Chapter 3. Industrial Hygiene
The learning objectives for this chapter are:
Learn the four steps in an industrial hygiene study.
Learn the Globally Harmonized System (GHS) for Safety Data Sheets (SDS) and labels.
Evaluate the magnitude of exposures and responses.
Develop and evaluate control techniques to prevent exposures.
Learn to use the National Fire Protection Association (NFPA) diamond.
Industrial hygiene is the science devoted to the anticipation, identification, evaluation, and control of occupational conditions that cause sickness and injury. Such exposures may involve chemicals, dusts, noise, and thermal radiation, to name a few possible conditions.
Typical projects involving industrial hygiene are monitoring toxic airborne vapor concentrations, reducing toxic airborne vapors through the use of ventilation, selecting proper personal protective equipment, developing procedures for the handling of hazardous materials, and monitoring and reducing noise, heat, thermal radiation, and other physical factors to ensure that workers are not exposed to harmful levels.
Chemical process technology is so complex that preventing hazardous workplace exposures requires the concerted efforts of industrial hygienists, engineers, process designers, operators, laboratory personnel, and management. The industrial hygienist works closely with these personnel as an integral part of a safety and loss prevention program. Engineers are very likely to work with industrial hygienists so they must have a basic understanding of industrial hygiene to be an effective member of the team.
After identifying and evaluating the hazards, the industrial hygienist makes recommendations relevant to control methods. The industrial hygienist and plant personnel work together to ensure that the control measures are applied and maintained.
The major responsibility of the industrial hygienist is to prevent hazardous workplace exposures. This is done using four traditional steps in an industrial hygiene study:
Anticipation: expectation of the presence of workplace hazards and worker exposures
Identification: determination of the presence of worker exposures
Evaluation: determination of the magnitude of the exposure
Control: application of appropriate technology to reduce workplace exposures to acceptable levels
3-1 Anticipating and Identifying Hazardous Workplace Exposures
Anticipating potential exposures takes into account potential hazards, entry modes of toxicants into the human body, and potential human injury. Table 3-1 lists some of these potential hazards; note that it is not inclusive.
Table 3-1 Identification of Some Potential Hazards
Potential hazards |
|
Toxicity |
Flammability |
Chemical reactivity |
Noise |
Thermal radiation |
Ionizing radiation |
Mechanical |
Temperature, either high or low |
Entry mode of toxicants |
|
Inhalation |
Ingestion |
Skin absorption |
Injection |
Potential human injury |
|
Lungs |
Skin |
Ears |
Eyes |
Nervous system |
Liver |
Kidneys |
Reproductive organs |
Circulatory system |
Other organs |
Industrial hygiene studies are only as good as the information available. Table 3-2 provides a list of potential information that would be valuable for an industrial hygiene study. Of course, this information must be up to date and reliable. Other information will likely be required depending on the specific situation.
Table 3-2 Data Useful for an Industrial Hygiene Study
Chemicals |
Physical state: solid, liquid, or gas Threshold limit values (TLVs), permissible exposure levels (PELs) Odor threshold for vapors Vapor pressure of liquids |
Chemical reactions |
Reaction rate Heat of reaction Hazardous properties of by-products Reactivity with other chemicals Thermal stability Sensitivity of chemical to temperature or impact |
Flammability |
Flammability limits Flash point temperature Auto-ignition temperature (AIT) Minimum explosible concentration (MEC) for dusts Minimum ignition energy (MIE) |
Physical conditions |
Noise Heat Humidity Thermal radiation Ionizing radiation |
Equipment |
Mechanical hazards Operating limits Design specifications Plant layout Materials of construction |
Note: This list will vary based on the particular situation.
The anticipation step involves understanding the potential hazards that may exist in the workplace. For example, the industrial hygienist could develop a list of all chemicals used in a plant. However, only some of these chemicals will have hazardous properties, and even fewer will be handled or used in such a way to result in a hazardous exposure.
The identification step determines whether hazardous exposures actually exist. These hazards are numerous, and they can also act in combination. Table 3-1 provides a list of some potential hazards that are commonly found during the identification step of industrial hygiene projects.
One approach to identify the presence of chemical vapors in the workplace is to list the odor thresholds for the chemicals present (Table 3-3). Individuals vary greatly with respect to odor detection so variability in the actual levels is expected. Also, some chemicals, such as methyl ethyl ketone, anesthetize the olfactory organs with continued exposure, reducing the ability to detect the odor. In many cases, the odor threshold is below the threshold limit value (TLV). For instance, chlorine has an odor threshold of 0.05 ppm, while its TLV is 0.5 ppm (see Appendix E). In this case, the odor is noticed at a concentration well below the TLV. For other chemicals, the reverse case is true: Ethylene oxide has an odor threshold of 851 ppm, while its TLV is 1 ppm. In this case, by the time the odor is detected, the exposure limit has been greatly exceeded.
Table 3-3 Odor Thresholds for Selected Chemicals
Chemical species |
Odor threshold (ppm) |
|---|---|
Acetaldehyde |
0.186 |
Acetic acid |
0.016 |
Acrolein |
0.174 |
Acrylic acid |
0.4 |
Acrylonitrile |
16.6 |
Ammonia |
5.75 |
Aniline |
0.676 |
Bromine |
0.066 |
Butane |
204 |
Butyraldehyde |
0.009 |
Camphor |
0.051 |
Chlorine |
0.05 |
Chloroform |
11.7 |
Cumene |
0.024 |
Diethylamine |
0.186 |
Ethyl alcohol |
0.136 |
Ethylamine |
0.324 |
Ethylene oxide |
851 |
Ethyl ether |
2.29 |
Ethyl mercaptan |
0.001 |
Fluorine |
0.126 |
Hydrogen chloride |
0.77 |
Hydrogen sulfide |
0.0005 |
Isopropyl ether |
0.055 |
Methyl alcohol |
141 |
Methylene chloride |
0.912 |
Methyl ethyl ketone (MEK) |
0.27 |
Methyl isocyanate |
2.1 |
Methyl mercaptan |
0.001 |
Ozone |
0.061 |
Phenol |
0.011 |
Phosgene |
0.55 |
Styrene |
3.44 |
Toluene |
0.16 |
Trichloroethylene |
1.36 |
Vinyl acetate |
0.603 |
Vinyl chloride |
0.253 |
Source: Data from 3M Respirator Selection Guide (St. Paul, MN: 3M Corporation, 2010).
The evaluation step obtains actual data to characterize the exposure. This could include chemical vapor concentrations, noise levels, temperatures, and thermal radiation levels, among other information. The industrial hygienist is invaluable in helping with these measurements. The evaluation step is discussed in detail in Section 3-3.
Control is the final step in industrial hygiene studies. This step is undertaken after potential health hazards are identified and evaluated, and then appropriate control techniques are developed and installed to reduce workplace exposures. The control step is discussed in detail in Section 3-4.
3-2 Globally Harmonized System
The Globally Harmonized System (GHS)1 is an international system that the United Nations created for the unified development of Safety Data Sheets (GHS SDS) and for unified label development for substances and mixtures (GHS label). The GHS is intended to be a worldwide system that all countries can use to identify the hazardous properties of chemicals and to provide unified labeling to facilitate shipping chemicals between countries.
1 Globally Harmonized System of Classification and Labelling of Chemicals (GHS), 7th rev. ed., Part 1, 2017. www.unece.org/trans/danger/publi/ghs/ghs_rev07/07files_e0.html.
