Chapter 14
Photographic Emulsions, Films, and Papers

Photograph by Josh Shagam, Biomedical Photographic Communications student, Rochester Institute of Technology.
Photographic Emulsions
A photographic emulsion consists of silver halide crystals suspended in gelatin. The emulsion is usually coated on a support that may be clear, as in the case of negative films, or opaque, as for photographic prints. The emulsion has the most significant controlling effect on the photographic and physical properties of the final product.
To visualize the emulsion, think of a chocolate candy bar with peanuts imbedded in it as shown in Figure 14 1. The peanuts are suspended in the chocolate medium. Similarly, the silver halide particles are suspended in gelatin. There are many different recipes for making photographic emulsions. Choice of gelatins and different combinations of salts (sodium, potassium, ammonium, or calcium and chloride, bromide, or iodide, for example), along with other chemical ingredients, impart characteristics to the emulsion such as speed, spectral sensitivity, contrast, resolution, graininess, and physical properties. The emulsion is applied to the film or paper in a liquid state, and thickness of the emulsion layer is adjusted by controlling viscosity and speed of coating. The gelatin is then chilled (just as a gelatin dessert is when placed in the refrigerator), and then slowly dried to remove most of the water.
Silver is used in photographic emulsions because its compounds are more sensitive to light than any other photochemical material.
Cellulose nitrate, which is chemically similar to guncotton, was not the safest support for photographic films.
The silver halides respond to light to produce a latent image that is later developed to produce a visible silver image. The gelatin also acts as a binder, serving to protect the silver halide from abrasion and other mechanical and chemical influences, and in some cases the gelatin can even serve as the support for the image. Through impurities and other characteristics, the gelatin contributes to the photographic and chemical performance of the silver halides in the emulsion. Silver is used because its compounds are more sensitive to light than any other photochemical material.
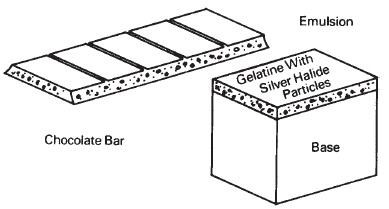
Figure 14-1 The photographic emulsion can be compared to the chocolate nut bar. The nuts are suspended and separated by the chocolate as the silver halide grains are in the emulsion.
Safety Base Films
Very early cellulose nitrate film bases were highly flammable. Frequent fires occurred where large amounts of film were stored, such as movie theaters and X-ray films in hospitals. The Eastman Kodak Company developed a nonflammable cellulose acetate safety film very early in the 1900s, but it was not accepted by the motion picture industry because of cost; and some of the other physical characteristics, such as wear on repeated showings, were not as good as those of the nitrate film. With more experience and further research and development, improved versions of acetate films were produced, and they are still the best support for commercial motion picture film.
Nitrate base of greater thickness was also used for early sheet films, replacing glass plates. These nitrate films were supplanted by acetate bases when this type of material became available. While acetate bases had better dimensional stability during processing and storage than did nitrate bases, they were still far from ideal for some applications. They were less flexible, more brittle, and did not wear as well as the nitrate films.
Polyester Film Bases
In recent years many of the products previously manufactured with acetate base have been produced on polyester bases, which are made in an entirely different manner. These are manufactured by extruding the heated polyester material through a slot, stretching it in a longitudinal direction at the same time that it is stretched by means of grippers in a widthwise direction while still heated and soft, as shown in Figure 14-2. This orients the molecules of plastic, yielding a base material that is free from stresses and strains, and that has very good dimensional stability and certain other characteristics.
One problem with this type of base is the need of a suitable subbing so that the emulsion will adhere properly during the life of the material. While the techniques of subbing for good adhesion have been perfected for the old solvent cast base materials, there have been some lingering problems with the newer polyester materials, leaving some doubt as to their suitability for archival storage of images. These problems have been resolved, and newer polyester materials are considered to be suitable for archival storage. Another early problem with the polyester films for motion picture applications was that of splicing, but this has been solved by new heat and tape splicing techniques (in those applications where the stronger base might be required).
Such polyester films can act like fiberoptics and pipe light in a transverse direction, thus not serving well as a self leader when the film itself is intended to protect the image forming part of the film from ambient light when on a daylight loading spool. In many applications, light piping of films is eliminated by introducing dyes or pigments into the base itself, as shown in Figure 14-3. Some light is absorbed in the viewing direction, where the density of the base may be on the order of 0.1 to 0.2, and the thickness of the base may be around 0.002 to 0.004 inch. In the crosswise direction, the density adds up very rapidly, and thus absorbs most of the light traveling in this direction.

Figure 14-2 Polyester film base manufacture.
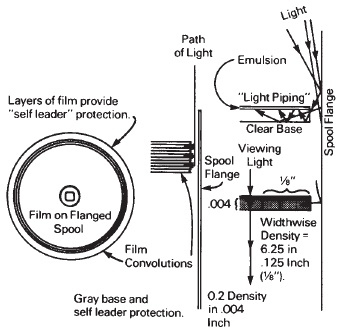
Figure 14-3 Daylight loading spools depend on the protection provided by several convolutions of film (4 to 7 feet in length). The necessary light stopping density is provided by the combination of emulsion, antihalation protection, and base density. Light entering between the film and the spool flange may be decreased by incorporating a pigment or dye in the base. The density in the viewing direction is relatively low, but adds up rapidly in the widthwise dimension.
Polyester films also have a greater tendency to generate static electricity— they are good insulators—and this makes them more susceptible to picking up dust and lint from the atmosphere. They are also more susceptible to scratching, and readily show scratches on viewing the photographic images if the base is not protected by a gelatin NC (non-curl) or antihalation coating. In the beginning, their very high tensile strength prevented them from breaking when trouble occurred in processing machines and similar equipment, and therefore the machinery was damaged rather than the film. The solvent cast acetate types of film are still largely used for commercial and motion-picture applications, and for most roll films.
Polyester film base materials have high strength, good dimensional stability, and other desirable characteristics, but they also tend to generate more static electricity, scratch more easily, and pipe light in from the edges.
The resin coating on the paper base of RC papers protects it from processing solutions and therefore shortens processing time.
Film Structure
A photographic film can be very simple in structure—a coating of a gelatin—silver halide emulsion on a transparent base, such as positive film, used for printing black-and-white motion pictures. Negative films used for pictorial photography are generally more complex in their structure. In many films two or more coatings of emulsion are used to derive the sensi-tometric characteristics that yield good pictorial tone rendition. On top of this there is generally an overcoating or surface layer that controls many of the physical characteristics of the film.
Paper Bases
Most photographic prints are made on some form of paper base. Traditionally this type of base was manufactured with pulp made from old rags, but in more recent years practically all of it has been made from a high-quality wood pulp (alpha cellulose). These are referred to as fiber-base papers. Many photographs are made on paper that has been coated with a plastic or resin (such as polyethylene) on both sides. These are referred to as RC (resin-coated) papers. The resin surface coating of the stock protects it from the chemical solutions and water of processing, so that processing, washing, and drying times can be much shorter than with the fiber-base papers. Because of the problems of emulsion adhesion to polyethylene coatings, fiber-base papers are still preferred for producing photographs with archival permanence.
Several paper surfaces are produced by embossing the stock with an engraved roller under pressure to impart a distinctive textured surface pattern. These patterns can be irregular, but some are geometric in nature, for example, “silk" and “linen" surfaces. Any of the above can be coated with one or more layers of barium sulfate (baryta) and/or other white pigments (sometimes before embossing) before coating with photographic emulsion. This imparts a better, brighter color to the base, and also limits the penetration of the emulsion into the base, which gives a more uniform coating and a more even image, with more uniform blacks. The nature of the baryta coating also affects the reflectivity of the coating's surface. The baryta coating is omitted from photographic papers that may be folded in use, to prevent the emulsion layer from cracking.
Brighteners
It is common to incorporate optical brighteners in both nonbaryta-coated and baryta-coated paper surfaces, producing the effect of intensifying the paper's brightness. These are similar to laundry brighteners. The effect is produced by using a dye or other substance that fluoresce upon exposure to ultraviolet energy (available to some extent from most light sources) to convert the invisible ultraviolet radiation to light.
