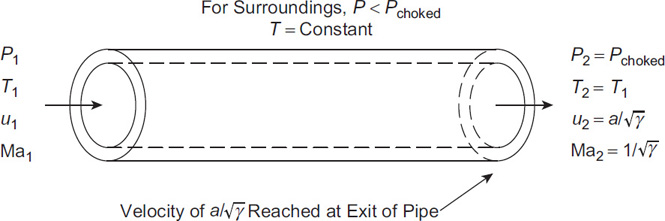
For long pipes or for large pressure differences across the pipe, the velocity of the gas can approach the sonic velocity. This case is shown in Figure 4-12.

When the sonic velocity is reached, the gas flow is called choked. The gas reaches the sonic velocity at the end of the pipe. If the upstream pressure is increased or if the downstream pressure is decreased, the gas velocity at the end of the pipe remains constant at the sonic velocity. If the downstream pressure is decreased below the choked pressure Pchoked, the flow through the pipe remains choked and constant, independent of the downstream pressure. The pressure at the end of the pipe will remain at Pchoked even if this pressure is greater than the ambient pressure. The gas exiting the pipe makes an abrupt change from Pchoked to the ambient pressure. For choked flow, Equations 4-56 through 4-60 are simplified by setting Ma2=1.0. The results are
TchokedT1=2Y1γ+1(4-63)
PchokedP1=Ma1√2Y1γ+1(4-64)
Pchockedρ1=Ma1√γ+12Y1(4-65)
Gchoked = ρˉu = Ma1P1√γgcMRgT1 = Pchoked√γgcMRgTchoked(4-66)
γ + 12ln[2Y1(γ + 1)Ma21] − (1Ma21−1) + γ(4fLd) =0(4-67)
Choked flow occurs if the downstream pressure is less than Pchoked. This is checked using Equation 4-64.
For most problems involving choked adiabatic flows, the pipe length (L), inside diameter (d), and upstream pressure (P1) and temperature (T1) are known. To compute the mass flux G, the procedure is as follows:
Determine the Fanning friction factor f using Equation 4-34. This assumes fully developed turbulent flow at high Reynolds numbers. This assumption can be checked later but is usually valid.
Determine Ma1 from Equation 4-67.
Determine the mass flux Gchoked from Equation 4-66.
Determine Pchoked from Equation 4-64 to confirm operation at choked conditions.
Equations 4-63 through 4-67 for adiabatic pipe flow can be modified to use the 2-K method discussed previously by substituting ∑Kf for 4fL/d.
The procedure can be simplified by defining a gas expansion factor Yg. For ideal gas flow, the mass flow for both sonic and non-sonic conditions is represented by the Darcy formula:11
11Crane Co. Flow of Fluids through Valves, Fittings, and Pipes, Technical Paper 410 (New York, NY: Crane Co., 2009). www.flowoffluids.com.
G=˙mA=Yg√2gcρ1(P1−P2)ΣKf(4-68)
where
G is the mass flux (mass/area-time),
˙m is the mass flow rate of gas (mass/time),
A is the area of the discharge (length2),
Yg is a gas expansion factor (unitless),
gc is the gravitational constant (force/mass-acceleration),
ρ1 is the upstream gas density (mass/volume),
P1 is the upstream gas pressure (force/area),
P2 is the downstream gas pressure (force/area), and
∑Kf are the excess head loss terms, including pipe entrances and exits, pipe lengths, and fittings (unitless).
The excess head loss terms ∑Kf are found using the 2-K method presented in Section 4-4. For most accidental discharges of gases, the flow is fully developed turbulent flow. Thus, for pipes, the friction factor is independent of the Reynolds number, and for fittings, Kf = K∞ and the solution is direct.
The gas expansion factor Yg in Equation 4-68 depends only on the heat capacity ratio of the gas γ and the frictional elements in the flow path ∑ Kf. An equation for the gas expansion factor for choked flow is obtained by equating Equation 4-68 to Equation 4-59 and solving for Yg. The result is
Yg= Ma1√γ∑Kf2(P1P1−P2)(4-69)
where Ma1 is the upstream Mach number.
The procedure to determine the gas expansion factor is as follows. First, the upstream Mach number Ma1 is determined using Equation 4-67. ∑Kf must be substituted for 4fL/d to include the effects of pipes and fittings. The solution is obtained by trial and error by guessing values of the upstream Mach number and determining whether the guessed value meets the equation objectives. This can be easily done using a spreadsheet.
The next step in the procedure is to determine the sonic pressure ratio, using Equation 4-64. If the actual ratio is greater than the ratio from Equation 4-64, then the flow is sonic or choked; the pressure drop predicted by Equation 4-64 is then used to continue the calculation. If the actual ratio is less than the ratio from Equation 4-64, then the flow is not sonic and the actual pressure drop ratio is used. Finally, the expansion factor Yg is calculated from Equation 4-69.
The calculation to determine the expansion factor can be completed once γ and the frictional loss terms ∑Kf are specified. This computation can be done once and for all with the results shown in Figures 4-13 and 4-14. As shown in Figure 4-13, the pressure ratio (P − P2)/P1 is a weak function of the heat capacity ratio γ. The expansion factor Yg has little dependence on γ, with the value of Yg varying by less than 1% from the value at γ = 1.4 over the range from γ = 1.2 to γ = 1.67. Figure 4-14 shows the expansion factor for γ = 1.4.

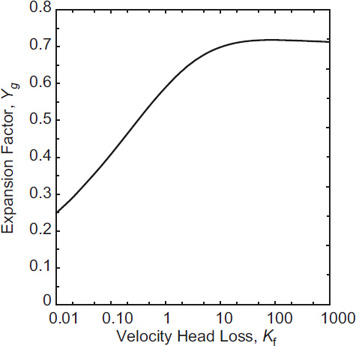
The functional results of Figures 4-13 and 4-14 can be fitted using an equation of the form ln Yg = A(ln Kf)3 + B(ln Kf)2 + C(ln Kf) + D, where A, B, C, and D are constants. The results are shown in Table 4-4 and are valid for the Kf ranges indicated, within 1%.
