Smart Inorganic and Organic Pretreatment Coatings for the Inhibition of Corrosion on Metals/Alloys
Peter Zarras; John D. Stenger-Smith Naval Air Warfare Center Weapons Division (NAWCWD), Polymer Science and Engineering Branch (Code 4L4200D), 1900N. Knox Road (Stop 6303), China Lake, CA, USA
Abstract
This chapter provides a comprehensive review of both inorganic and organic pretreatment coatings for metals and alloys. Aqueous and nonaqueous corrosion are introduced as well the economic costs associated with corrosion for several industrialized countries. Smart coating strategies currently employed to mitigate corrosion damage are shown as well as future materials and film development. Conversion coatings including chromate and phosphate conversion coatings are discussed as early examples of “smart coatings” for the inhibition of corrosion; and the latest use of lanthanide-based conversion coatings is discussed. The chapter also discusses the corrosion-inhibiting abilities of sol-gel and conductive polymer smart coatings. An examination of new methods to inhibit corrosion highlights self-assembling (SA) molecules, polyelectrolyte (PE) multilayers, the incorporation of “nanocontainers” in a coating formulation, and protective biofilms. These new innovative coatings can provide efficient barrier protection and/or controlled release of corrosion inhibitors, reducing the effects of corrosion via a self-healing mechanism.
Acknowledgments
The authors would like to acknowledge the financial support of the Office of the Director, Defense Research and Engineering.
3.1 Introduction
Metals and alloys play a significant role in today’s modern world. Metals and alloys have always had a role in past civilizations, but today’s complex world requires a variety of coated metals/alloys having multifunctional properties (smart properties). In order for metals and their alloys to effectively survive in many harsh operating conditions, coatings must be used to provide not only surface protection but also a decorative finish. In fact most everyday products are made usable and saleable by having a suitable coating via a surface treatment. Treatments of metals/alloys can impart surface protection of the material, which renders the product/material useful, inhibiting corrosion while retaining economic value. All of these factors have lasting economic impact that contributes to the health and economic well-being of today’s modern societies.
3.1.1 Corrosion—definition
Corrosion is normally defined as the “attack on a metallic material by reaction with its environment.”1 Usually one refers to metals when describing corrosion, but nonmetals such as ceramics, plastics, rubber, and so on, can also undergo destruction or deterioration when exposed to various environments. For purposes of this review we will restrict our examination to aqueous/wet environments and methods used to mitigate their corrosive effects.
As a general review we will introduce the several types of corrosion for metals/alloys and their various forms. Corrosion can be divided into three main groups:
■ Corrosion in other fluids
■ Dry corrosion
“Wet corrosion” refers to metal corrosion occurring in a wet/aqueous environment.2 The process is almost always electrochemical, and it occurs when two or more electrochemical reactions take place on a metal surface. When a metal is exposed to a corrosive wet/aqueous environment, the metallic nature of the metal is fundamentally changed into a nonmetallic form becoming dissolved species or solid corrosion products. The energy of the system is lowered as the metal converts to a more stable form, in this case nonmetallic corrosion products. This electrochemical process can occur uniformly or nonuniformly across the metal surface, which is called the “electrode” and the ionically conducting liquid is called the “electrolyte.” A classic example of this process is the rusting of a steel substrate: the iron metal is converted into a nonmetallic form called “rust.”
“Corrosion in other fluids” refers to the corrosion of metals/alloys in nonaqueous environments, such as fused salts sometimes referred to as molten salts.3 In addition corrosion can also occur in liquid metals.4 Corrosion in fused salts such as nitrates, halides, carbonates, sulfates, hydroxides, and oxides can cause profound attack on metal alloys via several mechanisms: (1) pitting because of electrochemical attack, (2) mass transport due to thermal gradients, (3) reaction of the constituents of the fused salt with the metal alloy, and/or (4) reactions with impurities present in the fused salt. In addition to fused salts, exposure of metal alloys to liquid-metal environments can cause severe corrosion. Liquid metals are used in industrial applications such as high temperature reducing agents or as coolants due to their excellent heat transfer properties. Corrosion as a result of exposure to liquid-metals can be caused by dissolution, impurity or interstitial reactions, alloying, and compound reduction.
“Dry corrosion” refers to corrosion affecting metals/alloys that are exposed to air or other aggressive gases.5 For a relatively small number of metals, exposure to gases does not result in corrosion; however, for most metals, exposure to very high temperature gases increases the corrosion rate resulting in corrosion failure. The figure below shows the elements necessary for corrosion under different environments (Figure 3.1).

