Chapter 15
Black-and-White Photographic Development

"Self-Portrait" by Robert Lussen, Advertising Photography student, Rochester Institute of Technology.
Negative Development
When a silver halide emulsion on film or paper has been exposed to a light, a latent image is formed in the emulsion and in most circumstances this image would not be visible because of the small amount of silver produced. The energy required to produce such a latent image is relatively small, permitting exposure times of 1/20,000 second or shorter under daylight illumination. The development process amplifies this image by a factor of up to 109 or a billion times producing the final silver image. Assuming that optical density is approximately proportional to the amount of silver per unit area of the image, it is possible to calculate the density of a latent image. For example, the maximum density that can be obtained in a reflection print is about 2.0. This density divided by 109, the maximum development amplification factor, equals 0.000000002, the calculated density of the corresponding latent image. The smallest density difference that can be measured with conventional densitometers is 0.01.
Development amplifies the latent image by a factor of up to a billion times.
The subsequent fixing step converts the unexposed, undeveloped silver halide remaining in the film to soluble silver complexes that are washed away to leave only the silver image. The photographic process is a negative-working one, in that dark areas in the scene record as light areas on the film, and light areas record as dark on the film. In the case of the original camera exposure, a negative image is created that serves as a light modulator to produce a positive image on another piece of sensitized material for viewing.
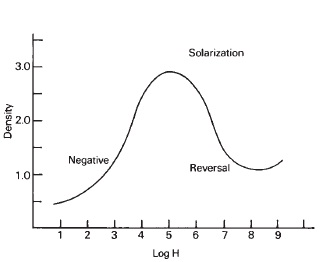
Figure 15-1 Solarization
Direct-Positive Images
A positive image can be produced on some types of emulsions by overexposing them to such an extent that the reversal region of the characteristic curve is reached (see Figure 15-1). X-ray film, for example, could be used as a contact-printing medium for reproduction of an original negative X-ray image as a negative image simply by grossly overexposing it, and processing in the usual way. Emulsions have been produced that give positive images with normal development for copying and duplicating negatives.
Reversal Processing
The basic negative-working silver halide emulsion system can be used to produce positive images by means of reversal processing (see Figure 15-2). Some emulsions are more suited to this type of processing than are others. Reversal processing begins with a negative image, as in normal processing after developing but before fixing. The silver image is then removed in a bleach, such as potassium permanganate, which reacts with the metallic silver to form soluble silver ions that are washed away, leaving the undeveloped silver halide in the emulsion. The remaining silver halide is then exposed to a strong light source, and developed again to produce a reversed or positive image. The regions that do not receive much original image exposure have very little density after the first development, but a great deal of halide is available to the second developer to produce a high density. Similarly, where there was high exposure in the beginning, there is little silver halide remaining for the second exposure and development, and low density is produced, as shown in Figure 15-3.
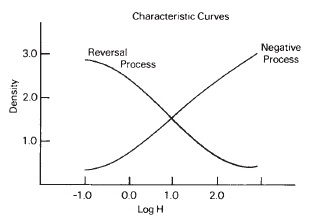
Figure 15-2 Densities increase as exposure increases in a negative process while densities decrease with increased exposure in a reversal process.
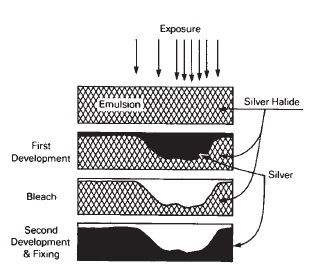
Figure 15-3 Reversal process.
More recently, fogging developers have been used for the second or reversal development, which eliminates the need for second exposure.
Mechanism of Development
Latent images can be divided into surface latent images and internal latent images. Most of the development takes place with the surface latent image, and the internal latent image normally contributes little to the total image. The latent image is formed when a certain number of photons have produced a nucleus of silver atoms in or on the surface of a silver halide grain. Four atoms of silver are considered to be the minimum number that constitutes a latent image.