Table 4-4 Correlations for the Expansion Factor Yg and the Sonic Pressure Drop Ratio (P1 − P2)/P1 as a Function of the Pipe Loss ΣKf for Adiabatic Flow Conditionsa
Expansion Factor: ln Yg=A(ln Kf)3+B(ln Kf)2+C(ln Kf)+DPressure Drop Ratio: [(P1−P2/P1)−1=A+B ln Kf+C/K0.5f
Function value |
Range of validity, ∑Kf |
A |
B |
C |
D |
|---|---|---|---|---|---|
Expansion factor, Yg |
0.2−1000 |
0.00129 |
−0.0216 |
0.116 |
−0.528 |
Sonic pressure drop ratio, γ = 1.2 |
0.01−1000 |
0.943 |
0.00762 |
1.12 |
– |
Sonic pressure drop ratio, γ = 1.4 |
0.2−1000 |
0.965 |
0.00461 |
0.944 |
– |
Sonic pressure drop ratio, γ = 1.67 |
0.01−1000 |
0.988 |
0.00113 |
0.768 |
– |
aJ. Keith and D. A. Crowl. “Estimating Sonic Gas Flow Rates in Pipelines,” Journal of Loss Prevention in the Process Industries 18 (2005): 55–62.
The procedure to determine the adiabatic mass flow rate through a pipe or hole is as follows:
Given M, γ based on the type of gas; pipe length, diameter, and type; pipe entrances and exits; total number and type of fittings; total pressure drop; and upstream gas density.
Assume fully developed turbulent flow to determine the friction factor for the pipe and the excess head loss terms for the fittings and pipe entrances and exits. The Reynolds number can be calculated at the completion of the calculation to check this assumption. Sum the individual excess head loss terms to get ∑Kf.
Calculate (P1 − P2)/P1 from the specified pressure drop. Check this value against Figure 4-13 to determine whether the flow is sonic. All areas above the curves in Figure 4-13 represent sonic flow. Determine the sonic choking pressure P2 by using Figure 4-13 directly, interpolating a value from the table, or using the equations provided in Table 4-4.
Determine the expansion factor from Figure 4-14. Either read the value off of the figure, interpolate it from the table, or use the equation provided in Table 4-4
Calculate the mass flow rate using Equation 4-68. Use the sonic choking pressure determined in step 3 in this expression
This method is applicable to gas discharges through piping systems and holes.
Isothermal Flows
Isothermal flow of gas in a pipe with friction is shown in Figure 4-15. For this case, the gas velocity is assumed to be well below the sonic velocity of the gas. A pressure gradient across the pipe provides the driving force for the gas transport. As the gas expands through the pressure gradient, the velocity must increase to maintain the same mass flow rate. The pressure at the end of the pipe is equal to the pressure of the surroundings. The temperature is constant across the entire pipe length.

Isothermal flow is represented by the mechanical energy balance in the form shown in Equation 4-54. The following assumptions are valid for this case:
ggc dz = 0
is valid for gases, and, by combining Equations 4-29 and 4-30 and differentiating,
dF = 2fˉu2gcd dL
assuming constant f, and
δWS = 0
because no mechanical linkages are present. A total energy balance is not required because the temperature is constant.
By applying the assumptions to Equation 4-54 and manipulating them considerably, we obtain12
12Octave Levenspiel. Engineering Flow and Heat Exchange, 2nd ed. (New York, NY: Springer, 1998), p. 46.
T2 = T1(4-70)
P2P1 = Ma1Ma2(4-71)
ρ2ρ1 = Ma1Ma2(4-72)
G=ρˉu=Ma1P1√γgcMRgT(4-73)
where G is the mass flux with units of mass/(area-time), and
2 lnMa2Ma1−1γ(1Ma21−1Ma22) + 4fLd = 0Kinetic Compressibility Friction(4-74)
The various energy terms in Equation 4-74 have been identified.
A more convenient form of Equation 4-74 is expressed in terms of pressure instead of Mach numbers. This form is derived using Equations 4-70 through 4-72. The result is
2 lnP1P2−gcMG2RgT(P12−P22)+4fLd= 0(4-75)
A typical problem is to determine the mass flux G given the pipe length (L), inside diameter (d), and upstream and downstream pressures (P1 and P2). The procedure is as follows:
Determine the Fanning friction factor f using Equation 4-34. This assumes fully developed turbulent flow at high Reynolds numbers. This assumption can be checked later but is usually valid.
Compute the mass flux G from Equation 4-75.
Levenspiel13 showed that the maximum velocity possible during the isothermal flow of gas in a pipe is not the sonic velocity, as in the adiabatic case. In terms of the Mach number, the maximum velocity is
13Octave Levenspiel. Engineering Flow and Heat Exchange, 2nd ed. (New York, NY: Springer, 1998), p. 46.
Machoked = 1√γ(4-76)
This result is shown by starting with the mechanical energy balance and rearranging it into the following form:
−dPdL=2fG2gcρd[11−(ˉu2ρ/gcP)]=2fG2gcρd(11−γMa2)(4-77)
The quantity – (dP/dL)→ ∞ when Ma→1/√γ. Thus, for choked flow in an isothermal pipe, as shown in Figure 4-16, the following equations apply:
Tchoked = T1(4-78)
PchokedP1 = Ma1√γ(4-79)
ρchokedρ1=Ma1√γ(4-80)
ˉuchokedˉu1 = 1Ma1√γ(4-81)
Gchoked = ρˉu = ρ1ˉu1 = Ma1P1√γgcMRgT = Pchoked√gcMRgT(4-82)
where Gchoked is the mass flux with units of mass/(area-time), and
ln(1γMa21)−(1γMa21−1)+4fLd=0(4-83)
For most typical problems, the pipe length (L), inside diameter (d), upstream pressure (P1), and temperature (T) are known. The mass flux G is determined using the following procedure:
Determine the Fanning friction factor using Equation 4-34. This assumes fully developed turbulent flow at high Reynolds numbers. This assumption can be checked later but is usually valid.
