Chapter 13
Chemistry for Photographers
Photograph by Jessica Peterson, Biomedical Photographic Communication student, Rochester Institute of Technology.
Edited by Paul A. Schwartz
Introduction
Photographers today have many choices regarding the materials and technologies available to them for exposing, processing, and printing images. In each of these steps, chemical substances play a key role in defining both the characteristics and quality of the images generated. At the time the camera shutter opens, light energy is focused on the image plane where a sensor is positioned. Digital cameras rely on an image sensor, a microelectronic semiconductor device, which converts the image to an electronic signal and begins the series of conversions known as image processing. The image data is sent to magnetic storage such as a flash card or other portable media. Film camera systems use silver halide films that capture latent image that will be amplified and stabilized via chemical processes. The final image is retained in the film.
Image Capture
Currently three main types of digital image sensors are available.
Charge Coupled Device (CCD)—A specialized semiconductor material that produces high-quality images. It was invented at Bell Labs in the late 1960s. Like almost all semiconductors, they are produced from doped-silicon wafers. Functionally, light is converted to charge; photons to electrons.
Complementary Metal Oxide Semiconductor (CMOS)—A standard semiconductor material that has been developed into image sensors. These sensors generally require less power than CCD and have found application in scanners, cell phone cameras, and digital and video camera systems. CMOS sensors have also reduced the costs of digital imaging as they are able to be fabricated with lower cost.
Foveon Sensors—A structured CMOS imaging semiconductor designed using three distinct layers which aims to improve light sensitivity and color reproduction. Foveon sensors were announced in the 2002.
In film camera systems, silver halide salts represent a category of chemical substances which have high reactivity to light in the visible and ultraviolet regions of the electromagnetic spectrum. Photographic films have allowed for many types of photographic innovations to occur. The fundamental reaction of silver halide photographic materials is the reaction of light to form latent image; photons to silver specks.
Silver halide salts serve as the image sensor. Film speed is defined by the sensitivity of these silver halide grains to light energy. Commonly, in the preparation film emulsions, an intentional doping of trace metal can improve the light sensitivity of the silver halide crystals. These dopants are often referenced as chemical sensitizers. Gold salts and certain rhodium and iridium complexes are among the materials used for chemical sensitization of silver halide film emulsions.
Silver catalyzed dye formation in structured color films and color papers is another image capture method which has benefits in many photographic applications. The color film and paper structures have been optimized to produce accurate color reproduction and other image quality characteristics, such as sharpness and grain have also been co-optimized.
Image storage onto magnetic media such as hard drives and flash storage is common for photographic image storage and data storage of other information. The materials and devices for data storage are continually improving in both convenience and reliability. Optical storage media such as CD or DVD are also convenient data storage and backup materials. The organic dyes in these media, define how permanent the data written onto these materials will be. The capacity and density of the data is defined by the storage media. Image retrieval and image stability are additional factors to be considered in setting a method for storing images both for near-term use and for archiving images.
Image Display
Final image presentation on paper, dye or ink on paper is also connected to the chemicals used in the process. Currently, the three main methods of printing images to paper in photographic systems are:
Inkjet printing, which uses a set of cyan, magenta, yellow, and black dyes or pigments to print onto coated paper for image display.
Chromogenic dye formation from silver halide photographic prints is a well-established method of forming image onto paper.
Thermal dye transfer methods produce dye onto paper prints.
Also, silver, platinum, palladium, cyanotype, and other variations of early photographic technologies continue to interest printmakers for their proven longevity and unique image tones.
Basic Chemistry
In order to understand photographic chemistry, we must review the basic concepts of general chemistry. The universe consists of a number of elements. Ninety-two of these occur naturally, but elements heavier than uranium have been created by means of nuclear bombardment and radioactive decay. These elements have distinct characteristics and can be divided into two groups—metals and nonmetals. Each element is composed of atoms, which are the practical minimum particle that has the characteristics of an element. Atoms consist of the subatomic particles of protons, neutrons, and electrons. Electrons are generated in many photochemical reactions which produce latent images in silver halide systems and, more directly, voltage in digital imaging sensors. Physicists have broken atoms into still smaller subatomic particles, but it is not necessary to go deeply into this to understand photographic chemistry.
The periodic table is a logical arrangement of the elements according to their chemical and physical properties.
