9.4. Diffusion-Controlled Burning Rate
This situation, as discussed in the last section, closely resembles that of the droplet diffusion flame, in which the oxygen concentration approaches zero at the flame front. Now, however, the flame front is at the particle surface, and there is no fuel volatility. Of course, the droplet flame discussed earlier had a specified spherical geometry and was in a quiescent atmosphere. Thus, hD must contain the transfer number term because the surface regresses, and the gas-phase product formed (e.g., a carbon oxide for a carbon surface) will diffuse away from the surface. For the diffusion-controlled case, however, one need not proceed through the conductance hD, as the system developed earlier is superior.
Recall for the spherical symmetric case of particle burning in a quiescent atmosphere that
![]() (9.25)
(9.25)
The most convenient B in liquid droplet burning was
![]() (9.26)
(9.26)
since, even though Ts was not known directly, cp(T∞ − Ts) could always be considered much less than imo∞ H and hence could be ignored. Another form of B, however, is
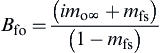 (9.27)
(9.27)
Indeed, this form of B was required in order to determine Ts and mfs with the use of the Clausius–Clapeyron equation. This latter form is not frequently used because mfs is essentially an unknown in the problem; thus it cannot be ignored as the cp(T∞ − Ts) term was in Eqn (9.26). It is, of course, readily determined and necessary in determining Gf. But observe the convenience in the carbon surface problem. Since there is no volatility of the fuel, mfs = 0, so Eqn (9.27) becomes
![]() (9.28)
(9.28)
Thus, a very simple expression is obtained for surface burning with fast kinetics:
![]() (9.29)
(9.29)
Whereas in liquid droplet burning B was not explicitly known because Ts is an unknown, in the problem of heterogeneous burning with fast surface reaction kinetics, B takes the simple form of imo∞, which is known provided the mass stoichiometric coefficient i is known. For small values of imo∞, Eqn (9.29) becomes very similar in form to Eqn (9.22) where for the quiescent case hD = D/rs = α/rs.
9.4.1. Burning of Metals in Nearly Pure Oxygen
The concept of the B number develops from the fact that in the quasi-steady approach used for droplet burning rates, the bulk flow was due not only to the fuel volatilization but also to the formation of product gases. This flow, represented by the velocity υ in the conservation equations, is outward directed; that is, the flow is in the direction of increasing r. In the case of metal combustion in pure oxygen and at relatively high pressures, the possibility arises that heterogeneous processes may occur with no product gas volatilization. Thus the B number effect disappears and the bulk velocity is inward directed. Indeed, it has been noted experimentally [22] that under these conditions small amounts of impurities in oxygen can reduce the burning rates of metals appreciably.
The extent of this impurity effect is surprising and is worthy of examination. Consideration of Eqn (6.111) in Chapter 6 readily shows [23] for heterogeneous oxidation that there is no apparent gas-phase reaction. This equation is now written in the form
![]() (9.30)
(9.30)
where the symbol Yo is now used for the mass fraction of oxygen to distinguish this unique case of droplet burning from all the others. Integrating Eqn (9.30) yields
![]() (9.31)
(9.31)
where the constant of integration is by definition the net mass flow rate of oxygen 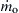 . Oxygen is the only species that has a net mass flow in this case, so
. Oxygen is the only species that has a net mass flow in this case, so
![]() (9.32)
(9.32)
where the subscript s, as before, designates the particle surface. The negative sign in Eqn (9.32) indicates that 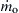 is inward directed. Integrating Eqn (9.31) from r = rs to r = ∞ then yields
is inward directed. Integrating Eqn (9.31) from r = rs to r = ∞ then yields
![]() (9.33)
(9.33)
where 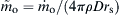 and (1 − Yo∞) represents the initial impurity mass fraction. The metal surface reaction rate
and (1 − Yo∞) represents the initial impurity mass fraction. The metal surface reaction rate  is now written as
is now written as
![]() (9.34)
(9.34)
which is another representation of Eqn (9.17). Since mf = imo, it is possible to define from Eqns (9.32) and (9.34) a nondimensional rate constant 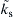 such that
such that
![]() (9.35)
(9.35)
so that
![]() (9.36)
(9.36)
One can see that 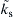 is a form of a Damkohler number (ks/(Ds/rs)), which indicates the ratio of the kinetic rate to the diffusion rate.
is a form of a Damkohler number (ks/(Ds/rs)), which indicates the ratio of the kinetic rate to the diffusion rate.