Determine Ma1 from Equation 4-83.
Determine the mass flux G from Equation 4-82.
The direct method using Equations 4-68 and 4-69 can also be applied to isothermal flows. Table 4-5 provides the equations for the expansion factor and the pressure drop ratio. Figures 4-17 and 4-18 are plots of these functions. The procedure is identical to the procedure for adiabatic flows.


Table 4-5 Correlations for the Expansion Factor Yg and the Sonic Pressure Drop Ratio (P1 − P2)/P1 as a Function of the Pipe Loss ΣKf for Isothermal Flow Conditionsa
Expansion Factor:ln Yg=A(ln Kf)3+B(lnkf)2+C(lnKf)+DPressure Drop Ratio:[(P1−P2)/P1]−1=A+B ln kf+C/K0.5f
Function value |
Range of validity, ∑Kf |
A |
B |
C |
D |
|---|---|---|---|---|---|
Expansion factor Yg |
0.2−1000 |
0.00130 |
−0.0216 |
0.111 |
−0.502 |
Sonic pressure drop ratio, all γ |
0.01−1000 |
0.911 |
0.0118 |
1.38 |
− |
aJ. Keith and D. A. Crowl. “Estimating Sonic Gas Flow Rates in Pipelines,” Journal of Loss Prevention in the Process Industries 18 (2005): 55–62.
Keith and Crowl14 found that for both the adiabatic and isothermal cases, the expansion factor Yg exhibits a maximum value. For isothermal flows, this maximum is the same for all values of the heat capacity ratio γ and occurs at a velocity head loss of 56.3 with a maximum expansion factor of 0.7248. For adiabatic flows, the maximum value of the expansion factor is a function of the heat capacity ratio γ. For γ = 1.4, the expansion factor has a maximum value of 0.7182 at a velocity head loss of 90.0.
14J. Keith and D. A. Crowl. “Estimating Sonic Gas Flow Rates in Pipelines,” Journal of Loss Prevention in the Process Industries 18 (2005): 55–62.
For both the adiabatic and isothermal flow cases, the expansion factor approaches an asymptote as the velocity head loss becomes large. This asymptote, 1/√2 = 0.7071, is the same for both the adiabatic and isothermal flow cases. Comparison of detailed calculations with the asymptotic solution show that for velocity head loss values of 100 and 500, the differences between the detailed and asymptotic solutions are 2.2% and 0.2%, respectively.
The asymptotic solution can be inserted into Equation 4-68 to result in the following simplified equation for the mass flow:
G=˙mA=√gcρ1P1∑Kf(4-84)
For gas releases through pipes, the issue of whether the release occurs adiabatically or isothermally is important. For both cases, the velocity of the gas increases because of the expansion of the gas as the pressure decreases. For adiabatic flows, the temperature of the gas may increase or decrease, depending on the relative magnitude of the frictional and kinetic energy terms. For choked flows, the adiabatic choking pressure is less than the isothermal choking pressure. For real pipe flows from a source at a fixed pressure and temperature, the actual flow rate is less than the adiabatic prediction and greater than the isothermal prediction.
Example 4-5 shows that for pipe flow problems, the difference between the adiabatic and isothermal results is generally small. Levenspiel15 showed that the adiabatic model always predicts a flow larger than the actual flow, provided that the source pressure and temperature are the same. Crane16 reported that adiabatic conditions are valid for short pipes of constant cross-sectional are discharging into a pipe or area of larger cross-section. Crane supported this conclusion with experimental data on pipes having lengths of 130 and 220 pipe diameters discharging air to the atmosphere. Finally, under choked sonic flow conditions, isothermal conditions are difficult to achieve practically because of the rapid speed of the gas flow. As a result, the adiabatic flow model is the model of choice for compressible gas discharges through pipes.
15Octave Levenspiel. Engineering Flow and Heat Exchange, 2nd ed. (New York, NY: Springer, 1998), p. 45.
16Crane Co. Flow of Fluids through Valves, Fittings, and Pipes, Technical Paper 410 (New York, NY: Crane Co., 2009). www.flowoffluids.com.
Example 4-5
The vapor space above liquid ethylene oxide (EO) in storage tanks must be purged of oxygen and then padded with 81 psig nitrogen to prevent explosion. The nitrogen in a particular facility is supplied from a 200 psig source. It is regulated to 81 psig and supplied to the storage vessel through 33 ft of new commercial steel pipe with an internal diameter of 1.049 in. The temperature is 80°F.
In the event that the nitrogen regulator fails, the vessel will be exposed to the full 200 psig pressure from the nitrogen source. This will exceed the pressure rating of the storage vessel. To prevent rupture of the storage vessel, it must be equipped with a relief device to vent this nitrogen. Determine the required minimum mass flow rate of nitrogen through the relief device to prevent the pressure from rising within the tank in the event of a regulator failure.
Determine the mass flow rate assuming (a) an orifice with a throat diameter equal to the pipe diameter, (b) an adiabatic pipe, and (c) an isothermal pipe. Decide which result most closely corresponds to the real situation. Which mass flow rate should be used?