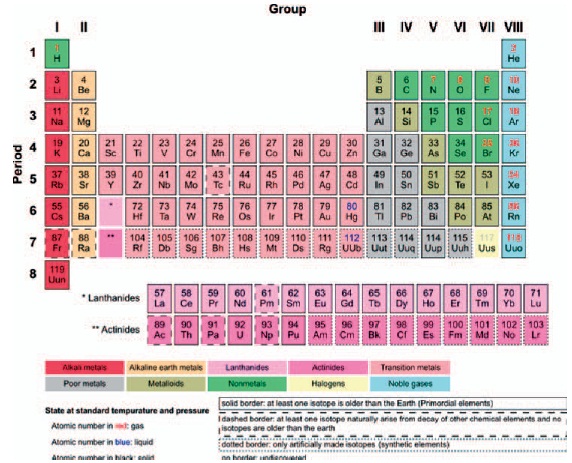
Figure 13-1 One form of the periodic table in which the atoms are arranged according to their chemical and physical properties. As the atomic numbers increase, so do the atomic weights.
The Periodic Table
In 1869, Mendeleev arranged the then-known atoms into a table, according to their chemical and physical properties. At first there were 89 elements in the table. A few years ago this number was extended to 103. The standard table as of October 2006 has 117 elements. Figure 13-1 has 118 elements present, but element 118, ununoctium, has been discovered. The name ununoctium, which is a systematic element name, is being used until the International Union of Pure and Applied Chemistry (IUPAC) can determine a permanent name for this element. When more recent information concerning the atomic structure became available, it gave further support to the arrangement of the table. The atomic number is equal to the positive charge of the nucleus in the atom, that is, the number of protons in the nucleus. The first column of the table contains the alkali metals—lithium, sodium, potassium, rubidium, cesium, and francium. Near the other side of the table can be found the halogens—fluorine, chlorine, bromine, iodine, and astatine. Other groups of elements are similarly grouped in the table.
Atoms
Atoms are the smallest bits of elements that can combine chemically.
The smallest atom is that of hydrogen, which consists of a single positive nucleus surrounded by one electron. All of the other atoms have a nucleus of higher positive charge, with corresponding electrons orbiting around the nucleus, and which have a total negative charge equal to the positive charge of the nucleus. Thus a stable atom is neutral. The atomic number is the number by which the charge on the hydrogen nucleus must be multiplied to equal the nuclear charge of the atom. These numbers determine the chemical behavior of the elements, and also support the arrangement of the atomic table (see Figure 13-2).
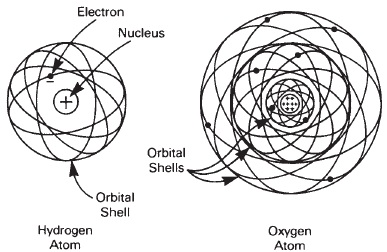
Figure 13-2 The hydrogren atom has 1 electron in orbit around a nucleus with a single positive charge. The oxygen atom has 8 electrons orbiting in shells around its nucleus, with an equal number of positive charges.
The various elements are designated by their symbols; that is, the symbol for hydrogen is H, He for helium, Na for sodium (for natrium, its Latin name), K for potassium (kalium). The element names and symbols are defined in the periodic table. When two or more atoms are combined, such as O2 and O3 to become molecules of oxygen and ozone, each molecule of O2 contains two atoms of oxygen. Each molecule of ozone O3 contains three atoms of oxygen. Most molecules are made of different atoms and they are called chemical compounds. Potassium bromide is a compound commonly used in photography.
Many compounds are made up of more than two elements, in which case two or more of each of the elements assume the charge characteristic of a single element. For example, ammonium chloride (NH4Cl) in solution ionizes into NH+ and Cl-. The NH+ is a positively charged ion, a cation.
Atoms are neutral—the balance of electrons orbiting the nucleus has a negative charge equal to the positive charge of the nucleus. Compounds formed from a metal and a non-metal are defined as ionic compounds. They will dissociate into separate atoms, each having a positive or negative electronic charge. Sodium chloride (NaCl), for example, when heated to make it molten, forms sodium ions (Na+) and chlorine ions (Cl-); also a solution of sodium chloride in water, dissociates into ions.
