Fluids
This chapter discusses the influence of fluid characteristics on flow measurement. A meter’s accuracy depends on the fluid’s physical properties. If these change considerably with small changes in temperature and pressure, then the meter will not achieve its potential accuracy.
The parameters that define low risk fluids are listed, as are the factors that cause a fluid to be classified as “high risk to measure”. Fluid flow must be single phase for basic meter accuracy to be meaningful. The problems with two-phase and multiphase fluids are discussed. Data on the physical properties of the fluid are needed for measurement. Many sources are available in the industry, and their use is discussed. Problems unique to some commonly measured gases and precautions that need to be taken for optimal flow measurement of liquids are outlined.
Keywords
natural gas; fluid; flow measurement; accuracy; low risk fluid; high risk; single phase; sampling error; data source; gas; liquid; mixture
Fluids—Liquids and Gases
If the fluid is water at ambient conditions, then its influence on flow measurement can be easily calculated from known and accepted data. However, if it is a mixture near its critical temperature and critical pressure, then accepted data may not be available, and the volume change with minor changes in temperature and pressure may make fluid definition the most important consideration in obtaining accurate flow measurements. This is one of the considerations most often overlooked in selecting and using a meter.
A meter’s commercially advertised accuracy normally allows for no error in determining the influence of a fluid’s physical properties, and users are misled into believing that simply buying a potentially accurate meter will take care of all problems. If the fluid is not adequately conditioned for flow measurement, then no meter will achieve its potential accuracy. It is of value, therefore, to review important fluid characteristics in order to know how to design an optimum metering system.
Low Risk Flow Measurement Fluids
1. Are not near their flash point (for liquids) or condensing points (for gases);
2. Are clean fluids without other phases present, with a composition whose PVT (pressure/volume/temperature) relationships are well documented with industry-acceptable data;
3. Are not exceptionally hot or cold, since temperature may limit the ability to use certain meters;
4. Have minimal corrosive, erosive, or depositing characteristics.
Considering these characteristics and answering related concerns minimizes the influence of fluids on measurement accuracy, and simplifies metering.
High Risk Flow Measurement Fluids
Many fluids classified as “high risk to measure” become the main consideration in the choice of a meter and in determining the potential for measurement accuracy.
1. Two or more phases in the flow stream;
3. Flows near fluid critical points;
4. Flows with temperatures over 120° or under 32°F;
5. Highly corrosive or erosive fluids;
Specific meters may react differently to the problems listed above, and there may be one that works better than others for the specific problem presented. It should be recognized that the fluid sometimes must be measured even if it is a “high risk” fluid, and the cost of making it a “low risk” fluid is prohibitive in a cost/value study. The preferred fluid conditions are sometimes simply not available at the point of measurement (Figure 6-1). On the other hand, these characteristics may result in a measurement uncertainty that is no better than ± 20 to ± 30%. An example is the measurement of carbon dioxide for injection into oil reservoirs for tertiary oil recovery. Removing oil from the formation efficiently requires the CO2 to be in the “dense phase” stage (near the critical point). Under these conditions—where the temperature and pressure are near the critical points of 88°F and 1,087 psia—the CO2 density variation may be in the order of one percent per degree Fahrenheit. In this high risk fluid situation, the flow measurement practitioner might prefer to change the temperature, or the pressure, or both, but the successful use of the fluid to remove the oil precludes such changes, so wider limits must be put on this measurement.
Basic Requirements and Assumptions
Fluid flow must be single phase for basic meter accuracy to be meaningful. There are two problems with a two-phase fluid. One is its effect on the meter mechanics, and the other is difficulty of obtaining a truly representative sample to determine the composition for calculating the reduction to base conditions. Studies are under way to determine whether there is a recommended way to sample two-phase fluids. Currently, sampling under these fluid conditions is less than optimal. This is particularly important in oil and gas production operations (Figure 6-2).
