Wearing Sensors Inside and Outside of the Human Body for the Early Detection of Diseases
Carmen C.Y. Poon1,2, Yali Zheng2, Ningqi Luo2, Xiaorong Ding2 and Yuan Ting Zhang2,3, 1Department of Surgery, The Chinese University of Hong Kong, Hong Kong SAR, China, 2Department of Electronic Engineering, The Chinese University of Hong Kong, Hong Kong SAR, China, 3Key Laboratory for Health Informatics of Chinese Academy of Science (HICAS), Shenzhen, China
Wearable systems have emerged as a promising area in healthcare research for the management of cardiovascular diseases such as atrial fibrillation and coronary heart diseases, as well as neurological diseases such as Parkinson’s disease, stroke, and epilepsy. Traditionally, wearable sensors were used on the body surface. Recent advancements in flexible electronics, however, have opened up a new era for wearing sensors inside the human body non-invasively, a research direction subtly but distinctively different from the invasive implantable systems. This new concept is particularly useful for sensing information inside the gastrointestinal (GI) tract because sensors designed and supported by flexible optoelectronic technologies are capable of conforming well and adhering to the irregular surface wall of the GI tract. Compared to sensors with traditional invasive designs, which require pins to anchor them on the GI wall, the new concept allows sensors to be worn non-invasively on the GI wall for the early and prompt detection of bleeding as well as better diagnosis and treatment of gastroesophageal reflux diseases. In summary, wearable sensory systems have huge potentials that are yet to be fully explored. It is anticipated that the advancements in this area will continue to transform how healthcare is delivered in the future.
Keywords
Wearable device; flexible electronics; mobile health (m-Health); health informatics; cuffless blood pressure; pulse transit time
1 Introduction
Advancing health informatics has been identified by the U.S. National Academy of Engineering as one of the fourteen grand engineering challenges of the twenty-first century, with wearable sensors and devices being highlighted as one of the key technologies in this field [1]. Wearable sensors and devices can be used to monitor chronic diseases continuously for early detection of symptoms as well as to capture acute and transient features that are difficult to be picked up during infrequent and ad hoc hospital visits.
The most classical example of wearable sensors for disease diagnosis is the Holter monitor or ambulatory electrocardiogram (ECG) monitoring device, which captures a subject’s electrical activities of the heart continuously over a 24-hour or even longer periods [2]. These systems have huge clinical applications since heart abnormalities are often paroxysmal and asymptomatic, and therefore, short-time and sporadic recording of ECG is often inadequate for detecting symptoms, while longer term ECG monitoring over several hours or even several days will provide better and accurate diagnosis. Since the Holter monitor was developed over 50 years ago, wearable sensors are advancing at a tremendously fast speed, demonstrating their potentials in various medical fields. In this chapter, recent advancements in wearable sensors for cardiovascular, neurological, and gastrointestinal diseases will be reviewed and discussed. In particular, the last section of this chapter outlines a promising future direction of wearable sensors, which by using recent flexible technologies, sensors can be worn non-invasively inside the human body for sensing information and dispensing drugs in the gastrointestinal (GI) tracts.
2 Cardiovascular Diseases
Cardiovascular diseases (CVDs) are the leading cause of death worldwide. About 17.3 million people died from CVDs in 2008, representing 30% of global deaths. Over 80% of deaths resulting from CVDs occurred in developing countries. It is anticipated that the number of people who die from CVDs will increase to 23.3 million by 2030, as reported by the World Health Organization [3]. The social and economic burden to society resulting from CVDs is huge worldwide.
CVDs are a collection of disorders of the cardiac system and blood vessels, including coronary heart disease, atrial fibrillation, heart failure, and peripheral arterial diseases. CVDs are mainly attributable to conventional risk factors, such as unhealthy diet, physical inactivity, tobacco use, and harmful use of alcohol [4]. These factors may present as high blood pressure (BP), raised blood glucose, and elevated blood lipids in the individuals. In particular, high BP, which is also known as hypertension, has been identified as a major dominant risk factor of CVDs, affecting 1 billion people globally [5]. One of the difficulties in controlling high BP is it usually develops without obvious symptoms or warning signs, hence people are usually oblivious and unaware of their high BP until severe symptoms appear. Hypertension is therefore known as the silent killer. In addition to absolute BP, BP variability within 24 hours has been reported to be an independent predictor of incidence of cardiac events [6]. Hence, long-term and continuous monitoring of BP can bring new insights into the cause of sudden cardiac deaths.