Chemical compounds have different physical characteristics from those of the atoms that are combined to make the compounds. Such properties as melting points, solubility in water or other solvents, crystalline form and color, and specific gravity or density are some of the characteristics that differentiate various compounds. Inorganic compounds are those containing metals, and do not contain the element carbon, with the exception of the oxides of carbon compounds containing the carbonate (CO2-) ion. Organic compounds are those containing carbon with the exceptions noted above usually along with hydrogen, oxygen, sulfur, nitrogen, iodine, phosphorous, and other elements. The benzene ring, a ring of 6 carbon atoms with hydrogen atoms attached, is the basis for most photographic developing agents and colored dyes and pigments. Organic compounds are often not very soluble in water, and they are combined with sodium or similar alkali to form a salt that is more soluble in aqueous solutions, with the compound retaining most of its desirable photographic characteristics.
Valence
Depending on the number of electrons in their outer orbitals, elements combine to form compounds in various whole-number proportions. Thus a sodium atom with a valence of one electron will readily form sodium ion (Na+) and a chlorine atom with a valence of seven electrons will readily form chloride ion (Cl-) and they can combine to form NaCl, sodium chloride. Gold, with a valence of one or three, takes a valence of three (Au+++) and each atom requires three chlorine atoms to form gold chloride (AuCl3). Nitrogen can have a valence of three or five. Oxygen has a valence of two, so two atoms of nitrogen can unite with three atoms of oxygen to form N2O3 (nitrous anhydride). When nitrous anhydride combines with a molecule of water (H2O), it forms two molecules of nitrous acid (2HNO2).
The benzene ring, a ring of 6 carbon atoms with hydrogen atoms attached, is the basis for most developing agents.
Atomic Weight
The atomic weight is the relative weight of the atom or very near to the weight of the nucleus (protons + neutrons) of the atom. For example, the atomic weight of oxygen is 16. The molecular weight of a compound is the total of the weights of the atoms making up the molecule. To form a given quantity of a compound without any of the atomic or molecular components left over, the reaction is carried out with amounts that are proportional to their atomic or molecular weights. When 23 grams of sodium (atomic weight— 23) are combined with 35.5 grams of chlorine (atomic weight—35.5), 58.5 grams of sodium chloride (molecular weight—58.5) are formed. A molar solution is one that has the same number of grams as the molecular weight dissolved in one liter of water. Such solutions would have equal numbers of molecules of chemicals dissolved in them, and equal numbers are then available for reaction. A 1 molar solution of sodium chloride would contain 58.5 grams per liter.
Ions are atoms that have a positive or negative charge.
Atoms can combine to form compounds. The number of each type of atom required depends on its valence.
Atomic weights are the relative weights of atoms with the weight of oxygen taken as 16.
A molar solution is one that has the same number of grams as the molecular weight dissolved in one liter of water.
Chemical Reactions
When chemical compounds are mixed, and the conditions favor forming new compounds a chemical reaction takes place; reactants form products. If a chemical reaction occurs in a liquid solution the compounds may remain in solution, or they may precipitate as a solid out of the solution. If a product of the reaction is a gas, it is given off and removed from the reaction. In either case, the reaction proceeds in the direction of the compound that is being removed, until the quantity that can remain in solution under the conditions is reached. If the reaction products, precipitate or gas, are removed, the reaction can continue as long as the unreacted chemicals are added.
When oxidation occurs, reduction also occurs.
For example, a solution of silver nitrate added to a solution of sodium chloride will form poorly soluble silver chloride and soluble sodium nitrate. (Chemical reactions are expressed by means of balanced chemical equations.) Silver nitrate added to sodium chloride can be indicated by the following (see Figure 13-3):
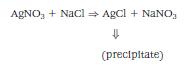
or more precisely,
The silver nitrate and sodium chloride in solution have dissociated into their ions. When the silver and chlorine ions combine, they no longer exist as ions (are not dissociated), but form an insoluble precipitate that is removed from the reaction. Na+ and NO- ions remain in the solution.
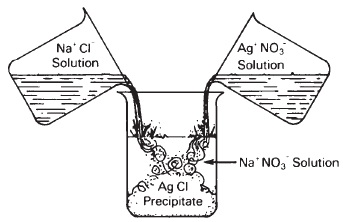
Figure 13-3 When clear, colorless solutions of sodium chloride and silver nitrate are added together a white insoluble precipitate of silver chloride is formed, along with a clear solution of sodium nitrate.
Oxidation/Reduction
Chemical reactions can take place without the ingredients being in solution—that is, between solids, liquids, and gases. When something burns, that means oxygen has combined with it, giving off heat at the same time. This is referred to as oxidation. Whenever oxygen unites with any other material, oxidation takes place. Oxygen is called the oxidizing agent. The material with which the oxygen combines is referred to as the reducing agent. Thus, reduction also occurs when a material takes on oxygen—one balances the other.