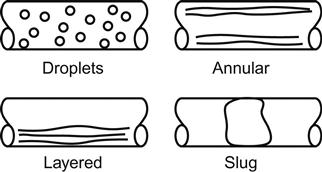
For liquids in a gas, the two-phase flow may affect flow profile configurations. Such flow patterns may conform to one of a number of regimes in a pipeline. The first phase tends to be droplets; if they are small enough, they form a homogeneous mixture so both the flow effects and sampling errors for composition mentioned above are minimized and may not be significant. The second regime occurs when there are sufficient droplets for them to begin to accumulate in non-flowing areas, including in the area of the meter, where flow distortion may occur. Also, a sample can be inaccurate. The third regime occurs when additional liquid is present. For example, there may be annular liquid flow with a core flow of gas. In this case, the mechanical configuration and the sampling are in trouble.
The addition of even more liquid means that there will then be two separate flowing streams (usually flowing at different velocities) forming layered flow (Figure 6-3).
The next regime is slug flow, which occurs as liquids collect until the lines are filled to a point where they “burp” the liquids. In such cases, there will be no way to take care of mechanical or sampling problems since liquid slugs will be followed by gaseous pockets with no indication of which fluid is causing the meter to indicate flow. Separation must be done prior to attempting measurement in any of the cases where two-phase flow exists at the meter.
In the reverse case—gas in liquids—similar problems exist, and de-aerators must be used. With solids in liquids, filtering is required to correct measurement and to minimize meter damage.
Some meters that react to mass can be successfully used for two-phase measurement provided they are not used to attempt to calculate volume without additional information on the fluid composition or density.
Some fluids are unstable and may present measurement problems when they break down into other products during interrupted flow conditions, or during exposure to conditions along a line. Hydrates in natural gas measurement are an example. Hydrates, a mechanical combination of hydrocarbons and water that forms an ice-like material, can block off main lines or gauge lines so that readings of differential or static pressure are impossible. Likewise, crude oil can form an emulsion with water, which does not lend itself to flow measurement. Some liquid plastics and such fluids as molten sulfur set up if they are not kept flowing or the meters are not heated. In these cases, the fluids must be removed from the lines when shutdown occurs. The mechanical problems these fluids create may prevent measurement if these precautions are not taken.
Although the problems caused by two phases have been outlined, some users are not aware that condensation of gases often may take place in a meter, since this may be the point with the lowest pressure and temperature in the system. Although the fluid may be above saturation upstream in the system, conditions at the meter may be below the condensing point.
The same is true with flashing liquids; the pressure within the meter may be lower than the pressure existing after flow goes through the meter to “recovery.” If there is any question, a higher back pressure should be planned, rather than risking the fluid flashing (Figure 6-4).
Fluids that are measured near their critical point have variations in relationships which are so large that the measurement of pressures and temperature within tolerances close enough to predict the effect on volume is beyond the ability of measuring equipment. Likewise, the accuracy of PVT correlation data deteriorates as the critical pressures and temperatures are approached. Because of these problems, flow measurement should not be attempted in such areas of operation if this can be avoided. The problem may also be addressed by moving the meter station to a better location in terms of fluid properties.
A number of meters have limitations for lower Reynolds numbers, and such limits should be checked in the standards prior to attempting to use a meter. With new meters the information on these limits is often quite general, and the user may be left in a quandary as to its meaning. If there is any question, a meter should be operated well away from such limits for best accuracy.
In summary: from a measurement standpoint, no fluid should be measured near a point of phase change, fluid characteristics change, near condensation, at too low a Reynolds number, or near critical pressure or temperature. Good flow measurement practice requires that these conditions are recognized and proper precautions are taken to minimize their effects.
Data Sources
Many sources of data for fluid physical properties characteristics are available in the industry. The particular industry standards for specific applications should be the first place to look. There are general fluid specifications available from universities, national standards organizations, and from suppliers that handle certain products; manufacturers of fluid products have their own data. The Flow Measurement Engineering Handbook has accumulated much data that is useful for general flow measurement.
