Chapter 37
Batteries
Battery power is mainly used for portability or stand-by (float) purposes. All batteries operate on one or another variant of the principle of electrochemical reaction, in which anode (negative) and cathode (positive) terminals are separated by an electrolyte, which is the vehicle for the reaction. This basic arrangement forms a “cell,” and a battery consists of one or more cells. The chemistry of the materials involved is such that a potential is developed between the electrodes which is capable of sustaining a discharge current. The voltage output of a particular cell type is a complex function of time, temperature, discharge history and state of charge.
The basic distinction is between primary (nonrechargeable) and secondary (rechargeable) cells. This section will survey the various types of each shortly, but first we shall make a few general observations on designing with batteries.
37.1 Initial Considerations
When you know you are going to use a battery, select the cell type as early as possible in the circuit and mechanical design. This allows you to take the battery’s properties into account and increases the likelihood of a cost-effective result, as otherwise you will probably need a larger, or more expensive, battery or will suffer a reduced equipment specification. Having made the selection, you can then design the circuit so that it works over the widest possible part of the battery’s available voltage range. Some of the cheaper types deliver useful power over quite a wide range, with an endpoint voltage of 60–70% of nominal, and some of this energy will be lost if the design cannot cope with it. Also, check that the battery can deliver the circuit’s load current requirements over the working temperature. This capability varies considerably for different chemical systems. Rechargeable batteries can often be recharged only over a narrower temperature range than they can be discharged.
Always aim to use standard types if your specification calls for the user to be able to replace the battery. Not only are they cheaper and better documented, but they are widely distributed and are likely to remain so for many years. You should only need to use special batteries if your environmental conditions or energy density requirements are extreme, in which case you have to make special provisions for replacement or else consider the equipment as a throwaway item.
37.1.1 Voltage and Capacity Ratings
Different types of battery have different nominal open-circuit voltages, and the actual terminal voltage falls as the stored energy is used. Manufacturers provide a discharge characteristic curve for each type which indicates the behavior of voltage against time for given discharge conditions. Note that the open-circuit voltage can exceed the voltage under load by up to 15%, and the operating voltage may be significantly less than the nominal battery voltage for some of the duration.
The capacity of a battery is expressed in ampere-hours (Ah) or milliampere-hours (mAh). It may also be expressed in normalized form as the “C” figure, which is the nominal capacity at a given discharge rate. This is more frequently applied to rechargeable types. Capacity will be less than the C rating if the battery is discharged at a faster rate; for instance, a 15 Ah lead-acid type discharged at 15 amps (1C) will only last for about 20 minutes (Figure 37.2).
Three typical modes under which a battery can be discharged are constant resistance, constant current and constant power. For batteries with a sloping discharge characteristic, such as alkaline manganese, the constant power mode is the most efficient user of the battery’s energy but also needs the most complex voltage regulating system to power the actual circuit.
37.1.2 Series and Parallel Connection
Cells can be connected in series to boost voltage output, but doing so decreases the reliability of the overall battery and there is a risk of the weakest cell being driven into reverse voltage at the end of its life. This increases the likelihood of leakage or rupture, and is the reason why manufacturers recommend that all cells should be replaced at the same time. Good design practice minimizes the number of series-connected cells. There are now several ICs which can be used to multiply the voltage output of even a single cell with high efficiency. It is not difficult to design a switching converter that simultaneously boosts and regulates the battery voltage.
Parallel connection can be used for some types to increase the capacity or discharge capability, or the reliability of the battery. Increased reliability requires a series diode in each parallel path to isolate failed cells. Recharging parallel cells is rarely recommended because of the uncertainty of charge distribution between the cells. It is therefore best to restrict parallel connection to specially-assembled units.
On the same subject, reverse insertion of the whole battery will threaten your circuit, and if it is possible, the user will do it. Either incorporate assured polarity into the battery compartment or provide reverse polarity protection, such as a fuse, series diode or purpose-designed circuit, at the equipment power input.