In 2013, the U.S. Occupational Safety and Health Administration (OSHA) adopted the GHS for classification and labeling of chemicals.2 The new GHS requirements are as follows:
2 A Guide to The Globally Harmonized System of Classification and Labeling of Chemicals (GHS) (The Purple Book), pp. 40, 47. www.osha.gov/dsg/hazcom/ghsguideoct05.pdf.
Hazard classification: The standard requires chemical manufacturers and importers to determine the hazards associated with the chemicals they produce or import. Information on the specific health and physical hazards of these chemicals must be provided to the consumer.
GHS SDS: The standard requires SDSs to have 16 specific sections, ensuring consistency in the presentation of important safety information about a chemical.
GHS labels: Chemical labels must include the name of the chemical as well as a signal word, pictograms, hazard statements, and precautionary statements that describe the hazards associated with the chemical.
Globally Harmonized System for Safety Data Sheets
The SDS has 16 sections that give a clear description of the hazards—that is, classify the hazards of substances and mixtures according to their health, environmental, and physical hazards. The sections of the SDS are as follows:
Identification
Hazard identification
Composition/information on ingredients
First aid measure
Firefighting measures
Accidental release measures
Handling and storage
Exposure controls/personal protection
Physical and chemical properties and safety characteristics
Stability and reactivity
Toxicological information
Ecological information
Disposal consideration
Transport information
Regulatory information
Other informatio.
Sections 1 through 8 provide general information about the chemical. This includes the chemical’s name, hazards, composition, safe handling practices, and emergency response measures. These first eight sections are helpful when information is required quickly in an emergency situation.
Sections 9 through 11 and section 16 contain other technical and scientific information, such as the chemical’s physical and chemical properties, information about the chemical’s stability and reactivity, toxicological information, and the date the safety data sheet was prepared or last revised.
Sections 12 through 15 contain information related to the chemical’s effect on the environment, its proper disposal, transport of the chemical, and additional regulations governing its use. These sections are considered “nonmandatory” by OSHA because the content of these sections is enforced by other federal and state government agencies.
The minimum required information for the 16 sections of the GHS SDS is identified in the “Purple Book.”3 A detailed description of the GHS SDS and actual SDSs for chemicals are readily available on the Internet.
3Occupational Safety and Health Administration. “Purple Book” www.osha.gov/dsg/hazcom/ghsguideoct05.pdf.
The GHS includes nine pictograms and 29 hazard classes, as shown in Table 3-4. The hazard classes include 17 physical hazard classes, 10 health hazard classes and 2 environmental hazard classes.
Table 3-4 GHS Pictograms and Hazard Classes
Group pictograms |
||
Health Hazard
|
Flammability
|
Compressed Gas
|
Corrosive
|
Explosive
|
Oxidizers
|
Environmental
|
Acute Toxicity
|
Other Hazards
|
Hazard classes – www.un.org for specific details on each class. |
||
Physical Hazards (17 classes) |
Explosives Flammable gases Flammable liquids – see Table 3-5 Flammable solids Aerosols Gases under pressure Self-reactive substances Pyrophoric liquids Pyrophoric solids Self-heating substances |
Substances which, in contact with water emit flammable gases Oxidizing gases Oxidizing liquids Oxidizing solids Organic peroxides Corrosive to metals Desensitized explosives |
Health Hazards (10 classes) |
Acute toxicity (oral / dermal / inhalation) – see Table 3-6 Skin corrosion / irritation Serious eye damage / eye irritation Respiratory or skin sensitization Germ cell mutagenicity Carcinogenicity Reproductive toxicology Target organ systemic toxicity – single exposure Target organ systemic toxicity – repeated exposure Aspiration toxicity |
|
Environmental Hazards (2 classes) |
Hazardous to aquatic environment (acute / chronic) Hazardous to ozone layer |
|
Each of the 29 hazard classes has a detailed specification for that class. Table 3-5 provides the specification for flammable liquids and Table 3-6 provides the specification for acute toxicity – inhalation. Refer to the Purple Book for specifications on the other hazard classes.
Table 3-5 GHS SDS Information for One Hazard Class: Flammable Liquid
Signal word |
GHS hazard statement/criteria |
GHS hazard category |
pictogram |
|---|---|---|---|
Danger |
Extremely flammable liquid and vapor Flash point < 23°C, boiling point < 35°C |
1 |
|
Danger |
Highly flammable liquid and vapor Flash point < 23°C, boiling point > 35°C |
2 |
|
Warning |
Flammable liquid and vapor 23°C < Flash point < 60°C |
3 |
|
Warning |
Combustible liquid 60°C <Flash point < 93°C |
4 |
No symbol |
Table 3-6 GHS SDS Information for Hazard Class: Acute Toxicity – Inhalation
Signal word |
GHS hazard statement/criteria |
GHS hazard category |
pictogram |
|---|---|---|---|
Danger |
Fatal If Inhaled: Dusts and mists: LC50 < 0.05 mg/L Gases: LC50 < 100 ppm Vapors: LC50 < 0.5 mg/L |
1 |
|
Danger |
Fatal If Inhaled: Dusts and mists: 0.05 mg/L < LC50 < 0.5 mg/L Gases: 100 ppm < LC50 < 500 ppm Vapors: 0.5 mg/L < LC50 < 2 mg/L |
2 |
|
Danger |
Toxic If Inhaled: Dusts and mists: 0.5 mg/L < LC50 < 1 mg/L Gases: 500 ppm < LC50 < 2500 ppm Vapors: 2 mg/L < LC50 < 10 mg/L |
3 |
|
Warning |
Harmful If Inhaled: Dusts and mists: 1 mg/L < LC50 < 5 mg/L Gases: 2500 ppm < LC50 < 5000 ppm Vapors: 2 mg/L < LC50 < 10 mg/L |
4 |
|
Warning |
No Specified Label Elements: Dusts and mists: LC50 > 5 mg/L Gases: LC50 > 5000 ppm Vapors: LC50 > 20 mg/L |
5 |
No symbol |
LC50 50% lethal concentration
Each hazard class specification has four elements: signal word, classification, hazard number, and pictogram, as shown in Figures 3-4 and 3-5. The hazard classes are numbered from 1 to 4 or 5, with the number 1 denoting the most severe hazard. As an example, gasoline has a signal word of “Danger” and a GHS hazard category of 2, while diesel fuel has a signal word of “Warning” and a GHS hazard category of 4 (see Table 3-5).
Example 3-1
A survey of a laboratory identifies the following chemical species: sodium chloride, sodium hydroxide (0.5M), and phenol. Using online GHS SDSs, identify for each chemical: (a) two hazard classes with corresponding categories, (b) signal words, (c) two hazard statements, (d) two precautionary statements, and (e) the manufacturer’s information. Note that a chemical may have more than two hazard classes.
Solution
SDS information for each chemical can be found on the Internet. The following table summarizes the results:
Chemical |
Description and potential hazard |
|---|---|
Sodium chloride |
|
Sodium hydroxide (0.5M) |
|
Phenol |
|
Globally Harmonized System for Labeling
The GHS label has six elements, as shown in Figure 3-1. The label format is not specified in the GHS; however, it is recommended that the product name or identifier, signal word, and the GHS pictograms should be located together on the label.4-7

4A Guide to The Globally Harmonized System of Classification and Labeling of Chemicals (GHS) (The Purple Book), pp. 33–38. www.osha.gov/dsg/hazcom/ghsguideoct05.pdf.