Prevention of CVDs partly relies on whether these abovementioned risk factors can be identified and controlled at an early stage. Wearable sensors, if designed to be worn unobtrusively, will encourage individuals to use them more frequently and will therefore increase the chance of detecting symptoms at an earlier stage. They also have the ability to disclose dynamic changes in physiological status and sense and monitor health status of individuals remotely.
2.1 Monitoring Risk Factors of Cardiovascular Diseases
In this subsection, monitoring of BP will be used as an example for discussion. It is now widely accepted that 24-hour ambulatory monitoring gives a better prediction of risk than office measurements and is useful for diagnosing white-coat hypertension, a phenomenon in which elevated BP is observed during clinical visits, but not in other settings [7]. Furthermore, continuous BP measurements have the advantage of detecting BP variability. Although the volume-clamp method has been used for beat-to-beat non-invasive BP monitoring, its cuff-based design prevents it from being used over extensive periods and is considered undesirable. Alternatively, a cuff-less BP measurement approach based on the measurement of pulse transit time or pulse arrival time has been proposed as a method for measuring BP without a cuff [8,9]. The method utilizes the fact that the transmission speed of the pulse along the arteries is related to pressure-dependent mechanical properties of the artery wall and therefore can be used as an estimate of BP. As shown in Figure 1, the sensors required for this method can be designed into a wearable garment or armband for the measurement of blood pressure [10,11].

In addition to using wearable sensors to monitor the cardiovascular parameters, there are also wearable sensors developed for fitness control and smoking supervision to alert the user to change his lifestyle as obesity and smoking are two major risk factors of CVDs. The mobile personal trainer (MOPET) system [12] has been investigated to supervise a physical fitness activity with motivation and health advice for the user. Lopez et al. [13] have recently developed an automatic wearable cigarette tracker. The tracker can detect smoking events through monitoring the cigarette-to-mouth hand gestures and recognize characteristic patterns of respiration during smoke inhalations. The system is flexible and non-invasive with applicability in free-living conditions over extended periods of time.
To summarize, wearable systems and related wireless technologies can offer the optimal solution for detecting the key risk factors of CVDs because they can provide continuous service almost anytime at anyplace with convenience, comfort and low cost, and summon medical assistance when required.
2.2 Diagnosis of Cardiovascular Diseases
Given the transient nature of symptoms of CVDs, wearable sensors and systems are also useful for improving the diagnosis of CVDs. Atrial fibrillation (AF) is the most common type of cardiac arrhythmia, caused by rapid, disorganized electrical impulses of the heart. AF can lead to stroke and heart failure. The incidence of AF is higher in elderly adults, and is often a complication after cardiac surgery without accompanying symptoms [14]. AF can be diagnosed from ECG, usually with the absence of P waves, rapid heart rate and irregular heart rhythms. The incidence, time, symptoms, and risk factors of AF can be monitored and analyzed by using a wearable cardiac event recorder [15]. Equipped with an automatic warning expert system, it can activate the emergency medical alarm system, and thus allow pre-emptive actions to be taken with an aim of preventing sudden deaths.
In addition to the diagnosis of AF, ECG can also provide important information for diagnosing and assessing other major risk factors of chronic cardiac diseases. Wearable systems for collecting ECG have been developed from e-textile materials, e.g., in the form of smart shirts [11]. The most important components of wearable monitoring systems are the wearable sensors, which are attached to the individual by integration to the wearable garment [16]. The fabric-based active electrodes have been developed to embed into clothing for ECG monitoring [17], e.g., based on a compact planar-fashionable circuit board [18]. Figure 2 shows a number of wearable ECG systems that have been developed, e.g., Biotex [19], Smart Vest [20], Lobin [21], Wealthy [22], and Protex [23]. Many of these systems have integrated sensor design to allow them to measure not only ECG but also other physiological signals such as photoplethysmogram (PPG) and respiration.