37.1.3 Mechanical Design
Choose the battery contact material with care to avoid corrosion in the presence of moisture. The recommended materials for primary cells are nickel-plated steel, austenitic stainless steel or inconel, but definitely not copper or its alloys. The contacts should be springy in order to take up the dimensional tolerances between cells. Single-point contacts are adequate for low current loads, but you should consider multiple contacts for higher current loads. The simplest solution is to use ready-made battery compartments or holders, provided that they are properly matched to the types of cell you are using. PCB-mounting batteries have to be hand soldered in place after the rest of the board has been built, and you need to liaise well with the production department if you are going to specify these types.
Rechargeable batteries when under charge, and all types when under overload, have a tendency to out-gas. Always allow for safe venting of any gas, and since some gases will be flammable, don’t position a battery near to any sparking or hot components. In any case, heat and batteries are incompatible: service life and efficiency will be greatly improved if the battery is kept cool. If severe vibration or shock is part of the environment, remember that batteries are heavy and will probably need extra anchorage and shock absorption material. Organic solvents and adhesives may affect the case material and should be kept away.
Dimensions of popular sizes of battery are shown in Table 37.1.
Table 37.1 Sizes of popular primary batteries


37.1.4 Storage, Shelf Life, and Disposal
Maximum shelf life is obtained if batteries are stored within a fairly restricted temperature and humidity range. Self-discharge rate invariably increases with temperature. Different chemical systems have varying requirements, but extreme temperature cycling should be avoided, and you should arrange for tight stock control with proper rotation of incoming and outgoing units, to ensure that an excessively aged battery is not used. Rechargeable types should be given a regular top-up charge.
In the early 90s, legislation appeared in many countries banning the use of some substances in batteries, particularly mercury, for environmental reasons. Thus mercuric oxide button cells were effectively outlawed and are not now obtainable. In Europe, this was achieved through the Batteries and Accumulators Directive (91/157/EEC).
This Directive also encourages the collection of spent NiCad batteries with a view to recovery or disposal, and their gradual reduction in household waste. In fact, what it has achieved is rather the development of alternative rechargeable technologies to NiCad, particularly NiMH and lithium. NiCads, though, are still widely used, despite the technical advantages of NiMH. The Batteries Directive is about to be updated and it is likely to propose the following changes:
• EU member states to collect and recycle all batteries, with targets of 75% consumer (disposable or rechargeable) and 95% industrial batteries
• no less than 55% of all materials recovered from the collection of spent batteries to be recycled.
In the UK in 1999, 654 million consumer batteries were sold, but the rate for recycling consumer rechargeables is a mere 5%, and less than 1% of consumer batteries are collected for recycling. On the other hand, more than 90% of automotive batteries are recycled and 24% of other industrial batteries. Clearly, for consumer batteries at least, a sea change in disposal habits is expected.
37.2 Primary Cells
The most common chemical systems employed in primary, nonrechargeable cells are alkaline manganese dioxide, silver oxide, zinc air and lithium manganese dioxide. Figure 37.1 compares the typical discharge characteristics for lithium and alkaline types of roughly the same volume on various loads.

Figure 37.1 Load discharge characteristics for lithium and alkaline manganese primary cells
37.2.1 Alkaline Manganese Dioxide
The operating voltage range of this type, which uses a highly conductive aqueous solution of potassium hydroxide as its electrolyte, is 1.3 to 0.8V per cell under normal load conditions, while its nominal voltage is 1.5V. Recommended end voltage is 0.8V per cell for up to 6 series cells at room temperature, increasing to 0.9V when more cells are used. The alkaline battery is well suited to high-current discharge. It can operate between -30 and +80°C, but high relative humidity can cause external corrosion and should be avoided. Shelf life is good, typically 85% of stored energy being retained after 3 years at 20°C. Standard types are now widely and cheaply available in retail outlets and it can therefore be confidently used in most general-purpose applications.
37.2.2 Silver Oxide
Zinc-silver oxide cells are used as button cells with similar dimensions and energy density to the older and now withdrawn mercury types. Their advantage is that they have a high capacity versus weight, offer a fairly high operating voltage, typically 1.5 V, which is stable for some time and then decays gradually, and can provide intermittent high pulse discharge rates and good low temperature operation. They are popular for such applications as watches and photographic equipment. Typical shelf life is two years at room temperature.