Development is the selective reduction to silver of additional silver halide molecules around the site of the silver latent image nuclei, with no appreciable reduction in those areas that have not been exposed to light. As development continues, the amount of metallic silver grows, and if carried far enough the entire silver halide grain is converted to metallic silver. There are basically two types of development—chemical development and physical development. In physical development, the silver is provided by a silver salt in the developing solution, whereas in chemical development the silver comes from the reduction of silver halide grains in the emulsion. In most instances, some physical development takes place along with chemical development as a result of the silver provided by the solvent action of sodium sulfite on silver halide grains, and the amount varies with the type of developer. Physical development can be accomplished after fixing the exposed but undeveloped image, in which case the silver nuclei not removed by fixing serve as the focal points for accumulation of silver from the developer, which contains a soluble silver compound. The acid in conventional fixing baths will destroy the latent image, however.
Some physical development usually occurs with so-called chemical development.
Most developers contain a reducing agent, an alkali, a preservative, and a restrainer.
Constitution of Typical Developers
The most important ingredient in a chemical developer is the developing agent or chemical reducer agent that converts the exposed silver halide to metallic silver. Most developing agents require a pH higher than 7 to function, and for this reason the developer also contains an alkali, also called a base. In order to minimize oxidation of the developing agent by oxygen in the air, the solution also usually contains a preservative, most often a sulfite salt. A restrainer, usually a bromide salt, is also part of most developers. The restrainer has the effect of slowing the rate of development, but this effect is greater in the unexposed areas than in the exposed areas of the emulsion, thereby limiting spontaneous development, or chemical fog. The presence of a restrainer in the developer formula also tends to minimize variations due to the release of halide ions during development, which would themselves act as restrainers (see Figure 15-4). Bromides or other halides are also sometimes referred to as anti-foggants, but this term is usually applied to a number of organic compounds that are used at much lower concentrations than bromide.
Developing agents provide electrons to reduce silver ions to silver atoms.

Figure 15-4 The restraining effect of reaction products of development is much greater when the developer formula contains a low amount of restrainer. A larger amount of restrainer in the developer formula makes the developer more tolerant of increases in restrainer because of the by-products of development.
A developer formula may also include other compounds that accelerate development, provide more even development, prevent the formation of insoluble compounds, etc.
Developing Agents
Common developers today make use of organic developing agents, although in the past inorganic metallic agents such as ferrous sulfate have been used. Development takes place as a result of the transfer of an electron to the silver halide. With ferrous sulfate, the iron of the developing agent goes to a higher valence in the presence of an organic ion such as oxalate. (The loss of an electron to Ag+ converts Fe++ to Fe+ ++.)
Ferrous sulfate developers were most commonly acid with a pH range of 4 to 6, but they can operate in an alkaline solution.
Hydroquinone
The majority of black and white developers utilize hydroquinone and Metol as their developing agents. Hydroquinone, discovered in 1881 by Abney, is probably used more than any other developing agent although usually in combination with another developing agent. It requires strongly alkaline solutions, since it has relatively low energy. Hydroquinone is slow to take effect but, once it has started, proceeds to develop rapidly and produces high contrast. It is quite sensitive to changes in temperature, and is practically inactive at temperatures below 15° C (59° F). In strongly alkaline solutions, it is a good developer for high-contrast and line copy work. Developers made with hydroquinone tend to produce stain and fog unless sufficient sulfite and bromide are included in the formula. Both hydroquinone and pyro-gallol tan the gelatin of an emulsion adjacent to those areas where development takes place; and either of these developing agents, or a combination of both (sometimes with other agents), is used in tanning developers. Tanning developers are used to produce matrices that can be dyed, with the dye being transferred to a mordanted paper or film as in the dye transfer or, previously, the Technicolor motion-picture film processes.
Metol
Metol, introduced by Hauff about 10 years after the discovery of hydro-quinone, is another popular developing agent. It is packaged, sometimes with a modification of acidic counter-ion, under a variety of proprietary names other than Metol—such as Elon, Pistol, and Photol. Metol is unlike hydroquinone in its developing action. It is a soft-working developer, development is initiated almost from the start of the developing time, it retains much detail in the images produced, it is not very sensitive to restrainers such as bromide, it has good shelf life, and it is capable of developing relatively large quantities of photographic emulsion before becoming exhausted.