Coronary heart disease (CHD), also known as coronary artery atherosclerosis, is caused by the blockages of blood vessels of the heart resulting in chest pain due to a lack of blood supply to the heart, i.e., angina. With uncontrolled blockage, parts of the heart may die, resulting in myocardial infarction (MI). Although many people with CHD have symptoms such as chest pain or shortness of breath, CHD can also be asymptomatic and can lead to sudden deaths as a result of rupture of atherosclerotic plaque. Major risk factors of CHD include age, high BP, smoking, and diabetes. Identification of individuals subject to high risks of CHD helps to create management plans for controlling the risk factors for early intervention. It has been reported that the baseline ECG abnormalities [24] and pulse pressure [25] can be used for screening risks of CHD.
Peripheral arteries disease (PAD) is a slowly progressive disease mainly due to atherosclerosis and further affects vessels such as the aorta and arteries of the lower extremities. PAD can lead to CAD, stroke, MI, and death from other vascular causes. Hypertension, diabetes, and smoking are the key risk factors associated with PAD [26]. Ankle-brachial index (ABI), the ratio of the BP in the lower legs to the BP in the arms, is a widely used index to assess asymptomatic PAD. PAD is determined with ABI<0.9 in either of the lower limbs. Early determination of PAD is necessary for timely intervention to improve prognosis. As technology for sensing PPG becomes mature, it is possible to build wearable systems for diagnosis of PAD [27], which is a promising alternative to the more costly and less convenient measurement method of ABI by continuous-wave Doppler.
In addition to measuring ABI, wearable systems comprised of PPG sensors can also provide a patient’s heart rate, oxygen saturation, and heart rate variability. PPG detects blood-volume changes in the micro-vascular bed of tissue. It has the advantages of being miniature and lightweight. Various PPG wearable systems have been developed based on different configurations, e.g., in the form of a ring, ear-wearable gadget, eyeglass, and garment. One of the pioneer developments in wearable devices is the PPG sensor ring developed by Asada et al. [28]. Several studies focused on PPG monitoring systems as ear-worn devices, such as the wearable in-ear measuring system (IN-MONIT) with micro-optic reflective sensor [29], a motion-tolerant magnetic earring sensor with an adaptive noise cancellation method [30], and an earphone system connected to a mobile phone [31] have been done. Wearable systems in the form of garments with sensors embedded in a hat, glove, and sock have also been developed [32]. Objects such as eyeglasses [33] are also a good platform for PPG monitoring with discomfort reduced by clips to the finger or ear. Figure 3 shows examples of some of the state-of-the-art wearable sensors for PPG measurement.
2.3 Summary and Future Development
Despite advancements in medicine and technologies, CVDs remain one of the major leading causes of death worldwide. CVDs such as AF and MI are transient in nature and therefore the use of unobtrusive wearable systems in an out-of-hospital setting to collect information for diagnosis has obvious advantages over the classic bulky alternatives that can be used only in the hospital. In the future, it is anticipated that these sensors will be seamlessly fused with our daily lives to enable prompt and pervasive management of cardiovascular diseases as well as many other abnormal health threatening conditions.
3 Neurological Diseases
Neurological diseases such as Parkinson’s disease and stroke are amongst the major causes of disability. Neurological rehabilitation provides therapeutic exercise to help patients to restore motor functions. Due to the shortage of hospital-centered rehabilitation resources, home-based rehabilitation therapies have gained interest. In this respect, wearable devices can play a role by providing remote monitoring in home settings of the mobility and physical functioning of patients with neurological disorders. It can significantly reduce healthcare cost and is useful in early symptom detection, which makes prompt and effective intervention possible. Since the present clinical tools for the assessment of these patients’ physical performance are either based on self-report or observation, wearable sensors can also provide an objective alternative to assessing the condition of these patients by monitoring their daily activities. Most importantly, incorporated with actuators, wearable devices (or wearable robots) can be used as rehabilitative and assistive devices for disabled people for the treatment of motor disorders. Furthermore, wearable devices can be used for the prediction of sudden unexpected events related to chronic neurological diseases such as seizures. By continuous monitoring with wearable devices, some transient and covert features or symptoms related to these acute events can be captured for the understanding of pathology and the prediction of incidents.