The oxidation/reduction concept can be applied to elements other than oxygen. Any element that gains electrons more rapidly than another element is said to have higher oxidizing power. Conversely, any element that loses electrons more readily than another element is said to have higher reducing power. When silver metal is subjected to chlorine gas, the two elements combine to form silver chloride (see Figure 13-4). This is an oxidation/reduction reaction where the silver is oxidized (and the chlorine is reduced), even though no oxygen is involved.
When treated with a reducing agent, such as a solution of ferrous sulfate, the silver chloride will be converted to metallic silver to form ferric sulfate and ferric chloride (see Figure 13-5). The iron, in oxidizing from the ferrous form with a valence of two (Fe++) to the ferric form with a valence of three (Fe+ + +), provides the electrons that reduce the positive ionic charge of silver:
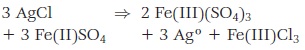

Figure 13-4 Oxidation occurs when a positively charged atom attracts a negatively charged atom, and reduction occurs when a negatively charged atom attracts a positively charged atom. In this reaction, silver has been oxidized, and chlorine has been reduced to form silver chloride.
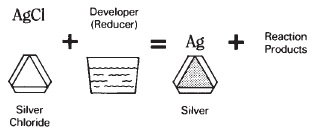
Figure 13-5 Silver chloride in a photographic emulsion is acted on by a reducer, the developer, to form metallic silver along with the other products of the reaction.
Development of silver chloride occurs in a photographic emulsion, when a mild reducing solution, the developer, provides electrons that selectively reduce only that part of the silver chloride that has been exposed to light. The latent image is converted into a real, visible silver image. Oxidation/reduction concepts can become rather involved.
Acid/Base
An acid is a compound containing hydrogen ion and another element or ion. Hydrochloric acid, for example, is a compound containing hydrogen and chlorine, HCl. This is a strong acid. HCl can exist as a gas, but as an acid it is an aqueous solution of that gas in water. Sulfuric acid is made up of hydrogen ion and the SO4- ion (SO3 [gas] + H2O [water] = H2SO4 [sulfuric acid]). Like hydrochloric acid, this is also a strong acid, which means that it is highly ionized or dissociated in solution—that is, H+ SO4-, and H+Cl-. Acetic acid, an organic compound, CH3COOH, is a relatively weak acid by comparison. Acids are formed when oxides of nonmetal-lic elements are dissolved in water, and bases are formed when oxides of the metallic elements are dissolved in water. Essentially, a base is a compound containing a hydroxyl (OH-) ion and another element or radical. Calcium oxide, CaO, when dissolved in water, yields calcium hydroxide, Ca(OH)2, a base. A base is also known as an alkali. Practically all developers are basic in composition. When placed in water, metallic sodium produces a violent reaction in which one of the hydrogen atoms of water is given off as a gas, which ignites from the heat of the reaction and burns, leaving a strong alkali, NaOH (Na+ + OH-), as shown in Figure 13-6. Ammonium hydroxide, NH4OH, is also a base, and has the characteristic ammonia odor. (Ammonia with moisture, ammonium hydroxide, is the developing agent for diazo papers.)
When an acid and an alkali combine, water is formed along with a salt, gas, or precipitate.
Acids are sour to the taste (such as acetic acid, the chief component of vinegar). Bases are characterized by their “slippery" feeling. For example, a weak solution of lye, NaOH, feels very slippery, as do most developer solutions that contain an alkali. When an acid solution is combined with a solution of a base, in equal molecular amounts, a salt is formed. For example, if hydrochloric acid is added to sodium hydroxide, sodium chloride, a salt, and water are formed. The water of formation is simply added to the water containing the acid and the base:


Figure 13-6 When the metal sodium is placed in water a violent reaction takes place in which one of the hydrogen atoms of water is released. The hydrogen burns while the remaining single atom each of oxygen and hydrogen combine with the sodium to form strongly alkaline sodium hydroxide.
Ionization
The atoms of salts dissociate when dissolved in water, forming negative and positive ions.
Acids have pH values below 7 and alkalis have pH values above 7.
Pure water is made up of equal parts of H+ (hydrogen) and OH- (hydroxyl) ions. When a salt is dissolved in water, it dissociates into the ions of the elements or polyatomic ions making up the salt. Sodium chloride, for example, ionizes into Na+ and Cl- ions. The presence of these free ions permits the passage of an electric current through the solution, and it becomes known as an electrolyte (see Figure 13-7). A solution of sugar, an organic compound that does not dissociate into ions, therefore does not carry an electric current.