Solution
The maximum flow rate through the orifice occurs under choked conditions. The area of the pipe is
A=πd24=(3.14)(1.049 in)2(1 ft2/144 in2)4 =6.00 × 10−3 ft2
The absolute pressure of the nitrogen source is
P1=200 + 14.7=214.7 psia=3.09×104 lbf/ft2
The choked pressure from Equation 4-49 is, for a diatomic gas,
Pchoked=(0.528)(214.7 psia)=113.4 psia=1.63×104 1bf/ft2
Choked flow can be expected because the system is venting to atmospheric conditions. Equation 4-50 provides the maximum mass flow rate. For nitrogen, γ = 1.41 (Table 4-3) and
(2γ+1)(γ+1)/(γ−1) = (22.41)2.41/0.41 = 0.335
The molecular weight of nitrogen is 28 lbm/lb-mol. Without any additional information, assume a unit discharge coefficient C0=1.0. Thus
Qm= (1.0)(6.00 × 10−3 ft2)× (3.09 × 104 1bf/ft2) × √(1.41)(32.17 ft 1bm/1bf s2)(28 1bm/1b-mole)(1545 ft 1bf/1b-moleoR)(540oR)(0.335)= (185 1bf)√5.08 × 10−4 lb2m/1b2f s2Qm= 4.17 1bm/s
Assume adiabatic choked flow conditions. For commercial steel pipe, from Table 4-1, ε = 0.046 mm. The diameter of the pipe in millimeters is (1.049 in) (25.4 mm/in) = 26.6 mm. Thus
εd=0.046 mm26.6 mm=0.00173
From Equation 4-34,
As stated previously for nitrogen, γ = 1.41.
The upstream Mach number is determined from Equation 4-67:
with Y1 given by Equation 4-56. Substituting the numbers provided gives
This equation is solved by trial and error or a solver program or spreadsheet for the value of Ma. The results are tabulated as follows:
Guessed Ma
Value of left-hand side of equation
0.20
−8.34
0.25
0.128
0.249
0.0093
This last guessed Mach number gives a result close to zero. Then, from Equation 4-56,
and from Equations 4-63 and 4-64,
The pipe outlet pressure must be less than 48.9 psia to ensure choked flow. The mass flux is computed using Equation 4-66:
The simplified procedure with a direct solution can also be used. The excess head loss resulting from the pipe length is given by Equation 4-30. The friction factor f has already been determined:
For this solution, only the pipe friction will be considered and the exit effects will be ignored. The first consideration is whether the flow is sonic. The sonic pressure ratio is given in Figure 4-13 (or the equations in Table 4-4). For γ = 1.41, Kf = 8.52 and P2 = 214.7 psia:
It follows that the flow is sonic because the downstream pressure is less than 49.4 psia. From Figure 4-14 (or Table 4-4), the gas expansion factor Yg = 0.69. The gas density under the upstream conditions is
By substituting this value into Equation 4-68 and using the choking pressure determined for P2, we obtain
This result is very close to the previous result, but requires a lot less effort to obtain.
For the isothermal case, the upstream Mach number is given by Equation 4-83. Substituting the numbers provided, we obtain
The solution is found by trial and error:
Guessed Ma |
Value of left-hand side of equation |
|---|---|
0.25 |
0.601 |
0.24 |
−0.282 |
0.245 |
0.174 |
0.244 |
0.085 |
0.243 |
−0.005← Final result |
The choked pressure is, from Equation 4-79,
The mass flow rate is computed using Equation 4-82:
Using the simplified, direct solution, from Table 4-5 or Figure 4-15,
It follows that the flow is sonic. From Table 4-5 or Figure 4-16, Yg = 0.70. Substituting into Equation 4-68, and remembering to use the choking pressure given earlier, gives . This is also very close to the more detailed method.
The results are summarized in the following table:
Case |
Pchoked (psia) |
Qm (lbm/s) |
|---|---|---|
a. Orifice |
113.4 |
4.17 |
b. Adiabatic pipe |
48.9 |
1.80 |
c. Isothermal pipe |
62.0 |
1.76 |
A standard procedure for these types of problems is to represent the discharge through the pipe as an orifice. The results obtained earlier show that this approach results in a large mass flow rate for this case. The orifice method always produces a larger value than the adiabatic pipe method, ensuring a conservative safety design. The orifice calculation, however, is easier to apply, requiring only the pipe diameter and the upstream supply pressure and temperature. The configurational details of the piping are not required, as in the adiabatic and isothermal pipe methods.
Also note that the computed choked pressures differ for each case, with a substantial difference between the orifice and the adiabatic and isothermal cases. A choking design based on an orifice calculation might not be choked in reality because of high downstream pressures.
Both the adiabatic and isothermal pipe methods produce results that are reasonably close. For most real-world situations, the heat transfer characteristics cannot be easily determined. Thus, the adiabatic pipe method is the method of choice; it will always produce the larger number for a conservative safety design.
Finally, the simplified adiabatic and isothermal methods produce almost the same results as the more detailed approach.
4-7 Flashing Liquids
Liquids stored under pressure above their normal boiling point temperature present substantial problems because of flashing. If the tank, pipe, or other containment device develops a leak, the liquid will partially flash into vapor, sometimes explosively.
Flashing occurs so rapidly that the process is assumed to be adiabatic. The excess energy contained in the superheated liquid vaporizes the liquid and lowers the temperature to the new boiling point. If m is the mass of original liquid, Cp is the heat capacity of the liquid (energy/mass degrees), To is the initial temperature of the liquid before depressurization, and Tb is the depressurized boiling point of the liquid, then the excess energy contained in the superheated liquid is given by
This energy vaporizes the liquid. If ΔHv is the heat of vaporization of the liquid, the mass of liquid vaporized mv is given by
The fraction of the liquid vaporized is
Equation 4-87 assumes constant physical properties over the temperature range To to Tb. A more general expression without this assumption is derived as follows. The change in liquid mass m resulting from a change in temperature T is given by
Equation 4-88 is integrated between the initial temperature To (with liquid mass m) and the final boiling point temperature Tb (with liquid mass m − mv):
where and are the mean heat capacity and the mean latent heat of vaporization, respectively, over the temperature range To to Tb. Solving for the fraction of the liquid vaporized, fv = mv/m, we obtain
Example 4-6
One lbm of saturated liquid water is contained in a vessel at 350°F. The vessel ruptures and the pressure is reduced to 1 atm. Compute the fraction of material vaporized using (a) the steam tables,
(b) Equation 4-87, and (c) Equation 4-91.