Gas and liquids are usually considered as either “pure products” or “commercial products” in most references. Mixture laws may be used to estimate the combined characteristics of uncommon fluids, but caution should be exercised if the mixtures contain widely varying molecular weights or involve extreme conditions of pressure and/or temperature. In these cases, actual PVT tests should be run over the ranges of operation and a specific set of tables or equations should be set up for the particular mix—if such information can be derived from the results obtained.
It is important to avoid the tendency in industry to specify a mixed fluid only by its major component. Mixture characteristics must be considered, not just the PVT relationships of the pure product. Any data correlation must be examined to determine the fluid data parameters involved before applying the data to a specific metering system (Figure 6-5).

Liquid propane is an example of a liquid that falls into this area of concern. Commercial propane contains 95% propane, while the percentages of the other constituents may vary; this variation will affect the correction factors used. Reagent grade propane, however, allows the use of “pure” correction factors such as those available from the National Institute of Standards and Technology (NIST).
Another way to approach the problem of variable fluid characteristics is to use densitometers or mass meters, and then measure mass. The measured mass can then be converted to an equivalent volume at base conditions using an analysis and the density of the individual components at base conditions. (Note: This approach will be correct to determine a contract volume. But if there are significant differences in the flowing and base volumes, some means of deriving an approximate line condition flow volume may be required for operations.) The contract volume is necessary for changing custody, but the actual volume at line conditions is needed for operational control.
For any petroleum fluid, Chapter 11 of the API Manual of Petroleum Measurement Standards is an excellent data source.
The ASTM, in conjunction with both the API and the GPA, also publishes common standards for petroleum-related fluids. International standards organizations also publish fluid data documents. (See Chapter 4, “Basic Reference Standards” in addition to the list below.)
Fluid Characteristics
Gases
The following section discusses problems unique to some commonly measured gases (Figure 6-6).

Natural gas is one of the gases most commonly measured, since it is used both as a fuel and a feedstock in many industries. It represents the largest daily dollar volume of any gas routinely bought and sold. The natural gas industry has been the leader in developing gas measurement technology and its standards are used in related areas of gas measurement.
The ANSI/API Manual of Petroleum Measurement Standards, Chapter 14, “Natural Gas Fluids Measurement,” Section 3, “Concentric, Square Edged Orifice Meters” Parts 1–4 (also entitled “American Gas Association Report Number 3,” Parts 1–4; and “Gas Processors Association 8185” Parts 1–4) is the most common standard used for gas measurement of all kinds. Representing over 70 years of study of gas measurement with the orifice meter, the standard continues to be upgraded further by additional work.
Natural gas as a fluid varies from an easily measured fluid to a very difficult fluid to measure to close limits. Separated, dehydrated, “pipeline quality” natural gas is normally easy to measure since it is a well defined fluid for which very precise data is available for relating the pressure, volume, and temperature from flowing conditions to base conditions. It is normally very clean, with a minimum of solid “pipeline dust” and compressor or dehydration plant oils present. It is normally handled at temperatures and pressures that cause minimal meter design limitations or operating concerns.
Pipelines typically measure the gases within tenths of a percent in their pipeline balances between purchased gas, operating use gas, and gas sold (Figure 6-7).
On the other hand, produced gas is often handled as a saturated fluid (separated to single phase but not dried), and the problems of flow measurement increase. The ability to balance a production field (with multiple wells) is thought to be within the industry norm if this balance is within 3 to 5%.
Other than some operating problems and poor maintenance that may affect measurement, the main cause of error is the fluid characteristics that cause both mechanical problems (liquid in the meter) and errors in fluid density calculation (determining the proper specific gravity or relative density from a sample). Quite often, the volumes measured are not used directly for custody transfer, but for allocation in determining the percent of total volume contributed by each well’s flow.
At the present time, the measurement of two-phase fluid (gas and gas liquids and/or water) is not attempted because of the problems caused in a meter. There is a great deal of work being done on multiphase measurement that may, in time, resolve these problems. Getting a material balance in a system or processing plant can be one of the most frustrating flow measurement jobs. Careful attention must be paid to all of the concerns outlined here; even then, getting a close balance is difficult.