5Occupational Safety and Health Administration. “Labels.” www.osha.gov/Publications/OSHA3636.pdf.
6“Six Elements of GHS Label.” www.bradyid.com/en-us/applications/ghs-labeling-requirements.
7U.S. Department of Transportation. “Hazardous Materials Regulations.” https://hazmatonline.phmsa.dot.gov/services/publication_documents/howtouse0507.pdf.
Six Elements of the GHS Label
Although the SDS is the major source of information about the hazardous properties of the chemical, the GHS label is designed to give the worker the most important information related to safe handling of the chemical. Each GHS label includes six elements:
Product name or identifier. This provides information on the chemical substance, or mixture, that is in the container.
Signal word. There are only two words used as signal words: “Danger” and “Warning.” “Danger” is used for the most severe circumstances, and “warning” for less severe conditions
GHS pictograms. Refer to Table 3-4
Hazards statements. These phrases describe the nature of the hazardous material and the degree of the hazard. Hazardous statements include “highly flammable liquid and vapor,” “may be fatal if swallowed and enters airways,” “causes skin irritation,” “causes serious eye irritation,” and “may cause cancer,” to name a few.
Precautionary statements/first aid. These instructions (and/or pictograms) are intended to prevent or minimize the effects of exposure to the hazardous product. Examples include special instructions (from manufacturer) before use—for example, “do not handle until all safety precautions have been read and understood,” “keep away from heat/sparks/open flames/hot surfaces,” “no smoking in the area,” “wear protective gloves/eye protection/face protection,” and “if swallowed—immediately call a poison center or doctor.”
Manufacturer information. This element identifies the manufacturer’s company name, address, and telephone number.
Additional label regulations apply when shipping materials by ground, air, or water transportation.8
8U.S. Department of Transportation. “Hazardous Materials Regulations.” https://hazmatonline.phmsa.dot.gov/services/publication_documents/howtouse0507.pdf.
Primary and Secondary Containers
Primary containers are the bags, barrels, bottles, and cans that are received from the manufacturer. These containers should have GHS labels. The supplier labels cannot be removed, altered, or defaced. If the label needs to be replaced, then the new label must contain the same information as the original.
Secondary containers are usually smaller than primary containers; for example, they may include spray bottles, jugs, and jars. These containers hold the material taken from the primary container. Secondary containers must comply with the GHS label requirements except when the following criteria are met: (1) the material is used within the work shift of the person making the transfer, (2) the worker making the transfer remains in the work area the entire time during use, and (3) the container stays within the work area and in the possession of the worker who filled the container. These requirements are so rigorous that labeling of all chemicals at all times is the best approach.
3-3 Evaluate the Magnitude of Exposures and Responses
The industrial hygiene evaluation phase determines the extent and degree of employee exposure to toxicants and physical hazards in the workplace environment. The various types of existing control measures and their effectiveness are also studied during this phase. (The industrial hygiene control techniques are presented in more detail in Section 3-4.)
During the evaluation study, the likelihood of large and small leaks must be considered. Sudden exposures to high concentrations, through large leaks, may lead to immediate acute effects, such as unconsciousness, burning eyes, or fits of coughing. There is rarely lasting damage to individuals if they are removed promptly from the contaminated area. In this case, ready access to a clean environment is important.
Chronic effects, however, arise from repeated exposures to low concentrations, mostly by small leaks or volatilization of solid or liquid chemicals. Many toxic chemical vapors are colorless and odorless (or the toxic concentration might be below the odor threshold). Small leaks of these substances might not become obvious for months or even years, but such exposures may lead to permanent and serious impairments. Special attention must be directed toward preventing and controlling low concentrations of toxic gases. In these circumstances, some provision for continuous evaluation is necessary; that is, continuous or frequent periodic sampling and analysis is important.
To establish the effectiveness of existing controls, samples are taken to determine the workers’ exposure to conditions that may be harmful. If problems are evident, controls must be implemented immediately; temporary controls such as personal protective equipment can be used in these circumstances. Longer-term and permanent controls, however, must be subsequently developed.
After the exposure data are obtained, it is necessary to compare actual exposure levels to acceptable occupational health standards, such as TLVs, PELs, or IDLH concentrations. These standards, together with the actual concentrations, are used to identify the potential hazards requiring improved or additional control measures.
Evaluating Exposures to Volatile Toxicants by Monitoring
A direct method for determining worker exposures is continuous monitoring of the air concentrations of toxicants online in a work environment. For continuous concentration data C(t), the TWA (time-weighted average) concentration is computed using the equation
where
C(t) is the concentration (in ppm or mg/m3) of the chemical in the air and
tw is the worker’s shift time in hours.
The integral is always divided by 8 hours, independent of the length of time actually worked in the shift. Thus, if a worker is exposed for 12 hours to a concentration of chemical equal to the TLV-TWA, then the TLV-TWA has been exceeded, because the computation is normalized to 8 hours.
Continuous monitoring is rarely performed because most facilities do not have the necessary equipment available. Instead, intermittent samples are typically obtained, representing worker exposures at fixed points in time. If we assume that the concentration Ci is fixed (or averaged) over the period of time ti, the TWA concentration is computed by
All chemical monitoring methods have drawbacks because (1) the workers move in and out of the exposed workplace, (2) the concentration of toxicants may vary at different locations in the work area, and (3) the concentrations will change with time depending on work activities. Industrial hygienists play an important role in the selection and placement of workplace monitoring equipment and the interpretation of the data.
If more than one chemical is present in the workplace, one procedure is to assume that the effects of the toxicants are additive (unless other information to the contrary is available). The combined exposures from multiple toxicants with different TLV-TWAs is determined with the equation
where
n is the total number of toxicants,
Ci is the concentration of chemical i with respect to the other toxicants, and (TLV-TWA)i is the TLV-TWA for chemical species i.
If the sum in Equation 3-3 exceeds 1, then the workers are overexposed.
The mixture TLV-TWA can be computed with the equation
If the sum of the concentrations of the toxicants in the mixture exceeds this amount, then the workers are overexposed.
For mixtures of toxicants with different effects (such as an acid vapor mixed with lead fumes), the TLVs cannot be assumed to be additive.
Example 3-2
Air contains 5 ppm of diethyl amine (TLV-TWA of 5 ppm), 20 ppm of cyclohexanol (TLV-TWA of 50 ppm), and 10 ppm of propylene oxide (TLV-TWA of 2 ppm). What is the mixture’s TLV-TWA, and has this level been exceeded?
Solution
From Equation 3-4,
The total mixture concentration is 5 + 20 + 10 = 35 ppm. The workers are overexposed under these circumstances.
An alternative approach is to use Equation 3-3:
Because this quantity is greater than 1, the TLV-TWA has been exceeded.
Example 3-3
Determine the 8-hour TWA worker exposure if the worker is exposed to toluene vapors as follows:
Duration of exposure (hr) |
Measured concentration (ppm) |
|---|---|
2 |
110 |
2 |
330 |
4 |
090 |
Solution
Using Equation 3-2,
Because the TLV for toluene is 20 ppm (Appendix E), the worker is overexposed. Additional control measures must be developed. On a temporary and immediate basis, all employees working in this environment need to wear the appropriate respirators.