Paper Weight and Thickness
Photographic papers are manufactured in various “weights." These are referred to in such terms as single weight, document weight, light weight, medium weight, and double weight (see Figure 14-4). Paper stock is customarily manufactured, controlled, and sold in terms of weight, but photographers are more aware of the different thicknesses of photographic paper bases. Hence, international standards designate papers in terms of their thicknesses. American National Standard PH1.1-1974, Thickness of Photographic Paper, Designation For, lists nine groups of paper thicknesses, with ranges for each group both in English and in metric units, and gives common trade designations (in terms of weights) for each of the groups.
A standard on photographic papers lists nine different categories of paper thickness.

Figure 14-4 Paper thickness.
The Gelatin Colloid
Gelatin is a colloid. A colloid is a particulate material that can be suspended in water or other solute without settling out. The sizes of the particles range from approximately 1 to 1000 nm, or intermediate between visibly suspended particles and invisible molecules. (One nanometer is one billionth of a meter, or one millionth of a millimeter.) Other colloids have been used for photography, and colloids that are a more similar substitute for gelatin have been tried, but gelatin has been, is, and will continue, for the near future at least, to be the best material for the preparation of photographic emulsions. (Albumen and collodion are examples of other colloids that have been important in photography.)
An important feature of gelatin is that it has a large molecular structure.
Properties of Gelatin
Photographic gelatin has many desirable characteristics in addition to being transparent.
Gelatin tolerates both acid and alkaline solutions, prolonged washing, and changes in temperature.
The gelatin colloid used to make photographic emulsions has several important properties:
- Gelatin disperses the light-sensitive silver halide crystals and prevents them from adhering to one another, or coagulating, and thus forming, in effect, larger crystals or grains.
- When wet, gelatin can be changed to a solid gel or liquid reversibly by changing the temperature.
- When dry, gelatin is reasonably stable, and thus protects the silver halide grains.
- Gelatin serves as a receptor for halogen atoms in some aspects of latent image formation.
- Gelatin has no effect on the silver halide, other than the protective function, although impurities found in many gelatins contribute to the photographic result (sensitivity, fog, etc.), sometimes beneficially, at other times in a manner that degrades the image.
- Gelatin permits the processing solutions to penetrate and chemically react with the silver halide grains in development, fixing, etc.
- Gelatin can be produced uniformly and inexpensively, and stored for long periods prior to use in manufacture.
- Gelatin is transparent.
To help ensure uniformity, numerous batches of gelatin of a given type are blended so that when a new batch is added it contributes only a relatively small amount to the overall characteristics of the blend. (American table wines are often blends of various batches of grapes for the same reason.)
Chemical Nature of Gelatin
Gelatin is an organic compound or, more precisely, a group of compounds (they are derived from organic materials—living animals—and can be burned) largely made up of carbon, hydrogen, oxygen, and nitrogen atoms having a composition of approximately 50, 7, 25, and 18 parts of these atoms, respectively. It has a very complex molecular structure made up of various amino acids. The average molecular weight of gelatin is about 27,000 or some multiple of this. (Molecular-weight values determined in a variety of different ways have ranged from 768, corresponding to a formula C32H52O12N10, to 96,000.) Since the amino acid molecules contain both acid carboxyl groups and basic amino groups, they are amphoteric—that is, they can act either as an acid or a base. Since the acidic and basic characteristics are not strong, it acts as a buffer. Large additions of either an acid or a base do not have a large effect on the hydrogen ion concentration (pH).
Physical Properties of Gelatin
Dry gelatin contains about 10% water and is a tough material with great mechanical strength. To prepare an emulsion, dry gelatin is soaked in water, which penetrates into the gelatin structure and causes it to swell many times its original dimension. When thus wet, it is soft and easily damaged.
When the soaked gelatin is heated to about 40° C (100° F), it melts and can be further diluted with water indefinitely. If the concentration of gelatin in water is greater than 1%, it will set when cooled, just as dessert gelatin does, to become a gel that can be dried with dry air without remelting. Before drying, the set gelatin can be remelted by raising the temperature, and reset by cooling, repeatedly but the setting and melting temperatures do not coincide.
Emulsion Making
Throughout the history of photography, the theory of the emulsion-making process has been difficult to understand. The characteristics of the final emulsion are governed by many factors, including the choice of soluble halides and gelatin, the method of silver halide precipitation, the cooking or ripening processes, and the choice of chemical additives to the emulsion. Since many of these processes cannot be patented, photographic manufacturers take great pains to maintain the secrecy of their emulsion-making techniques. The following basic considerations, however, are well known. The basic emulsion-making steps for a typical black-and-white film emulsion are precipitation, ripening, washing, digestion, additions, and coating.
A photographic emulsion is formed by treating a soluble silver salt with a soluble halide or halides in the presence of gelatin in solution; for example, Silver nitrate plus potassium chloride yields silver chloride plus potassium nitrate.
The light-sensitive silver halide, in this example silver chloride, exists in the form of crystals or grains that are one micrometer (one thousandth of a millimeter) in diameter or less. Mixtures of halides (chloride, bromide, iodide) are commonly in the form of mixed crystals containing two or three halides in each crystal (see Figure 14-5). The gelatin acts as the protective colloid in that it prevents the crystals from coalescing, and also controls the size and distribution of the crystals to some extent. Silver halides are primarily sensitive to ultraviolet radiation and blue light. Small amounts of compounds that react with the silver halide to increase sensitivity may be present in the gelatin, or they may be added separately to inert gelatin. Dyes may also be added to extend sensitivity to other regions of the spectrum than the ultraviolet and blue.
Basic emulsion making consists of precipitating a silver halide in a gelatin solution.
The size and size distribution of silver halide grains have an important effect on the sensitivity and contrast of photographic emulsions.

Figure 14-5 Silver-halide crystals.
Emulsion Characteristics
The photographic properties of an emulsion depend on a number of factors including its silver halide composition; the shape, average size, and size distribution of the crystals, and the presence of substances that affect sensitivity. All of this is governed by the amount and kind of gelatin(s) in the original solution, the choice of halide compounds, the way in which they are mixed together, the way they are treated following this precipitation, what other substances are added, and the coating procedure. These factors control photographic properties such as the speed, characteristic-curve shape, spectral sensitivity, and exposure latitude of the emulsion and image characteristics such as graininess, sharpness, and resolving power.
Grain Size, Sensitivity, and Contrast
Controls such as choice of silver halides, type of gelatin, method of mixing, additives, and cooking time make it possible to produce a great variety of film and paper emulsions.
The grains of the emulsion can be considered as individual units as far as exposure and development are concerned. A latent image formed in one of the grains does not ordinarily spread to the other grains unless they are touching. For a given exposure there is a greater probability that a large grain will absorb a quantum of light than will a small grain. If the grains were uniform in size, and in a single layer (one grain thick), the probability that individual grains would be exposed and made developable would depend on the random distribution of photons reaching the grains. Since such grains are uniform in size, density after development will vary with the fraction absorbing at least the number of photons required to become developable. When several photons are required, most of the grains will receive enough at the same time and thus the characteristic curve will have a steep slope, indicating high contrast. The toe of the curve represents the region of exposure where only a small fraction of the grains receive enough photons to become developable.

Figure 14-6 Emulsions having grains of nearly equal size will produce steep gradation, while those having a wide variety of grain sizes will produce low gradation.
If the grains are large, they provide a greater area for receiving photons, are thus more likely to be exposed, have a greater number of silver ions available for reduction, and after development they have a greater light-absorbing capability. This has the effect of giving the larger grains a greater amplification effect than smaller grains.
Intermediate-size grains would produce an amplification effect between those of the largest and smallest sizes (see Figure 14-6). Emulsions with a wide variety in grain sizes will have inherently lower contrast than emulsions having equal grain sizes, and will have greater sensitivity due to the availability of larger grains. The resulting characteristic curve will have a lower slope than in the case where the grains are all nearly the same size.
Grain Composition
The composition of the silver halide grains plays an important part in the emulsion. The presence of iodide in small amounts enhances the sensitivity of silver bromide grains. The composition of the grains formed by precipitation of a silver salt and two or three halogen salts depends on the solubility characteristics of the precipitated halides. The three light-sensitive silver halides used in photography are relatively insoluble compared to the salts from which they are formed.