Mixtures of gases are more easily measured if the mixture has a relatively constant composition. This allows specific PVT tests to be run, or data may be available for common mixtures from prior work. The ability to use the mixture laws successfully has been previously discussed. If the mixture is changing rapidly, use of a densitometer or a mass meter may be required to determine an accurate quantity.
Ethane, a common chemical building block, may be a measured product or a mixture of an enriched stream from a processing plant. Data are available on ethane as a pure gas product. Except at critical points, measuring ethane is fairly straightforward. However, ethane’s critical temperature of 90.1°F at a critical pressure of 667.8 psia may easily occur in normal pipeline and process plant operations. For the best measurement, some heating or compression may be required for conditions near critical to make them more favorable for measurement.
From a measurement standpoint, it is worth remembering that though classified as a gas, ethane mixtures have the same characteristics as dense fluids up to approximately 1,000 psia and at temperatures from 90° to 120°F. In this area of operation (which may exist quite often in a pipeline, or as part of a pipeline or processing measurement requirement), the density changes significantly with small changes of temperature and/or pressure. Because of this, flow metering accuracy relates to these and proper selection of the equation of state for the ethane. At pressures below 670 psia, if the temperature drops below 90°F, two-phase flow can be encountered. When either of these situations is likely, consideration should be given to adding heat or pressure to the system prior to attempting flow measurement.
Measurement of ethane as a pure liquid is not very common. Since the liquid has to be handled at a low temperature, it presents unique problems with meters. However, the data for making PVT corrections are available and accepted.
The measurement of ethane liquid mixtures is more common. As noted above, data on pure ethane are readily available, whereas data on ethane-rich streams are limited, and metering accuracy will suffer accordingly. This is particularly true for variable mixtures. At times the better option is to use a densitometer with careful attention to proper sampling to minimize sample errors due to temperature and pressure variations in the stream.
Propane can be handled as a liquid or a gas since its critical temperature is 206°F at a pressure of 616 psia. At normal ambient temperature, it can be a gas or liquid, depending on the pressure. However, to solve measurement problems, the phase relationship must be known so that a single phase of liquid or gas is considered. The meter can then be properly sized, and the two-phase or phase-changing regions can be avoided. As with any fluid, the closer to phase change the measurement is attempted, the more difficult measurement becomes.
Ethylene is a popular hydrocarbon feed stock used in the chemical industry. It is difficult to measure in a number of industrial cases since its critical temperature is 48.5°F at 731 psia, which means that it is handled in an area of greatest density sensitivity (i.e., the measurement problem becomes one of the correct density measurement). Near this point in the vapor phase, the compressibility factor changes as rapidly as 1% per degree Fahrenheit and 0.5% per 1 psi pressure. This problem is significant enough to, at times, require heating prior to flow measurement to obtain sufficient accuracy. Heating to 80° to 90°F will minimize these density changes. This represents an example where the effects of the fluid characteristics on measurement accuracy are so significant that they are changed prior to measurement.
Another characteristic of ethylene is that a very fine carbon dust is produced during processing, which cannot be removed with a 5 micron filter. Depending on the frequency of buildup, the meter and meter piping must be cleaned so that the flow characteristics are not changed. This also affects measurement transducers and their lead lines, which must be cleaned.
Ethylene often contains small quantities of hydrogen. This can affect filled differential transducers; an internal pressure builds up over a period of time to the point that the unit will not be capable of meeting calibration, and replacement is required. How often this happens varies with pressure and hydrogen content.
Ethylene requires that special elastomer materials are used for those parts of a meter that come in contact with the fluid (“wetted” parts). Meter materials should be checked, and meters should be ordered to accommodate these requirements.
Propylene is another popular feed stock for the chemical industry. It is somewhat easier to measure than ethylene. This is true because its critical temperature is 197°F at a critical pressure of 667 psia. Propylene is less reactive with most meter materials, but materials in meter seals should be checked carefully for reaction with propylene.