The three main types of corrosion have been identified and described in detail; we can now turn our attention to the most common forms of corrosion, which are found in wet or aqueous environments. There are eight forms of corrosion, which can be identified based on the appearance of the corroded metal or alloy. These corrosion types are listed below:
■ Uniform or general corrosion
■ Pitting corrosion
■ Crevice corrosion (includes corrosion under tubercles or deposits and filiform and poultice corrosion)
■ Galvanic corrosion
■ Erosion-corrosion (cavitation and fretting corrosion)
■ Intergranular corrosion (sensitization and exfoliation)
■ Dealloying corrosion (dezincification and graphite corrosion)
■ Environmentally assisted cracking corrosion (stress-corrosion cracking, corrosion fatigue, and hydrogen damage)
The above listed forms of corrosion do not take place independently; there is normally overlap and concurrent forms of corrosion occurring at any time during the corrosion process. Corrosion can also occur at the macroscopic level, which is visible to the naked eye and microscopic level where corrosion occurs in minute amounts and is not visible to the naked eye. In the latter case to examine these effects requires higher magnification before the damage is so severe that it is visible to the naked eye (Figure 3.2). Structural integrity is often compromised after the effects of corrosion become visible to the naked eye.

3.1.2 Costs of metallic corrosion/prevention
Metallic corrosion affects almost every U.S. industrial sector ranging from infrastructure to manufacturing.6 Aging infrastructure is currently the most important sector affected by corrosion. Recently state and federal agencies have made it a priority to extend their useful lifetimes.7 Additional sectors (including infrastructure) in today’s modern society affected by corrosion are:
■ Infrastructure—highway bridges, gas/liquid transmission pipelines, waterways/ports, airports, railroads
■ Utilities—gas distribution, drinking water/sewer systems, electrical utilities, telecommunication
■ Transportation—motor vehicles, ships, aircraft, railroad cars, hazardous materials transport
■ Production/Manufacturing—oil/gas exploration/production, mining, petroleum refining, chemical/petrochemical/pharmaceutical production, pulp/paper, agricultural production, food processing, electronics, home appliances
■ Government—defense, nuclear waste storage
The cost for corrosion repair, maintenance, and replacement affects all facets of the United States and the industrialized world’s infrastructure. These costs are not only limited to severe damage compromising public safety, but also environmental damage. Corrosion can disrupt operations requiring extensive repair and replacement of failed assets. Direct corrosion costs in the United States have been estimated at ~$300 billion U.S. dollars annually, accounting for nearly 3.1% of gross domestic product (GDP).8 Extrapolating to the industrialized world, corrosion costs account for nearly $2.2 trillion U.S. dollars, which represents over 3% of the world’s GDP.
3.1.3 Corrosion costs to national economies
As was detailed above in Section 3.1.2, corrosion costs are estimated at ~$300 billion U.S. dollars annually for the United States. This often cited number produced by the National Association of Corrosion Engineers (NACE) Corrosion study of 1998 has been reviewed and updated.7 G2MT Laboratories have updated this estimate by including both the direct and indirect costs of corrosion and have estimated that all corrosion costs to the U.S. economy are over $1 trillion U.S. dollars for 2013.9 Additional studies have been conducted throughout the industrialized world. Similar percentage costs to GDP (2-5%) have been found across various economies.10 Table 3.1 lists several national economies and their associated corrosion costs.
Table 3.1
Cost of Corrosion for Several National Economies
| Country | Direct Costs of Corrosion ($US Dollars) | Percent (%) of GNP |
| United Kingdom11 | $3.2 Billion dollars (1969 US Dollars) | ~ 3.5% |
| Australia12 | $0.5 Billion dollars (1973 US Dollars) | ~ 3% |
| Japan13 | $9.2 Billion Dollars (1974 US Dollars) | ~ 1.8% |
In order to reduce the costs of corrosion affecting various national economies, effective corrosion strategies need to be employed. These existing corrosion-inhibiting technologies include: (1) proper design; (2) proper selection of materials (alloys, metals, plastics, and so on); (3) cathodic protection; and (4) coatings, inhibitors, and surface treatment. The focus of this review is to provide the reader with an overview of “smart pretreatment coatings,” a necessary first step in the prevention and control of corrosion for metals/alloys.