Solution
The initial state is saturated liquid water at To = 350°F. From the steam tables:
The final temperature is the boiling point at 1 atm, or 212°F. At this temperature and under saturated conditions, from the steam tables,
Because the process occurs adiabatically, Hfinal = Hinitial and the fraction of vapor (or quality) is computed from
That is, 14.6% of the mass of the original liquid is vaporized.
For liquid water at 212°F,
From Equation 4-87,
The mean properties for liquid water between To and Tb are
Substituting into Equation 4-91 gives
The two methods using the equations produce results that are comparable to the actual value from the steam table. This agreement is not expected as one nears the critical temperature since the properties of the steam are changing rapidly.
For flashing liquids composed of many miscible substances, the flash calculation is complicated considerably, because the more volatile components flash preferentially. Procedures are available to solve this problem.17
17J. M. Smith and H. C. Van Ness. Introduction to Chemical Engineering Thermodynamics, 6th ed. (New York, NY: McGraw-Hill, 2000).
Flashing liquids escaping through holes and pipes require special consideration because two-phase flow conditions may be present. Several special cases may arise.18 If the fluid path length of the release is short (through a hole in a thin-walled container), nonequilibrium conditions exist, and the liquid does not have time to flash within the hole; instead, the fluid flashes external to the hole. The equations describing incompressible fluid flow through holes apply in this scenario (see Section 4-2).
18Hans K. Fauske. “Flashing Flows or: Some Practical Guidelines for Emergency Releases.” Plant/ Operations Progress (July 1985): 133.
If the fluid path length through the release is greater than 10 cm (through a pipe or thick-walled container), equilibrium flashing conditions are achieved and the flow is choked. A good approximation is to assume a choked pressure equal to the saturation vapor pressure of the flashing liquid. The result will be valid only for liquids stored at a pressure higher than the saturation vapor pressure. With this assumption the mass flow rate is given by
where
A is the area of the release,
Co is the discharge coefficient (unitless),
ρf is the density of the liquid (mass/volume),
P is the pressure within the tank, and
Psat is the saturation vapor pressure of the flashing liquid at ambient temperature.
Example 4-7
Liquid ammonia is stored in a tank at 24°C and a pressure of 1.4 × 106 Pa. A pipe of diameter 0.0945 m breaks off a short distance from the tank, allowing the flashing ammonia to escape. The saturation vapor pressure of liquid ammonia at this temperature is 0.968 × 106 Pa, and its density is 603 kg/m3. Determine the mass flow rate through the leak. Equilibrium flashing conditions can be assumed.
Solution
Equation 4-92 applies for the case of equilibrium flashing conditions. Assume a discharge coefficient of 0.61. Substituting into Equation 4-92,
For liquids stored at their saturation vapor pressure, P = Psat, and escaping through a longer pipe, the liquid flashes in the pipe as the pressure in the pipe decreases. Equation 4-92 is no longer valid. An example of this would be liquid propane stored under its own vapor pressure escaping to atmospheric pressure through a pipe section. A much more detailed approach is required for this case.
Consider a fluid that is initially quiescent and is accelerated through the leak. Assume that kinetic energy is dominant and that potential energy effects are negligible. Then, from a mechanical energy balance (Equation 4-1), and realizing that the specific volume (with units of volume/mass) v = 1/ρ, we can write
A mass velocity G with units of mass/(area-time) is defined by
Combining Equation 4-94 with Equation 4-93 and assuming that the mass velocity is constant results in
Solving for the mass velocity G and assuming that point 2 can be defined at any point along the flow path, we obtain
Equation 4-96 contains a maximum, at which choked flow occurs. Under choked flow conditions, dG/dP = 0. Differentiating Equation 4-96 and setting the result equal to zero gives
Solving Equation 4-98 for G, we obtain
The two-phase specific volume is given by
where
vfg is the difference in specific volume between vapor and liquid,
vf is the liquid specific volume, and
fv is the mass fraction of vapor.
Differentiating Equation 4-100 with respect to pressure gives
But, from Equation 4-87,
and from the Clausius–Clapeyron equation, at saturation
Substituting Equations 4-103 and 4-102 into Equation 4-101 yields
The mass flow rate is determined by combining Equation 4-104 with Equation 4-99:
Note that the temperature T in Equation 4-105 is the absolute temperature from the Clausius–Clapeyron equation and is not associated with the heat capacity.
Small droplets of liquid also form in a jet of flashing vapor. These aerosol droplets are readily entrained by the wind and transported away from the release site. A frequently made assumption in such circumstances is that the quantity of droplets formed is equal to the amount of material flashed.19
19Trevor A. Kletz. “Unconfined Vapor Cloud Explosions.” In Eleventh Loss Prevention Symposium (New York, NY: American Institute of Chemical Engineers, 1977).
Example 4-8
Propylene is stored at 25°C in a tank at its saturation pressure. A 1-cm-diameter hole develops in the tank below the liquid level. Estimate the mass flow rate through the hole under these conditions for propylene:
Solution
Equation 4-105 applies to this case. The area of the leak is
Using Equation 4-105, we obtain
4-8 Liquid Pool Evaporation or Boiling
The case for evaporation of a volatile from a pool of liquid has already been considered in Chapter 3. The total mass flow rate from the evaporating pool is given by Equation 3-12, reproduced here as Equation 4-106:
where
Qm is the mass vaporization rate (mass/time),
M is the molecular weight of the pure material,
K is the mass transfer coefficient (length/time),
A is the area of exposure (area),
Psat is the saturation vapor pressure of the liquid (force/area),
Rg is the ideal gas constant, and
TL is the temperature of the liquid (degree).
For liquids boiling from a pool, the boiling rate is limited by the heat transfer from the surroundings to the liquid in the pool. Heat is transferred (1) from the ground by conduction, (2) from the air by conduction and convection, and (3) by radiation from the sun and/or adjacent sources such as a fire.