37.2.3 Zinc Air
This type has the highest volumetric energy density, but is very specialized and not widely available. It is activated by atmospheric oxygen and can be stored in the sealed state for several years, but once the seal is broken it should be used within 2 months. It has a comparatively narrow environmental temperature and relative humidity range. Consequently its applications are somewhat limited. Its open circuit voltage is typically 1.45V, with the majority of its output delivered between 1.3 and 1.1V. It cannot give sustained high output currents.
37.2.4 Lithium
Several battery systems are available based on the lithium anode with various electrolyte and cathode compounds. Lithium is the lightest known metal and the most electro-negative element. Their common features are a high terminal voltage, very high energy density, wide operating temperature range, very low self discharge and hence long shelf life, and relatively high cost. They have been used for military applications for some years. If abused, some types can be potentially very hazardous and may have restrictions on air transport. The lithium manganese dioxide (LiMnO2) couple has become established for a variety of applications, because of its high voltage and “fit-and-forget” lifetime characteristics. Operating voltages range from 2.5 to 3.5V. Very high pulse discharge rates (up to 30A) are possible. Widely available types are either coin cells, for memory back-up, watches and calculators and other small, low power devices; or cylindrical cells, which offer light weight combined with capacities up to 1.5 Ah and high pulse current capability, together with long shelf life and wide operating temperature range.
Other primary lithium chemistries are lithium thionyl chloride (Li-SOCl2) and lithium sulfur dioxide (Li-SO2). These give higher capacities and pulse capability and wider temperature range but are really only aimed at specialized applications.
37.3 Secondary Cells
There have historically been two common rechargeable types: lead-acid and nickel-cadmium. These have quite different characteristics. Neither of them offer anywhere near the energy density of primary cells. At the same time, their heavy metal content and consequent exposure to environmental legislation have spurred development of other technologies, of which NiMH and lithium ion are the frontrunners.
37.3.1 Lead-Acid
The lead-acid battery is the type which is known and loved by millions all over the world, especially on cold mornings when it fails to start the car. As well as the conventional “wet” automotive version, it is widely available in a valve-regulated “dry” or “maintenance-free” variant in which the sulfuric acid electrolyte is retained in a glass mat and does not need topping-up. This version is of more interest to circuit designers as it is frequently used as the standby battery in mains-powered systems which must survive a mains failure.
These types have a nominal voltage of 2V, a typical open circuit voltage of 2.15V and an end-of-cycle voltage of 1.75V per cell. They are commonly available in standard case sizes of 6V or 12V nominal voltage, with capacities from 1 to 100 Ah. Typical discharge characteristics are as shown in Figure 37.2. The value “C”, as noted earlier, is the ampere-hour rating, conventionally quoted at the 20-hour discharge rate (5-hour discharge rate for nickel-cadmium and nickel metal hydride). Ambient temperature range is typically from −30 to +50°C, though capacity is reduced to around 60%, and achievable discharge rate suffers, at the lower extreme.
Valve regulated lead-acid types can be stored for a matter of months at temperatures up to 40°C, but will be damaged, perhaps irreversibly, if they are allowed to spend any length of time fully discharged. This is due to build-up of the sulfur in the electrolyte on the lead plates. Self discharge is quite high—3% per month at 20°C is typical—and increases with temperature. You will therefore need to ensure that a recharging regime is followed for batteries in stock. For the same reason, equipment which uses these batteries should only have them fitted at the last moment, preferably when it is being dispatched to the customer or on installation.