3.2 Designing Smart Coatings for Corrosion Protection
Over the past several decades, coatings have undergone a transformation from simple barrier/decorative coatings to more specialized responsive coatings. These types of coatings that can react or respond to external stimuli are called “smart coatings.”14 Smart coatings have evolved over time from:
■ modified coatings with specialized additives, to
■ coatings that contain inert ingredients to impart unique properties normally not found in conventional coatings, and finally to
■ coatings that can sense and respond to their environment in a consistent and predictable manner.15
Nature is the best example of stimuli responsiveness in which living cells have evolved with the ability to respond to continuous and/or intermittent forms of external stimuli. Cells have the ability through reorganization, rebuilding, signaling, and/or reshaping to survive permanent or temporary changes in their environment. This feat is accomplished by the cell membrane as the result of its hierarchically organized multifunctional molecular system.16 Current research efforts in the coating industry, academia, and government laboratories are trying to develop and adapt the unique features of nature into innovative “smart coatings” for the twenty-first century. These innovative “smart coatings” can have profound effects on everyday living to the most complex of industrial settings.17
3.3 Pretreatment Coatings
Pretreatment coatings such as conversion coatings are applied to various metal/alloy surfaces as a first defense against corrosion, but also as an adhesion promoting coating for primers and topcoats.18 Typically, primers and topcoats do not adhere very well to metal/alloy surfaces without a pretreatment coating. Therefore the following sections will deal exclusively with the pretreatment coating and its ability to act as a “smart coating.”
3.3.1 Selecting the proper metal alloy
Prior to depositing the pretreatment coating, the proper metal alloy must be selected. Different metal alloys are known to have various susceptibilities to corrosion depending on their composition. In order to mitigate these effects, different levels of corrosion protection are needed. The selection criteria are based on the most optimal material required, but this is never a straightforward process. There is the balance of adequate performance versus cost-effectiveness. The selection process is dependent on the type of industry and the corrosive environment that the metal/alloy will be exposed to. Figure 3.3 provides a flow diagram to follow for material selection criteria with the caveat that costs often outweigh the purchase of highly corrosion-resistant metal/alloys in most cases.19

3.3.2 Surface modification
Before we can discuss various “smart pretreatment coatings” on metals/alloys, we must first examine the methods to clean a surface. Before a coating is applied, the surface must be rendered suitable for the pretreatment coating (e.g., conversion coating). This process involves several steps: (1) assessment of surface condition; (2) inspecting for fabrication defects (3) pre-cleaning; and (4) final surface inspection.20
The assessment of the surface condition requires inspection of the entire surface to determine the level of contaminants present. These contaminants can be smut, grease, oil, dirt, rust, corrosion products, salt deposits, and die-release compounds. The presence of these contaminants can dramatically alter the adhesion properties of applied pretreatment coats and subsequent primers and topcoats.
Prior to surface cleaning, any design and/or fabrication defect in the part or substrate to be coated must be removed or repaired. These defects can include excessive pitting, dents, or defects in the metal/alloy component. There are several methods available to remove contaminants from the surface of metals/alloys, which include (1) solvent cleaning, (2) etching cleaners (alkaline and acid etch), (3) desmut or deoxidation, (4) physical cleaning (blasting, abrasion, and/or polishing), (5) acid pickling, and (6) rinsing.18 Solvent cleaning removes the bulk of smut, grease, oil, dirt, rust, corrosion products, salt deposits, and die-release compounds, but usually requires an additional step such as acid or alkaline etch to remove reactive species. Further cleaning via physical methods is required for the harder steel surfaces where thick or scaly residues are present. This process is not normally used for softer aluminum surfaces. After multiple cleaning processes are complete, a desmut, deoxidation, or pickling step is sometimes used on the metal/alloy to remove any contaminating species rendering an acceptable surface. This provides a surface without weak boundary layers or damage in a reasonable time and cost. A rinsing step is often employed in industrial settings to remove excess reagent(s) and/or solvent(s) from various bath steps and to prevent cross-contamination of the baths. Now that we have reviewed corrosion, metal alloy selection, and cleaning procedures, our next step is to focus on the core of this chapter, which are the various pretreatment coatings commercially available and under development that can act as “smart coatings” as the first line of defense for corrosion inhibition.