3.1 Motor Activity Monitoring and Intervention for Neurological Rehabilitation
Micro-electromechanical inertial sensors such as accelerometers (ACCs) and gyroscopes are widely adopted for the monitoring of motor activities. ACC sensors measure changes in velocity and displacement while gyroscopes measure changes in orientation such as rotational displacement, velocity, and acceleration. These sensors have been extensively used for the continuous and automatic monitoring of movement disorders and functional activities [34]. Patel et al. first developed an integrated platform (SHIMMER) with a wearable system and algorithm to estimate the severity of three different Parkinson’s symptoms (i.e., tremor, bradykinesia, and dyskinesia) [35], as shown in Figure 4(a). Each sensor node in the SHIMMER platform consists of a triaxial ACC sensor, a microprocessor, a radio transmitter, and a MicroSD card slot. Three feature types, including root-mean-square value, the data range value, and two frequency-domain features, were extracted to estimate the clinical score from the ACC signals recorded from patients during ten different motor tasks. The platform showed promising estimation results in terms of clinical score compared to that derived from visual inspection of video recordings. Recently, the same research team developed a Web-based system (MercuryLive) based on SHIMMER for the monitoring of patients with Parkinson’s disease in home settings [36]. It contains three tiers, including central server, patient host, and clinician host. The clinician tier can access the sensor data to estimate the clinical score remotely. Rigas adopted a set of wearable sensors to detect and assess tremor in a ubiquitous environment [37]. Two sets of features extracted from tremor activity (3–12 Hz component in the measured ACC signals) were incorporated into a hidden Markov model for tremor severity recognition, and the results showed high accuracy in tremor quantification and high specificity in distinguishing tremor activity from other motor symptoms. A wearable shoe-based device shown in Figure 4(b) has also been designed for the rehabilitation of stroke patients [38].

Other novel-sensing methods have also been proposed to measure motor activities. A wearable system using a capacitive sensing method for locomotion monitoring and classification has been developed in [39]. The wearable strain-sensing shirt in Figure 5 with conductive elastomers distributed over arm, forearm, and shoulders was designed for motion analysis in the context of neurological rehabilitation [40]. A set of representative rehabilitation exercises (i.e., gleno-humeral flexion of shoulder on a sagittal plane, lateral abduction, and external rotation) was designed to evaluate the performance of the system in terms of posture recognition and classification.

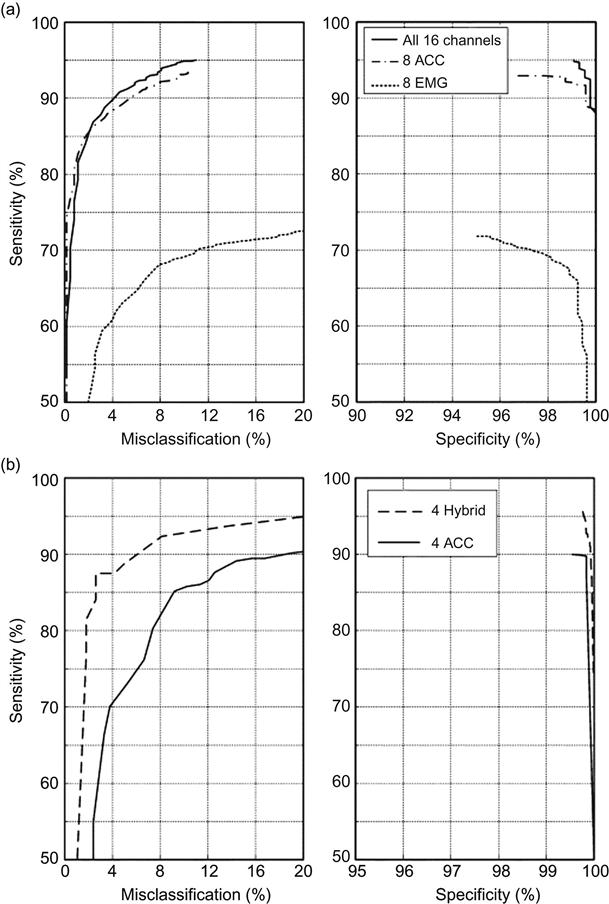
In terms of the classification of various motor activities, ACC sensors have inherent limitations in differentiating between an active versus a passive performance of a movement. EMG, which is monotonically related to muscle torque, has the advantage to distinguish between active and passive movements. Therefore, the combination of EMG and ACC information is expected to significantly improve the classification of performance. Roy et al. investigated the feasibility of a hybrid-surface EMG (sEMG) and ACC wearable sensor system for automatic classification of daily activities in patients with stroke [41]. The results showed that the hybrid configuration can achieve a mean sensitivity and specificity of 95% and 99.7% for the identification tasks and misclassification error of less than 10% for non-identification tasks. A comparison of the classification performance of the different configurations is shown in Figure 6. The results of this study proved that the inclusion of both EMG and ACC can achieve higher classification accuracy.