The overall solubility of the silver halides in emulsions is increased by virtue of the small size of the crystals, 1 micrometer or less, which increases the surface-to-weight ratio significantly. This difference in solubility according to grain size accounts for the increase in average grain size during ripening, in which the smallest grains become smaller and the dissolved silver halide comes out of solution as an addition to the larger grains.
Spectral Sensitivity
The fine division of halides in emulsions provides a greater amount of surface for adsorption of materials to the silver halide grains. Adsorption is the adherence of atoms, ions, or molecules to the surface of another substance. First, silver halide grains adsorb additional halide ions, with the greatest adsorption occurring with iodide and the least with chloride ions. In addition, the grains adsorb gelatin, sensitizing dyes, and other compounds. These all contribute to the overall sensitivity of the emulsion. The silver halide grains themselves have varying sensitivities to light of different wavelengths, depending on the halide. Silver chloride, which is colorless, is sensitive at the shortest wavelengths, mostly ultraviolet energy, extending up to about 420 nm. Silver bromide, which is more yellowish in appearance, has sensitivity extending to about 500 nm. Additions of iodide, which has a strong yellow color, in amounts ranging from 0.1% to 1.0% of the chloride crystals, extends the sensitivity to 450 to 475 nm. A similar extension is achieved when about 40% bromide is added to chloride. Bromide with about 3% iodide extends the sensitivity to about 525 nm (see Figure 14-7). Even without further extensions of sensitization, such as with dyes, the safelight filter must not have any transmission below 550 nm.
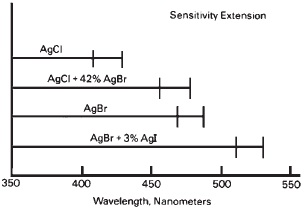
Figure 14-7 In silver halide emulsions, grains of silver chloride are sensitive to the shortest wavelengths of light, silver bromide to considerably longer wavelengths, and combinations of bromide and iodide to the longest wavelengths. (Chateau et al., The Theory of the Photographic Process, 1966, p. 6.)
Silver bromide, chloride, and iodide have different ranges of spectral sensitivity in the short wavelength part of the spectrum.
Crystal Structure
Defects in the crystals of silver halides are an important aspect of light-sensitive emulsions. Silver halide crystals are usually composed of ions in a cubic lattice so that each halide ion is surrounded by six silver ions, and each silver ion is surrounded by six halide ions, as shown in Figure 14-8. This arrangement can exist within the interior of the crystal, but at the outer surface there has to be one of each with only five of the other surrounding it. Even in the cubic system, crystals can be formed that are octahedral in shape, or in the form of hexagonal plates (see Figure 14-5). Different photographic effects are produced by the surfaces presented by these different crystal shapes.
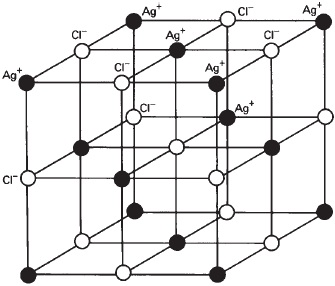
Figure 14-8 Structure of silver halide crystal.
Defects
The shape of silver halide crystals and the presence of defects in the crystals affect the photographic characteristics of the emulsion.
The rate of mixing emulsion ingredients affects the speed and contrast of the emulsion.
The defects in the crystal lattice can be divided into extended imperfections and point defects. Whereas the faces of the crystal have only five ions around each one of opposite charge, at the corners there can be only three, and at the edges only four. Adsorption is greater at these positions and reactions may begin here. These positions are supplemented by other dislocations that can occur in the crystal's formation. All are important in the photoconductive and photochemical processes. The most important type of point defect is interstitial silver ions—a minute fraction of the silver ions escape from their positions in the crystal lattice and can move through the spaces in the lattice, as shown in Figure 14-9.
Defects in the crystal act as locations for the formation of sub-image centers during latent image formation. Silver and halogen ions are not free to move, except to adjacent positions in the lattice, but electrons are free to move throughout the crystal. These motions manifest as electrolytic conductivity, which plays an important part in the photolytic process. One kind of defect arises from a vacancy in the crystal lattice, such as would occur if a silver ion were removed to an interstitial position. This would leave a silver ion vacancy that would have a negative charge. The interstitial silver ion itself would have a positive charge. An extra electron would produce a negative charge, and a positive charge would occur when an electron is removed from the valence band of the crystal.

Figure 14-9 The point defect of interstitial silver ions in a silver-halide crystal. Lattice ions themselves cannot move throughout the lattice, but an interstitial ion (defect) can. It can also push a lattice silver ion to a new position.
Emulsion Precipitation
A typical emulsion-making process starts with a relatively dilute solution of gelatin in water (about 1%). Soluble halide salts (one or more) are added to this solution (potassium, sodium, or ammonium chloride, bromide, or iodide), as shown in Figure 14-10. Then, at a selected temperature, a soluble silver salt, such as silver nitrate, is added at a controlled rate and with controlled stirring. The silver halide or halides are precipitated out, and the crystals are first formed in a strong solution of soluble halide, in which the silver halide is much more soluble than in pure water. Under these conditions, the crystals first formed grow rapidly and continue to grow throughout the precipitation.
Even a relatively dilute gelatin solution provides protection to the silver halide crystals formed. If it were not for the gelatin, the particles of the precipitate would coalesce and rapidly fall to the bottom of the reacting vessel. The growth of crystals to grains of the desired size with good structure would be difficult.

Figure 14-10 Emulsion precipitation.
Washed Emulsions
When an emulsion is coated on a porous support, such as a baryta-coated or otherwise uncoated paper base, the excess alkali nitrate after the precipitation reaction can be absorbed by the base. If the base or support is a film, or a paper base with a water-impermeable surface such as resin coated (RC), these excess salts have to be removed by washing to prevent them from crystallizing out on the surface (see Figure 14-11). It is also desirable in the emulsion-making process to remove excess salts to prevent further growth of the silver halide grain before continuing the process. This is accomplished by coagulating the gelatin, washing the emulsion, then re-dispersing (dissolving) the gelatin and halides. At its isoelectric point, the gelatin can be coagulated by adding a salt such as a sulfate. There are also other methods of accomplishing this coagulation.
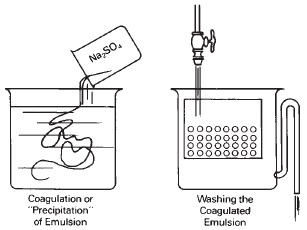
Figure 14-11 The gelatin emulsion is coagulated while at its isoelectric point by the addition of a salt solution such as sodium sulfate. It is then washed to remove the salts including those that were formed during the emulsion-making process. The washed grains can then be redispersed by raising the pH, along with the addition of more gelatin.
After the precipitation and ripening steps, excess soluble salts are removed by washing to prevent crystallization and excessive growth of the silver halide grains.
Historically the emulsion, with added gelatin, was chilled and set in a manner similar to setting food dessert gelatin. This set emulsion was then extruded into noodles, or sometimes cut into cubes (the noodling procedure was established by F.C. Wratten—of Wratten Filters—around 1878), and washed with chilled water until conductivity or pH tests showed that enough of the soluble salts had been removed.
Multiple Coatings
To achieve the desired sensitivity and tone-reproduction characteristics, black-and-white films often will have two different emulsions coated on top of the other. A slow, relatively short scale, or high gamma emulsion, is coated first, then a faster, coarsegrained, long-scale emulsion is coated on top of it to provide a characteristic curve with increasing slope in the upper midtone or lower highlight regions (see Figure 14-12). This provides better speed and scale characteristics than would be achieved by blending the emulsions. Color films are made up of at least three emulsions, one for recording each of the primary colors—red, green, and blue. One or more of these three, in turn, may be made up of multiple coatings to provide required tonal response, such as required by internegative films and those for other special applications.
Multiple emulsion coatings are used on some black-and-white films to modify the tone-reproduction characteristics of the film.
Effects of Development on Developers
The reaction products of development include soluble salts of the halides, along with modified developer components. The soluble halides act as restrainers, and thus curtail development. The kind and quantity of restrainer included in the developer formula is intended to minimize, as far as practicable, the effects of these reaction products of development.
Dye-sensitized emulsions become sensitized to the colors of light that are absorbed by the dyes.
It is possible to dissolve all of the silver-halide grains in an exposed film with hypo without destroying the latent image.