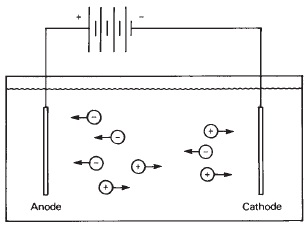
Figure 13-7 A solution of sodium chloride contains negatively charged chlorine ions and positively charged sodium ions. Electrodes placed in the solution and connected to a battery will cause the negatively charged ions to move to the anode where they lose their charge and become chlorine gas. The positively charged sodium ions will move to the cathode to form sodium metal, which reacts with the water to form sodium hydroxide and hydrogen gas.
pH
Chemists commonly specify the hydrogen ion concentration in terms of pH. pH is the negative logarithm of the hydrogen ion concentration. A strong acid with a hydrogen ion concentration of 10-2 (that is, 1/100) has a pH of 2, and a strong alkali with a hydrogen ion concentration of 10-12 (that is, 1/1,000,000,000,000) has a pH of 12. Pure water or a neutral salt solution that has an equal number of positive and negative ions has a pH of 7. The higher negative exponents, representing lower hydrogen ion concentrations, and thus higher hydroxyl concentrations, range from 7 through 14, and are the alkaline side of the scale. (D-72 developer for paper has sodium carbonate as the alkali, and a pH in the vicinity of 10.) Conversely, lower negative exponents, representing higher hydrogen ion concentrations, are on the acid side of the scale, and range from 1 through 7. (An acetic acid stop bath, for neutralizing the alkali of the developer after development, might have a pH in the vicinity of 3.5.) Or this can be restated as follows:
where H+ is the hydrogen ion concentration.
pH Meters
Since pH indicates hydrogen ion concentration, it is also related to electrical potential. That is, if suitable electrodes are placed in such an ionized solution, it acts as a battery or galvanic cell, and produces a voltage, E. The relationship between pH and the voltage (or electrical potential) is rather complex, and therefore several qualifications have to be made when using this potential to measure pH. Since we are interested in the potential without the passage of current, which would have the effect of immediately lowering the voltage, the voltage is usually measured in comparison to a standard cell incorporated in the meter. Some modern meters, however, with very little internal resistance, are direct reading. The relationship between the measured potential and pH is as follows:
where E0 is a constant.
pH meters are calibrated directly in pH values, and it is not necessary to read a voltage and then convert it to pH. The measurements are directly affected by temperature, which has to be taken into consideration and adjustments made if it is different from 25° C.
pH Indicators
Many dyes change their color under the influence of a particular pH condition.

Figure 13-8 Dye-treated pH indicator paper. A small strip of the paper is taken from the package and dipped in the solution to be tested. The dye in the paper changes color according to the pH of the solution. The pH value is then assessed by comparing it with the color references on the package.
These are referred to as pH indicators. Papers that have been impregnated with the dyes are available for indicating in various pH ranges (see Figure 13-8). These are generally more broad in their measure than pH meters, and there is a limit to the number of useful indicators that are available. Also, some judgment may be required in the interpretation of the colors produced. However, they are useful under many circumstances.
A pH meter is a voltmeter calibrated in terms of pH.
The performance of various photographic chemical solutions is influenced to a great extent by pH, and the control of this factor in one way or another is important. For example, the rate of development tends to increase as the pH, or alkalinity, of the developer is increased. Bleaches and other solutions used in color photography require control of pH to make sure that the bleaching action is adequate while at the same time dyes formed by the process are not adversely affected. The acid strength of stop baths and fixers has to be maintained to ensure that their neutralizing effects are adequate without producing other problems. Rate of fixing, hardening, and stability of the fixing solution are influenced by pH.
Complexes
Some dyes change color in response to a particular pH condition and can be used to measure pH.
A complex compound is one that is made up of two or more compounds or compounds and ions. In the fixing process used in photography, for example, silver complexes are formed when the unexposed and undeveloped silver halide salt is treated with the fixing agent, sodium thiosulfate, Na2S2O3. The fixing reactions pass through several complexes of varying solubility before one that is easily soluble is reached. The following represent several complexes that are part of the fixing reactions of silver halide photographic materials.
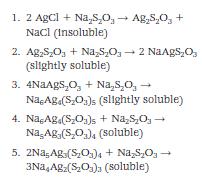
During the fixation process, different silver complexes are formed in sequence with increasing degrees of solubility.