The initial stage of boiling is usually controlled by the heat transfer from the ground. This is especially true for a spill of liquid with a normal boiling point below ambient temperature or ground temperature. The heat transfer from the ground is modeled with a simple one–dimensional heat conduction equation, given by
where
qg is the heat flux from the ground (energy/area-time),
ks is the thermal conductivity of the soil (energy/length-time-degree),
Tg is the temperature of the soil (degree),
T is the temperature of the liquid pool (degree),
αs is the thermal diffusivity of the soil (area/time), and
t is the time after spill (time).
Equation 4-107 is not considered conservatiave.
The rate of boiling is determined by assuming that all the heat transferred is used to boil the liquid. Thus
where
Qm is the mass boiling rate (mass/time),
qg is the heat transfer for the pool from the ground, determined by Equation 4-107 (energy/area-time),
A is the area of the pool (area), and
ΔHv is the heat of vaporization of the liquid in the pool (energy/mass).
At later times, solar heat fluxes and convective heat transfer from the atmosphere become important. For a spill onto an insulated dike floor, these fluxes may be the only energy contributions. This approach seems to work adequately for liquefied natural gas (LNG) and perhaps for ethane and ethylene. The higher hydrocarbons (C3 and above) require a more detailed heat transfer mechanism. This model also neglects possible water freezing effects in the ground, which can significantly alter the heat transfer behavior. More details on boiling pools are provided elsewhere.20
20Center for Chemical Process Safety. Guidelines for Consequence Analysis of Chemical Releases (New York, NY: American Institute of Chemical Engineers, 1999).
4-9 Realistic and Worst-Case Releases
Table 4-6 lists a number of realistic and worst-case releases. The realistic releases represent the incident outcomes with a high probability of occurring. Thus, rather than assuming that an entire storage vessel fails catastrophically, it is more realistic to assume that a high probability exists that the release will occur from the disconnection of the largest pipe connected to the tank. The worst-case releases are those that assume almost catastrophic failure of the process, resulting in near-instantaneous release of the entire process inventory or release over a short period of time.
Table 4-6 Guidelines for Selection of Process Incidents
Incident characteristic |
Guideline |
|---|---|
Realistic release incidentsa |
|
Process pipes |
Rupture of the largest diameter process pipe as follows: |
|
For diameters smaller than 2 in (5.08 cm), assume a full-bore rupture. |
|
For diameters 2–4 in (5.08–10.16 cm), assume rupture equal to that of a 2-in (5.08 cm) diameter pipe. |
|
For diameters greater than 4 in (10.16 cm), assume rupture area equal to 20% of the pipe cross-sectional area. |
Hoses |
Assume full-bore rupture. |
Pressure relief devices relieving directly to the atmosphere |
Use calculated total release rate at set pressure. Refer to pressure relief calculation. All material released is assumed to be airborne. |
Vessels |
Assume a rupture based on the largest-diameter process pipe attached to the vessel. Use the pipe criteria. |
Other |
Incidents can be established based on the plant’s experience, or the incidents can be developed from the outcome of a review or derived from hazard analysis studies. |
Worst-case incidentsb |
|
Quantity |
Assume release of the largest quantity of substance handled on-site in a single process vessel at any time. To estimate the release rate, assume the entire quantity is released within 10 min. |
Wind speed/stability |
Assume F stability, 1.5 m/s wind speed, unless meteorological data indicate otherwise. See Chapter 5. |
Ambient temperature/humidity |
Assume the highest daily maximum temperature and average humidity. |
Height of release |
Assume that the release occurs at ground level. |
Topography |
Assume urban or rural topography, as appropriate. |
Temperature of release substance |
Consider liquids to be released at the highest daily maximum temperature, based on data for the previous three years, or at process temperature, whichever is higher. Assume that gases liquefied by refrigeration at atmospheric pressure are released at their boiling points. |
aDow’s Chemical Exposure Index Guide (New York, NY: American Institute of Chemical Engineers, 1994).
bU.S. Environmental Protection Agency. RMP Offsite Consequence Analysis Guidance (Washington, DC: U.S. Environmental Protection Agency, 1996).
The selection of the release case depends on the requirements of the consequence study. If an internal company study is being completed to determine the actual consequences of plant releases, then the realistic cases would be selected. However, if a study is being completed to meet the requirements of the U.S. EPA Risk Management Plan, then the worst-case releases must be used.
4-10 Conservative Analysis
All models, including consequence models, have uncertainties. These uncertainties arise because of (1) an incomplete understanding of the geometry of the release (that is, the hole size), (2) unknown or poorly characterized physical properties, (3) a poor understanding of the chemical or release process, and (4) unknown or poorly understood mixture behavior, to name a few factors.
Uncertainties that arise during the consequence modeling procedure are treated by assigning conservative values to some of these unknowns. By doing so, a conservative estimate of the consequence is obtained, defining the limits of the design envelope. This ensures that the resulting engineering design to mitigate or prevent the hazard is overdesigned. Every effort, however, should be made to achieve a result consistent with the demands of the problem.
For any particular modeling study, several receptors might be present that require different decisions for conservative design. For example, dispersion modeling based on a ground-levelrelease will maximize the consequence for the surrounding community but will not maximize the consequence for plant workers at the top of a process structure.
To illustrate conservative modeling, consider a problem requiring an estimate of the gas discharge rate from a hole in a storage tank. This discharge rate is used to estimate the downwind concentrations of the gas, with the intent of estimating the toxicological impact. The discharge rate depends on a number of parameters, including (1) the hole area, (2) the pressures within and outside the tank, (3) the physical properties of the gas, and (4) the temperature of the gas.
The reality of the situation is that the maximum discharge rate of gas occurs when the leak first occurs, with the discharge rate decreasing as a function of time as the pressure within the tank decreases. The complete dynamic solution to this problem is difficult, requiring a mass discharge model cross-coupled to a material balance on the contents of the tank. An equation of state (perhaps nonideal) is required to determine the tank pressure given the total mass. Complicated temperature effects are also possible. A modeling effort of this detail is not necessarily required to estimate the consequence.
