Assessment of Physical, Chemical, and Tribological Properties of Different Biodiesel Fuels
Abstract
Fuel properties of biodiesels are influenced by the physical features of the fatty acid composition, such as the degree of unsaturation, the percentage of saturated fatty acid, monounsaturated fatty acid, and polyunsaturated fatty acid. Fuel properties are the key factors in determining the suitability of any fuel as an alternative fuel. In this study, biodiesels from five different feedstocks have been characterized for their physical and chemical properties. Gas chromatography has been carried out to find out the ester composition of these five biodiesels, and correlation between composition and fuel properties of these five biodiesels have been developed. Fuel properties were measured according to standard procedure ASTM D6751 and EN 14214 and estimated based on the previously published correlation. Also, the quality of these biodiesels was assessed and compared with commercially available biodiesels through multivariate data analysis using PROMETHEE-GAIA software. In the last part, wear and friction of selected biodiesel fuels have been studied and compared with diesel fuel. The result shows that the properties of produced biodiesel are within the acceptable limit of ASTM and EN standards. Highly linear correlations were found between the composition and cetane number, iodine value, oxidation stability, and cold flow plugging point with the regression value of 0.9965, 0.9983, 0.7044, and 0.9985, respectively. Overall, this study found that, among the biodiesels studied, the palm biodiesel was the most suitable alternative followed by the macadamia, moringa, and jatropha, and beauty leaf biodiesel.
Keywords
14.1. Introduction
14.2. Materials
14.2.1. Biodiesel Production
Table 14.1
Properties of Crude Oils Used in This Study
| Properties | Units | Standards | Macadamia Oil | Palm Oil | Jatropha Oil | Moringa Oil | Beauty Leaf Oil |
| Dynamic viscosity | mPa.s | ASTM D445 | 35.23 | 36.30 | 31.52 | 38.90 | 48.73 |
| Kinematic viscosity at 40°C | mm2/s | ASTM D445 | 39.22 | 40.40 | 34.93 | 43.33 | 52.13 |
| Density at 15°C | kg/m3 | ASTM D4052 | 898.60 | 898.4 | 902.5 | 897.5 | 922.2 |
| Flash point | °C | ASTM D93 | 167.5 | 165 | 220 | 268.5 | 195.5 |
| Pour point | °C | ASTM D97 | 8 | 9 | −3 | 11 | 8 |
| Cloud point | °C | ASTM D2500 | 0 | 8 | −2 | 10 | 8 |
| Calorific value | MJ/kg | ASTM D240 | 39.89 | 39.44 | 38.66 | 38.05 | 38.51 |
| Acid value | mg KOH/g oil | ASTM D664 | 4 | 3.47 | 10.7 | 8.62 | 40 |

14.2.2. Determination of Fatty Acid Composition
14.2.3. Fuel Properties
![]() (14.1)
(14.1)
![]() (14.2)
(14.2)
Table 14.2
| Property | Equipment | Standard Method | Accuracy |
| Kinematic viscosity | NVB classic (Norma Lab, France) | ASTM D445 | ±0.01 mm2/s |
| Density | DM40 LiquiPhysics density meter (Mettler Toledo, Switzerland) | ASTM D127 | ±0.1 kg/m3 |
| Flash point | NPM 440 Pensky-martens flash point tester (Norma Lab, France) | ASTM D93 | ±0.1°C |
| Cloud and pour point | NTE 450 cloud and pour point tester (Norma Lab, France) | ASTM D2500 | ±0.1°C |
| Higher heating value | IKA C 2000 calorimeter, United Kingdom | ASTM D240 | ±0.001 MJ/kg |
| Acid number | Automation titration rondo 20 (Mettler Toledo, Switzerland) | ASTMD664 and EN 14111 | ±0.001 mg KOH/g |
| Oxidation stability, 110°C | 873 Rancimat (Metrohm, Switzerland) | EN 14112 | ±0.01 h |

![]() (14.3)
(14.3)
![]() (14.4)
(14.4)
![]() (14.5)
(14.5)
14.2.4. Equipment for Tribological Study
Table 14.3
The Variables and Preference Used in PROMETHEE-GAIA Analysis
| Variables | Preference for PROMETHEE-GAIA |
| KV | Min |
| D | Min |
| HHV | Max |
| OS | Max |
| AV | Min |
| FP | Max |
| CP | Min |
| CFPP | Min |
| CN | Max |
| IV | Min |
| DU | Min |
| MUFA | Min |
| PUFA | Min |
14.2.5. Determination of the Coefficient of Friction
![]() (14.6)
(14.6)
![]() (14.7)
(14.7)
14.2.6. Determination of Flash Temperature Parameter
![]() (14.8)
(14.8)
14.3. Results and Discussion
14.3.1. Fatty Acid Profile of Biodiesels
14.3.2. Analysis of Fuel Properties of Biodiesel Samples
Table 14.4
Fatty Acid Compositions of Biodiesel Fuels
| Fatty Acids | Molecular Weight | Structure | MaBD (Wt%) [18] | JBD (Wt%) | PBD (Wt%) | MoBD (Wt%) | BBD (Wt%) |
| Lauric | 200 | 12:0 | 0.100.6 | 0.1 | 0 | 0 | 0 |
| Myristic acid | 228 | 14:0 | 0.6 | 0.1 | 0 | 0.1 | 0 |
| Palmitic | 256 | 16:0 | 7.9 | 14.6 | 40.3 | 7.9 | 14.9 |
| Palmitoleic | 254 | 16.1 | 16.2 | 0.6 | 0 | 1.7 | 0.2 |
| Stearic | 284 | 18:0 | 3.2 | 7.6 | 4.1 | 5.5 | 17.2 |
| Oleic | 282 | 18:1 | 61.3 | 44.6 | 43.4 | 74.1 | 38.2 |
| Linoleic | 280 | 18:2 | 2.1 | 31.9 | 12.2 | 4.1 | 27.6 |
| Linolenic | 278 | 18:3 | 0.1 | 0.3 | – | 0.2 | 0.3 |
| Arachidic | 312 | 20:0 | 2.7 | 0.3 | – | 2.3 | 0.9 |
| Eicosenoic | 310 | 20:1 | 2.6 | – | – | 1.3 | 0.3 |
| Behenic | 340 | 22:0 | 0.9 | – | – | 2.8 | 0.3 |
| Erucic | 338 | 22:1 | 0.3 | – | – | – | 0 |
| Lignoceric | 368 | 24:0 | 0.4 | – | – | – | 0.1 |
| Total saturated fatty acid | 15.80 | 22.6 | 44.6 | 18.6 | 33.4 | ||
| Total monounsaturated fatty acid | 80.40 | 45.2 | 43.4 | 77.1 | 38.7 | ||
| Total polyunsaturated fatty acid | 2.20 | 32.2 | 12 | 4.3 | 27.9 | ||
| Others | 1.6 | 0 | 0 | 0 | 0 | ||
| Degree of unsaturation (DU) | 84.50 | 109.60 | 67.80 | 85.70 | 94.50 | ||
| Long-chain saturated factor (LCSF) | 7.24 | 5.26 | 6 | 10.04 | 11.64 | ||

