Chapter 5
Detonation
Abstract
Detonation phenomena are introduced by differentiation between explosions, deflagrations, and detonations followed by a discussion of the onset or transition of deflagration to detonation. The Hugoniot relationships are derived and the detonation velocity calculated. Experimental results are compared with theory and the effects of pressure and temperature discussed. The structure of the detonation wave is analyzed and concepts of a cellular detonation front and detonation limits presented. A brief description of detonations in nongaseous media is also presented.
Keywords
C-J point; Cellular front; Detonation limits; Detonation phenomena; Detonation velocity; Hugoniot curve; ZND structure5.1. Introduction
Established usage of certain terms related to combustion phenomena can be misleading, for what appear to be synonyms are not really so. Consequently, this chapter begins with a short digression into the semantics of combustion, with some brief mention of subjects to be covered later.
5.1.1. Premixed and Diffusion Flames
The previous chapter covered primarily laminar flame propagation. By inspecting the details of the flow, particularly high-speed or higher Reynolds number flow, it was possible to consider the subject of turbulent flame propagation. These subjects (laminar and turbulent flames) are concerned with gases in the premixed state only. The material presented is not generally adaptable to the consideration of the combustion of liquids and solids, or systems in which the gaseous reactants diffuse toward a common reacting front.
Diffusion flames can best be described as the combustion state controlled by mixing phenomena—that is, the diffusion of fuel into oxidizer, or vice versa—until some flammable mixture ratio is reached. According to the flow state of the individual diffusing species, the situation may be either laminar or turbulent. It will be shown later that gaseous diffusion flames exist, that liquid burning proceeds by a diffusion mechanism, and that the combustion of solids and some solid propellants fall into this category as well.
5.1.2. Explosion, Deflagration, and Detonation
Explosion is a term that corresponds to rapid heat release (or pressure rise). An explosive gas or gas mixture is one that will permit rapid energy release as compared to most steady, low-temperature reactions. Certain gas mixtures (fuel and oxidizer) will not propagate a burning zone or combustion wave. These gas mixtures are said to be outside the flammability limits of the explosive gas.
Depending on whether the combustion wave is a deflagration or detonation, there are limits of flammability or detonation.
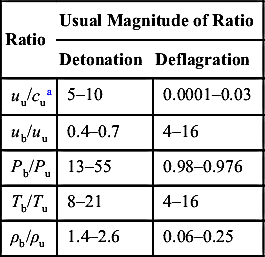
Table 5.1
Qualitative Differences between Detonations and Deflagration in Gases
| Ratio | Usual Magnitude of Ratio | |
| Detonation | Deflagration | |
| uu/cua | 5–10 | 0.0001–0.03 |
| ub/uu | 0.4–0.7 | 4–16 |
| Pb/Pu | 13–55 | 0.98–0.976 |
| Tb/Tu | 8–21 | 4–16 |
| ρb/ρu | 1.4–2.6 | 0.06–0.25 |
Although the detonation wave is another class of the combustion wave, in general the combustion wave is considered as a deflagration only. The detonation wave is, in essence, a shock wave that is sustained by the energy of the chemical reaction in the highly compressed explosive medium existing in the wave. Thus, a deflagration is a subsonic wave sustained by a chemical reaction and a detonation is a supersonic wave also sustained by chemical reaction. In the normal sense, it is common practice to call a combustion wave a “flame,” thus combustion wave, flame, and deflagration have been used interchangeably.
It is a very common error to confuse a pure explosion and a detonation. An explosion does not necessarily require the passage of a combustion wave through the exploding medium, whereas an explosive gas mixture must exist to have either a deflagration or a detonation. That is, both deflagrations and detonations require rapid energy release; but explosions, although they also require rapid energy release, do not require the presence of a waveform.
5.1.3. The Onset of Detonation
Depending on various conditions, an explosive medium may support either a deflagration or a detonation wave. The most obvious conditions are confinement, mixture ratio, and ignition source.