3.4 Nonmetallic-Inorganic Pretreatment Coatings
Nonmetallic-inorganic coatings consist of ceramic coating materials, conversion, and anodized coatings. Ceramic coatings such as chemical setting silicate cement lining and porcelain enamels are used for corrosion inhibition. Conversion coats are coatings that convert the natural oxide coating present on a metal surface into a different metal oxide or metal salt. Anodization refers to the oxidation of a metal surface either chemically or electrochemically to generate a thick, dense, passive oxide layer on the metal surface.21,22 We shall briefly expand on the anodization process for metals because of its importance to conversion coatings. Anodizing has been known since the 1930s, but did not gain widespread and commercial success until the 1970s.23 There are several metals that can be protected via anodic protection: steels, stainless steels, nickel, nickel alloys, chromium, and aluminum. The “hard anodization” process refers to electrochemically driven surface treatment,24 and the “standard” process refers to solutions of chromic, sulfuric, or phosphoric acids.25–27 In response to current environmental and health warnings about chromium, specifically hexavalent chromium [Cr(VI)]28 nonchromium alternatives have been investigated and commercialized for the anodization of metals. These alternatives comprise lead-tin, cobalt, zirconium, titanium, and silicate processes.29–32 Anodization converts the existing metal surface to its oxide form or increases the oxide layer thickness, resulting in the formation of a thicker coating. The anodization process provides improved corrosion resistance and paint adhesion, permits subsequent plating, and provides a lustrous decorative appearance to most surfaces. Its facile method, reproducibility, and cost-effectiveness have resulted in its industrywide adaption.
3.4.1 Conversion coatings
Conversion coatings include chromate conversion coatings (CCCs), phosphate conversion coating, and lanthanide-based conversion coating and are deposited onto various metal/alloy substrates such as steel, zinc, aluminum, magnesium, copper, tin, silver, and nickel. Chemical conversion coatings processes are classified by their primary constituent. For example CCCs are mainly composed of chromates, whereas phosphate conversion coatings have as their main component phosphates.33 The metal/alloy surface is converted into coatings via a chemical or electrochemical process. These types of coatings are used for corrosion protection, increased surface hardness, and added decorative finish and/or as paint primers. Conversion coatings can be very thin 0.00001″ (0.254 μm) or thick coatings 0.002″ (50.8 μm) and can be grown either anodically or by repeated exposure in conversion baths.
Conversion coatings are adherent, insoluble, inorganic crystalline, or amorphous surface coatings that are an integral part of the metal/metal oxide surface. In such films a portion of the base metal is converted into a metal oxide that is less reactive to corrosion than the original metal and improves adhesion for primers. Conversion coatings have gained widespread industrial use due to their strong adhesion properties, high speed of coating formation, and very favorable economics, while providing reproducible and consistent coatings for large-scale industrial use.34
3.4.1.1 Chromate conversion coatings
CCCs are normally formed by either chemical or electrochemical treatment of metals/alloys in a solution containing hexavalent chromium [Cr(VI)] with other components.19,35 This process produces an amorphous protective coating that can be applied to various metals (e.g., steel or aluminum) via immersion or spraying. CCCs are applied to enhance bare or painted corrosion resistance, improve the adhesion of paint or primer coatings, and provide the metallic surface with an appealing decorative finish. The main components of the solutions used for forming CCC films are trivalent chromium [Cr(III)], Cr(VI), the base metal, various oxides, water, and several additional components such as phosphates, sulfates, and fluorides. CCCs provide excellent bare or painted corrosion protection to the metal. The level of protection afforded by CCCs depends on several factors: (1) type of substrate metal, (2) type of chromate coating used, and (3) chromium coating weight. The mechanism by which Cr(VI) provides corrosion protection has been investigated by numerous researchers over the past several decades. Over the past decade, a general consensus on the mechanism by which CCCs inhibit corrosion has been proposed by several researchers.36–41 Their research supports the idea that soluble Cr(VI) in CCCs is released to the defect areas in a coating, providing a “self-healing” mechanism that thereby inhibits further corrosion. The soluble Cr(VI) present in CCCs and to a lesser degree in chromated primers acts as a reservoir capable of migrating to defects and inhibiting corrosion. The mechanism of CCCs consists of both a cathodic, inhibiting, insoluble Cr(III) oxide and an anodic, inhibiting, soluble, transportable Cr(VI) species acting in concert with each other. Cr(III) acts as the insoluble, durable, inert coating, and when damage occurs a pH-controlled transport mechanism is enabled releasing soluble Cr(VI) species. The release of the active Cr(VI) inhibitor species in CCC involves the reversible formation of a Cr(VI)-O-Cr(III) mixed oxide. This system is one of the earliest examples of “smart coatings” for corrosion control on various metal alloys.