 (9.37)
(9.37)
Knowing Yo∞ and 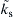 , one can iteratively determine
, one can iteratively determine 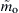 from Eqn (9.37); and knowing
from Eqn (9.37); and knowing 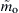 one can determine the metal burning rate from
one can determine the metal burning rate from 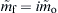 . The surface oxidizer concentration is given by
. The surface oxidizer concentration is given by
![]() (9.38)
(9.38)
The next consideration is how small amounts of inert affect the burning rate. Thus, Eqn (9.37) is considered in the limit Yo∞ → 1. In this limit, as can be noted from Eqn (9.37),  . Thus rewriting Eqn (9.37) as
. Thus rewriting Eqn (9.37) as
![]() (9.39)
(9.39)
and solving for 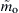 , one obtains
, one obtains
![]() (9.40)
(9.40)
Two observations can be made regarding Eqn (9.40). First, differentiating Eqn (9.40) with respect to the oxygen mass fraction, one obtains
![]() (9.41)
(9.41)
Thus one finds that 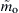 varies in an exponentially sensitive manner with the ambient oxygen concentration, Yo∞, and consequently with the impurity level for a sufficiently fast surface reaction. Second, since
varies in an exponentially sensitive manner with the ambient oxygen concentration, Yo∞, and consequently with the impurity level for a sufficiently fast surface reaction. Second, since 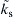 is an exponential function of temperature through the Arrhenius factor, the sensitivity of the oxidation rate to the oxygen concentration, and hence the impurity concentration, depends on the metal surface condition temperature in an extremely sensitive, double exponentiation manner.
is an exponential function of temperature through the Arrhenius factor, the sensitivity of the oxidation rate to the oxygen concentration, and hence the impurity concentration, depends on the metal surface condition temperature in an extremely sensitive, double exponentiation manner.

The variation of the oxygen concentration at the surface can be clearly seen by rearranging Eqn (9.41) after recalling Eqn (9.38) to give
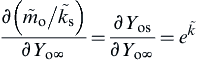 (9.42)
(9.42)
Figure 9.16 shows a plot of  versus Yo∞ for different values of
versus Yo∞ for different values of 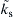 . The points in this figure were extracted from experimental data obtained by Benning et al. [22] for the burning of aluminum alloy rods in oxygen with an argon impurity. These data correspond to a
. The points in this figure were extracted from experimental data obtained by Benning et al. [22] for the burning of aluminum alloy rods in oxygen with an argon impurity. These data correspond to a 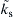 close to 36. Large values of
close to 36. Large values of 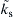 specify very fast surface reaction rates. For a value of
specify very fast surface reaction rates. For a value of 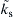 of 50, an impurity mass fraction of 0.5% reduces the oxygen mass fraction at the surface to 0.1, a 10-fold decrease from the ambient.
of 50, an impurity mass fraction of 0.5% reduces the oxygen mass fraction at the surface to 0.1, a 10-fold decrease from the ambient.
9.4.2. Burning of Small Particles—Diffusion versus Kinetic Limits
Recall the diffusion-controlled burning rate of a particle with fast heterogeneous reactions at the surface was given by (Eqn (9.29)),
![]()
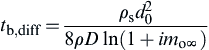
where d0 is the initial particle diameter, ρs is the particle density, and ρD is the product of the gas density and the diffusivity. This relationship is the “d2” relationship discussed in Chapter 6 for droplet combustion. As with droplet combustion, changes in oxidizer type are observed to affect the burning rate primarily by the diffusivity of the oxidizer and the overall reaction stoichiometry.
For kinetically controlled combustion, Eqns (9.9), (9.17), and (9.34) yield for the mass burning rate
![]()
The combustion time from the initial particle size to burnout is
![]()
Thus, tb in a kinetically controlled regime is described by a “d1” law. Furthermore, tb is found to be inversely proportional to pressure (for a first-order reaction) under kinetically controlled combustion, and in contrast, independent of pressure under diffusionally controlled combustion (since D ∝ P−1). In the kinetically controlled regime, the burning rate depends exponentially on temperature.