14.3.2.1. Density
Table 14.5
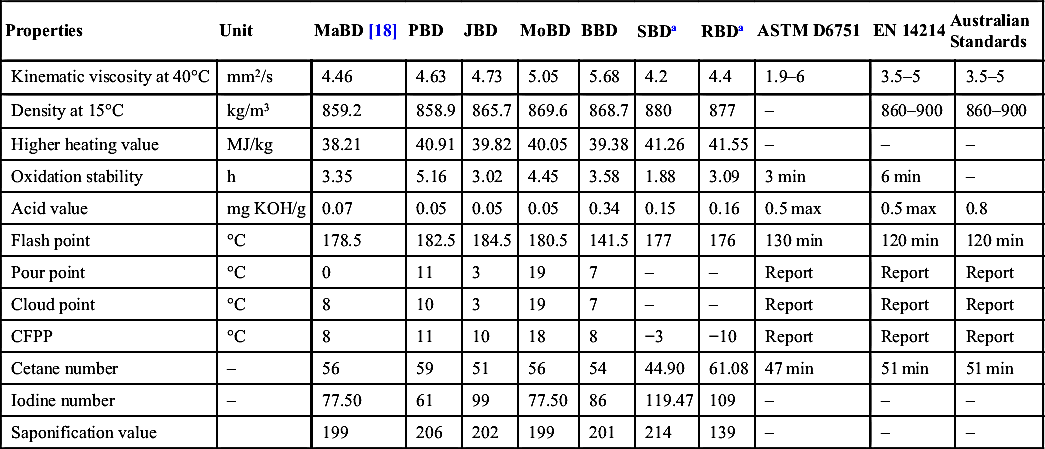
Properties of Produced Biodiesel Compared With Other Commercial Biodiesel
| Properties | Unit | MaBD [18] | PBD | JBD | MoBD | BBD | SBDa | RBDa | ASTM D6751 | EN 14214 | Australian Standards |
| Kinematic viscosity at 40°C | mm2/s | 4.46 | 4.63 | 4.73 | 5.05 | 5.68 | 4.2 | 4.4 | 1.9–6 | 3.5–5 | 3.5–5 |
| Density at 15°C | kg/m3 | 859.2 | 858.9 | 865.7 | 869.6 | 868.7 | 880 | 877 | – | 860–900 | 860–900 |
| Higher heating value | MJ/kg | 38.21 | 40.91 | 39.82 | 40.05 | 39.38 | 41.26 | 41.55 | – | – | – |
| Oxidation stability | h | 3.35 | 5.16 | 3.02 | 4.45 | 3.58 | 1.88 | 3.09 | 3 min | 6 min | – |
| Acid value | mg KOH/g | 0.07 | 0.05 | 0.05 | 0.05 | 0.34 | 0.15 | 0.16 | 0.5 max | 0.5 max | 0.8 |
| Flash point | °C | 178.5 | 182.5 | 184.5 | 180.5 | 141.5 | 177 | 176 | 130 min | 120 min | 120 min |
| Pour point | °C | 0 | 11 | 3 | 19 | 7 | – | – | Report | Report | Report |
| Cloud point | °C | 8 | 10 | 3 | 19 | 7 | – | – | Report | Report | Report |
| CFPP | °C | 8 | 11 | 10 | 18 | 8 | −3 | −10 | Report | Report | Report |
| Cetane number | – | 56 | 59 | 51 | 56 | 54 | 44.90 | 61.08 | 47 min | 51 min | 51 min |
| Iodine number | – | 77.50 | 61 | 99 | 77.50 | 86 | 119.47 | 109 | – | – | – |
| Saponification value | 199 | 206 | 202 | 199 | 201 | 214 | 139 | – | – | – |
14.3.2.2. Flash Point
14.3.2.3. Viscosity
14.3.2.4. Cold Flow Properties
14.3.2.5. Cetane Number
14.3.2.6. Higher Heating Value
14.3.2.7. Oxidation Stability
14.3.3. Effect of Fatty Acid Composition on Fuel Properties
14.3.4. Validation of Biodiesel Properties
14.3.5. Study of Tribological Characteristics
14.3.5.1. Friction Behavior
14.3.5.2. Wear Scar Diameter and Flash Temperature Parameter
14.4. Conclusions











