3.7. The Oxidation of Methane
3.7.1. Low-Temperature Mechanism
Methane exhibits certain oxidation characteristics that are different from those of all other hydrocarbons. Tables of bond energy show that the first broken C–H bond in methane takes about 40 kJ more than the others, and certainly more than the C–H bonds in longer-chain hydrocarbons. Thus, it is not surprising to find various kinds of experimental evidence indicating that ignition is more difficult with methane–air (oxygen) mixtures than it is with other hydrocarbons. At low temperatures, even oxygen atom attack is slow. Indeed, in discussing exhaust emissions with respect to pollutants, the terms total hydrocarbons and reactive hydrocarbons are used. The difference between the two terms is simply methane, which reacts so slowly with oxygen atoms at atmospheric temperatures that it is considered unreactive.
The simplest scheme that will explain the lower-temperature results of methane oxidation is the following:

There is no H2O2 dissociation to OH radicals at low temperatures. H2O2 dissociation does not become effective until temperature reaches about 900 K.
As before, reaction (3.76) is slow. Reactions (3.77) and (3.78) are faster, since they involve a radical and one of the initial reactants. The same is true for reactions (3.80)–(3.82). Reaction (3.80) represents the necessary chain branching step. Reactions (3.79) and (3.83) introduce the formyl radical known to exist in the low-temperature combustion scheme. Carbon monoxide is formed by reaction (3.81), and water by reaction (3.78) and the subsequent decay of the peroxides formed. A conversion step of CO to CO2 is not considered because the rate of conversion by reaction (3.49) is too slow at the temperatures of concern here.
It is important to examine more closely reaction (3.77), which proceeds [30,31] through a metastable intermediate complex—the methyl peroxy radical—in the following manner:
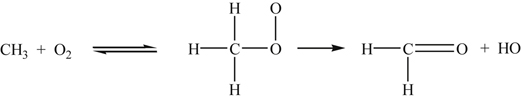 (3.77)
(3.77)At lower temperatures, the equilibrium step is shifted strongly toward the complex, allowing the formaldehyde and hydroxyl radical formation. The structure of the complex represented in reaction (3.77) is well established. Recall that when O2 adds to the carbon atom in a hydrocarbon radical, it forms about a 90° bond angle. Perhaps more important, however, is the suggestion [30] that at temperatures of the order of 1000 K and above, the equilibrium step in reaction (3.77) shifts strongly toward the reactants so that the overall reaction to form formaldehyde and hydroxyl cannot proceed. This condition would therefore pose a restriction on the rapid oxidation of methane at high temperatures. This possibility should come as no surprise, as one knows that a particular reaction mechanism can change substantially as the temperature and pressure change. There now appears to be evidence that another route to the aldehydes and OH formation by reaction (3.77) may be possible at high temperatures [9,31]; this route is discussed in the next section.
3.7.2. High-Temperature Mechanism
Many extensive models of the high-temperature oxidation process of methane have been published [32–35]. Such models are quite complex and include hundreds of reactions. The availability of sophisticated computers and computer programs such as those described in Appendix I permits the development of these models, which can be used to predict flow reactor results, flame speeds, emissions, etc., and to compare these predictions with appropriate experimental data. Differences between model and experiment are used to modify the mechanisms and rate constants that are not firmly established. The purpose here is to point out the dominant reaction steps in these complex models of methane oxidation from a chemical point of view, just as modern sensitivity analysis [32–34] as shown earlier can be used to designate similar steps according to the particular application of the mechanism. The next section will deal with other higher-order hydrocarbons.
In contrast to reaction (3.76), at high temperatures the thermal decomposition of the methane provides the chain initiation step, namely
![]() (3.87)
(3.87)
With the presence of H atoms at high temperature, the endothermic-initiated H2–O2 branching and propagating scheme proceeds, and a pool of OH, O, and H radicals develops. These radicals, together with HO2 (which would form if the temperature range were to permit reaction (3.76) as an initiating step), abstract hydrogen from CH4 according to
![]() (3.88)
(3.88)
where again X represents any of the radicals. The abstraction rates by the radicals OH, O, and H are all fast, with OH abstraction generally being the fastest. However, these reactions are known to exhibit substantial non-Arrhenius temperature behavior over the temperature range of interest in combustion. The rate of abstraction by O compared to H is usually somewhat faster, but the order could change according to the prevailing stoichiometry; that is, under fuel-rich conditions the H rate will be faster than the O rate owing to the much larger hydrogen atom concentrations under these conditions.