To determine the dominant combustion mechanism for a given set of conditions, the Damkohler number (Da) is given as
![]()
If Da = 1 is defined as the transition between diffusionally controlled and kinetically controlled regimes, an inverse relationship is observed between the particle diameter and the system pressure and temperature for a fixed Da. Thus, for a system to be kinetically controlled, combustion temperatures need to be low (or the particle size must be very small, so that the diffusive time scales are short relative to the kinetic time scale). Often for small particle diameters, the particle loses so much heat, so rapidly, that extinction occurs. Thus, the particle temperature is nearly the same as the gas temperature, and to maintain a steady-state burning rate in the kinetically controlled regime, the ambient temperatures need to be high enough to sustain reaction. The above equation also shows that large particles at high pressure likely experience diffusion-controlled combustion, and small particles at low pressures often lead to kinetically controlled combustion.
Another length scale of importance to the combustion of small particles is the mean free path in the surrounding gas phase. A comparison of this length scale to the particle diameter defines whether continuum conditions exist (i.e., the particle may be distinguished separately from the gas molecules). The key dimensionless group that defines the nature of the surrounding gas to the particle is the Knudsen number,
![]()
where λ is the mean free path of the gas phase. The mean free path for like molecules is given by
![]()
where σ is the molecular diameter of the molecule and N is the gas concentration (number density). Using kinetic theory to relate the gas viscosity to the mean free path yields

where μ is the gas viscosity and MW is the molecular weight of the gas. At atmospheric pressure, particles of dimensions 100 nm and smaller are characterized by Knudsen numbers greater than unity for the entire temperature range from room temperature to combustion flame temperatures, indicating that they can no longer be considered as macroscopic particles in a continuum gas. For Knudsen numbers of the order of 1 (the transition regime between continuum and free molecular flow), expressions used to model heat and mass transfer in the continuum limit are no longer applicable, and simple corrections to such models to account for rarified flow effects are only of limited utility. As the Knudsen number increases toward the transition regime, one of the first observable effects in the flow is the appearance of a discontinuity in temperature, velocity, and concentration profiles at the gas–surface interface, often referred to as slip or jump. The magnitude of this slip for each parameter depends on the efficiency of exchange of energy and momentum between the gas and the surface. This efficiency is quantified in accommodation coefficients α of a gas–surface interface. For example, the thermal accommodation coefficient αT is defined as
![]()
where Ts is the surface temperature, Tg is the temperature of the gas incident on the surface, and Tf is the temperature of the gas after scattering from the surface. The value of αT depends on the gas and surface temperatures in addition to the nature of the gas and surface. The convective heat transfer coefficient h can then be related to αT in the transition regime by
![]()
where c is the mean speed of the gas. Experimental measurements of thermal accommodation coefficients suggest that αT values at elevated temperatures for some systems are of the order of 0.1, and some may be much lower. Thus, heat and mass transfer to a nanoparticle do not increase linearly with specific surface area of the particle. As the Knudsen number increases, heat and mass transfer are reduced significantly by noncontinuum effects. As a result, the combustion of nanoscale metals may exhibit combustion characteristics that are not consistent with the classical kinetic limit. In the kinetic limit, transport of mass (and heat for Le ∼1) is rapid, and concentration and temperature profiles are relatively flat with mos = mo∞ and Ts = T∞. However, for isolated burning aluminum nanoparticles, particle temperatures can rise hundreds of degrees above ambient during combustion at elevated pressures, whereas for lower pressures the temperature overshoot (as well as the combustion rate) is reduced. The abundance of gas-phase intermediates such as Al and AlO is greatly reduced, but not eliminated. These observations suggest that although Kn > 1 for some nanometal systems, the combustion has not reached the kinetic limit due to inhibited transport in the noncontinuum regime. Such particles can be considered to be burning in a “transition” regime between the diffusion and the kinetic limit, in which neither the d2 nor the d burning rules apply.
Furthermore, the surface process that limits the combustion in the kinetic limit may not be surface reaction kinetics, but solid-phase transport. Since nanoaluminum is known to ignite and burn at temperatures well below the melting point of the aluminum oxide coating, reaction of the metal with ambient oxidizer requires either that oxidizer diffuses inward through the solid oxide shell or that aluminum fuel diffuses out. Solid state diffusion processes are generally much slower than the metal/oxygen reaction. Therefore, the gas-phase temperature and oxidizer concentration profiles characteristic of a particle burning in the classical kinetic limit may be generated by a nanoscale particle in which diffusion of fuel or oxidizer through an oxide product at the particle surface is the true limiting process.