MQ Developers
Developer formulas made with both Metol and hydroquinone (MQ) produce an effect superior to either one used alone or the sum of the two used separately. This super-additive effect seems to have the advantage of an earlier induction period, allowing the hydro-quinone to come into play sooner than it otherwise would. The super-additivity, however, exists not only during the induction period, but also at any degree of development. One explanation is that Metol is the primary developing agent and its oxidation product is reduced back to Metol by the hydro-quinone as long as any hydroqui-none exists in the developer solution. Several other developer combinations also produce an improved working characteristic, but Metol and hydroquinone are the best known. Phenidone and hydroquinone used together produce a similar super-additivity effect.
Accelerators
In order for the developing agent to function properly, the pH of the solution must be stable and alkaline, usually around the pH range 8 to 12 for with organic developing agents. This is accomplished by adding an alkaline buffer, or a mixture of compounds designed to maintain the pH at the desired level over the life of the developer, or at least during the development time of a given film. Sodium hydroxide is a strong alkali that is used for some types of developers, but the amount required to produce the required pH for most developers would be small, and the hydrogen ions liberated during development would rapidly change the pH. If the required pH is above 12 or 13, however, the quantity would be sufficient to maintain the pH even after the hydrogen has neutralized a substantial proportion of the hydroxyl OH~ ions to H2O.
Most developers require that the alkali be buffered so as to maintain the availability of OH~ ions over a period of use. The buffering compound is usually a salt of a weak acid, such as sodium phosphate, sodium metaborate, sodium sulfite, or sodium bicarbonate. Several factors, including other chemicals in the formula, affect the pH of the developer but the choice of alkali is the most significant one. The pH, then, is an indicator of the relative activity of the developer; the higher the pH, the higher the activity. Some types of developing agents require a strong activator to make them function, whereas others can function with relatively weak activators, and a few with none at all. The following typical accelerators are arranged in order of decreasing alkalinity:
Metol and hydroquinone used together in a developer develop more silver than the sum of the silver produced by the two used separately.
The activity of most developers increases as the pH is increased by adding alkali.
Sodium hydroxide, pH |
12+ |
Sodium carbonate, pH |
11.5 |
Sodium metaborate |
10.8 |
Borax, pH |
9.6 |
Sodium sulfite |
(weak alkali) |
Without a restrainer, most developers would develop an excessive number of the unexposed silver halide grains.
Preservatives
Being a reducing agent, the developer will also react with oxygen in the air and thus lose its capability to develop a silver image. A preservative is added to the solution to prevent aerial oxidation. Sodium sulfite is the most commonly used preservative for developers utilizing organic developing agents. In the case of Metol-hydroquinone developers, the sulfite also serves the purpose of removing the reaction products of regeneration of the Metol by hydro-quinone, so that the process can go on without slowing down. Sulfite also acts as a weak silver halide solvent, forming complexes that provide for a degree of physical development—the effect being influenced by other ingredients in the formula such as bromide, certain developing agents, etc. A small amount of physical development contributes to “fine-grain" development. In color developers, where the formation of dyes requires further reactions with oxidation products, the amount of sulfite must be kept to a minimum to prevent removal of the intermediate reaction products.
Restrainers
A simple developer formula (developing agent, activator, and preservative) may not differentiate adequately between the exposed and unexposed grains of silver halide in the emulsion. That is, in addition to developing the image grain, it may also tend to develop the non-image grains to produce silver or fog. (Re strainers sometimes cause some reduction in emulsion speed, especially if used in larger amounts.) A soluble halide, usually bromide, is added to the solution to act as a restrainer. It has the effect of controlling the reduction process so that there is a better differentiation between exposed and unexposed grains. In addition, since one of the reactions of development is the release of bromide or other halide into the solution, these ions will act as a restrainer. As more and more film is processed in the developer, additional halide will be added to the solution, thus increasing the restraining effect. A quantity of bromide in the developer formula minimizes the variability produced by the addition of relatively much smaller quantities of halide resulting from development (see Figure 15-4).