In addition to motor activity monitoring, wearable systems can also be designed with real-time feedback to control human-machine interaction to assist daily activities for patients suffering from motor disorders. Tremor is considered the most common motor disorder caused by neurological diseases, and a variety of wearable systems for tremor assessment and suppression have been previously developed [42,43]. A representative wearable robotic exoskeleton for orthotic tremor suppression (WOTAS) [44] was designed to measure and suppress tremor by using two gyroscopes placed distally and proximally to the elbow joint. To depress tremor without affecting concomitant voluntary movement, one critical issue is tremor characterization, i.e., extracting instantaneous tremor parameters from the raw motion data to generate control command to drive a neuroprothesis or the human muscle itself in real time. Tremor was modeled as a sinusoidal signal of frequency ω0 plus M harmonics to calculate the estimation error εk as follows:
(1)
The tremor frequency and amplitude can then be estimated by recursive method, i.e.,
(2)
 (2)
(2)
Separation of the voluntary and tremorous movement is needed before the characterization. Active and passive tremor reduction strategies, i.e., impedance control and notch filtering, were implemented and validated on the system and can achieve 40% of tremor power reduction for all users [44]. Similar wearable biofeedback systems have also been developed, such as an EMG-controlled exoskeletal orthosis wearable system for exercise training after stroke [45], wearable intelligent systems with real-time vibro-tactile feedback for the training of motor functions [46], and for posture correction [47] in rehabilitative and protective applications.
3.2 Seizure Activity Monitoring for Epilepsy Patients
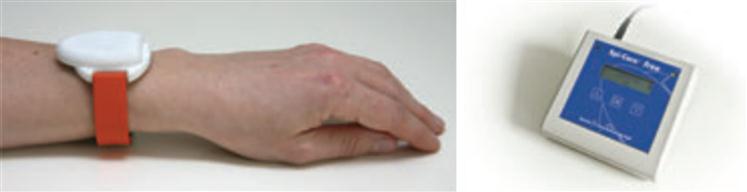
Mechanisms leading to sudden and unexpected death following a seizure in patients with chronic epilepsy are not fully understood in medicine [48]. Cardiac arrhythmia, respiratory dysfunction, dysregulation of systemic or cerebral circulation, and seizure-induced hormonal and metabolic changes can be potential pathomechanisms of the disease. The occurrences of seizures are unpredictable and random. For inpatients, the forewarning of seizure activity is often monitored by EEG video hospital systems, which is a bulky system that greatly restricts the activities of the patient. Wearable devices with ACC sensors are another promising alternative for seizure-activity monitoring with predefined algorithms for real-time analysis of the ACC signals. Sensitivity and false alarm rate are the most important indices of wearable systems for these applications. A user-friendly designed wrist-type device for seizure detection was developed by Danish Care Technology ApS, which contains a three-axis ACC sensor, a microprocessor, and battery, as shown in Figure 7 [49]. A number of clinical studies have been conducted to validate the feasibility and accuracy of this sort of wearable device. In a prospective multi-center study including 73 patients of generalized tonic-clonic seizures (GTCS), this wrist-type device showed a mean sensitivity of 91% and a false alarm rate of 0.2/day. Another clinical study [50] showed that a bracelet alarm device with a three-axis for epilepsy monitoring could identify tonic, clonic, and tonic-clonic seizures with similar sensitivity (20 of 22 seizures) and low false alarm rate (8 false alarms during 1,692 hours of monitoring).