Much of the highly desirable traits of nanosized metal powders in combustion systems have been attributed to their high specific surface area (high reactivity) [24,25a,25b] and potential ability to store energy in surface defects [26]. In addition, nano-sized powders are known to display increased catalytic activity [27], superparamagnetic behavior [28], superplasticity [29], lower melting temperatures [30,31], lower sintering temperatures [32], and higher theoretical densities compared to micrometer and larger sized materials. Ignition temperatures of nAl particles have been observed to be as low as 1000 K (versus ignition temperatures closer to the melting temperature of alumina, common for micrometer-sized particles) [33,34], though the reasons for this ignition point suppression are not well understood. The combustion rates of materials with nanopowders have been observed to increase significantly over similar materials with micrometer-sized particles. For example, SHS reactions with nanopowders can support fast deflagrations and detonations (with combustion speeds of over 1000 m/s), which are several orders of magnitude greater than the propagation speeds of SHS reactions with micrometer and larger sized particles. A lower limit in size of nano energetic metallic powders in some applications may result from the presence of their passivating oxide coating. For example, Al particles typically have an oxide coating with a limiting thickness of about 3 nm at room temperature. With a 100-nm-diameter particle having a 3-nm-thick coating, the energy loss per unit volume due to the presence of the oxide layer is 10%. A particle with a diameter of 10 nm would have an energy loss per unit volume of approximately 60%. Consequently, coatings, self-assembled monolayers, and the development of composite materials that limit the volume of nonenergetic material in the powders have been under development in recent years. An introduction to the combustion of nano metallic particles may be found in a special issue of the Journal of Propulsion and Power [35].
9.4.3. The Burning of Boron Particles
In certain respects, the combustion of boron is different from that of carbon because, under normal temperature and pressure conditions, the product oxide, B2O3, is not a gas. Thus, a boron particle normally has an oxide coat that thickens as the particle is heated in an oxidizing atmosphere. This condition is characteristic of most metals, even those that will burn in the vapor phase. For the efficient combustion of the boron particle, the oxide coat must be removed. The practical means for removing the coat is to undertake the oxidation at temperatures greater than the saturation temperature of the boron oxide B2O3. This temperature is about 2300 K at 1 atm.
The temperature at which sufficient oxide is removed so that oxidation can take place rapidly is referred to as the metal ignition temperature. The rate of oxidation when the oxide coat persists has been discussed extensively in Refs. [36,37]. Nevertheless, what does control the burning time of a boron particle is the heterogeneous oxidation of the clean particle after the oxide has been evaporated. Thus, for efficient burning the particle and oxidizing medium temperatures must be close to the saturation temperature of the B2O3. Then the burning rate of the particle is given by Eqn (9.29), the same as that used for carbon except that the mass stoichiometric coefficient i is different. Even though the chemical reaction steps for boron are quite different from those of carbon, i is a thermodynamic quantity, and the atomic weight 10 for boron is comparable to 12 for carbon; consequently, it is not surprising that the i values for both materials are nearly the same.
Just as the surface oxidation chemistry makes it unlikely that carbon would yield CO2, it is also unlikely that boron would yield B2O3. Gaseous boron monoxide BO forms at the surface, and this product is oxidized further to gaseous B2O3 by vapor-phase reactions. The gaseous B2O3 diffuses back to the clean boron surface and reacts to form three molecules of BO. The actual reaction order is most likely given by the sequence of reactions [37] discussed in the following paragraphs.
In a high-temperature atmosphere created by the combustion of a host hydrocarbon fuel, there will be an abundance of hydroxyl radicals. Thus, boron monoxide reacts with hydroxyl radicals to form gaseous metaboric oxide HOBO.
![]()
It is postulated that HOBO then reacts with BO to form gaseous boron oxide hydride HBO and boron dioxide BO2 (OBO).
![]()
The boron dioxide then reacts with another BO to form boron oxide B2O3.
![]()
This route is consistent with the structure of the various boron oxide compounds in the system [37].
In a hydrogen-free oxidizing atmosphere, a slower step forms the boron dioxide,
![]()
whereupon B2O3 again forms via the reaction above.