In the processing of multilayer color films where at least three emulsions have to be quite precisely controlled relative to one another, the maintenance of the halogen ions in the developer may have to be closely monitored. The restraining action of various halides is considerably different—iodide, for example, requiring one-thousandth the quantity of bromide required to produce a given restraining action. On the other hand, the amount of chloride required is so high that it is not useful as a restraining agent.
Anti-Foggants
Anti-foggants are halides such as potassium bromide that restrain the development of fog in non-image areas during development. However, the term is usually reserved for another class of developer additives that minimize fog, usually with less effect on the overall speed of the emulsion. The term is applied to a group of organic compounds sometimes used in the preparation of emulsions, as well as developers, although the specific compounds are not necessarily interchangeable in the two applications. Some high-energy developers that require a high pH, for example, must of necessity incorporate an anti-foggant of this type to keep the fog at an acceptable level. Since the organic anti-foggants usually require lower concentrations than does bromide, they may be used where high concentrations of bromide are out of the question because of its effect on the other characteristics of the emulsion. Typical anti-foggants used in developers include 6-nitrobenzimidazole and benzotriazole.
Other Development Accelerators
Numerous compounds increase the rate of development when added to developer formulas due to effects other than the effect of the pH increase produced by the developer accelerator. These compounds work in a variety of ways. Some wetting agents, for example, have a positive charge (cationic), and decrease the induction period by canceling out the negative charge on the silver grain. Some sensitizing dyes with positive charges can have a similar effect. Certain neutral salts such as potassium nitrate and sodium sulfate also have an accelerating effect, which is thought to be caused by the improved diffusion of the developer ions through the gelatin of the emulsion. Another class of compounds that are weak silver solvents also act as development accelerators through the extra density provided by physical development (e.g., sodium thiocy-anate). Wetting agents are sometimes added also for the purpose of smoothing out the flow of developer over the emulsion surface to produce more uniform development.
The concentration of developer solutions has a positive but not always predictable effect on the rate of development.
Other substances, manufactured under proprietary names, are often added to developer formulas to increase shelf life, lower the freezing temperature of liquid developers, improve mixing and solution capabilities, etc. Thus a packaged formula may be considerably different from a mixed formula used for the same purpose, or sometimes even have the same name. These proprietary formulations often provide competitive advantages in handling, and they are not disclosed to the general public.
Concentration of Developer
The components of a developer interact in a variety of ways. The develop-ing-agent concentration, the alkali and resulting pH, the restrainers, and the sulfite or preservative concentration influences the development rate. The interaction of all these components is not constant, and adds further to the complexity. For example, it is often difficult to predict the results of dilution of many developer formulas. Some of them are designed to minimize these interrelating effects, and carry recommendations for diluting to various strengths to meet specific photographic requirements. In general, these developers are such that the product of dilution and development time to provide a given degree of development is not always constant, and each dilution should be tested with the type of film being used. Many developer formulas are such that a concentrated stock solution is prepared, which is diluted to various strengths as required.
Other factors affecting the degree of development are time, temperature, and agitation. These are interrelated so that a change in one can, to a considerable degree, be compensated for by a change in the other. Thus, different developing times are recommended for tray development of film with constant agitation and tank development with intermittent agitation. Agitation should be selected to produce uniform development over the picture area, not to control the degree of development. Most photographic processes, however, are designed to function best at a prescribed combination of time, temperature, and agitation. This is particularly important for color processes, where the relationship of reproduction characteristics between the red, green, and blue records has to be precisely maintained.
The temperature coefficient is the development time factor that compensates for a decrease in the temperature of 10° C.
Development Time
The chemical effects of development are not constant throughout the total time of the process. In the beginning there are no reaction products, and the developer has to penetrate the emulsion to gain access to the silver halide grains. As soon as the development process starts, reaction products begin to form and a restraining action comes into play, which is controlled to some extent by the removal of these products and their replacement with fresh developer by means of agitation. In the beginning there is also a relatively low concentration of silver ions present as the result of the solvent action of the developer, and it is some time before any physical development begins to take place. Rapid-acting developers, therefore, have a minimum of physical development components, whereas developers requiring extended times tend to have greater physical development components. Most fine-grain developers require relatively long developing times. The composition of the developer, the proportions of the developing agents, etc., have significant effects on the development characteristics with time. Most developer formulas in common use have been optimized for performance throughout the range of times that produce the desired contrast indexes for films.
