9.7. Catalytic Combustion
While not a process involving combustion of a nonvolatile fuel, combustion of gaseous fuels can also occur at a surface with no significant gas-phase reaction in the catalytic combustion process. A brief introduction to the field is provided here. For more detailed information, the reader can consult several excellent reviews [67–69]. Catalytic combustion is widely considered to have been identified by Davy who identified flameless combustion with a platinum catalyst [70]. In a catalytic process, a mixture that is below its ignition temperature and/or outside its flammability limit may still react to completion through interactions with a catalytic surface. It is important to recognize that the presence of the catalyst does not affect the equilibrium product distribution; it does, however, affect the kinetic pathways and effective activation energy for a given oxidation process.
Catalytic oxidation processes involve three stages. Initially, fuel and oxidizer from the gas phase adsorb on the surface. Decomposition of both fuel and oxidizer occurs through interaction of these adsorbed species with other surface species or gas-phase species. Product is formed through similar surface interactions and reenters the gas phase through the process of desorption. The global stoichiometry of a catalytic reaction is the same as a gas-phase reaction.
Several fundamental processes may occur in a catalytic reaction. Adsorption involves a gas-phase species colliding with the surface and forming a surface species that adheres to the surface by a chemical bond (chemisorption) or by van der Waals forces (physisorption). In many cases, the adsorption process can lead to the breakup of the adsorber into multiple surface species (dissociative adsorption). Chemisorbed and physisorbed species can interact with one another to form different surface species. For example, chemisorbed O and OH can interact to form physisorbed H2O. In addition, surface species can migrate by surface diffusion to other locations on the surface. In this fashion, species do not necessarily need to be created at adjacent sites to undergo surface reaction. Just as species can adsorb, they can also desorb back into the gas phase. Physisorbed species have very weak interactions, and therefore have short lifetimes on the surface before desorption. Finally, gas-phase species may interact with surface species to generate other surface species or new gas-phase species. For example, gas-phase radical species can abstract chemisorbed hydrogen in the same fashion as they might abstract hydrogen from gas-phase species.

An example of the proposed reaction mechanism for the hydrogen/oxygen system is shown in Figure 9.26. This approach follows that of Rinnemo et al. [71]. The initial steps in the mechanism involve dissociative adsorption of both fuel and oxidizer onto the surface:
![]() (9.52)
(9.52)
![]() (9.53)
(9.53)
where the (s) notation specifies a surface species. Species O(s) and H(s) react to form OH(s), which further reacts by two possible paths to form the physisorbed water:
![]() (9.54)
(9.54)
![]() (9.55)
(9.55)
![]() (9.56)
(9.56)
The water product desorbs as the final step of the series.
![]() (9.57)
(9.57)
The reaction rate of each reaction in a surface mechanism is characterized by a preexponential term and an activation energy. In some reactions, especially the dissociative adsorption reactions, the activation energy may be a function of the reactive site fraction of the surface. Whereas the activation energy for catalytic combustion is lower than gas-phase combustion, the process of catalytic reaction remains strongly temperature dependent, though ignition or “light off” of hydrocarbon oxidation often occurs at much lower temperatures, often well below 300 °C.
In the same fashion as for gas-phase combustion, reaction in a catalytic combustion system can be modeled by numerical approaches using explicit kinetic rate data. These approaches require both surface kinetic rate expressions, as well as the thermodynamics of surface species involved. In general, rate data for surface reactions are much less abundant than gas-phase data. In addition, for many catalysts, rates depend on the crystal orientation exposed to the surface, which complicates calculations in practical systems. In addition, the evaluation of the thermodynamics of surface species is not as simple as for gas-phase species, and databases of surface species data contain limited data at present.