After the gaseous reaction system is established, the B2O3 diffuses back to the nascent boron surface to form BO, just as CO2 diffuses back to the carbon surface to form CO. The reaction is
![]()
Thus, the overall thermodynamic steps required to calculate the mass stoichiometric index in Eqn (9.29) are
![]()
![]()
![]()
and i = 10/16 = 0.625 compared to the value of 0.75 obtained for the carbon system.
Boron does not meet Glassman's criterion for vapor-phase combustion of the metal. Thus, the boron surface remains coated with a vitreous B2O3 layer, and boron consumption becomes extremely slow; consequently, boron is not burned efficiently in propulsion devices.
9.4.4. Carbon Particle Combustion (C. R. Shaddix)
The appropriate stoichiometric coefficient for oxidation of carbon is not readily apparent, because there are two different oxidation states of carbon, namely carbon monoxide and carbon dioxide, that may be present when a carbon surface is oxidized. These products form according to the overall reaction steps
![]()
![]()
The large differences in the heat release of these reactions and the twofold difference in the carbon gasification rate per reacted O2 molecule make it imperative to properly understand the oxidation reaction. Consequently, a number of experiments have been performed to attempt to shed light on this reaction path. Complicating the interpretation of these experiments is the fact that CO readily oxidizes to CO2 at high temperatures (particularly if there is any moisture present, as discussed in Chapter 3). The dominant viewpoint arising from these studies is that the direct production of CO2 from carbon oxidation is only significant at fairly low temperatures, such that it is usually safe to assume under combustion conditions that only CO is produced. With this assumption, the value of i in Eqn (9.28), that is, the mass stoichiometric coefficient for fuel to oxygen, is equal to 24/32 or 0.75.
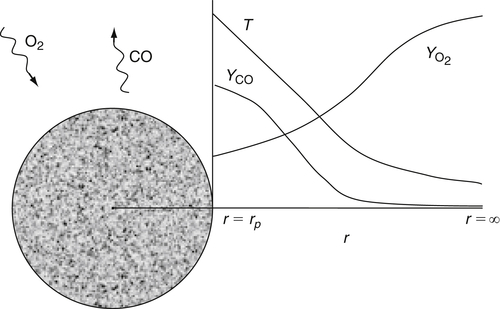
In the simplest situation, the CO produced at the particle surface diffuses away from the surface without further reactions. This assumption, known as the single-film or frozen boundary layer model, results in the species profiles shown in Figure 9.17.
For sufficiently high temperatures (greater than 1000 K) and sufficiently large particles, the CO will oxidize in the particle boundary layer according to the overall reaction
![]()
When this happens, the oxygen flux to the particle surface is cut off because of its consumption in oxidizing CO, and CO2 becomes the relevant gasification agent for the particle surface according to the Boudouard reaction:
![]()
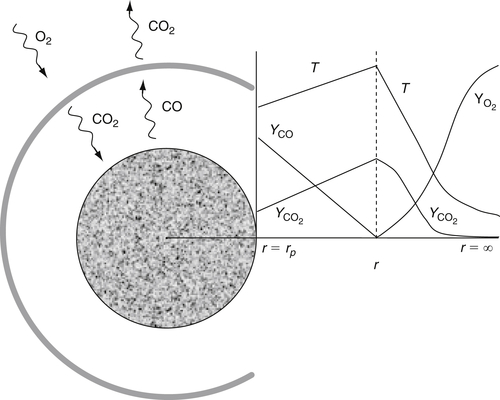
When these conditions exist the carbon is consumed according to a double-film model with species profiles as indicated in Figure 9.18.
Carbon dioxide oxidizes carbon at a substantially slower rate than O2 at normal combustion temperatures. As a consequence, the transition from single-film combustion of a carbon particle to double-film combustion typically involves a strong reduction in the carbon oxidation rate, as eloquently demonstrated by Makino and coworkers in a series of experiments in which graphite rods were oxidized in air at different temperatures and flow rates [38].
For oxidation of solid carbon to produce CO (either by O2 or CO2), 2 mol of gaseous combustion products is produced per mole of gaseous reactant, resulting in a net gas flow away from the particle surface. This Stefan flow reduces the rates of heat and, especially, mass transfer from the boundary layer to the particle surface (akin to drag reduction on a flat surface with active blowing). Consequently, accurate modeling of carbon oxidation must account for Stefan flow reduction of the diffusion rate of oxygen to the particle surface.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.