Patients with GTSC have higher risk for injuries and sudden deaths that cannot be detected by the abovementioned devices [49]. Poh et al. recently developed a wrist-worn device with skin conductance electrodes for long-term electrodermal activity (EDA) recordings that reflected sympathetic nerve activities [51]. The performance of this device was compared to an FDA-approved device during the baseline, task, and recovery conditions and found high correlations in all states. The performance of the device with two different electrode materials, i.e., Ag/AgCl and conductive fabric electrodes, was also compared and the results showed that the fabric electrodes performed as well as Ag/AgCl under baseline state, but were less promising during stressor task. This wearable device opens up opportunities to monitor autonomic nerve activities unobtrusively during daily activities, which would be very meaningful for the long-term monitoring of psychological and neurological conditions. The author conducted a clinical study to explore the clinical value of the EDA recordings in the prediction of sudden death in epilepsy [52]. As shown in Figure 8, high correlation (r=0.81, p=0.003) was found between the increase in EDA amplitude and the duration of EEG suppression, which has been reported in a previous study being significantly prolonged in earlier GTCS of patients who died later of epilepsy-related sudden death. The findings of this study indicate that sudden death in epilepsy may be correlated with postictal autonomic dysfunction, and autonomic parameters such as EDA amplitude might be able to provide valuable information regarding the severity of the seizure for prompt decision and action.

3.3 Summary and Future Development
This section presents an overview of recent developments in the field of wearable sensors and systems related to the monitoring and intervention of neurological diseases such as stroke, Parkinson’s disease, and epilepsy. At present, applications mainly focus on the remote monitoring and assessment of motor functions as well as real-time intervention during the rehabilitation process. An emerging application in this area of wearable devices is to capture critical moments and extract information related to the occurrences of acute events for predicting future events. For future developments, these systems should be designed to be applied comfortably on a daily basis. A leading EU-funded project in this area was launched aiming to develop unobtrusive systems for monitoring of daily activities and training of motor functions for stroke survivors using smart-textile technology implemented on shoes, trousers, shirts, and gloves [53]. With recent advances in flexible electronics, skin-attachable devices such as the epidermal electronics, which can measure EMG and other electrophysiological signals on the skin [54], and the electronic artificial skin (e-skin) using organic materials, also provide promising solutions for unobtrusive implementations of these systems [55].
4 Gastrointestinal Diseases
Although the gastrointestinal (GI) tract is an organ situated inside the human body, it is exposed to the external environment as much as the human skin. Conventionally, sensing inside the GI tract is accomplished by implantable devices, e.g., esophageal pH monitoring, which is the gold standard for diagnosing gastroesophageal reflux disease (GERD) [56]. GERD is a chronic symptom of mucosal damage caused by stomach acid coming up from the stomach into the esophagus. It is increasingly recognized worldwide for its linkage to the development of esophageal carcinoma. The majority of GERD cases do not have endoscopic abnormalities and a complete evaluation with biopsies, esophageal motility, and 24-hour ambulatory pH monitoring are frequently needed before therapy is initiated. An ambulatory wireless pH monitoring system called Bravo™ (Given Imaging Ltd.) can be clipped to the lining of the esophagus for continuously measuring pH over a 48-hour period in an ambulatory setting such that acid reflux variables, including total reflux time, number of reflux episodes, and total percentage time of pH<4, can be estimated and collected as the symptom-association probability scores for the diagnosis of GERD [57]. The sensor eventually falls off the esophageal lining after several days and is passed in the stool.
In order to anchor the sensor to the esophagus, the sensing capsule is designed with a pin. Recent advances in flexible optoelectronics, however, have opened up a new direction for designing wearable sensors to be worn non-invasively on irregular body surfaces or the GI wall for the early and prompt detection of diseases. In this section, designing flexible wearable sensors will be briefly discussed.
4.1 On-Body Wearable Sensor Design Based on Flexible Electronics
Wearable sensors can be fabricated on flexible substrate with a reasonable degree of stretchability such that they can conform to the complex and intricate body surface [55]. At present, studies in this area are focused on the design of on-body sensors, e.g., a high-pressure sensitivity sensor based on flexible polymer transistor for measuring blood pressure [58,59] and multi-sensor epidermal electronics system (EES) based on flexible silicone substrate [54,60]. The integrated EES is designed with temperature sensor and electrodes for measuring ECG/EMG with an aim to adhering to the human skin for monitoring health conditions in our daily lives. An ultra-thin sensing film for mobile electronics application, healthcare, and biomedical systems has also been proposed [61].