Developer Temperature
Development time is also a function of developer temperature. But again, there is no hard and fast rule for quantifying the relationship that would apply to all developer formulas. In practical situations with well-designed developers, and within the range encountered in normal practice, the rate of development approximately doubles for every 10° C (18° F) increase in temperature. The temperature coefficient is the ratio of the increase in development time that occurs when the temperature is decreased by 10° C (18° F). It can be determined by observing the times for first appearance of the image in the developer at the two different temperatures and applying the following formula (Eq. 15-1):
When the developer contains two developing agents, the coefficient is useful over only a limited range. Some typical coefficients are:
| Metol | 1.3 |
| Pyrogallol | 1.9 |
| Hydroquinone | 2.5 |
| Metol-hydroquinone | 1.9 |
If the time to achieve a constant gamma at two temperatures is plotted on a graph in which the time scale is a ratio scale, a chart is produced that will show the time of development for any temperature required to produce the gamma, as shown in Figure 15-5.
Developers with hydroquinone as a developing agent have a relatively high response to changes in temperature, whereas those with Metol or Phenidone have a lower response. With the combination of Metol and hydroquinone this is evened out to some extent, but at lower temperatures there is less developing effect of the hydroquinone than Metol, and at higher temperatures the reverse is true. The concentration of bromide in the developer affects its response to temperature variations, as does pH. A higher pH value or a lower bromide concentration makes the developer less responsive to temperature variation (it has a lower temperature coefficient).

Figure 15-5 Time-temperature graph.
Developer Agitation
Agitation affects both the degree and the uniformity of development. When development starts, the solution penetrates into the emulsion to react with the silver halide grains. As this proceeds, the developer is used up, and has to be replaced with fresh developer. The presence of the gelatin retards the diffusion, but in time the partially exhausted developer diffuses to the surface of the emulsion. If development is stagnant, this exhausted developer accumulates at the surface and slows down development. Agitation of the developer removes the exhausted developer and replaces it with fresh solution that diffuses into the emulsion to continue the development process. Agitation rate should be geared to obtain maximum uniformity of development. With high-acutance developers, where the image enhancement is achieved by the accumulation of exhausted developer and of fresh developer, at the edges of high densities and low densities, respectively, little or no agitation is recommended. With short developing times, agitation has a greater effect on degree of development and overall density than it does with long developing times.
The temperature coefficient is the development time factor that compensates for a decrease in the temperature of 10° C.
To ensure uniformity of development, the manner of agitation is important. There should be a nonlinear flow of the developer over the film; otherwise large areas of density differing from the surrounding area will allow exhausted (or relatively fresh) developer to be swept across adjacent areas of differing exposure. A motion-picture film, for example, that is carried through the process in a linear fashion will show drag, areas of light density trailing dark areas on the film. The effect would be more prominent with short developing times. Likewise, intermittent agitation in trays can produce standing waves that will cause patterns on the developed film. Constant gas-burst agitation will tend to produce linear patterns, but if the bursts are intermittent, the combination of the bubbles' sweeping action followed by a period of stagnation will produce a more uniform result on the developed film. With tray processing constant agitation is recommended, but the tray or the materials in it should be moved in a random way (the tray is rocked, first one corner down, then the second) so as to minimize standing waves or directional effects.

Figure 15-6 Some film and paper processing configurations.
The type of processing apparatus used has a strong influence on the degree of agitation (see Figure 15-6). Deep tanks in which the film is manipulated by hand can produce minimum agitation, as well as can stagnant tray development. Shallow tanks, spiral reels, and trays offer an intermediate degree of agitation and require well-defined agitation techniques to minimize non-uniformity problems. Processing drums, on which the film or paper is held in close proximity to the rotating drum surface with the developer carried between the drum surface and the emulsion surface, produces close to maximum agitation— the developer is exchanged at a very high rate. The roller transport processor produces a similar high degree of agitation. Tubes that carry the sensitized material inside, with the developer and other solutions flowing over the emulsion as the result of rotating or tilting the tube, also produce a high degree of agitation. Processing machines for long lengths like motion picture films with spiral configurations also provide good agitation, but these can lead to unevenness because of the linear passage of the film through the solution. Here, extra agitation of the solution by means of circulating pumps and jets can be made to sweep the film to minimize these problems.