Carbon dioxide, commonly measured, is used in the oil and gas industry for the recovery of crude oil. It also has a troublesome critical temperature of 88°F at a critical pressure of 1,071 psia that is significant when attempting flow measurement. The compressibility factors in these ranges may represent as much as a 200% correction and will be the controlling factor in achieving accurate flow measurement. Carbon dioxide is quite often handled as a mixture, which further complicates the measurement of density. Data are available from the NIST in Boulder, Colorado, for mixtures of CO2 from 94 to 99.7% containing small amounts of methane (0 to 2%), ethane (0 to 1%), propane (0 to 2%), and nitrogen (0 to 2%), as well as pure CO2 (Figure 6-8).
Once again, carbon dioxide measurement is not easy because of the density sensitivity at normal operating conditions—even if well removed from the two-phase flow region. The solution requires continual integration of density with the flow device because of the rapid changes. A computer is needed to do this calculation rapidly enough.
Another measurement problem is CO2 wetness. If water is present at a sufficient level, a hydrate may be formed at temperatures well above freezing (32°F). In addition, wet CO2 is very corrosive, and a large amount of corrosion products will move with the gas; this often results in deposits that can cause problems with meters and other equipment such as densitometers. Likewise, CO2 causes most standard seal materials, such as rubber and Teflon, to break down, and lubricants rapidly deteriorate in the presence of CO2. All these factors should be taken into account before designing a carbon dioxide metering station.
Steam flow measurement is often one of the most misunderstood of all flow measurements made in industry. There are many reasons for this, but fluid problems are the most important. Measuring steam as a fluid is fundamentally the same as measuring any flowing fluid. If the fluid dynamics and the thermodynamics are known, then the first steps toward accurate measurement have been taken. However, these two areas are not particularly well known or understood in many of the applications in which steam measurement is required.
As commonly used, the term “steam” is meaningless when considering measurement. In the industrial world, the definition narrows somewhat, but even here it covers a variety of flowing conditions. The following sections cover the three possible steam flowing conditions: wet, saturated, and superheated.
Wet (quality) steam is usually the most difficult fluid to measure. A wet steam is a fluid that contains both condensed hot water and steam. In the two-phase portion of the phase diagram (see Figure 6-9), with the same temperature and pressure there is a different density. Therefore a third parameter, quality, must be added to correct a measurement for the right density. Quality is defined as the ratio of percent flow that is steam to the percent flow that is free water, in mass. For example, 95% quality means that 95% of the flow is steam and 5% is water by mass.
Quality can be determined by a calorimeter test (batch operation), which is valid only until changes in the system take place (i.e., flow rate or density change). In addition to these fluid identification problems, the effect of two phases on the meter mechanism can create errors. Because of these problems, quality steam measurement is inaccurate and should be attempted only as a last resort, recognizing that it will have very wide accuracy tolerances.
Saturated steam has no free water present and exists only at one pressure and a corresponding temperature. However, water is not always pure; therefore the exact saturation point, pressure and temperature may be difficult to define. At the same pressure and a higher temperature, the steam is superheated; at a lower temperature, condensation takes place, and the fluid becomes quality steam. Saturated steam exists at a boiler, but when steam leaves the boiler (assuming no superheat is added), the flow creates a pressure drop, and there is the possibility of a temperature drop, depending on flow line insulation. Therefore, steam traveling through a plant will normally be superheated (i.e., the pressure drops, but the temperature is relatively constant and the steam is almost never saturated away from the boiler). Table 6-1 shows the measurements required to determine steam density.
Table 6-1
| Temperature | Pressure | Quality | |
| Wet | Yes | Yes | Yes |
| Saturated | T or Ρ | T or Ρ | — |
| Superheated | Yes | Yes | — |

Most designers state that they will be handling “saturated steam” and may not allow for temperature and pressure measurement at the meter. As the flow rate varies, the pressure will change at the meter, and sometimes the temperature will also change. To determine the density, the temperature and pressure must be known (measured) as shown in Table 6-1. If true saturated steam exists at the meter, then measuring the temperature or pressure will define the density.