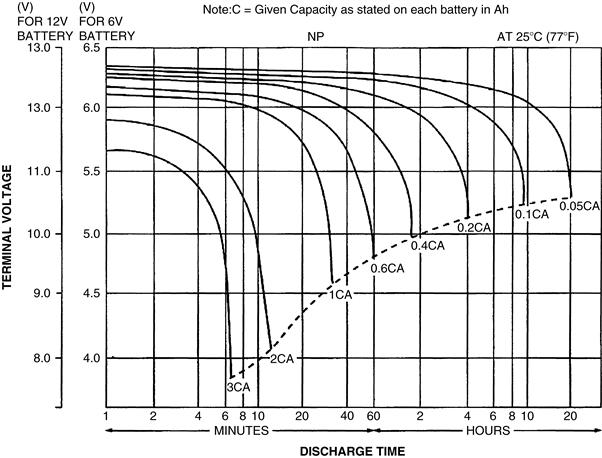
Figure 37.2 Discharge characteristics for sealed lead-acid batteries
Source: Yuasa (dotted line indicates the lowest recommended voltage under load)
Typical operational lifetime in standby float service is four to five years if proper float charging is followed, although extended lifetime types now claim up to fifteen years. When the battery is frequently discharged a number of factors affect its service life, including temperature, discharge rates and depth of discharge. A battery discharged repeatedly to 100% of its capacity will have only perhaps 15% of the cyclic service life of one that is discharged to 30% of its capacity. Overrating a battery for this type of duty has distinct advantages.
37.3.2 Nickel-Cadmium
NiCads, as they are universally known, are comparable in energy density and weight to their lead-acid competitors but address the lower end of the capacity range. Typically they are available from 0.15 to 7 Ah. Nominal cell voltage is 1.2V, with an open circuit voltage of 1.35–1.4V and an end-of-cycle voltage of 1.0V per cell. This makes them comparable to alkaline manganese types in voltage characteristics, and you can buy NiCads in the standard cell sizes from several sources, so that your equipment can work off primary or secondary battery power.
NiCads offer an ambient temperature range from −40 to +50°C. They are widely used for memory back-up purposes; batteries of two, three or four cells are available with PCB mounting terminals which can be continuously trickle charged from the logic supply, and can instantly supply a lower back-up voltage when this supply fails. Self-discharge rate is high and a cell which is not trickle charged will only retain its charge for a few months at most. Unlike lead-acid types they are not damaged by long periods of full discharge, and because of their low internal resistance they can offer high discharge rates. On the other hand they suffer from a “memory effect”: a cell that is constantly being recharged before it has been completely discharged will lose voltage more quickly, and in fact it is better to recharge a NiCad from its fully discharged condition.
However, NiCads are now frowned upon because of their heavy metal content and hence the environmental consequences of their disposal to landfill. They are largely being superseded by nickel metal hydride.
37.3.3 Nickel Metal Hydride
The discharge characteristics of NiMH are very similar to those of NiCad. The charged open circuit, nominal and end-point voltages are the same. The voltage profile of both types throughout most of the discharge period is flat (Figure 37.3). NiMH cells are generally specified from −20°C to +50°C. They are around 20% heavier than their NiCad equivalents, but have about 40% more capacity. Also, they suffer less from the “memory effect” of NiCads (see above). On the other hand, they are less tolerant of trickle charging, and only very low trickle charge rates should be used if at all.

Figure 37.3 NiCad and NiMH discharge characteristics
NiMH cells are available in a wide range of standard sizes, including button cells for memory back-up, and are also frequently specified in multi-cell packs for common applications such as mobile phones, camcorders and so on.
37.3.4 Lithium-Ion
The Lithium-ion cell has considerable advantages over the types described above. Principally, it has a much higher gravimetric energy density (available energy for a given weight)—see Figure 37.5, which compares approximate figures for the three types, drawn from various manufacturers’ specifications. But also, its cell voltage is about three times that of nickel batteries, 3.6–3.7V versus 1.2V. Its discharge profile with time is reasonably flat with an endpoint of 3V (Figure 37.4), and it does not suffer from the NiCad memory effect.