Replenishment
When processing small volumes of photographic materials, it is best to use the developer once and to then discard it. In this way uniform fresh developer is always used, and the resulting development is more uniform from one time to the next.
When larger quantities of materials are being processed, it is not economical to discard the relatively large volumes of solutions required, and so replenishment can maintain the constant activity of the developer (or other solutions used in the photographic process). In determining the makeup of the replenisher formula and the rate of addition, several factors have to be considered. These include: (1) the loss of developer resulting from carry-out by the film or paper as it is removed from the solution, (2) loss in developer activity caused by accumulation of reaction products of development in the solution, mainly soluble bromide, and to some extent other halides, (3) loss of activity casued by exhaustion of developing agent and sulfite consumed in the developing process, (4) increase in developer concentration caused by evaporation, and sometimes (5) carry-in of water or other chemicals if a pre-bath or pre-rinse is used prior to development. (Other steps in a process will also have to take into account the carry-over of the previous solution or rinse.)
The replenishment formula is calculated to accommodate these changes caused by processing, and to maintain the activity of the developer at a constant level. Such a formula is established by measuring the changes in the developer's chemical composition as measured quantities of film or paper are processed, calculating the amount of each chemical required to maintain the developer at its original strength, and adjusting this formula by actual performance tests. Because there are differences in the variables, it is often necessary to adjust the replenishment rate and/or formulation, especially if the process is to be maintained over a long period of time.
The temperature coefficient is the development time factor that compensates for a decrease in the temperature of 10° C.
Some moderate volume replenishment systems are designed to be replenished over a period that would require the addition of an amount of replen-isher equal to the volume of the original developer solution. Then the developer is discarded, and a new developer, along with an equal volume of replenisher, is prepared for use with additional films. This generally avoids or minimizes the problems that can arise when attempting to maintain the process over a long period without re-mixing. With some systems, only a replenisher chemistry set is sold, and a starter solution is provided that adjusts the replenisher to make it similar to a seasoned developer by adding bromide and reducing pH with an acid, and without processing any film to condition the developer. This becomes the properly compounded developer, and the replenisher itself is used for replenishment as intended.
Replenisher formulas generally supply additional sulfite and developing agent that are consumed in the process, provide additional alkali to make up for loss, but reduce the amount of or omit bromide to compensate for that added to the developer as the result of the development reaction. In general, long-running processes can be maintained by suitable control of the chemistry through sensitometric monitoring and analysis of the constituents of the developer in use. In many instances, the carry-over of solution from the tank is sufficient to accommodate the replenisher solution introduced into the tank, but in some instances control is achieved by having the formulas adjusted so that some additional developer has to be withdrawn to permit the introduction of a greater amount of replenisher. This makes it possible to adjust the withdrawal rate as another means of controlling the developer's chemical composition. In some cases, it may be necessary to remove more than is carried over by the film to compensate for excessive buildup of halides beyond that required in the original formula.
Large-scale operations with continuous processing require constant chemical and sensitometric monitoring, as well as careful adjustments in the replenisher formula and rate in order to maintain the result within narrow limits. Since the rate of replenishment depends upon the type of film being processed, and the type of images on the film (high-density vs. low-density, etc.), these factors must be considered in maintaining uniformity—particularly in the case of color processes.
Paper Processing
Process control procedures used to maintain consistent quality involve sensitometric tests and chemical analysis of the processing solutions.
Selecting a film developer commonly involves a trade-off, such as fine-grain versus short developing time.
The developers used for papers generally follow the same principles as those for films, with additional problems because of the physical differences (which govern carry-over, among other things). Alkalinity and other aspects of the developer may affect the sizing, curl, and/or brittleness of the paper. The content of the developer—restrainers and reaction products—can have a significant effect on the tone of the image produced, and this can become more apparent if the prints are subsequently chemically toned.