Example 3-4
Determine the mixture TLV at 25°C and 1 atm pressure of a mixture derived from the following liquid:
Component |
Mole percent |
Species TLV (ppm) |
|---|---|---|
Heptane |
50 |
400 |
Toluene |
50 |
20 |
Solution
The solution requires the concentrations of the heptane and toluene in the vapor phase. Assuming that the composition of the liquid does not change as it evaporates (the quantity is large), the vapor composition is computed using standard vapor–liquid equilibrium calculations. Assuming that Raoult’s and Dalton’s laws apply to this system under these conditions, the vapor composition is determined directly from the saturation vapor pressures of the pure components. Saturation vapor pressure equations are provided in Appendix C. From these equations the saturation vapor pressure is calculated at 25°C:
Using Raoult’s law, the partial pressures in the vapor are determined:
The total pressure of the toxicants is (23.2+14.1) = 37.3 mm Hg. From Dalton’s law, the mole fractions on a toxicant basis are
The mixture’s TLV is computed using Equation 3-4:
The TLVs for the individual species in the mixture are
If the actual species concentrations exceed these levels, more control measures will be needed.
Evaluating Worker Exposures to Dusts
Dusts are also a contaminant that may cause health exposures. According to toxicological theory, the dust particles that present the greatest hazard to the lungs are normally in the respirable particle size range between 2 and 5 μm (see Chapter 2). Particles larger than 5 μm are usually unable to reach the lungs, whereas those smaller than 0.2 μm settle out too slowly and are mostly exhaled with the air.
The main reason for sampling for atmospheric particulates is to estimate the concentrations that are inhaled and deposited in the lungs. Sampling methods and the interpretation of data relevant to health hazards are relatively complex; industrial hygienists, who are specialists in this technology, should be consulted when confronted with this type of problem.
Dust evaluation calculations are performed in a manner identical to that used for volatile vapors. Instead of using ppm as a concentration unit, mg/m3 or mppcf (millions of particles per cubic foot) is more convenient.
Example 3-5
Determine the TLV for a uniform mixture of dusts containing the following particles:
Type of dust |
Concentration (wt.%) |
TLV (mppcf) |
|---|---|---|
Dust A |
70 |
20 |
Dust B |
30 |
2.7 |
Solution
From Equation 3-4:
Special control measures will be required when the actual particle count (of the size range specified in the standards or by an industrial hygienist) exceeds 6.8 mppcf.
Evaluating Worker Exposures to Noise
Noise problems are common in chemical plants; this type of problem is also evaluated by industrial hygienists. If a noise problem is suspected, the industrial hygienist should immediately make the appropriate noise measurements and develop recommendations.
Noise levels are measured in decibels. A decibel (dB) is a relative logarithmic scale used to compare the intensities of two sounds. If one sound is at intensity I and another sound is at intensity Io, then the difference in intensity levels in decibels is given by
Thus, a sound 10 times as intense as another has an intensity level 10 dB.
An absolute sound scale (in dBA for absolute decibels) is defined by establishing an intensity reference. For convenience, the hearing threshold is set at 0 dBA. Table 3-7 contains dBA levels for a variety of common activities.
Table 3-7 Sound Intensity Levels for a Variety of Common Activities
Source of noise |
Sound intensity level (dB) |
|---|---|
Riveting (painful) |
120 |
Punch press |
110 |
Passing truck |
100 |
Factory |
90 |
Noisy office |
80 |
Conventional speech |
60 |
Private office |
50 |
Average residence |
40 |
Recording studio |
30 |
Whisper |
20 |
Threshold of good hearing |
10 |
Threshold of excellent youthful hearing |
0 |
Permissible noise exposure levels for single sources are provided in Table 3-8. Noise evaluation calculations are performed identically to calculations for vapors, except that dBA is used instead of ppm and hours of exposure is used instead of concentration.
Table 3-8 Permissible Noise Exposures
Sound level (dBA) |
Maximum exposure (hr/day) |
|---|---|
85 |
16 |
88 |
10.5 |
90 |
8 |
91 |
7 |
92 |
6 |
94 |
4.8 |
95 |
4 |
97 |
3 |
100 |
2 |
102 |
1.5 |
105 |
1 |
110 |
0.5 |
115 |
0.25 |
Source: “Permissible Noise Exposures,” www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=9735.
Example 3-6
Determine whether the following noise level is permissible with no additional controls:
Noise level (dBA) |
Duration (hr) |
Maximum allowed (hr) |
|---|---|---|
85 |
3.6 |
16 |
95 |
3.0 |
4 |
110 |
0.5 |
0.5 |
Solution
From Equation 3-3:
Because the sum exceeds 1.0, employees in this environment are immediately required to don hearing protection. On a longer-term basis, noise reduction control methods should be developed for the specific pieces of equipment producing excessive noise levels.
Evaluating Worker Exposures to Thermal Radiation
Thermal radiation also impacts workers. In this case, the effect on the worker is a function of both the thermal radiation intensity and the time of the exposure. Table 3-9 shows the effects on workers and Table 3-10 shows the effects on various materials. The effects may vary considerably depending on actual circumstances.
Table 3-9 Effects of Thermal Radiation on Workers
Thermal radiation intensity (kW/m2) |
Time for severe pain (s) |
Time for second-degree burn (s) |
|---|---|---|
1 |
115 |
663 |
2 |
45 |
187 |
3 |
27 |
92 |
4 |
18 |
57 |
5 |
13 |
40 |
6 |
11 |
30 |
8 |
7 |
20 |
10 |
5 |
14 |
12 |
4 |
11 |
Source: Handbook of Chemical Hazard Analysis Procedures (Washington, DC: U.S. Federal Emergency Management Agency, U.S. Department of Transportation, and U.S. Environmental Protection Agency, 1988).
Table 3-10 Effects of Thermal Radiation on Materials
Thermal radiation intensity (kW/m2) |
Effects |
|---|---|
4 |
Glass ruined |
12 |
Plastic melting |
15 |
Wood firing, when there is an ignition source |
18-20 |
Destruction of thermal insulation |
25 |
Automatic firing of wood |
37.5 |
Damage to process equipment |
100 |
Damage and breaking of steel. |
Sources: F. P. Lees, Loss Prevention in the Process Industries (Oxford, UK: Butterworth-Heinemann, 1980);
Methods for the Determination of Possible Damage to People and Objects Resulting from Releases of Hazardous Materials, 1st ed. (Radarweg, Netherlands: TNO, 1992).
First-degree burns are the least severe. With these burns, the skin is red and painful and swells slightly. Second-degree burns have blisters and are painful. Third-degree burns damage all the layers of the skin and the skin looks white or charred; these burns may cause little or no pain if the nerves are damaged.
Example 3-7
Flares are used to burn off vapors from a plant. The flare burns the vapors using a flame at the top of a stack. One design criterion for a flare is that the thermal radiation intensity in the vicinity of the flare on the ground should not exceed 1500 BTU/hr ft2. What thermal effect will this have on the workers?
Solution
First convert to metric units:
From Table 3-9, this will cause second-degree burns in about 40 s, with severe pain occurring in 13 s. Access to the ground area around the flare must be controlled to prevent burns.
Estimating Worker Exposures to Toxic Vapors
The best procedure to determine exposures to toxic vapors is to measure the vapor concentrations directly. For design purposes, estimates of vapor concentrations are frequently required in enclosed spaces, above open containers, where drums are filled, and in the area of spills.