When a substance, a solute, is dissolved in a solvent, a solution is formed.
When the silver halide has been converted to complexes 4 and 5, they can be washed out of the emulsion and/or paper to increase the image permanence. Complexes 1, 2, and 3, while they are transparent and give the film or paper the appearance of being fixed, are insoluble or only slightly soluble, and thus are not removed by washing.
Solutions
The weight of a given volume of a substance compared to that of an equal volume of water is known as specific gravity.
Most of the chemical processing of photographs is accomplished with various solutions. A solution consists of the solvent water in most photographic applications, with various chemical compounds dissolved in it. A chemical in solution is referred to as the solute. Various compounds have varying degrees of solubility—that is, capability of being dissolved in water, the solvent, until no more will dissolve. Such a solution is known as a saturated solution. The solubility of most chemicals varies with the temperature of the solution—a greater amount of the chemical can be dissolved in water at higher temperatures. When a solution has as much solute dissolved in it as is possible at a higher temperature, and the solution is cooled, the solute is thrown out of solution, usually in the form of crystals. A supersaturated solution is one that contains more solute dissolved in it than would normally be possible at that temperature. The addition of a small crystal of the solute, or some other disturbance, will cause the excess to be crystallized out rapidly. If a chemical compound in solution is mixed with another one also in solution, the product of the chemical reaction may be insoluble, and will be thrown out as a precipitate.
Other liquids can also be considered to be solvents, and indeed in chemistry there are many systems in which solvents other than water are used.
Rate of solution also depends on the size of the particles being dissolved, as shown in Figure 13-9. Small particles or crystals have a much higher ratio of surface to volume, hence the solvent can act over a larger area in a given time and thus the particles go into solution faster. (Extremely fine powders may provide excess surface and permit such factors as hydroly-sis—reactions with water—to decrease solubility. The chemistry of solubility is complex and involves rates of diffusion, degree of dissociation, and many other factors.) Chemical compounds may either give off heat— exothermic—or absorb heat—endothermic—when they are dissolved. Anhydrous sodium thio-sulfate (Na2S2O3) gives off heat when it goes into solution but the compound of crystallization with water (Na2S2O3 • 5H2O) absorbs heat when it goes into solution—that is, it is endothermic and the solution becomes cooler as the thiosulfate is dissolved. Monohydrated sodium carbonate (Na2CO3 • H2O) gives off heat when it is dissolved, and is thus exothermic—but sodium carbonate with 10 molecules of water of crystallization (Na2CO3 • 10H2O) absorbs heat, cools the solution, and is endothermic.

Figure 13-9 Volume of large cube (hence weight) is equal to that of all the smaller cubes, but surface area of large cube is one-third that of the smaller cubes.
Specific Gravity
Specific gravity is the weight of a given volume of a substance, such as a solution, compared to that of an equal volume of water at a given temperature (which has to be taken into account for precise measurements). Specific gravity can be measured with a hydrometer, a graduated weighted tube that floats to a greater or lesser degree depending on the specific gravity of the solution in which it is floated. Specific gravity is read from a scale inside the tube.
REVIEW QUESTIONS
- An element that is not a member of the halogen family is …
- chlorine
- fluorine
- helium
- iodine
- bromine
- Atoms normally have …
- a positive electrical charge
- a negative electrical charge
- a neutral electrical charge
- The chemical formula for water is H2O. If it is known that hydrogen has a valence of 1, it can be assumed that oxygen has a valence of …
- 0
- 1
- 2
- 3
- 4
- The light-sensitive compound silver bromide (AgBr) could be produced with a chemical reaction between …
- silver nitrate and sodium chloride
- silver chloride and sodium nitrate
- silver nitrate and silver bromide
- silver nitrate and sodium bromide
- Developers for black-and-white films and papers are …
- reducing agents
- oxidizing agents
- nonparticipatory agents
- A tray of developer could be identified by touch in a darkroom sink that also contained trays of stop bath, fixer, and water because the developer would feel …
- warm
- slippery
- sticky
- stingy
- gritty
- An ion is an atom that has …
- no electrical charge
- a positive charge
- a negative charge
- either a positive or a negative charge
- A photographic processing liquid that has a pH of 9 is probably …
- a developer
- a stop bath
- a fixing bath
- a hypo clearing agent bath
- the wash water
- Indicator papers identify pH by means of a change in …
- lightness
- hue
- conductivity