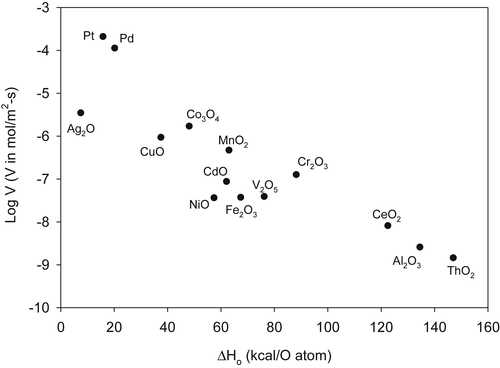
Several materials have shown catalytic activity for combustion of hydrogen and hydrocarbons. The most widely examined materials are the noble metals such as platinum, palladium, and rhodium. Catalytic activity of a surface has been semiquantitatively associated with the bond strength of the oxide formed by the catalytic metal. Species which form strong oxides, such as aluminum, oxidize quickly and thus generate inert surfaces. Species such as gold (or fully oxidized metals) do not interact strongly enough with gas-phase species, and the lifetimes of physisorbed species are too short to allow for surface reactions to take place. Metals such as platinum and palladium, which form weak oxides have an appropriate balance, allowing for effective adsorption of gas-phase species, as well as desorption of products. This balance is shown in the classic “volcano” plot shown in Figure 9.27 following the data of Ref. [72].
In practice, the reactivity of a catalytic surface is affected by many parameters, and different catalysts are more active for different fuel/oxidizer systems. In addition, most catalytic combustion applications involve the dispersal of a catalytic metal onto a ceramic surface (the “support”), where the catalytic metal is present as generally discrete and very fine particles, with high specific surface area. The support material can have a significant effect on the reactivity by generating an interface that is more reactive than either the base material or the support itself. A classic example of this interaction occurs with gold where a support interaction with one of several ceramic materials can lead to a highly efficient catalyst for CO oxidation, despite the negligible activity of pure gold for this reaction. Indeed, it is common to speak of the reactivity of a catalyst/support combination instead of only referring to the catalyst. In addition, the oxide of the noble metal may be a better catalyst than the pure metal itself. For example, with respect to methane oxidation, palladium (Pd), a common catalyst, oxidizes at elevated temperatures forming PdO which serves as the primary catalyst in the palladium system, though metal Pd sites may still play some role. Furthermore, some oxides, especially the transition metal and rare earth oxides, have been shown to be very active for hydrocarbon oxidation. A well-known example is cerium oxide which has been shown to be an active hydrocarbon oxidation catalyst as well as an oxygen-storing support material [73].
Catalyst lifetime is an important issue in practical systems. Some catalyst systems require an activation in either an oxidizing or a reducing atmosphere. Catalytic activity generally decays over time (deactivation) due to degradation of the catalyst material and reduction of active sites. Catalyst fouling or poisoning, which involves rapid loss of catalytic activity, can occur in the presence of certain species, especially sulfur, lead, phosphorous, and manganese compounds, which bind to surface sites rendering them inactive.
In practical applications, catalytic combustion most often occurs in monolith structures which consist of closely packed arrays of narrow passages. The monolith is commonly made of a ceramic or high-temperature metal. A ceramic washcoat is dispersed on the monolith, and the catalyst is impregnated onto the washcoat surface. The high surface area monolith structure ensures that the flow is exposed to the maximum surface area of a catalyst while maintaining as low a pressure drop as possible.
The most common applications for catalytic combustion have been power generation and small scale heating and cooking appliances. In the former case, catalytic combustion allows for combustion well outside the conventional, gas-phase flammability limits. Thus, a stable operation can be maintained with full conversion of the hydrocarbon at very low temperatures, typically less than 1400 °C at the monolith exit. Thus, CO and unburned hydrocarbon emissions are small due to the ultralean operation, and the low temperatures combined with low concentrations of radical species (e.g., O) in the gas phase result in very low NOx production from the thermal mechanism.
Catalytic radiant heaters and stoves tend to operate at even lower temperatures, burning mixtures that are well outside the conventional flammability limits and generating temperatures in the catalytic element (typically around 250 °C) that are below those required to ignite typical hydrocarbon air mixtures. Thus, the element provides radiant heat due to hydrocarbon oxidation, but maintains an intrinsically safe operation.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.