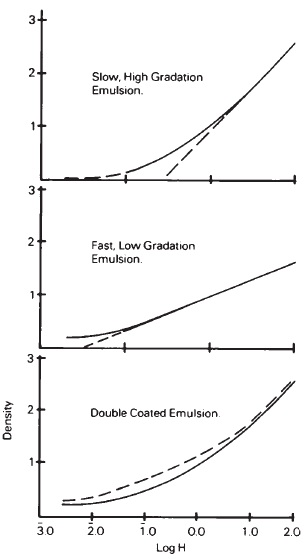
Figure 14-12 By coating a fast, low gradation emulsion over a slow, high gradation emulsion, the combination produces a sensitometric curve with increasing slope in the upper midtone and lower highlight regions.
Dye Sensitization
The basic silver halide emulsion is sensitive only to the blue and ultraviolet regions of the spectrum (about 180 nm to 520 nm) that it absorbs. The human visual response is highest in the green region, with a peak in the vicinity of 550 nm. Thus, photographs made with an unsensitized emulsion will have different black-and-white rendering of the brightnesses of colors than that perceived with the human visual system.
In 1873, the photographic scientist H.W. Vogel found that emulsions could be made to respond to wavelengths of light in the green region of the spectrum by adding a pink or red dye. The emulsion became sensitized to the color absorbed by the dye. When an emulsion sensitized to green by means of a red dye is used in the camera, with a yellow filter over the lens to absorb some of the blue light (to which the film remains sensitive), it gives a response in terms of black-and-white rendering that is somewhat closer to that observed by the eye and is thus described as an orthochromatic or “correct" color rendering. The term isochromatic has also been used to identify this characteristic.
Later, a green or cyan dye that absorbs red light extended the emulsion's sensitivity. Thus the emulsion is sensitized to red light by the addition of a cyan (red-absorbing) dye. Combined with the added green sensitivity, the emulsion responds to all of the colors of the visible spectrum, which includes blue, green, and red, and is termed panchromatic (see Figure 14-13). Spectral sensitization, while used in films mostly to provide appropriate tone reproduction of subject colors, also increases the film speed with white light, and some higher-speed films have a greater-than-normal sensitivity to red light.
These sensitizing dyes are sometimes retained by the film after processing, especially if a rapid processing technique is used, giving black-and-white negatives a pink cast. Normal development, fixation, and washing usually remove nearly all of the sensitizing and antihalation dyes from the negatives.
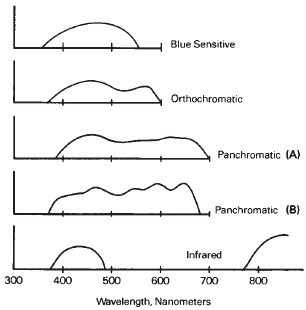
Figure 14-13 Traces from spectrograms for typical blue sensitive, orthochromatic, panchromatic, and infrared films. Panchromatic (A) has extended red sensitivity to nearly 700 nm; Panchromatic (B) is more representative of that used for pictorial photography.
While most photographic papers are manufactured with emulsions that have been made to meet the sensitometric requirements without further modification, some papers require a final speed adjustment that can be achieved by addition of sensitizing dyes to the emulsion. This extends the sensitivity to some of the longer wavelengths of light. This technique can be satisfactory until a change in the color of the light sources used for printing or recording reveals a greater-than-expected shift in speed from one emulsion coating to another. Some variable-contrast papers have mixed emulsions of different contrast with spectral sensitivities that can be used to control the contrast by changes in filtration. A typical vari-able-contrast enlarging paper produces high-contrast images when exposed with blue light (with a magenta filter), low-contrast images when exposed with green light (with a yellow filter), and medium-contrast images when exposed with white light.
Formation of the Latent Image
After the sensitized material is exposed in a camera, examination of the surface would not reveal an image. It is said to be a latent image. Some of the chemical characteristics of this image are as follows:
- It is weakened or destroyed by oxidizing agents such as chromic acid, which also oxidize metallic silver.
- This oxidizing reaction does not destroy the sensitivity of the emulsion, which after washing and drying can be used to expose a new image, although spectral sensitivity and speed may be degraded.
- It is not soluble in silver halide solvents. (If the exposed image is bathed in sodium thiosulfate solution and the remaining silver and halide ions are removed by subsequent washing, physical development can then be used to develop the latent image. Silver metal from silver ions in the developing solution plates out on image areas where exposure occurred.)
- The reduction of silver ions to silver metal by the developer is catalyzed by the presence of silver atoms. The latent image also increases the reduction of silver ions.
From the above it appears that the latent image and silver have the same reactions. This indicates that exposure sets into operation a mechanism within the crystal that in the end produces silver atoms, which distinguish exposed silver halide grains from those that are unexposed.
It is thought that a latent image must contain at least four atoms of silver to be stable and developable.
Thus, when the crystal of silver halide, made up of silver and halogen ions, is exposed to light, it becomes capable of being reduced by a developer to metallic silver, which along with all the other exposed crystals forms the image of the photograph. Among several theories that have been proposed for the mechanism of latent image formation, the Gurney-Mott hypothesis is a prominent one. It consists of two distinct steps in an exact order, but concluded in a short time interval. Using silver bromide as an example, they are:
- The radiation of the silver bromide crystal produces electrons that are raised to a higher energy level associated with the conductance band. The electrons move through the crystal by photoconductance until they are trapped by the sensitivity specks. The specks, or traps, then possess a negative electrical potential. This concludes the primary process, and its final effect is to initiate the secondary process.
- The secondary process involves the movement of the interstitial silver ions that are attracted to the negatively charged specks. The positive silver ions are neutralized by the negative charges, and the production of silver atoms is completed.
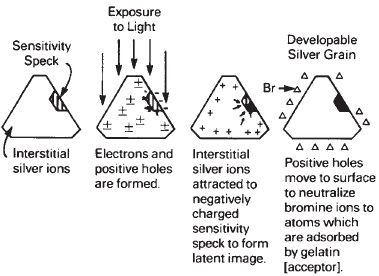
Figure 14-14 Gurney-Mott theory for latent image formation.
The hypothesis does not explain the fate of the halogen. It is possible that the halogen atoms may either recombine with an electron, may attack the silver atoms produced, or react with a halogen acceptor such as gelatin or sensitizers that reduce the atoms to halogen ions (see Figure 14-14).
Absorption of a quantum of light by the crystal excites an electron so that it is free to move through the lattice and may combine with an interstitial silver ion, thus yielding an atom of silver. This is most likely to occur at a nucleus produced by chemical sensitization, called a sensitivity center, which traps the electron and holds it until an interstitial silver ion arrives. There is a strong tendency for this atom of silver to give up an electron and thus return to the ionic state. It is also possible for the electron to recombine with the positively charged “hole” left when it was released from a halide ion. If exposure is sufficient, two silver atoms together form a sub-latent image that is not capable of development. However, a greater time will be required for the two atoms to give off electrons and return to the ionic state than is the case for a single atom. When sufficient exposure has been received to bring about four atoms of silver at the site, the grain may become developable. Further exposure of the grain to light adds to the number of silver atoms at the original site, and thus increases the developability of the grain. About 10 to 20% of the grains in a fast negative emulsion are rendered developable when four atoms of silver have been formed at the site, but the average number of atoms required for developability is considerably higher.
The latent image is usually formed on the surface of the crystal, but a latent image can also be formed in the crystal's interior on exposure to light. This internal image is usually protected from the developer, but it can be developed in a solution containing a silver halide solvent. In nearly all emulsions the contribution of the internal latent image to the developed image is negligible. A grain with an internal image usually has a surface image. For purposes of experimental research, the surface image can be destroyed to demonstrate the existence of the internal latent image.
The positive holes, formed by the loss of electrons to form silver atoms, move to the crystal surface to neutralize halogen ions to atoms that are adsorbed by the gelatin. The gelatin in this context is referred to as a halogen or bromine acceptor.
While most of the latent image in ordinary photographic materials is formed on the surface of the crystal, the interior latent image plays an important part in some photographic effects. In the Gurney-Mott hypothesis the electron “traps” are considered to be distributed throughout the crystal but are more effective on the crystal's surface.
Photographic Effects
The mechanism of latent image formation is closely related to six photographic effects. These six are: Reciprocity, Intermittency, Herschel, Clayden, Solarization, and Sabattier. It is worth noting that these effects do not happen in digital photography. While film will suffer reciprocity, extended exposures in the digital world will increase noise in the image only. There are however many techniques that can be applied to the digital image to simulate these effect.