Original studies of gaseous detonations have shown no single sequence of events leads to what is now known as the complex cellular structure of a detonation wave. The primary result of an ordinary thermal initiation always appears to be a flame that propagates with subsonic speed. When conditions are such that the flame causes adiabatic compression of the still unreacted mixture ahead of it, the flame velocity increases. According to some early observations, the speed of the flame seems to rise gradually until it equals that of a detonation wave. Normally, a discontinuous change of velocity is observed from the low flame velocity to the high speed of detonation. In still other observations, the detonation wave has been seen to originate apparently spontaneously some distance ahead of the flame front. The place of origin appears to coincide with the location of a shock wave sent out by the expanding gases of the flame. Modern experiments and analysis have shown that these seemingly divergent observations were in part attributable to the mode of initiation. In detonation phenomena, one can consider that two modes of initiation exist: a slower mode, sometimes called thermal initiation, in which there is transition from deflagration; and a fast mode brought about by an ignition blast or strong shock wave. Some [2] refer to these modes as self ignition and direct ignition, respectively.
When an explosive gas mixture is placed in a tube having one or both ends open, a combustion wave can propagate when the tube is ignited at an open end. This wave attains a steady velocity and does not accelerate to a detonation wave.
If the mixture is ignited at one end that is closed, a combustion wave is formed; and, if the tube is long enough, this wave can accelerate to a detonation. This thermal initiation mechanism is described as follows. The burned gas products from the initial deflagration have a specific volume of the order of 5–15 times that of the unburned gases ahead of the flame. Since each preceding compression wave that results from this expansion tends to heat the unburned gas mixture somewhat, the sound velocity increases and the succeeding waves catch up with the initial one. Furthermore, the preheating tends to increase the flame speed, which then accelerates the unburned gas mixture even further to a point where turbulence is developed in the unburned gases. Then, a still greater velocity and acceleration of the unburned gases and compression waves are obtained. This sequence of events forms a shock that is strong enough to ignite the gas mixture ahead of the front. The reaction zone behind the shock sends forth a continuous compression wave that keeps the shock front from decaying, and so a detonation is obtained. At the point of shock formation, a detonation forms and propagates back into the unburned gases [2,3]. Transverse vibrations associated with the onset of detonation have been noticed, and this observation has contributed to the understanding of the cellular structure of the detonation wave. Photographs of the onset of detonation have been taken by Oppenheim and coworkers [3] using a stroboscopic-laser Schlieren technique.
The reaction zone in a detonation wave is no different from that in other flames, in that it supplies the sustaining energy. A difference does exist in that the detonation front initiates chemical reaction by compression, by diffusion of both heat and species, and thus inherently maintains itself. A further, but not essential, difference worth noting is that the reaction takes place with extreme rapidity in highly compressed and preheated gases.
The transition length for deflagration to detonation is of the order of a meter for highly reactive fuels such as acetylene, hydrogen, and ethylene, but larger for most other hydrocarbon air mixtures. Consequently, most laboratory results for detonation are reported for acetylene and hydrogen. Obviously, this transition length is governed by many physical and chemical aspects of the experiments. Such elements as overall chemical composition, physical aspects of the detonation tube, and initiation ignition characteristics can all play a role. Interestingly, some question exists as to whether methane will detonate at all.
According to Lee [2], direct initiation of a detonation can occur only when a strong shock wave is generated by a source and this shock retains a certain minimum strength for some required duration. Under these conditions, “the blast and reaction front are always coupled in the form of a multiheaded detonation wave that starts at the (ignition) source and expands at about the detonation velocity.” [2] Because of the coupling phenomena necessary, it is apparent that reaction rates play a role in whether or not a detonation is established. Thus, ignition energy is one of the dynamic detonation parameters discussed in the next section. However, no clear quantitative or qualitative analysis exists for determining this energy, so this aspect of the detonation problem will not be discussed further.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.