Consider the enclosed volume shown in Figure 3-2. This enclosure is ventilated by a constant-volume airflow. Volatile vapors are evolved within the enclosure. An estimate of the concentration of volatile in the air is required.

Where
C is the concentration of volatile vapor in the enclosure (mass/volume),
V is the volume of the enclosure (volume),
Qv is the ventilation rate (volume/time),
k is the nonideal mixing factor (unitless), and
Qm is the evolution rate of volatile material (mass/time).
The nonideal mixing factor k accounts for conditions in the enclosure that are less than well mixed. It follows that:
Total mass of volatile in volume =VC
Accumulation of mass of volatile
Mass rate of volatile material resulting from evolution =Qm
Mass rate of volatile material out = k QVC
Because accumulation equals mass-in minus mass-out, the dynamic mass balance on the volatile species is
At steady state, the accumulation term is 0, and Equation 3-6 is solved for C:
Equation 3-7 is converted to the more convenient concentration units of ppm by direct application of the ideal gas law. Let m represent mass, ρ represent mass density, and the subscripts v and b denote the volatile and bulk gas species, respectively. Then
where
Rg is the ideal gas constant,
T is the absolute ambient temperature,
P is the absolute pressure, and
M is the molecular weight of the volatile species.
The term mv/Vb is identical to the concentration of volatile computed using Equation 3-7. Substituting Equation 3-7 into Equation 3-8 yields
Equation 3-9 is used to determine the average concentration (in ppm) of any volatile species in an enclosure given a source term Qm and a ventilation rate Qv. It can be applied to the following types of exposures: a worker standing near a pool of volatile liquid, a worker standing near an opening to a storage tank, or a worker standing near an open container of volatile liquid.
Equation 3-9 includes the following important assumptions:
The calculated concentration is an average concentration in the enclosure. Localized conditions could result in significantly higher concentrations; workers directly above an open container might be exposed to higher concentrations.
A steady-state condition is assumed; that is, the accumulation term in the mass balance is zero.
The nonideal mixing factor varies from 0.1 to 0.5 for most practical situations.9 For perfect mixing, k = 1.
9R. Craig Matthiessen. “Estimating Chemical Exposure Levels in the Workplace.” Chemical Engineering Progress (April 1986): 30.
Example 3-8
An open toluene container in an enclosure is weighed as a function of time, and it is determined that the average evaporation rate is 0.1 g/min. The ventilation rate is 100 ft3/min. The temperature is 80°F and the pressure is 1 atm. Estimate the concentration of toluene vapor in the enclosure and compare your answer to the TLV for toluene of 20 ppm.
Solution
Because the value of k is not known directly, it must be used as a parameter. From Equation 3-9,
From the data provided
Substituting into the equation for kC ppm:
Because k varies from 0.1 to 0.5, the concentration is expected to vary from 18.9 ppm to 94.3 ppm. Actual vapor sampling is recommended to ensure that the TLV of 20 ppm is not exceeded.
Estimating the Vaporization Rate of a Liquid
Liquids with high saturation vapor pressures evaporate more rapidly. As a result, the evaporation rate (mass/time) is a function of the saturation vapor pressure. In reality, for vaporization into stagnant air, the vaporization rate is proportional to the difference between the saturation vapor pressure and the partial pressure of the vapor in the stagnant air; that is,
where
Psat is the saturation vapor pressure of the pure liquid at the temperature of the liquid and
p is the partial pressure of the vapor in the bulk stagnant gas above the liquid.
A more generalized expression for the vaporization rate is available:10
10Steven R. Hanna and Peter J. Drivas. Guidelines for the Use of Vapor Cloud Dispersion Models, 2nd ed. (New York, NY: American Institute of Chemical Engineers, 1996).
where
Qm is the evaporation rate (mass/time),
M is the molecular weight of the volatile substance,
K is a mass transfer coefficient (length/time) for an area A,
Rg is the ideal gas constant, and
TL is the absolute temperature of the liquid.
For many situations Psat>>p, and Equation 3-11 is simplified to
Equation 3-12 is used to estimate the vaporization rate of a volatile from an open vessel or from a spill of liquid.
The vaporization rate or source term, Qm, determined by Equation 3-12, is used in Equation 3-9 to estimate the concentration (in ppm) of a volatile in a ventilated enclosure resulting from evaporation of a liquid:
For most situations, T = TL and Equation 3-13 is simplified to
As defined previously, P is the absolute pressure.
The gas mass transfer coefficient is estimated using the relationship11
11Louis J. Thibodeaux. Environmental Chemodynamics, 2nd ed. (New York, NY: Wiley, 1996), p. 85.
where
a is a constant and
D is the gas-phase diffusion coefficient.
Equation 3-15 is used to determine the ratio of the mass transfer coefficients between the species of interest K and a reference species Ko:
The gas-phase diffusion coefficients are estimated from the molecular weights M of the species:12
12Gordon M. Barrow. Physical Chemistry, 2nd ed. (New York, NY: McGraw-Hill, 1966), p. 19.
Equation 3-17 is combined with Equation 3-16, giving
Water is most frequently used as a reference substance; it has a mass transfer coefficient13 of 0.83 cm/s.
13R. Craig Matthiessen. “Estimating Chemical Exposure Levels in the Workplace.” Chemical Engineering Progress (April 1986): 33.
Example 3-9
A tank with a 1.5-m diameter opening contains toluene, C7H8. Estimate the evaporation rate from this tank assuming a temperature of 25°C and a pressure of 1 atm. If the ventilation rate is 85 m3/min, estimate the concentration of toluene in this workplace enclosure.
Solution
The molecular weight of toluene is 92. The mass transfer coefficient is estimated from Equation 3-18 using water as a reference:
The saturation vapor pressure is given in Example 3-4:
The pool area is
The evaporation rate is computed using Equation 3-12:
The concentration is estimated using Equation 3-14 with k as a parameter:
The concentration will range from 446 ppm to 2230 ppm, depending on the value of k (ranging from 0.1 to 0.5). Because the TLV for toluene is 20 ppm, additional ventilation is recommended, or the amount of exposed surface area should be reduced. The amount of ventilation required to reduce the worst-case concentration (2230 ppm) to 20 ppm is
This represents an impractical level of general ventilation. Potential solutions to this problem include containing the toluene in a closed vessel or using local ventilation at the vessel opening.
Estimating Worker Exposures during Vessel Filling Operations
For vessels being filled with liquid, volatile emissions are generated from two sources, as shown in Figure 3-3:
Evaporation of the liquid, represented by Equation 3-14
Displacement of the vapor in the vapor space by the liquid filling the vessel
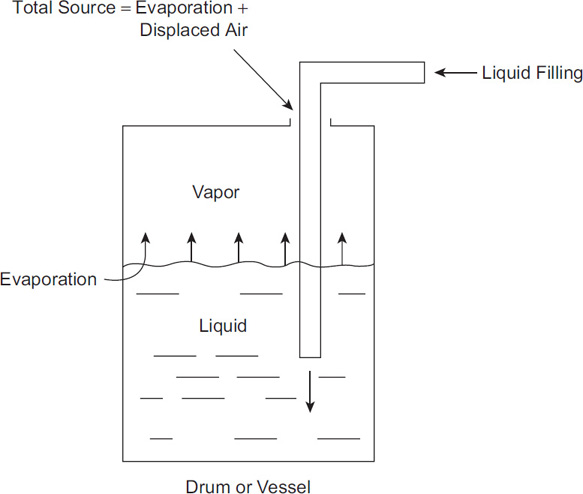
The net generation of volatile is the sum of the two sources:
where
(Qm)1 represents the source resulting from evaporation and
(Qm)2 represents the source resulting from displacement.