Reciprocity Effects
Photographic exposure is the amount of light falling on the emulsion (Exposure = Illuminance X Time). Reciprocal combinations of illumination and time will give the same exposure, but not necessarily the same density. This decrease in density with certain combinations of illuminance and time is called reciprocity law failure or reciprocity effect. When exposures are made at low light levels, the efficiency with which the sub-latent images are formed on the crystal is low because of the tendency of the silver atoms to give up an electron and return to the ionic state. Further exposure allows a greater number of silver atoms to accumulate around those few sub-images that survive, but these sub-images are relatively stable.
With long exposures at low light levels, silver atoms in the latent image can change back to silver ions.
When exposures are made at high illuminance, with correspondingly short exposure times, the electrons are released so rapidly that the relatively slower-moving silver ions cannot neutralize them fast enough for the centers to grow to the predicted size. A greater number of sub-image centers are spread over the crystal surface, and sometimes into the crystal's interior. There is more competition for the additional silver atoms formed, and thus a smaller number of them grow large enough to become permanent developable latent image centers.
Reciprocity law failure is caused by the relatively low efficiency of the formation of sub-image centers at low intensities with long exposure times and by the high efficiency of the formation of sub-image centers at high intensities, with corresponding wide distribution of competing sub-image centers. Emulsions for pictorial use are formulated so that the most efficient compromise between exposures made at low intensities and those made at high intensities generally occurs when the exposure times are in the vicinity of 1/10 to 1/100 second. The reciprocity law failure of an emulsion is the same for all wavelengths of light when compared on the basis of equal densities and equal times (see Figure 14-15).
A series of intermittent exposures may not produce the same density as a single exposure even though the total amounts of light are the same.
The reciprocity law is valid for the production of electrons in the primary process of the Gurney-Mott theory (Figure 14-14), but does not apply to the secondary and other processes that are necessary for the production of the final image.
The reciprocity failure of a photographic material is dependent on the temperature of the material during exposure. Tests show that the failure virtually disappears at temperatures of -186° C, with an accompanying loss of sensitivity. Temperature variation has different effects on low- and high-intensity reciprocity law failure.
Exposures made with X-rays and gamma rays show no reciprocity failure, due to the high velocity of the electrons first liberated, releasing large numbers of electrons on collision with ions in the grain. Print-out papers also do not show reciprocity failure.
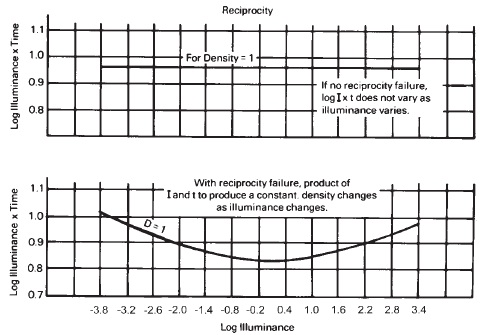
Figure 14-15 Reciprocity.
Intermittency Effect
The intermittency effect is closely associated with reciprocity failure. Intermittent exposure means exposures in discrete installments rather than in one continuous installment. If the intermittency rate is low, the intermittent exposure will produce the same photographic effect as a continuous exposure of equal total energy. As the frequency is increased at a given level of illumination, the photographic effect is decreased until with a further increase in frequency the loss in photographic effect becomes constant. The point at which this occurs is a critical value that varies with the illuminance level. If the intermittent exposure is made at a sufficiently high illuminance level, the photographic effect will be greater than that of a continuous exposure of equal energy. In relation to the U-shaped reciprocity-law-failure curve, the intermittency effect at the critical interruption frequency is equivalent to moving to the left on the curve. In the case of low illuminances on the left half of the curve, the move is upward, indicating that more exposure is required to produce a specified density. In the case of high illuminances on the right half of the curve, the move to the left is downward, indicating that less exposure is required to produce a specified density (see Figure 14-15).
Solarization
When a sensitometric curve is considered, solarization is the reversal or decrease in density with additional exposure increase beyond that required to produce maximum density on the film (see Figure 14-16). The maximum solarization effect is produced with moderate developing times, while extended development reduces, or even eliminates, the effect. In addition, the presence of silver halide solvents, such as sodium thiosulfate and sodium sulfite, in the developer inhibits or removes the solarization. Developers that do not contain silver halide solvents usually produce the effect.
If halogen acceptors are present during exposure, solarization may be diminished or even eliminated. Thus, solarization is considered to be the result of rehalogenation of the pho-tolytic silver formed at the sensitivity specks as the result of exposure. Normal exposure produces halogen at a rate that allows the halogen to react with acceptors such as gelatin. If the exposure is great, the production of the halogen proceeds at a rate beyond the capability of the acceptor, and thus may react with the latent-image silver to reform silver halide. This surface coating of silver halide, although it contains a latent image beneath it, will shield the latent image from the developer. This is sufficient to lower the number of developable crystals, and the result is a lower density. If the developer contains a silver halide solvent, it will remove the surface silver halide and thus expose the latent image for development.

Figure 14-16 The Sabattier effect is the result of arrested development, wash, re-exposure, and further development. Solarization is the result of extended exposure and moderate development in a developer without silver halide solvents. Both effects produce a reversal image.
With a solarized image, heavily exposed areas do not develop because rehalogenization produces a protective coating of silver halide on the latent images.
Sabattier Effect
The Sabattier effect is produced by developing an exposed photographic emulsion for a short time, washing it, and exposing the emulsion a second time. The film is then further developed, fixed, and washed (see Figure 14-16). The effect is sometimes confused with solarization, in that the final result is a partially reversed image. It appears to be caused by two mechanisms: (1) the image produced by the first development screens or acts as a negative and thus allows the exposure of the remaining silver halide to be modulated to produce a positive image during the second development, and (2) the by-products of the first development act as a restrainer in the developed areas. The migration of used and fresh developer across image boundaries may produce Mackie lines, a line of increased density just inside the denser area and a line of decreased density just inside the thinner area.
Latent Image Stability
The fact that amateur photographers sometimes allow months and even years to elapse between the time the first exposure is made on a roll of film and the time the film is processed attests to the stability of the latent image. Preservation has been exceptional in cases where exposed film has been frozen in ice, such as in the Arctic region, but images have been obtained with exposed film that has been stored for as long as 25 years under household conditions.
Latent images have produced usable images when development has been delayed for as long as twenty-five years.
These examples are not intended to suggest that the latent image is permanent and does not change with time. Since the latent image consists of a cluster of silver atoms, the loss of only a few atoms may render a silver halide grain undevelopable. An atom of silver can combine with an atom of bromine, for example, to form a molecule of silver bromide, reversing the effect of exposure. Images developed after long periods of time may have less contrast and be less distinct due to the spontaneous development of a larger number of unexposed silver halide grains. Delayed development of film increases fog. A point is reached where the developed latent image can no longer be detected. A small amount of development fog, however, can produce the effect of increasing the speed of the photographic emulsion and the density of the developed latent image in the same manner as latensification (see Figure 14-17).
For critical work, detectable changes in developed latent images sometimes occur in remarkably short times. Exposing a large number of black-and-white prints identically, and then developing part of the batch one day and the balance the following day, has been reported to produce a significant difference in density. Improvements in emulsion technology in recent years, however, has reduced the decay rate of the latent image in black-and-white printing papers.

Figure 14-17 Effect of age on a typical black-and-white film.
Changes in the latent image of color printing papers are more serious because they can affect the color balance of the print in addition to the density and contrast. For this reason, recommendations are made to store color paper for a fixed time after exposure prior to processing to make the latent image change relatively constant. If the exposed paper is to be held for more than the hour or two normally built into the schedule, recommendations are that it be stored at 0° F (-18° C) or below, and then for a period of no more than seventy-two hours. Film manufacturers recommend that color films be processed as quickly as possible after exposure or that they be stored at a low temperature, noting that storage in a closed automobile on a hot day for only a few hours can have a serious effect on the developed image. They also warn against allowing color films to come into contact with various fumes, including those of chemical solvents and mothballs.