Figure 37.4 Li-ion discharge characteristics

Figure 37.5 Comparison of energy density vs. weight (approximate values)
These advantages come at a price, and Li-ion batteries are more expensive than the others. Also, they are much more susceptible to abuse in charging and discharging. The battery should be protected from over-charge, over-discharge and over-current at all times and this means that the best way to use it is as a battery pack, purpose designed for a given application, with charging and protection circuits built in to the pack. This prevents the user from replacing or accidentally degrading individual cells, and gives the designer greater control over the expected performance of the battery. Since the high cost of a Li-ion battery pack makes it more suited to high value applications such as laptops and mobile phones, the extra cost of the integrated control circuitry is marginal and acceptable.
37.4 Charging
The recharging procedures are quite different for the various types of secondary cell, and you will drastically shorten their lifetime if you follow the wrong one. The greatest danger is of over-charging. Briefly, NiCad and NiMH require constant current charging, while lead acid needs constant voltage.
37.4.1 Lead-Acid
The recommended method for these types is to provide a current-limited constant voltage (Figure 37.6). The initial charging current is limited to a set fraction of the C value, generally between 0.1 and 0.25. The constant voltage is set to 2.25–2.5V per cell, depending on whether the intention is to trickle charge or recover from a cyclic discharge. The higher voltage for cyclic recharging must not be left applied continuously since it will over-cook the battery. The actual voltage is mildly temperature dependent and should be compensated by −4 mV/°C when operating with extreme variations. This charging characteristic can easily be provided by a current-limited voltage regulator IC.

Figure 37.6 Current limited constant-voltage charging
An elegant modification to this circuit is to arrange for the output voltage to drop from the cyclic charge level to the trickle charge level when the charging current has decreased sufficiently, typically to 0.05C. This two-step charging gives the advantages of rapid recovery from deep discharge along with the benefit of trickle charging without threat to the battery’s life. It does not work too well when the load circuit is connected, though.
The cheap-and-cheerful charging method is to charge the battery from a full-wave or half-wave rectified AC supply through a series resistance. This is known as “taper” charging because the applied voltage rises toward the constant voltage level as the charging current tapers away. Since it is cheap it is very popular for automotive use, but manufacturers do not recommend it because of the risk of over-charging, and the undesirable effects of the AC ripple current. Fluctuations in the mains supply can easily lead to overvoltage and the end current cannot be properly controlled. If you have to use it, include a timer to limit the overall charging time.
Lead-acid batteries can be charged from a constant current, typically between 0.05 and 0.2C, subject to monitoring the cell voltage to detect full charge. The technique is not often used, but can be effective for charging several batteries in series at once.
37.4.2 NiCad and NiMH
Because the voltage characteristic during charging varies substantially and actually drops when the cell is over-charged, NiCad and NiMH batteries can only be charged from a constant current. Continuous charging at up to the 0.1C rate is permissible without damage to the cell. A 0.1C charge rate will recharge the cell in 16 hours, not 10, due to the inefficiency of the charging process. An accelerated charge rate of up to 0.3C is permissible for long periods without harm, but the cell temperature will rise when charging is complete. Higher rates for a rapid charge will work, but in this case it is essential to monitor the charging progress and terminate it before the battery overheats.
A series resistor from a voltage source at a significantly higher level than the battery terminal voltage, which can rise to 1.55V per cell, is an adequate constant-current charger. For tighter control over the charging current, and especially for rapid charging, a voltage/current regulator with a battery temperature sensor is needed. Several dedicated battery charge controller ICs are available, such as the TEA1100, MC33340, and LT1510 series, and the MAX713.
Occasional overcharging, in which a partly discharged battery is put back on charge for longer than necessary, will not have much effect; but repeatedly recharging an already full battery will damage it and reduce its lifetime.
For NiMH, continuous long term “trickle” charging at 0.1C or 0.05C is not recommended. If trickle charging is necessary by design, it should be kept to C/250 or less, sufficient to replace losses due to self discharge, but not enough to severely degrade the lifetime.
37.4.3 Lithium Ion
Because of the high energy density of Li-Ion cells, charging regimes must be very carefully controlled, both to get the best out of the battery and to prevent degradation and possible serious damage. In general it is best to integrate a constant voltage/constant current controller together with over-charge, over-discharge and over-current protection, along with the battery pack.