4.2 In-Body Chemical and Biological Flexible Sensors and Systems
Chemical and biological sensors are widely used in many different medical fields, including the sensing of pH inside the GI tract [62]. Chemical sensors measure concentration of a specific component of a chemical reaction while a biological sensor is used to detect analytes, such as proteins, DNA, and antibodies based on physiochemical measurements. Figure 9 shows a typical sensor based on a field-effect transistor (FET).
The channel current of the sensor shown in Figure 9 can be described by the following equation [63]:
(3)
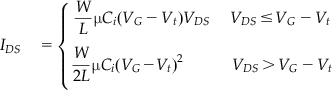 (3)
(3)
where IDS, VG, and Vt are channel current, gate voltage, and threshold voltage, respectively, W and L are the width and length of the channel, respectively, μ is the carrier mobility, and Ci is the capacitance of the gate insulator. When there is a change in chemical concentration or a component, drain and source current IDS will change, as the chemical diffuses into the semiconductor layer and changes the carrier mobility (μ) and threshold voltage (Vt). Information about the chemical reaction of a system can therefore be reflected by the chemical concentration or component. The conductivity of an organic semiconductor is sensitive to ion-doping. Therefore, some organic field-effect transistors have been used for ion sensing. For example, poly(3-hexylthiophene-2,5-diyl) can be used as an active layer of the field-effect transistor to detect the concentration of K+ and H+ ions. This type of sensor can be useful for measuring the acidity in the GI tract, where gastric acid, a digestive fluid formed in the stomach, is composed of hydrochloric acid (HCl) (around 0.5%, or 5,000 parts per million) as high as 0.1 M, potassium chloride (KCl), and sodium chloride (NaCl).
The sensor and supporting components together form an electronics system, as illustrated in Figure 10. The sensor is the key component of the electronics system, defining the function of it, while the interconnector connects the sensor to other sensors or contact pads, and the substrate supports the sensor and the device as a whole. Compared to integrated circuits, the sensor is analogous to the die, the substrate is analogous to the frame, and the interconnector is the wire bond.
4.2.1 Technical Challenges for Fabricating Flexible Sensory Systems
Two major challenges arise for fabricating flexible electronics systems: 1) to maintain high performances while keeping the system flexible, and 2) to transfer the system and adhere it to the body surface seamlessly and firmly.
4.3 Design of High-Performance Flexible Sensory Systems
Since bending or changes in the shape of the system will affect the performance of the sensor, the sensor should be designed as a rigid component. When the sensor is kept small enough, the irregular body surface will appear as a smooth plane to the sensor, even though the body surface such as the human skin will have wrinkles, creases, and pits with amplitudes and feature sizes of 15 to 100 µm and 40 to 1,000 µm, respectively.
In order to keep the entire system as flexible as possible, the substrate and interconnector should be made of flexible materials with a reasonable degree of stretchability. Polydimethylsiloxane (PMDS) is a suitable choice for use as flexible substrate. PDMS (A:B=30:1) has low Young’s modulus of 145 kPa at the thickness of 0.6 mm, which is similar to human epidermis modulus, 140 to 600 kPa [54]. Low modulus assures the PDMS film has high stretchability. Meanwhile, PDMS has good biocompatibility, which will ensure the flexible system can be used for in vivo applications.
The challenge for designing the interconnector is to ensure stretchability while maintaining conductivity. In particular, the stretching of solid material lengthens chemical bonds and results in changing the distance between atoms and decreasing conductivity. Although crystalline structures of metals make them a good electricity conductor, they are hard to mold since the internal bonds are unbendable. Therefore, good conductor materials do not stretch well and stretchable materials are not good conductors. Recent studies reported the use of cross-stacked super-aligned carbon nanotube films [64] and the polymer-embedded carbon nanotube ribbons [65] as the conductor materials. Another study reported the development of a high conductivity and stretchability polymer by doping Au nanoparticles in polyurethane and suggested that the high conductivity of the polymer under stretch results from the dynamic self-organization of the nanoparticles under stress [66]. A simpler way has also been proposed by using conventional gold conductor and changing the straight line to filamentary serpentine shape [60].