Some Alternative Systems
The familiar black-and-white and color films and papers that use gelatin as a suspension vehicle for the light-sensitive silver salts are but one of many ways to make a light-sensitive photographic system. Most of the older processes, such as calotype, daguerreotype, cyanotype, albumen, wet collodion, ambro-type, carbon, carbro, gum bichromate, platinum, and kallitype, use something other than silver salts and/or gelatin. None has the light-amplification ability of silver and therefore they all have very slow speeds. The required long exposure times or high light levels limit their use in a camera, so they are relegated to photographic printing or certain types of recording where high sensitivity is not required.
Many of these older processes are also being rediscovered by photographers who are exploring their delicate tones and esthetic qualities. Some processes utilize silver and/or gelatin only in an intermediate step. Many of the present-day color processes are examples of the latter where the final images consist of dyes. Newer processes such as electrophotography and television use electrical and magnetic fields for image recording and reproduction. Light-sensitive microchips containing many light-sensitive picture elements have sufficient sensitivity to be used as disks in cameras.
Calotype Process
Another early process utilizing silver but not involving gelatin was the calotype process, invented by William Henry Fox Talbot, and patented in 1841. Talbot had obtained negative silver images on silver chloride paper as early as 1839, with what was later called the Talbotype process. The negative and positive images formed the basis of modern photography. Paper was sensitized with silver iodide, silver nitrate, and gallic acid, and was developed in gallic acid. This paper was used for both the camera negative, and for printing a positive from the negative.

Figure 14-18 Calotype.
According to a modern version of the process, the paper is first iodized by coating it with an approximately 7% solution of silver nitrate in distilled water, as shown in Figure 14-18. After the paper is dried it is floated on a solution containing about 7% potassium iodide and 1% sodium chloride, and dried again. It is then sensitized by flowing onto the surface a solution containing about 10% silver nitrate, 7% acetic acid, and 1% gallic acid, producing light-sensitive silver halide. The sensitized paper is dried and kept in the dark until ready for camera exposure. The exposed paper is developed in a solution containing about 0.8% gallic acid and 2.5% silver nitrate, producing a silver image. The developed negative is fixed in a solution of about 30% sodium thiosulfate (hypo) solution, washed, and dried. Prints can be made by exposing this negative on a conventional modern printing paper, or on another sheet of calotype paper.
History has seen many photographic systems other than the silver halide gelatin processes in use today—some of which still have significance.
The negative-positive calotype process, patented in 1841, used silver halide but did not use gelatin.
Daguerreotype
The process announced by Louis Jacques Mande Daguerre on August 19, 1839, introduced practical photography to the world. No gelatin was involved. A sheet of copper or brass was plated on one side with silver, which was buffed to a high mirror-like polish. It was then sensitized by placing it in a light-tight box containing iodine crystals, which gave off iodine vapor that reacted with the silver to form a coating of silver iodide. Exposures were made in a camera, and since the image was viewed on the surface of the plate and not through it, it was reversed from left to right, a condition that was corrected in practice by placing a mirror at 45° to the lens in front of the camera.
The daguerreotype process used a silver surface that was treated with iodine vapor to form a light-sensitive silver halide.
After exposure, the plate was placed in a box containing a dish of mercury that was heated to about 75° C (165° F). The mercury adhered only to the exposed parts of the plate, giving a whitish amalgam of silver and mercury. The plate was fixed in a solution of sodium thiosulfate (hypo), washed, and dried. When the daguerreotype was viewed so that the unexposed, undeveloped areas reflected the dark surroundings of a room, a positive image was seen. The photographic speed of the plate was increased by adding bromine to the iodine vapor, and the image strength could be improved by toning with gold chloride after fixing. Since the silver image was readily tarnished or damaged by handling, the plate was protected by placing it in a decorative cutout frame and covering it with glass.
Cyanotype
The cyanotype process is a nonsilver printout process that requires only washing after exposure.
The cyanotype (or blueprinting) process, invented by Sir John Herschel in 1842, is a nonsilver, nongelatin process. The ferric iron salt used in coating the paper is reduced by light to the ferrous state, which is then precipitated to Prussian Blue (ferric ferro-cyanide, Fe4[Fe(CN)6]3), by the action of the potassium ferrocyanide, the second component of the coating solution. The image is made up of varying densities of the Prussian Blue. A good grade of sized paper should be used. The paper is sensitized by coating, brushing, floating, or swabbing, with a solution consisting of about 12.5% ferric ammonium citrate and 7.5% potassium ferrocyanide (usually prepared by mixing separate solutions of these two compounds). Ferric ammonium oxalate can be substituted for the ferric ammonium citrate for increased speed, and about 1% of potassium dichromate can be added for increased contrast.
After exposure, a pale image can be seen, and processing normally consists of washing the prints in plain water to remove the unexposed soluble ferric salt, followed by drying. Prussian Blue is a fairly stable compound but it is soluble in alkalis, and the image is affected by impurities in the atmosphere that sometimes cause the image to take on a metallic luster in the denser areas. The cyanotype process can be used for printing con-tinuous-tone photographs by contact from large negatives. It was also once used on a large scale for copying drawings from original tracings but has since been supplanted by the diazo process. (There are several other cya-notype formulas, but they all result in a Prussian Blue image.)
Cyanotypes can be converted to purplish-black images by bathing in a 10% sodium carbonate solution, which bleaches the image; then redeveloping in about 1% tannic acid solution. Cyanotype images can be inked over with waterproof ink, and the blue bleached out with about 5% oxalic acid, followed by washing and drying, to produce a pen-and-ink drawing. A modification of the process (pellet process) produces a faint image that can be developed to a reversal image (positive to positive) upon development with potassium ferrocyanide. Another version, the pointevin process, is a posi-tive-to-positive one that produces purplish-black lines on a light background.
Wet Collodion
The wet collodion process is a silver process, but it uses nitrocellulose as the binder for the halide crystals. It represents an important phase in the development of photography because it produces negative images on a transparent base, and was the principal method of making negatives from 1851, when it was introduced by Frederick Scott Archer, until the 1870s, when gelatin dry plates were introduced.
The cellulose nitrate is prepared by immersing cotton in a mixture of nitric and sulfuric acids, followed by washing in water. When dry, the nitrated cotton is dissolved in a mixture of ether and alcohol to produce collodion. (Prepared collodion can be obtained from chemical supply houses.) For photography, a small amount of sodium or potassium iodide or bromide is dissolved in the collodion. Some formulas have also made use of cadmium bromide and other halides. The prepared collodion is then flowed onto a clean glass plate until it is covered, and the excess collodion drained back into the bottle. The alcohol and ether evaporate and leave a tacky coating on the plate. Flow characteristics, which vary with atmospheric conditions, are adjusted by altering the relative amounts of ether and alcohol in the collodion. The tacky plate is immersed in the dark in a tank containing silver nitrate, about 65 grams per liter, for about 1 minute. The plate is then loaded into a special holder, while still wet, and inserted into the camera for exposure.
Following exposure the plate is developed before it has a chance to dry, by flowing a ferrous sulfate solution, or pyro solution, over the plate, and allowing the puddle to remain there during development. Some operators drain and replace the developer during development. However, it is thought that some physical development takes place, that is, some of the silver is plated back onto the image from the excess on the plate.
After development, the plates are fixed in a solution of potassium cyanide, or sodium cyanide, about 65 grams per liter, or with a sodium thiosul-fate (hypo) fixing bath. The collodion image could be stripped off the glass to provide a film negative and a reusable glass plate, but this was not done often in the field. The wet collodion process continued to be used in the graphic arts industry until well into the twentieth century. The images were routinely stripped off the glass support, and recemented onto another support, or “flat"—a number of images placed together on a single support. The term stripping is still applied to the removal and repositioning of images into a new layout.
The wet collodion process was the first to produce a negative image on a transparent glass support, but the sensitized plate had to be exposed before it had time to dry.
Gum Bichromate
With the gum bichromate process, light produces a hardened image that remains after the unhardened gum is removed by washing.
The gum bichromate process depends on the hardening effect produced by light on a bichromated colloid—in this case the colloid is gum arabic. Before the turn of the century the process enjoyed great popularity because of its adaptability to printing controls, and the wide choice of colored pigments that could be used. While it is considered obsolete today, it is enjoying revived popularity as a means of artistic expression. There are various formulas for preparing the sensitizer, depending on the tonal rendering desired, but a fairly standard formula consists of mixing equal parts of a 10% solution of potassium bichromate with a 30% solution of gum arabic. The potassium bichromate can be dissolved in hot water, but the gum arabic requires soaking overnight or longer at room temperature. A small amount of thymol or other preservative can be added to improve the keeping qualities of the gum solution. Ammonium bichromate is sometimes used in place of the potassium salt to produce increased sensitivity. Any of a wide variety of water-miscible colorants can be added to the mixture.