The source term (Qm)1 is computed using Equation 3-12. (Qm)2 is determined by assuming that the vapor is completely saturated with the volatile. An adjustment is introduced later for less than saturated conditions.
Define
Vc as the volume of the container (volume),
rf as the constant filling rate of the vessel (time−1),
Psat as the saturation vapor pressure of the volatile liquid, and
TL as the absolute temperature of the container and liquid.
It follows that rfVc is the volumetric rate of bulk vapor being displaced from the drum (volume/time). Also, if ρv is the density of the volatile vapor, then rfVc ρv is the mass rate of volatile displaced from the container (mass/time). Using the ideal gas law,
and it follows that
Equation 3-21 can be modified for container vapors that are not saturated with the volatile. Let φ represent this adjustment factor; then,
For splash filling (filling from the top of a container with the liquid splashing to the bottom), φ = 1. For subsurface filling14 (by a dip leg to the bottom of the tank), φ = 0.5.
14R. Craig Matthiessen. “Estimating Chemical Exposure Levels in the Workplace.” Chemical Engineering Progress (April 1986): 33.
The net source term resulting from filling is derived by combining Equations 3-12 and 3-22 with Equation 3-19:
This source term is substituted into Equation 3-9 to compute the vapor concentration (in ppm) in an enclosure resulting from a filling operation. The assumption that T = TL is also invoked. The result is
For many practical situations, the evaporation term KA is much smaller than the displacement term and can be neglected.
Example 3-10
Railroad cars are being splash-filled with toluene. The 10,000-gal cars are being filled at the rate of one every 8 hours. The filling hole in the tank car is 4 inches in diameter. Estimate the concentration of toluene vapor as a result of this filling operation. The ventilation rate is estimated at 3000 ft3/min. The temperature is 77°F and the pressure is 1 atm.
Solution
The concentration is estimated using Equation 3-24. From Example 3-9, K = 0.949 ft/min and Psat = 0.0371 atm. The area of the filling hole is
Thus
The filling rate rf
For splash filling, the nonideal filling factor ϕ is 1.0. The displacement term in Equation 3-24 is
As expected, the evaporation term is small compared to the displacement term. The concentration is computed from Equation 3-24, using k as a parameter:
The actual concentration could range from 69 ppm to 344 ppm, depending on the value of k. Sampling to ensure that the concentration is below 20 ppm is recommended. For subsurface filling, ϕ = 0.5, and the concentration range is reduced to 35–172 ppm.
With splash filling or filling with a dip pipe, if the addition rate is low, then the evaporation rate could be higher than the displacement rate.
3-4 Develop and Evaluate Control Techniques to Prevent Exposures
After potential industrial hygiene health hazards are identified and evaluated, the appropriate control techniques must be developed and installed. This requires the application of appropriate technology for reducing workplace exposures. The types of control techniques used in the chemical industry are described in Table 3-11.
Table 3-11 Industrial Hygiene Control Methods
Type and explanation |
Typical control techniques |
|---|---|
Inherently safer |
|
Eliminate or reduce hazard |
Eliminate chemical entirely. Reduce chemical inventories, including raw materials, intermediates, and products. Replace chemical with less hazardous chemical. Decrease temperature and pressure of chemical. Reduce pipeline size to reduce hold-up inventory. |
Enclosures |
|
Enclose room or equipment and place under negative pressure. |
Enclose hazardous operations such as sample points. Seal rooms, sewers, ventilation, and the like. Use analyzers and instruments to observe inside equipment. Shield high-temperature surfaces. |
Local ventilation |
|
Contain and exhaust hazardous substances. |
Use properly designed hoods. Use hoods for charging and discharging. Keep exhaust systems under negative pressure. Use ventilation at drumming station. Use local exhaust at sample points. |
Dilution ventilation |
|
Design ventilation systems to control low-level toxics. |
Design locker rooms with good ventilation and special areas or enclosures for contaminated clothing. Design filter press rooms with directional ventilation. Design ventilation to isolate operations from rooms and offices. |
Wet methods |
|
Use wet methods to minimize contamination with dusts. |
Clean vessels chemically versus sandblasting. Use water sprays for cleaning. Use water sprays to shield trenches or pump seals. Clean areas frequently. |
Good housekeeping |
|
Keep toxicants and dusts contained. |
Use dikes around tanks and pumps. Provide water and steam connections for area washing. Provide lines for flushing and cleaning. Provide well-designed sewer system with emergency containment. |
Personal protection |
|
As last line of defense |
Use aprons, arm shields, and space suits. Use safety glasses and face shields. Wear appropriate respirators; airline respirators are required when oxygen concentration is less than 19.5%. |
Designing control methods is an important and creative task. During the design process, the engineer must pay particular attention to ensure that the newly designed control technique provides the desired control and that the new control technique itself does not create another hazard, sometimes even more hazardous than the original problem.
The two major control techniques are environmental controls and personal protection. Environmental control reduces exposure by reducing the concentration of toxicants in the workplace environment. This includes enclosures, local ventilation, dilution ventilation, wet methods, and good housekeeping, as discussed previously.
Personal protection prevents or reduces exposure by providing a barrier between the worker and the workplace environment. This barrier is usually worn by the worker—hence the designation “personal.” Typical types of personal protective equipment (PPE) are listed in Table 3-12. The major problem with personal protection is that it frequently compromises the workers’ abilities to move and is frequently uncomfortable. As a result, PPE should always be considered as a last line of defense.
Table 3-12 Personal Protective Equipment, Not Including Respirators
Type |
Description |
|---|---|
Hard hat |
Protects head from falling equipment and bumps |
Safety glasses |
Impact-resistant lenses with side shields |
Chemical splash goggles, gas-tight |
Suitable for liquids and fumes |
Steel-toed safety shoes |
Protects feet against dropped equipment |
Wraparound face shield |
Provides additional face protection. Resistant to most chemicals |
Vinyl apron |
Resists most chemicals |
Splash suit |
Viton or butyl rubber for nonflammable exposures |
Umbilical cord suit |
Used with external air supply |
Rubber gloves over sleeves |
Protects forearms |
PVC-coated gloves |
Resists acids and bases |
PVC and nitrile knee boots |
Resists acids, oils, and greases |
Ear plugs |
Protects against high noise levels |
Respirators
Respirators are routinely found in chemical laboratories and plants. Respirators should be used only under the following conditions:
On a temporary basis, until regular control methods can be implemented
As emergency response equipment, to ensure worker safety in the event of an incident
As a last resort, in the event that environmental control techniques are unable to provide satisfactory protection
Respirators always compromise worker ability, so that a worker with a respirator is unable to perform or respond as well as a worker without one. Various types of respirators are listed in Table 3-13.