4.4 Transfer and Adherence of the Flexible System to Irregular Body Surface Seamlessly and Firmly
Ensuring good contacts to the body surface is another challenge in designing flexible electronics systems, such that they can adhere to the skin seamlessly and firmly to reduce the effect of motion artifacts.
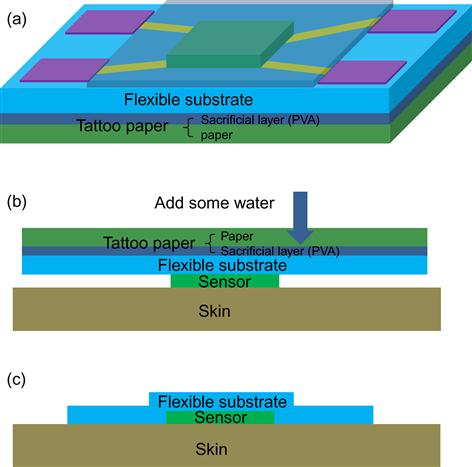
Since the substrate is flexible, if it is thin enough, it can be designed with an adhesive tape to attach the system to the body surface by van der Waals interactions. Nevertheless, the thin substrate will easily collapse if it is not supported by a strong material. Tattoo papers have been therefore proposed to be used as a support structure [67]. The tattoo paper is covered by a thin sacrificial layer of PVA, which is water soluble. The transferring process is illustrated in Figure 11. The device is fabricated on the flexible substrate, which is supported by tattoo paper and transferred onto the skin. When water is rushed on the surface, the water-soluble sacrificial layer of PVA will detach from the tattoo paper and adhere to the body surface.
Though this device-transferring technique is especially suitable for epidermal electronics, it can also be used for sensing inside the human body. For example, fabrication of a flexible electrode array on thin polyimide substrate for mapping brain activity in vivo has been reported [68].
Fabrication of flexible and wearable sensors to seamlessly and non-invasively attach to the GI tracts for detection of bleeding and pH is still at the research stage. There are several major technical challenges that need to be overcome. First, in order to ensure good contact with the irregular GI tract and to maintain robust measurement during GI peristalsis, fabrication of flexible and stretchable sensors of different types is needed, e.g., flexible and stretchable light-emitting diodes and optical sensors to detect bleeding. Second, materials used to support the flexible electronics specialized for adhesion to the mucosa layer of the GI tract need to be further studied. At present, there are several types of muco-adhesive materials commercially available on the market. Previous studies compared the performance of some of them, such as Carbopol 971 P, polycarbophil, Carrrageenan type λ, and Sodium carboxymethylcellulose [69]. Carbopol 971 P, a high molecular weight cross-linked polymer of acrylic acid (Noveon Inc.), is one of the outstanding materials. It can attach to the mucus via physical bonds [70], and its powdered polymers have a long history of safe use in cosmetic and pharmaceutical products [71]. Third, transfer of the sensors to the GI wall will require novel and skillful endoscopic techniques as this is clearly much more difficult than using them as EES. Overall, development of sensors worn on the inside of the body is a multi-disciplinary problem with high research potential.
5 Conclusion
Health informatics, particularly wearable sensors and systems, have emerged as a promising field of research. Conventionally, wearable and implantable sensory systems were thought to be designed for sensing information on the body surface and inside the human body, respectively. Recent advances in flexible optoelectronics have, however, opened up a new phase where sensors can be worn non-invasively inside the human body, particularly along the GI tract, for sensing information as well as dispensing drugs. This advancement will refine the scope of wearable systems and enhance the potential applications for early detection of cardiovascular, neurological, and gastrointestinal diseases.
Acknowledgment
This work was supported by the Hong Kong Innovation and Technology Commission (ITS/159/11 and ITS/197/12), CUHK Direct Grant (No. 4054058), and in part by the 973 Project Fund (2010CB732606) and the Guangdong LCHT Innovation Research Team Fund in China.