With thexerographic process, light reduces the electrical charge in the exposed areas of a charged plate. The remaining charged areas attract a pigment that forms the final image.
Thermographic materials form images by reacting to heat radiation in any of several ways, including decomposition, softening, and the production of a transferrable image.
The mixture should be applied to paper or another support, which is temporarily attached to stiff cardboard, using a brush. Experience will result in a technique that will produce a uniform coating. The coated material is dried in a dark room. Sensitivity is low until the material is nearly dry. The speed is similar to that of POP (print-ing-out-paper), but it is necessary to run an exposure test, as the speed depends on variations in the colorant.
The prints are made by contact, using sunlight, or some other source rich in actinic ultraviolet radiation, using a printing frame. A typical exposure is about 5 minutes. Development consists of dissolving the unhardened coating. The print is placed face-down in a tray of cold water for a time that may vary from 15 minutes to hours. The process can be speeded up with a gentle spray after the initial soaking. The image should not be touched until it is completely dry. During the drying stage, the print should be attached to a stiff support to prevent curling.
Electrophotography
There are many different electrophotographic processes (which use electricity to form images), some of which have not yet been fully developed. Electrostatic photography (xerography), which had its beginnings with the inventions of Chester Carlson in 1938, is now the most widely used electrophotographic system. The basic principle of image formation is that certain materials, such as selenium (early experiments were conducted with sulfur) and zinc oxide, will take on an electrical charge when passed near a source of high voltage, a corona charge. This is usually a positive charge in systems utilizing selenium, but the zinc oxide (in a resin binder) requires a negative charge. The action of light is to eliminate the charge, resulting in an image that can be made visible by dusting the plate with pigmented toner, which adheres to the charged (unexposed) areas. The toner also can be carried in a liquid.
With xerography (Xerography is a trade name, but xerography is now listed in dictionaries as a generic term), a drum plated or coated with selenium rotates first through an electric field (corona discharge) where it picks up a uniform positive electrical charge, as shown in planographic form in Figure 14-19. The image of the original is projected onto the charged drum, leaving an image consisting of positive ions in those areas that were not exposed. The drum is dusted with the toner, which adheres to the charged image. The drum then picks up a sheet of paper and both are passed through an electrical field that causes the toner to transfer from the drum to the paper. The paper is passed through heat to fix the pigmented toner permanently to the paper. If the exposure is made while the drum is rotating, a scanning system is used to synchronize the image with the rotation of the drum, but an alternative system uses a stationary charged surface with electronic flash illumination.
Good line copies are readily made with these processes, but one problem in their development has been to produce good continuous-tone reproductions. There have been efforts in this direction to devise methods that control the corona charging rates, among other things, which are meeting with continued success. Since the toner can be made in various colors, and since successive images can be made on the same sheet, it is possible to produce multicolored copies. Positive images are produced, since the toner adheres to the areas that are not exposed to light.
Thermography
Thermography is another nonsilver, nongelatin process. Images are formed by heat, usually by radiation from an infrared lamp that is modulated by the image on the original document. The denser areas absorb infrared radiation and become hotter than the surrounding less-dense areas. The heat is transferred to the heat-sensitive material, which is placed in contact with the original. The most common changes are a physical softening in the heated areas, or a chemical decomposition. Since printing and typing inks generally do not strongly absorb infrared, it is necessary first to make an electrostatic copy of such originals and then make a thermographic copy from that copy—not a practical procedure if a single copy is needed.
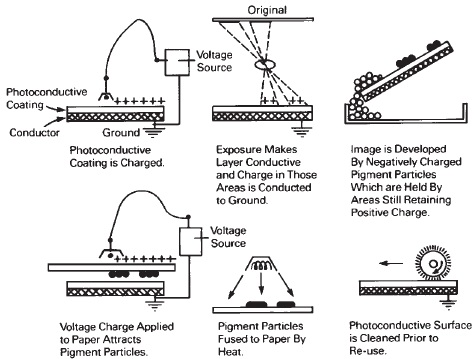
Figure 14-19 Electrophotography.
Diazo
The diazo family of nonsilver, nongelatin processes is mainly divided into two categories—one producing dye images, and the other vesicular images. With the dye image process, ultraviolet radiation decomposes compounds known as diazonium salts. The remaining salts are converted to azo dyes with ammonia or heat. The ammonia may be applied either in solution form or as ammonia fumes. Since dye is formed in the areas protected from radiation, a positive image is formed from a positive original. No fixing is needed because the sensitivity is destroyed in the non-image areas by the action of the exposing radiation.
With the diazo process, diazonium salts are decomposed by exposure to ultraviolet radiation and the remaining salts form a dye image when treated with ammonia.
A diazo material that produces black images on a white background is widely used for copying drawings, and the resulting prints are called white prints to distinguish them from blueprints. Diazo materials are available with paper, film, and foil bases and a wide variety of image colors. Limitations of the process are low sensitivity and the need for an ultraviolet-rich source of radiation for exposure. The high-contrast characteristics of the sensitized materials make them unsuited for copying continuous-tone originals. However, they are capable of high resolution, and they were at one time considered favorable for reproduction of microfilm images and optical sound recordings.
The image displayed on a television screen is produced by scanning a phosphor layer on the tube face with an electron beam, which causes the phosphors to emit light.
With the vesicular process, crystalline and non-crystalline diazonium salts are randomly dispersed in a thermo plastic film (see Figure 14-20). Upon exposure to ultraviolet-rich radiation, the diazonium salt is decomposed and nitrogen gas is released into the thermoplastic film. Development consists of heating the film to change the light dispersal pattern mainly due to the tiny bubbles that are formed as the gas expands. Upon cooling, these larger bubbles remain and produce an image by scattering light (as contrasted to silver and dye images, which produce visible images by absorbing light). Fixation consists of a uniform exposure to radiation without heat which decomposes the residual diazonium compounds and the released nitrogen gas is allowed to diffuse from the film.
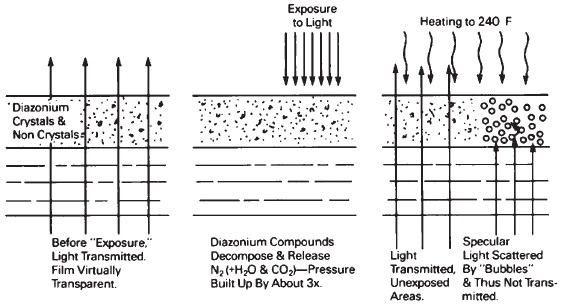
Figure 14-20 Vesicular film.
Although vesicular images appear low in contrast when viewed by diffuse illumination, the contrast is satisfactory when viewed by specular illumination. Since bubbles are formed where exposure occurs, a negative image is formed, but the processing procedure can be modified to produce a positive image. Typical applications are the production of positive images from microfilm negatives, black-and-white negatives from 35-mm color slides, and quick projectuals in audiovisual work. Before the displacement of black-and-white motion pictures with color, this process was a strong contender for making motion-picture release prints.
Electronic Image Display
There are multiple electronic processes that do not form permanent images, but when tied to a recording device such as digital video recorder produce images that can be modified and edited. Such devices include the cathode ray tube (CRT), rear projectors (RPTV), flat panel devices including liquid crystal and plasma displays, and LED and organic light-emitting diode (OLED) technology.
The CRT is an evacuated glass tube that has a fluorescent screen and a electron gun. When the electrons from the electron gun strike the fluorescent screen light is emitted. The electron beam can be modulated to display images on the screen. CRTs are used in televisions, computer screens, radar screens and oscilloscopes.
A rear projector screen uses very bright lights and the principles of additive color to produce images. These are very popular for large home theaters.
The LCD or liquid crystal display is a thin flat display device that consists of an array of pixels in front of a light source or reflector. The LCD has a layer of molecules aligned between two transparent electrodes and two polarizing filters. The polarizing filters are aligned perpendicular to each other, which should block all light passing through. The electrodes apply an electrical field to the liquid crystals which align to control the amount light that passes through the system.