Wet steam requires that temperature, pressure, and quality are measured to define the density. Saturated steam requires temperature or pressure, and superheated steam requires temperature and pressure. In each case, these measurements must be fed to a computer that calculates the density based on the equations in steam tables.
A problem unique to steam is the large difference between the temperatures of ambient air surrounding a meter and the flowing fluid. This makes the proper measurement of steam temperature a major concern. Without suitable insulation and special precautions, major errors in density will result. In a flowing stream at low velocity, steam tends to stratify by temperature; the steam must be mixed to get the temperature constant across the stream and allow accurate steam measurement (Table 6-1).
In summary, steam is the most difficult fluid to measure accurately. Even with the utmost care, a plant balance of steam and water flows in a power-generating or process plant is very difficult to do well.
Liquids
Liquids are generally reputed to be easier to measure than gases. That might be true if all liquids behaved like water at ambient temperatures. They do not, however, and certain precautions need to be taken for optimal flow measurement of each of the fluids that will be discussed in this section.
Crude oil has had as much research conducted on it as any liquid in terms of product value as well as related to the worldwide measurement and product exchange. The generic term “crude oil” covers a multitude of fluids that can be categorized by the following terms: light, sweet, sour, and waxy. These conditions all affect flow measurement procedures.
Light crude is the most desirable from a measurement point of view. Its viscosity range and wax content are the lowest, and both of these affect metering. Some heavy crudes cannot be measured without heating. The high wax content creates the possibility of deposits in lines and meters, and no common meter can operate properly if this occurs. Wax treatment or heating is required before measurement is attempted. As previously outlined, flowing temperatures must be known to define the magnitude of measurement problems. A statement about light crude must be accompanied by the operating temperature range to allow proper metering system design.
Sweet and sour crude oils typically affect meter materials and involve foreign materials introduced by corrosion. Knowledge of the fluid composition allows meter materials to be selected appropriately. Corrosive products may be treated or filtered out before they enter the meter.
Most meters are sensitive to viscosity, which limits the range some can handle. The effect of temperature on viscosity adds to the problem—plus the fact that the temperatures of crude oil measurement cover a wide range, and they are normally not controlled and are therefore determined by the situation. For example, storage tank oil may run at 120°F, whereas tanker oil will arrive at ocean temperature, and pipeline flows typically arrive at ground or ambient temperature.
Medium and heavy crudes have intermediate characteristics, but high viscosities and “crud” content can aggravate metering problems.
Complete pressure and temperature correction data are available in the literature and have been accepted by petroleum measurement groups across the world. These data were first accepted in early 1980 (in the United States, August 1980) and are referenced by most contracts. The data allow corrections from flowing conditions to base conditions not only for crude oil but also for all liquid hydrocarbons within the defined data base limits.
Dirty crude includes foreign material that will collect in a meter. This is often experienced in some production areas where the term “grass” is applied to the phenomenon. Such materials should be filtered out before metering to prevent meter stoppage or inaccuracy. If “pipeline quality” fluid is achieved, this problem seldom occurs.
Refined products, as the name indicates, are processed so that foreign materials have been eliminated. There are normally limited amounts of various components, since refined products must meet industry-established product specifications. A large quantity of industry data is available (as mentioned in the crude oil section). For new products, individual buyers and sellers will develop accepted data based on PVT tests covering the ranges of operation. Then, when the product becomes widely traded, the industry will correlate the various data, run additional tests as necessary, and establish industry correlations. Hence, most of these fluids have well-defined characteristics that can be used with confidence to get good measurement.
Ethylene and propylene liquids are well defined if they are pure products. Unless they are handled near their critical temperatures, they introduce no specific problems. Concerns about measurement near the critical points of these two fluids, or for their mixture problems when they are not pure, have been pointed out previously in the section relating to the individual compounds.
Gasoline is relatively easy to measure since it is stable over the temperature ranges at which is it normally handled, and has no tendency to flash to a gas. Correction factors are well established and accepted, and they are available for many meter readout systems with no special programming required. There are no viscosity or foreign material problems to cause special concern about a meter’s operation.