A much simpler procedure is to calculate the mass discharge rate at the instant the leak occurs, assuming a fixed temperature and pressure within the tank equal to the initial temperature and pressure. The actual discharge rate at later times will always be less, and the downwind concentrations will always be less. In this fashion, a conservative result is ensured.
For the hole area, a possible decision is to consider the area of the largest pipe connected to the tank, because pipe disconnections are a frequent source of tank leaks. Again, this maximizes the consequence and ensures a conservative result. This procedure continues until all model parameters are specified.
Unfortunately, this approach can result in a consequence that is many times larger than the actual outcome, leading to a potential overdesign of the safety systems. This occurs, in particular, if several decisions are made during the analysis, with each decision producing a maximum result. For this reason, consequence analysis should be approached with intelligence, tempered with a good dose of reality and common sense.
Suggested Reading
Consequence Modeling
Center for Chemical Process Safety. Guidelines for Consequence Analysis of Chemical Releases (New York, NY: American Institute of Chemical Engineers, 1999).
Center for Chemical Process Safety. Guidelines for Chemical Process Quantitative Risk Analysis (New York, NY: American Institute of Chemical Engineers, 2000).
Flow of Liquid through Holes
Sam Mannan, ed. Lees’ Loss Prevention in the Process Industries, 3rd ed. (London, UK: Butterworths, 2006), p. 15/6.
Flow of Liquid through Pipes
Ron Darby and Raj P. Chhabra. Chemical Engineering Fluid Mechanics, 3rd ed. (Boca Raton, FL: CRC Press, 2017).
Octave Levenspiel. Engineering Flow and Heat Exchange, 3rd ed. (New York, NY: Springer, 2014), ch. 2.
Warren L. McCabe, Julian C. Smith, and Peter Harriott. Unit Operations of Chemical Engineering, 7th ed. (New York, NY: McGraw-Hill, 2004), ch. 5.
E. Shashi Menon. Piping Calculations Manual (New York, NY: McGraw-Hill, 2005).
Flow of Vapor through Holes
Mannan. Loss Prevention, p. 15/10.
Levenspiel. Engineering Flow and Heat Exchange, ch. 3.
Flow of Vapor through Pipes
Jason Keith and Daniel A. Crowl. “Estimating Sonic Gas Flow Rates in Pipelines.” Journal of Loss Prevention in the Process Industries 18 (2005): 55–62.
Levenspiel. Engineering Flow and Heat Exchange, ch. 3.
Flashing Liquids
Steven R. Hanna and Peter J. Drivas. Guidelines for Use of Vapor Dispersion Models, 2nd ed. (New York, NY: American Institute of Chemical Engineers, 1996), pp. 24–32.
Mannan. Loss Prevention, p. 15/11.
Liquid Pool Evaporation and Boiling
Hanna and Drivas. Guidelines, pp. 31, 39.
Problems
4-1. An automobile tire is inflated to 3 atm gauge. What will be the initial gas discharge rate in kg/s due to a 1.0-mm-diameter hole? Assume a temperature of 30°C and an ambient pressure of 1 atm.
4-2. A pipeline of 300 m length supplies liquid chlorine from a regulated 20 barg source to a process through a horizontal, new commercial steel pipe of actual inside diameter of 2 cm. The ambient pressure is 1 atm and everything is at a temperature of 30°C.
If the pipe breaks off at the end of the 300 m length, estimate the flow in kg/s. Neglect entrance and exit effects and assume that the frictional loss is entirely due to the pipe length.
If the pipe breaks off at the regulated source, estimate the flow in kg/s.
For chlorine, the following properties are available:
Density: 1380 kg/m3
Viscosity: 0.328×10-3Pa-s
4-3. A round vented storage vessel with a 10-m inside diameter sits on top of a concrete pad within a 20-m2 diked area to contain any leaks. The tank contains runoff water from a process area. A leak of 10-cm diameter occurs on the side of the vessel located 1 m above the bottom. By the time the leaking flow was stopped, the liquid level in the diked area had reached a height of 0.79 m. Assume pure water for this calculation.
Calculate the total amount of water spilled into the dike in m3 and kg.
What was the original liquid level in the tank, in m?
If the liquid level in the tank at the end of the spill was 8.5 m above the bottom of the tank, estimate the length of time for the leak, in minutes.
4-4. Air flows from a regulated pressure source at 100 bara through a 100-m-long pipe into the atmosphere at 1atm pressure and 25°C. The pipe is new commercial steel pipe with an actual inside diameter of 0.5 cm. The pipe is horizontal. Neglect entrance and exit effects.
Determine if the flow is choked for both the adiabatic and isothermal cases.
Estimate the mass flow in kg/s for the adiabatic case.
For air, the molecular weight is 29.
Problems 4-5 through 4-7 involve liquid cyclohexane, C6H12. For liquid cyclohexane at 25°C, the density is 778.1 kg/m3 and the viscosity is 0.00102 Pa-s. The molecular weight is 84.16.
4-5. Cyclohexane liquid at 10 barg flows through a 1-cm-diameter hole to atmospheric pressure. Estimate the discharge rate if the ambient pressure is 1 atm.
4-6. A vertical cylindrical storage vessel is 10 m high and 2 m in diameter. The vessel contains liquid cyclohexane currently at a liquid level of 8 m. The vessel is vented to the atmosphere. A 1-cm-diameter hole develops in the bottom of the vessel. Calculate and plot the discharge rate of cyclohexane as a function of time until the vessel is completely empty. Show that the time to empty is the same as the time calculated using Equation 4-21.
4-7. Cyclohexane flows through 100 m of horizontal pipe. The pipe is 3-cm ID commercial steel pipe with light rust. The upstream supply pressure is 7 barg and the downstream pressure is 1 atm. Estimate the flow rate, in kg/s, through the pipe.