Analysis of Developers
The best control of processing operations involves the use of both sensito-metric tests and chemical analysis of the processing solutions, techniques of process control (see Chapter 3). However, the analysis of most photographic chemistries involves rather complicated procedures and requires a well-equipped laboratory operated by a trained chemist. In addition, some methods are subject to error due to the presence of other chemicals in the solution. It is best to continually make reference to a properly mixed operating solution that has not been used to make sure the calibrations of the analyses are accurate. Simple procedures include the measurement and control of pH, measurement of specific gravity, and notation or measurement of the color of the solutions. These can be plotted and serve as an indication of change that may call for further study or more advanced analysis. And, of course, because all chemical processes are a function of time, temperature, and, in the case of photography, agitation, carefully monitoring these aspects is probably the most important control that can be exercised.
The importance of good darkroom habits cannot be overstressed. Most failures of photographic processes can be attributed to accidents involving contamination of the processing solutions, even in trace amounts.
Specific Black-and-White Processes
Processing of black-and-white films, papers, and plates normally involves development, followed by a rinse in an acid stop bath, fixing in an acid thiosulfate solution, washing to remove the soluble silver complexes and processing chemicals, and then drying. The wide choice of developer formulas depends on the photographic objectives, economic considerations (time, overhead), quality advantages (real or imagined), safety, ecological considerations, etc.
Table 15-1 Common Acronyms

aKodak
bMonohydrated
Paper-processing formulas are designed to produce pleasing image tone and densities, both before and after chemical toning; to have good tray or machine tank life; and freedom from unwanted stain or fog in the whites of the print. Color formulas have to be capable of forming appropriate dyes of good density. In some applications, film speed is the primary criterion, and the formula may be chosen to maximize this characteristic, even at the sacrifice of some aspects of image quality. In most cases there has to be a trade-off between desired attributes and those that are required to meet some condition. Table 15-1 compares some typical developer formulas.
Practical Applications
Understanding the process by which both film and paper are developed is only part of picture. Understanding the effect it has on your photographs is the rest. Although the chapter on Tone Reproduction has gone into this in detail, it is worthy of a brief discussion here.
The assumption made here is that the starting negative is properly exposed. Let's begin with a normal reactive developer at the proper temperature with proper agitation.
The effect of underdeveloping film will cause an overall drop in contrast of the resulting negative and the final print. This is caused by an insufficient amount of time for the exposed silver halide crystal to be developed into metallic silver. The highlight areas of the image, which should have a high density in the negative, are not given sufficient time to build up the density required to produce bright, clean highlights in the final print. The shadow areas that have detail in them are lost in the final print also, again due to insufficient time for the developer to produce separation in densities in these areas.
Overdeveloping film will often increase the contrast of the negative, but still not produce a satisfactory result. Overdeveloping can allow the developer to start to react with nonexposed silver halide crystals, building up density where it should not be.
REVIEW QUESTIONS
- The development process amplifies the latent image silver by as much as …
- one hundred times
- one thousand times
- one million times
- one billion times
- one trillion times
- The basic steps in reversal processing, in the correct order, are …
- develop, fix, bleach, re-expose, redevelop, fix
- develop, bleach, re-expose, redevelop, fix
- re-expose, develop, bleach, redevelop, fix
- develop, re-expose, bleach, redevelop, fix
- In chemical development, the silver that forms the final image is provided by …
- a pre-development toning bath
- the developer
- the latent image
- the silver halide grains
- the fixer
- Developing agents are classified as being …
- oxidizers
- reducers
- ambivalent
- “Superadditivity" refers to the effect produced by using …
- Metol and Phenidone in the same developer
- Metol and hydroquinone in the same developer
- double the normal amount of Metol in a developer
- Metol and sodium sulfite in the same developer
- The chemical most commonly used as a preservative in developers is …
- potassium b romide
- sodium sulfate
- sodium sulfite
- sodium sulfide
- sodium thiosulfate
- Benzotriazole, which is sometimes used in developers, is classified as …
- a developing agent
- a preservative
- an alkali
- an anti-foggant
- an accelerator
- In comparison with the original developer, developer replenishers typically contain …
- less preservative
- less restrainer
- less alkali