Plasma display panels are also flat, as LCDs are. Plasma displays have many tiny cells that are placed between two glass panels. Each cell contains an inert mixture of noble gases, typically neon and xenon. These cells are turned into plasma with an electrical current. The plasma then excites the phosphors to emit light.
The organic light-emitting diode (OLED) are a type of light-emitting diode. The emissive electroluminescent layer in an OLED is made of a film of organic compounds. The layer is typically a polymer substance that allows the organic compound to be deposited in rows and columns on a flat carrier. This produces addressable pixels. OLED can be used in televisions and computer displays. The advantage of this technology is that they do not require a backlight to operate and use less energy than an LED type display. They are also easier to manufacture than LCDs and plasma displays.
Charge-Coupled Devices
There are several ways in which images formed by light can be electronically recorded, including charge-coupled devices (CCD) and complementary metal-oxide semiconductor (CMOS) imagers. Many digital cameras and scanners exist that record images using these devices for later use as computer images.
CCDs serve as the light sensor in many contemporary still digital cameras and digital backs for view cameras and some medium-format hand-held cameras. The CCDs are made up of a large number of minute individual sensors arranged either in a single row (linear array) that scans the light image in the camera or in a two-dimensional format (area or block array), the same as film, that permits instantaneous exposure of the entire light image (for black-and-white pictures, or, with a mosaic of minute red, green, and blue filters over the sensors, for color pictures). The individual picture elements, called pixels, are sensitive to all wavelengths of light and ultraviolet radiation, like panchromatic black-and-white film and color film, and are also sensitive to invisible infrared radiation. Since sensitivity to ultraviolet and infrared radiation is not wanted for most images, it can be reduced or eliminated with appropriate filters on the surface of the CCD or elsewhere in the light path.
When exposed to the light image in the camera, each pixel produces an electrical charge that is proportional to the illumination level. The light image formed on the surface of the CCD by the camera lens is in analog form, as with film, but since computers require the information to be in digital form the CCD is designed to move the individual pixel charges in sequence through an analog-to-digital-converter (ADC). The variations of voltage of the individual CCD pixels, which represent variations of image illumination, are converted to a sequence of 0s and 1s for each pixel by the ADC, the length of the sequence of 0s and 1s being determined by the bit depth of the CCD. An 8-bit-depth system, for example, would produce strings of eight 0s and 1s, which would represent 28 or 256 tones from black to white.
With the diffusion transfer process, the silver halides that remain after development of a negative image are used to form a positive image.
Dye Transfer
The gelatin-silver emulsion provides an intermediate matrix from which the image is produced by transferring dye to a mordanted gelatin-coated film or paper support. (A mordant is a substance that binds a dye to a given material, in this case, gelatin.) With the Technicolor motion picture process, now defunct in the United States, a set of matrices (one each dyed with cyan, magenta, and yellow to represent the red, green, and blue records of the scene) transferred the dyed images to a gelatin coated imbibition blank film. Beginning in the 1930s the images were transferred to a processed positive film carrying the silver sound track. For dye transfer prints on paper, see Figure 14-21.
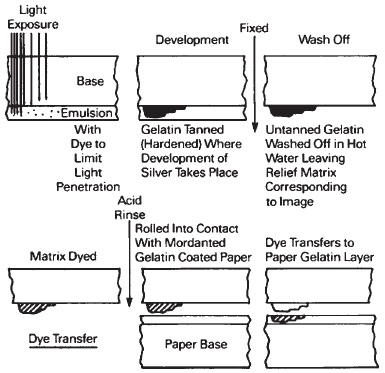
Figure 14-21 Dye transfer.
Diffusion Transfer
With diffusion transfer, an exposed gelatin-silver negative emulsion on either a film or paper base is brought into contact with a receiver sheet, with a viscous activator solution containing a strong alkali and a silver halide solvent such as thiosulfate placed between them. The negative emulsion also contains a developing agent, and the receiver sheet, which is not light sensitive, contains nucleating particles such as silver sulfide, along with a developing agent. The activator causes development to take place in the negative, and the halide is reduced to silver to form a negative. At the same time, the undeveloped silver halide in the negative is dissolved by the thiosulfate, and diffuses to the receiving sheet. The silver sulfide particles serve as nuclei for development of the halide that diffuses to the receiving sheet, and it is reduced by the developing agent, now activated, to metallic silver to form a positive image. When the two sheets are separated, the remaining viscous activator— developing/solvent solution—stays with the negative, leaving the clean positive image on the receiving sheet. No further fixing or washing is required. The process normally goes to completion, although varying the time and/or temperature can permit some variation in density or contrast.
REVIEW QUESTIONS
- The major disadvantage of cellulose nitrate as a support was that it …
- was expensive
- became brittle
- turned opalescent
- was combustible
- had a disagreeable odor
- A disadvantage of polyester as a photographic emulsion support for motion-picture film is that it …
- is difficult to perforate for sprocket holes
- acts as a light pipe
- has a strong inherent color
- tears easily
- curls excessively
- NC coatings on photographic films are applied to the …
- base side
- emulsion side
- edges
- The name of non-RC photographic papers could be abbreviated …
- RF
- FC
- FB
- FF
- Brighteners are used in …
- negative black-and-white films
- negative color films
- reversal color films
- black-and-white infrared films
- photographic papers
- The number of groups of photographic paper thicknesses listed in the American National Standard on paper thicknesses is …
- 3
- 4
- 8
- 7
- 9
- A statement that is not true for photographic gelatin is that gelatin …
- can be changed between liquid and solid states by changing the temperature
- has no effect on the sensitivity of the emulsion
- permits penetration by liquids
- serves as a receptor for silver ions in latent image formation
- is transparent
- The more uniform the silver halide grains are in size in an emulsion, the …
- higher the contrast
- lower the contrast
- higher the speed
- lower the speed
- The inherent spectral sensitivity of silver bromide is to …
- ultraviolet, blue, green, red, and infrared radiation
- ultraviolet, blue, green, and red radiation
- ultraviolet, blue, and green radiation
- ultraviolet and blue radiation
- blue radiation
- If the gelatin is omitted during the precipitation stage of emulsion making …
- the chemicals will not react
- the silver halide formed will decompose rapidly
- the silver halide will not stay in suspension
- the silver halide formed will rapidly dissolve
- Sensitizing dyes increase sensitivity of photographic emulsions to wavelengths of radiation …
- reflected by the dye
- absorbed by the dye
- transmitted by the dye
- When exposed but undeveloped film is placed in a sodium thiosulfate solution, the latent image is …
- unaffected
- destroyed
- converted to a visible image
- At the end of the primary process of latent image formation, the sensitivity center …
- has a positive electrical charge
- has a negative electrical charge
- is neutral
- Movement during the secondary process of latent image formation consists of movement of …
- electrons
- sensitivity specks
- positive ions
- negativeions
- all of the above
- The minimum number of atoms of silver required to be formed during exposure for a silver halide grain to be developable is considered to be …
- one
- two
- three
- four
- five
- A common characteristic of cyanotype, gum bichromate, and other nonsilver processes is that …
- none is capable of producing a neutral color image
- none of the images formed has as good archival permanence as silver images
- all are more expensive than silver processes
- all have lower film speeds than silver processes
- none is capable of producing an image of normal contrast
- The first negative-positive photographic process is considered to be the …
- daguerreotype process
- cyanotype process
- calotype process
- gum bichromate process
- electrostatic process
- An example of a photographic process that does not use silver is the …
- daguerreotype process
- cyanotype process
- wet collodion process
- printing-out-paper process
- The distinctive characteristic of the wet collodion process at the time it was introduced was that it was the first …
- negative-positive process
- silver-halide process
- process to use dyes
- process to use liquid development
- process to use a transparent base
- A photographic process that is based on the hardening effect of exposure to light is the …
- cyanotype process
- thermography process
- electrostatic photography process
- gum bichromate process
- diazo process
- In electrostatic photographic processes, light forms a latent image by…
- placing an electrical charge on a surface
- neutralizing an electrical charge on a surface
- hardening toner on a supporting surface
- creating a fluorescent image on the receiving surface
- A distinguishing characteristic of the diazo dye process is that the images
- have low contrast
- have high contrast
- have a magenta color
- are composed of silver dyes
- are fixed with ammonia gas