Table 3-13 Respirators Useful to the Chemical Industry
Exposure |
Type |
Limitations |
|---|---|---|
Dust |
Mouth and nose dust mask |
|
Chemical vapors |
Mouth and nose with chemical cartridge |
|
Chemical vapors |
Full face mask with chemical cartridge |
|
Chemical vapors and dusts |
Self-contained breathing apparatus (SCBA) |
|
Source: Information from following and National Institute for Occupational Safety and Health. “Respirator Selection Logic.” 2004. www.cdc.gov/niosh/docs/2005-100/
Selection guide: https://multimedia.3m.com/mws/media/639110O/3m-respirator-selection-guide.pdf
General information about masks: www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=12716
Gas mask description: www.osha.gov/dts/shib/respiratory_protection_bulletin_2011.html
Gas mask limitations: wwwn.cdc.gov/NIOSH-CEL/Limitations/G14#14G_1
Respirators can be used improperly and can be damaged to the extent that they do not provide the needed protection. OSHA and National Institute for Occupational Safety and Health (NIOSH) have developed standards for using respirators,15 including fit testing (to ensure that the device does not leak excessively), periodic inspections (to ensure that the equipment works properly), specified use applications (to ensure that the equipment is used for the correct job), training (to ensure that it is used properly), and record keeping (to ensure that the program is operating efficiently). All industrial users of respirators are legally bound to understand and fulfill these OSHA requirements. All respirators require training. Annual respirator medical exams are also required.
15National Institute for Occupational Safety and Health. “Respirator Selection Logic.” 2004. www.cdc.gov/niosh/docs/2005-100/.
Ventilation
For environmental control of airborne toxic material, the most common method is ventilation, for the following reasons:
Ventilation can quickly remove dangerous concentrations of flammable and toxic materials.
Ventilation can be highly localized, reducing the quantity of air moved and the equipment size.
Ventilation equipment is readily available and can be easily installed.
Ventilation equipment can be added to an existing facility.
The major disadvantage of ventilation is the operating cost. Substantial electrical energy may be needed to drive the potentially large fans, and the cost to heat or cool the large quantities of fresh air can be large. These operating costs need to be considered when evaluating alternatives.
Ventilation is based on two principles: (1) dilute the contaminant below the target concentration, and (2) remove the contaminant before workers are exposed. Thus, ventilation systems are composed of fans and ducts. The fans produce a small pressure drop (less than 0.1 psi) that moves the air. The best system is a negative-pressure system, in which the fans are located at the exhaust end of the system, pulling air out. This ensures that leaks in the dust system draw air in from the workplace rather than expel contaminated air from the ducts into the workplace. This arrangement is shown in Figure 3-4.

Two types of ventilation techniques are used: local and dilution ventilation.
Local Ventilation
The most common example of local ventilation is the hood. A hood is a device that either completely encloses the source of contaminant or moves the air in such a fashion as to carry the contaminant to an exhaust device. Several types of hoods are available:
An enclosed hood completely contains the source of contaminant.
An exterior hood continuously draws contaminants into an exhaust from some distance away.
A receiving hood is an exterior hood that uses the discharge motion of the contaminant for collection.
A push–pull hood uses a stream of air from a supply to push contaminants toward an exhaust system.
The most widely encountered enclosed hood is the laboratory hood, like the standard laboratory utility hood shown in Figure 3-5. Fresh air is drawn through the window area of the hood and is removed out the top through a duct. The airflow profiles within the hood are highly dependent on the location of the window sash. It is important to keep the sash open a few inches, at a minimum, to ensure adequate fresh air. However, the sash should never be fully opened because contaminants might escape. The baffle, which may be present at the rear of the hood, ensures that contaminants are removed from the working surface and the rear lower corner.

Another type of laboratory hood is the bypass hood, shown in Figure 3-6. In this design, bypass air is supplied through a grille at the top of the hood. This ensures the availability of fresh air to sweep out contaminants in the hood. The bypass air supply is reduced as the hood sash is opened.

Enclosed hoods offer the following advantages:
Completely eliminate exposure to workers
Require minimal airflow
Provide a containment device in the event of fire or explosion
Provide a shield to the worker by means of a sliding door on the hood
However, hoods also have some disadvantages:
Limit the workspace available
Can be used only for small, bench-scale or pilot plant equipment
Most hood calculations assume plug flow. For a duct of cross-sectional area A and average air velocity ū (distance/time), the volume of air moved per unit time Qv is computed as
For a rectangular duct of width W and length L, Qv is determined using the equation
Consider the simple box-type enclosed hood shown in Figure 3-7. The design strategy is to provide a fixed velocity of air at the opening of the hood. This face or control velocity (referring to the face of the hood) ensures that contaminants do not exit from the hood.

The required control velocity depends on the toxicity of the material, the depth of the hood, and the evolution rate of the contaminant. Shallower hoods need higher control velocities to prevent contaminants from exiting the front. However, experience has shown that higher velocities can lead to the formation of a turbulent eddy from the bottom of the sash; backflow of contaminated air is possible. For general operation, a control velocity between 80 and 120 feet per minute (fpm)—that is, 24.4 to 36.6 m3/min— is suggested.
The airflow velocity is a function of the sash height and the blower speed. Arrows are frequently used to indicate the proper sash height to ensure a specified face velocity. Instruments are available for measuring the airflow velocity at specific points of the hood window opening. Testing of this velocity is an OSHA requirement. Design equations are available for a wide variety of hood and duct shapes.16
16Industrial Ventilation: A Manual of Recommended Practice, 27th ed. (Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 2010).
Other types of local ventilation methods include “elephant trunks” and free-hanging canopies and plenums. The elephant trunk is simply a flexible vent duct with a diameter of 10 cm or more that is positioned near a source of contaminant. It is most frequently used for loading and unloading toxic materials from drums and vessels. Free-hanging canopies and plenums can be either fixed in position or attached to a flexible duct to enable movement. These methods will most likely expose workers to toxicants, but in diluted amounts.
Dilution Ventilation
If the contaminant cannot be placed in a hood and must be used in an open area or room, dilution ventilation is necessary. Unlike hood ventilation, where the airflow prevents worker exposure, dilution ventilation always exposes the worker to the chemical, but in amounts diluted by fresh air. Dilution ventilation always requires more airflow than local ventilation, so the operating expenses for such a system can be substantial.
Equations 3-9, 3-12, and 3-14 are used to compute the ventilation rates required. Table 3-14 1ists values for k, the nonideal mixing factor used with these equations.
Table 3-14 Nonideal Mixing Factor, k, for Various Dilution Ventilation Conditions
|
Vapor concentration (ppm) |
||
|---|---|---|---|
Ventilation condition |
0–100 |
101–500 |
More than 500 |
Poor |
1/11 |
1/8 |
1/7 |
Average |
1/8 |
1/5 |
1/4 |
Good |
1/7 |
1/4 |
1/3 |
Excellent |
1/6 |
1/3 |
1/2 |
For exposures to multiple sources, the dilution air requirement is computed for each individual source. The total dilution requirement is the sum of the individual dilution requirements.
The following restrictions should be considered before implementing dilution ventilation:
The contaminant must not be highly toxic.
The contaminant must be evolved at a uniform rate.
Workers must remain a suitable distance from the source to ensure proper dilution of the contaminant.
Scrubbing systems must not be required to treat the air before exhaust into the environment.