4-8. Oxygen flows through a 1-cm diameter hole in a storage vessel to atmospheric pressure. The ambient temperature is 298 K.
What minimum pressure in the tank is required to guarantee choked flow through the hole?
If the gas is stored in the vessel at 2 atm, what is the mass flow rate?
4-9. Natural gas flows from a source at 100 bara through 100 m of 0.5-cm ID new commercial steel pipe to atmospheric pressure. The pipe is horizontal. Determine if this flow is choked for the adiabatic and isothermal cases.
4-10. For sonic flow through a hole, the mass flow rate is a function of the upstream pressure only. This can be used to design a flow meter with the unique feature that the flow is not affected by downstream pressure fluctuations.
A laboratory experiment requires a constant mass flow rate of methane of 0.1 g/s. What is the largest hole diameter (in mm) required to just produce sonic conditions at this flow rate? Of course, sonic flow conditions will occur at any hole diameter smaller than this.
Assume the methane is available at a regulated pressure of 10 barg, an ambient pressure of 1 atm and a temperature of 25°C.
4-11. The TLV-TWA for hydrogen sulfide gas is 10 ppm. Hydrogen sulfide gas is stored in a tank at 100 psig and 80°F. Estimate the diameter of a hole in the tank leading to a local hydrogen sulfide concentration equal to the TLV. The local ventilation rate is 2000 ft3/min and is deemed average. The ambient pressure is 1 atm.
4-12. The morning inspection of the tank farm finds a leak in the turpentine tank. The leak is repaired. An investigation finds that the leak was 0.1 in. in diameter and 7 ft above the tank bottom. Records show that the turpentine level in the tank was 17.3 ft before the leak occurred and 13.0 ft after the leak was repaired. The tank diameter is 15 ft. Determine (a) the total amount of turpentine spilled, (b) the maximum spill rate, and (c) the total time the leak was active. The density of turpentine at these conditions is 55 lbm/ft3.
4-13. A home hot water heater contains 40 gal of water. Because of a failure of the heat control, heat is continuously applied to the water in the tank, increasing the temperature and pressure. Unfortunately, the relief valve is clogged and the pressure rises past the maximum pressure of the vessel. At 250 psig, the tank ruptures. Estimate the quantity of water flashed after the tank failure.
4-14. The distillation column in the Michigan Tech Unit Operations Lab distills a mixture of ethanol and water. The ethanol product is delivered to tank T-107 for temporary storage. The tank is a vertical cylinder in geometry with a cone-shaped bottom, as shown in Figure 4-19. A 2-in. schedule 40 pipe is connected to the bottom of the tank to pump the tank back to the raw material tank. We want to determine the consequences due to the rupture of the pipe at the very bottom of the vessel and spillage and evaporation of the ethanol.
Typically, during normal operation, tank T-107 is approximately 20% full. However, for our purposes, we will assume a more conservative 50% full. Also, the ethanol is about 90% in purity, but we will consider the more conservative case of 100% ethanol.

Supplemental information:
Ethanol: C2H6O
Specific gravity of ethanol: 0.7893
2-in. schedule 40 pipe: ID = 2.067 in., OD = 2.375 in.
Use Table 4-6 to determine an appropriate pipe diameter for the realistic release case of the pipe rupture at the base of the tank. Should you use the ID or OD of the pipe?
What is the initial discharge rate when the pipe initially ruptures? The tank is padded with nitrogen at a regulated pressure of 4 in. of water gauge.
If the initial discharge rate is maintained, how long will it take to drain all of the material from the tank? If the total draining time is less than 10 min, then the release can be considered instantaneous. Is this the case?
If an ethanol pool forms on the floor with an estimated liquid depth of 1 cm, what is the pool area, in ft2?
Estimate the evaporation rate of the ethanol from the pool, in lbm/s. Assume a temperature of 80°F and an ambient pressure of 1 atm.
Estimate the concentration of ethanol vapors in the UO lab (in ppm) from this evaporation. Assume a UO lab volume of 74,300 ft3 and a ventilation rate of 6 air changes per hour.
4-15. A 31.5% hydrochloric acid solution is pumped from one storage tank to another. The power input to the pump is 2 kW and is 50% efficient. The pipe is plastic PVC pipe with an internal diameter of 50 mm. At a certain time the liquid level in the first tank is 4.1 m above the pipe outlet. Because of an accident, the pipe is severed between the pump and the second tank, at a point 2.1 m below the pipe outlet of the first tank. This point is 27 m in equivalent pipe length from the first tank. Compute the flow rate (in kg/s) from the leak. The viscosity of the solution is 1.8 × 10–3 kg/m s, and the density is 1600 kg/m3.
4-16. One accident mitigation procedure is called emergency material transfer, in which the material is transported away from the accident site before it becomes involved. We plan on mitigating a crude oil tank fire scenario by pumping the tank empty in 1 hr total time.
The crude oil storage tank is 30 m in diameter, and the crude oil is typically at a level of 9 m. The transfer will be accomplished by pumping the crude oil through a 200-mm (internal diameter) new commercial steel pipe to another tank 40 m in diameter and 10 m high. The pipeline represents 50 m of equivalent pipe.
Estimate the minimum pump size (in HP) required to pump the entire tank empty in 1 hr. Assume a pump efficiency of 80%.
If a 100-HP pump (80% efficient) is available, how long will it take to empty the tank?
What conclusions can be drawn about the viability of this approach?
The density of the crude oil is 928 kg/m3 with a viscosity of 0.004 kg/m s.
4-17.
Show that for any pump the maximum liquid discharge velocity is given by
where u is the maximum liquid discharge velocity, Ws is the pump shaft work, ρ is the density of the liquid, and A is the pump outlet discharge area. Make sure you list your assumptions in your solution.
A 1-kW pump discharges water through a 50-mm (internal diameter) pump outlet. What is the maximum velocity of the liquid from this pump? What is the maximum discharge rate (in kg/s)?
Additional homework problems are available in the Pearson Instructor Resource Center.