The fact that reaction (3.77) may not proceed as written at high temperatures may explain why methane oxidation is slow relative to that of other hydrocarbon fuels and why substantial concentrations of ethane are found [4] during the methane oxidation process. The processes consuming methyl radicals are apparently slow, so the methyl concentration builds up, and ethane forms through simple recombination:
![]() (3.89)
(3.89)
Thus methyl radicals are consumed by other methyl radicals to form ethane, which must then be oxidized. The characteristics of the oxidation of ethane and the higher-order aliphatics are substantially different from those of methane (see Section H1). For this reason, methane should not be used to typify hydrocarbon oxidation processes in combustion experiments. Generally, a third body is not written for reaction (3.89), since the ethane molecule's numerous internal degrees of freedom can redistribute the energy created by the formation of the new bond.
Brabbs and Brokaw [36] were among the first who suggested the main oxidation destruction path of methyl radicals to be
![]() (3.90)
(3.90)
where 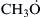 is the methoxy radical. Reaction (3.90) is very endothermic and has a relatively large activation energy (∼120 kJ/mol [4]); thus it is quite slow for a chain step. There had been some question [37] as to whether reaction (3.77) could prevail even at high temperature, but reaction (3.90) is generally accepted as the major path of destruction of methyl radicals. Reaction (3.77) can be only a minor contribution at high temperatures. Other methyl radical reactions are [4]
is the methoxy radical. Reaction (3.90) is very endothermic and has a relatively large activation energy (∼120 kJ/mol [4]); thus it is quite slow for a chain step. There had been some question [37] as to whether reaction (3.77) could prevail even at high temperature, but reaction (3.90) is generally accepted as the major path of destruction of methyl radicals. Reaction (3.77) can be only a minor contribution at high temperatures. Other methyl radical reactions are [4]
![]() (3.91)
(3.91)
![]() (3.92)
(3.92)
![]() (3.93)
(3.93)
![]() (3.94)
(3.94)
![]() (3.95)
(3.95)
![]() (3.96)
(3.96)
These are radical–radical reactions or reactions of methyl radicals with a product of a radical–radical reaction (owing to concentration effects) and are considered less important than reactions (3.77) and (3.90). However, reactions (3.77) and (3.90) are slow, and reaction (3.96) can become competitive to form the important methoxy radical, particularly at high pressures and in the lower-temperature region of flames (see Chapter 4).
The methoxy radical formed by reaction (3.90) decomposes primarily and rapidly via
![]() (3.97)
(3.97)
Although reactions with radicals to give formaldehyde and another product could be included, they would have only a very minor role. They have large rate constants, but concentration factors in reacting systems keep these rates slow.
Reaction (3.90) is relatively slow for a chain branching step; nevertheless, it is followed by the very rapid decay reaction for the methoxy (reaction (3.97)), and the products of this two-step process are formaldehyde and two very reactive radicals, O and H. Similarly, reaction (3.96) may be equally important and can contribute a reactive OH radical. These radicals provide more chain branching than the low-temperature step represented by reaction (3.77), which produces formaldehyde and a single hydroxyl radical. The added chain branching from the reaction path (reactions (3.90) and (3.97)) may be what produces a reasonable overall oxidation rate for methane at high temperatures. In summary, the major reaction paths for the high-temperature oxidation of methane are
![]() (3.87)
(3.87)
![]() (3.88)
(3.88)
![]() (3.90)
(3.90)
![]() (3.77)
(3.77)
![]() (3.97)
(3.97)
![]() (3.72)
(3.72)
![]() (3.74)
(3.74)
![]() (3.89)
(3.89)
![]() (3.49)
(3.49)
Of course, all the appropriate higher-temperature reaction paths for H2 and CO discussed in the previous sections must be included. Again, note that when X is an H atom or OH radical, molecular hydrogen (H2) or water forms from reaction (3.88). As previously stated, the system is not complete because sufficient ethane forms so that its oxidation path must be a consideration. For example, in atmospheric-pressure methane–air flames, Warnatz [38,39] has estimated that for lean stoichiometric systems about 30% of methyl radicals recombine to form ethane, and for fuel-rich systems the percentage can rise as high as 80. Essentially, then, there are two parallel oxidation paths in the methane system: one via the oxidation of methyl radicals and the other via the oxidation of ethane. Again, it is worthy of note that reaction (3.88) with hydroxyl is faster than reaction (3.49) so that early in the methane system CO accumulates; later, when the CO concentration rises, it effectively competes with methane for hydroxyl radicals and the fuel consumption rate is slowed.
The mechanisms of CH4 oxidation covered in this section appear to be most appropriate, but are not necessarily definitive. Rate constants for various individual reactions could vary as the individual steps in the mechanism are studied further.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.