Example 3-11
Xylene is used as a solvent in paint. A certain painting operation evaporates an estimated 10 liters of xylene in an 8-hour shift. The ventilation quality is rated as average. Determine the quantity of dilution ventilation air required to maintain the xylene concentration below 100 ppm, the TLV-TWA. Also, compute the air required if the operation is carried out in an enclosed hood with an opening of 5 m2 and a face velocity of 30 m/min. The temperature is 25°C and the pressure is 1 atm. The specific gravity of xylene is 0.864, and its molecular weight is 106.
Solution
The evaporation rate of xylene is
From Table 3-14, for average ventilation and a vapor concentration of 100 ppm, k = 1/8 = 0.125. Using Equation 3-9, we solve for Qv:
For a hood with an open area of 5 m2, using Equation 3-25 and assuming a required control velocity of 30 m/min, we get
The hood requires significantly less airflow than dilution ventilation and prevents worker exposure completely.
3-5 National Fire Protection Association Diamond
Another method that is used to characterize the hazardous properties of chemicals is the National Fire Protection Association (NFPA) diamond. The NFPA is a professional society that was established in 1896 to reduce worldwide fatalities and injuries due to fires and other hazards. Its primary function is to promote consensus codes and standards, including the National Electrical Code (NEC).
The NFPA diamond frequently appears on chemical containers and storage vessels. The main purpose of the diamond is to provide a quick means for emergency response personnel to recognize the chemical hazards that they may face during a fire or other emergency. However, it is also useful for routine operations where hazards recognition is important. NFPA diamonds are frequently found on chemical containers and process storage vessels.
The NFPA diamond consists of four separate areas, as shown in Figure 3-8. These areas correspond to health, fire, stability, and special hazards. In the past, the word “reactivity” was used instead of “stability,” but chemical stability is a more accurate description of the hazard facing emergency response personnel during a fire incident.

The simplicity of the NFPA diamond is that the respective hazards are indicated by a number, with the number 0 representing minimal hazard and the number 4 representing the greatest hazard. Figure 3-8 shows how the numbers are assigned. Note that the NFPA hazard numbers are the reverse of the GHS system. Appendix E contains NFPA numbers for a variety of common chemicals.
Online Resources
National Institute for Occupational Safety and Health (NIOSH), www.cdc.gov/niosh.
U.S. Code of Federal Regulations, www.gpoaccess.gov.
U.S. Occupational Safety and Health Administration (OSHA), www.osha.gov.
Suggested Reading
Industrial Hygiene
Roger L. Brauer. Safety and Health for Engineers, 3rd ed. (NY: Wiley Interscience, 2016).
Richard J. Lewis, ed. Sax’s Dangerous Properties of Industrial Materials, 11th ed. (Hoboken, NJ: John Wiley, 2005).
Barbara A. Plog and Patricia J. Quinlan. Fundamentals of Industrial Hygiene, 6th ed. (Itasca, IL: National Safety Council, 2012).
Vernon E. Rose and Barbara Cohrssen. Patty’s Industrial Hygiene, 6th ed. (Hoboken, NJ: John Wiley, 2011).
Ventilation
Industrial Ventilation: A Manual of Recommended Practice, 29th ed. (Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 2016).
Problems
3-1. Search the Internet and
Print a GHS label for phenol. If you are unable to find a label, then develop your own label.
Develop a few conclusions concerning the process of finding labels.
3-2. Copy the GHS label pictograms for (a) oxidizers, (b) flammables, (c) explosives, (d) acute toxicity, (e) corrosives, and (f) carcinogens.
3-3. An air mixture contains 4 ppm of carbon tetrachloride, 25 ppm of 1,1-dichloroethane, 5 ppm of diethyl amine, 20 ppm of cyclohexanol, and 10 ppm of propylene oxide. Determine the mixture TLV and determine if this TLV has been exceeded.
3-4. An open container was monitored and found to have a toluene evaporation rate of 0.1 g/min. The ventilation rate is 2.84 m3/min, the temperature is 27°C, and the pressure is 1 atm. Estimate the concentration of toluene vapor in the enclosure and compare it to the TLV of 50 ppm. Perform the calculation for a k of 1.0 and 0.1.
3-5. An ideal liquid mixture of benzene and toluene (50% by volume each) is used in a plant at 27°C and 1 atm. Determine (a) the mixture TLV, (b) the evaporation rate per unit area for this mixture, and (c) the ventilation rate required to keep the concentration below the TLV if the liquid is contained in a drum with a bung diameter of 5.1 cm. Assume the ventilation quality within the vicinity is average.
3-6. A drum contains 0.16 m3 of toluene. If the lid is left open (lid diameter is 0.92 m), determine the
Time required to evaporate all of the toluene.
Concentration of toluene (in ppm) near the drum if the local ventilation rate is 28.34 m3/min. The temperature is 30°C and the pressure is 1 atm.
3-7. A railcar is being splash filled with toluene. The car is 38 m3 in volume, and the filling hole is 0.10 m in diameter. It takes 30 min to fill the rail car. Estimate the concentration of toluene in this vicinity if the ventilation rate is 85 m3/min. The temperature is 25°C and the pressure is 1 atm.
3-8. Xylene is used in a hood that has a face area of 4.65 m2, with a temperature of 25°C and pressure of 1 atm. The evaporation rate of xylene is 11.6 liters in 8 hours.
Determine the dilution ventilation required to keep the area concentration below the TLV-TWA.
Compare this rate to the recommended face velocity for hoods.
3-9. Determine the recommended air volumetric flow rate, in m3/min, that is required for a hood having face dimensions of 1.22 m (height) and 0.91 m (width).
3-10. If the hood in Problem 3-9 is used for handling trichloroethylene, determine the rate of air passing into the hood face to keep the concentration (a) below the TLV-TWA and (b) below the lower explosive limit. Then (c) compare these rates with the recommended rate shown in Problem 3-9. The temperature is 25°C and the pressure is 1 atm. In all cases, the evaporation rate is 0.04 kg/min.
3-11. Use Safety Data Sheets to determine the TLV and NFPA ratings for ethanol, chlorine, and phosgene.
3-12. A worker is exposed to multiple sound sources: . Derive the following equation for the net sound exposure from these multiple sources:
Two sound sources are at 90 dBA. What is the net exposure from these two sound sources?
3-13. Two liters of a liquid chemical is spilled on the laboratory floor. Assume 1 atm of pressure and a temperature of 25°C.
If the liquid pool is 2 mm deep, calculate the area of the pool.
What ventilation rate, in m3/s, is required to prevent the air concentration from exceeding the TLV for the chemical of 100 ppm?
Ventilation air is provided for the lab via a 0.5-m-square duct. The air velocity exiting the duct is measured with a velometer at 30 m/s. Calculate the volumetric flow of air from this duct in m3/s. Is this adequate ventilation for this spill?
Data for the liquid chemical:
Molecular weight: 100
Saturation vapor pressure of chemical at 25°C: 100 mm Hg
3-14. A large workshop uses a 2:1 mixture by volume of liquid xylene and hexane to strip varnish from antique furniture. A total of 15 liters is used per 8-hour shift. The workshop is maintained at 25°C and 1 atm and has a ventilation system that provides 1 m3/s of fresh air. Is the ventilation system adequate to maintain the concentrations below the TLV-TWAs?
Xylene: molecular weight: 106.16, specific gravity: 0.870, TLV-TWA: 100 ppm
Hexane: molecular weight: 86.18, specific gravity: 0.657, TLV-TWA: 50 ppm
Additional homework problems are available in the Pearson Instructor Resource Center.