Heavier hydrocarbons (i.e., ![]() and heavier) present potential viscosity problems that must be addressed, since they are normally handled in smaller quantities, and small meters have more problems with viscosity. These fluids also may be unstable at normal handling temperatures and require controlled temperature and pressure conditions.
and heavier) present potential viscosity problems that must be addressed, since they are normally handled in smaller quantities, and small meters have more problems with viscosity. These fluids also may be unstable at normal handling temperatures and require controlled temperature and pressure conditions.
Natural gas liquid mixtures produced from natural gas contain variable amounts of light hydrocarbons (ethane through nonane). They are significant fluids in the oil and gas industry and have received a great deal of attention for flow measurement. As indicated in their definition, natural gas liquids are undefined mixtures that quite often change composition over time, pressure, and temperature. They are recovered from separators whose efficiencies relate to operating temperatures and pressures that change between night and day. The range of variations in flowing temperatures and pressures can be wide, which further complicates measurement. With the wide range of molecular weights of some of the components—particularly ethane (30) and pentane (72)—mixtures of the products have variable shrinkages with changing compositions (see Figure 6-5).
The problems above have given rise to a number of individual operating company pressure and temperature correction tables as well as tables available from the Gas Processors Association that apply to a restricted data set. Since these problems arise from changes in operating parameters, many operators have opted to use a densitometer with a volume flow meter to measure mass flow, or a mass meter that measures mass rate. A true mass meter does not require a densitometer. If an analysis is made, the mass flow can be converted to volume flow by knowing the cubic feet per pound at base conditions of the individual components (Figure 6-10).
The amount of mass or volume to be measured will define the equipment required. With wide variations in fluid characteristics, the procedure of using mass and analysis provides the most accurate way of measuring these flows, particularly at extreme temperatures or pressures.
When natural gas liquids are processed, they can be broken down into their pure components, which are more fully defined liquids. However, it is very important that quality requirements for the products be taken into consideration since they will affect the flow measurement. If the fluid is reagent grade ethane, propane, or butane, then industry-accepted pure product corrections may be used for correcting from flowing to base conditions. On the other hand, if commercial grade product mixtures of hydrocarbons are involved, then mixtures of hydrocarbons exist, and the correlations of the pure products must be adjusted to reflect corrections from the pure products’ specific gravities. Here again, industry-accepted correction tables may be used within the limits of their databases.
When a customer states he wants to measure “propane,” the meter designer does not know exactly what is required. Reagent grade is over 99.5% propane, but commercial propane requires only 95% propane. The amount of ethane and butane may vary and cause correction factors to vary. So-called propane-rich streams may have even lower propane percentages. Ethane-propane mixtures present a measurement problem with fluid PVT relationships, since normal handling conditions are near critical conditions.
There are numerous correction tables from operating companies based on their own databases. Some of these are available from the Gas Processors Association. There is also a study taking place to standardize the temperature correction values in liquid. The specific gravity range of 0.35 to 0.70, with a temperature range of roughly 50° to 150°F, generally covers the EP mix. The purpose of this study is to compare all standard procedures and, if possible, pull the industry together to agree on a single relationship. (Availability was imminent when this book was being published, in 2014.)
Two-phase flows fall into two general categories with most meters: measurable and not measurable. Since current techniques do not always provide the ability to prevent two-phase flow, studies have been made of handling the problem in limited ranges. Within these specified limits, the methods have been correlated based on the density of the two individual streams, so as to address the problem of up to 5% by volume of gas in liquids and up to 2% by weight of liquids in gas. These are very low limits and should not be stretched to higher-content mixed flow. In each of these cases, the accuracy tolerance of such measurement is at least double that expected by single phase measurement with a given meter. These procedures have been applied to steam and condensed water systems, natural gas and natural gas liquids, and crude oil and gas flows.
Some true direct mass meters can measure two-phase flows within design limits. Flows outside these limits are not measurable and should be separated and measured as individual liquid and gas flows.