CCCs have shown over these many decades outstanding corrosion inhibition, and their replacements have not performed as well. However, CCCs and chromate primers are all under increased scrutiny and regulatory oversight by both the U.S and international regulatory agencies. The Cr(VI) compound is a known carcinogenic and toxic material to both humans and the environment.42–46 Chronic inhalation of Cr(VI) compounds increases the risk for lung cancer, soluble species can cause or exacerbate contact dermatitis, and ingestion can cause irritation and ulcers of the stomach and/or intestine. Cr(VI) is transported into cells via the “sulfate transport” mechanism. As a result of its toxicity, new Cr(VI) OSHA regulations as defined in the February 28, 2006 Federal Register, now covers occupational exposure to Cr(VI) for workers during an 8-h TWA period of no more than 5 μg/m3.47 As of January 24, 2013, the National Institute for Occupational Safety and Health (NIOSH) had posted a document entitled “Criteria for a Recommended Standard: Occupational Exposure to Hexavalent Chromium.” NIOSH reviewed the critical health effect studies of Cr(VI) and updated its assessment of the potential health effects of occupational exposure to Cr(VI) compounds. The recommendation for the new PEL is 0.20 μg/m3 for a workplace environment.48 This recommendation by NIOSH was advisory only; NIOSH cannot issue a regulatory directive. The European Union (EU) Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulations (EC 1907/2006) were adopted in December 2006 and came into force in June 2007. REACH was introduced because many thousands of chemicals are used in the EU, some in very large quantities and with increased risks to human health and to the environment. Cr(VI) is one of the many substances that is highly regulated by the EU and is currently a substance of very high concern (SVHC). As a result of Cr(VI) toxicity and persistence in the environment, alternative nonmetallic pretreatment coatings have been developed and tested. We shall now review several of these coatings and compare them to CCCs.
3.4.1.2 Phosphate conversion coatings
The phosphating process has been known for over a century, and the bath process consists of dilute phosphoric acid based solutions.49,19,50 The formation of the phosphate coating requires the precipitation of a divalent metal and phosphate ions onto a metal surface. This treatment provides for a reasonably hard, electronically nonconducting surface coating composed of insoluble phosphate. The insoluble phosphate is contiguous and highly adherent to the underlying metal. The phosphate conversion coating is formed as a result of chemical reactions that transform the surface of the underlying base metal into a corrosion-resistant film. There are three types of phosphate conversion coatings:
■ iron phosphates—lightweight, amorphous phosphate coatings that do not contain significant amounts of divalent metal ions
■ zinc phosphates—medium-weight, crystalline phosphate coatings that contain divalent metal ions from solution and/or the metal surface
■ heavy phosphates—coatings that contain divalent metal ions from solution and from the metal surface
Phosphate conversion coats are applied either via spraying or immersion for bare corrosion protection and/or painted corrosion protection on ferrous and nonferrous metals/alloys. Phosphate conversion coatings can provide effective barrier protection to metal/alloys provided the coating is applied properly. Thicker phosphate conversion coatings can provide better corrosion protection when combined with an overcoat of paint.
3.4.1.3 Lanthanide-based conversion coatings
Nonhexavalent chromium pretreatment coatings have been investigated for the past several decades. The rare earth (lanthanide) elements have received considerable attention due to their unique properties.18,51,52 The rare earth elements such as cerium (Ce), yttrium (Y), lanthanum (La), neodymium (Nd), samarium (Sm), and praesodymium (Pr) have been investigated for their corrosion-inhibiting properties. The unique properties of the rare earth elements are:
■ diverse allowable electronic configurations
■ formation of multiple oxidation states such as + 3 and + 4 and occasionally + 2
■ reactivity with water to form neutral oxides
■ formation of stable insoluble mixed oxides
■ complex coordination chemistry
■ low reduction potential
■ instability of lower valence states in alkaline conditions
■ precipitates that contribute to the stability and protection of films
■ relatively inexpensive
■ low toxicity
■ low environmental impact18,53–55
The pioneering work of Hinton and Wilson used rare earth conversion coatings as alternatives to toxic CCCs.56–59 Their work focused on cerium salt conversion coats for corrosion inhibition of zinc and aluminum metal/alloys. A cathodic mechanism was proposed by these two researchers to explain the formation of the rare earth oxide coating. The mechanism was based on cathodic reactions generating an alkaline environment, which led to localized precipitation of the rare earth oxides. The precipitated oxides enabled surface protection for the underlying metal alloy, thereby inhibiting corrosion. Further studies by Montemor et al. showed that the mechanism of rare earth oxide formation was a two-stage process, in which Ce-based conversion coatings undergo oxidation from Ce (III) to Ce(IV) state.60–63
Cerium-based conversion coatings can be prepared via two routes: (1) deposition of conversion coating onto a metal/alloy substrate is achieved by immersion for several days in simple cerium salt solution at or near neutral pH, or (2) H2O2-assisted solution of acidified cerium salt solutions, which results in deposition of cerium-based conversion coating in ≤ 10 min.64–67 Ce-based conversion coating have been studied for their corrosion-inhibiting properties on various metals with mixed results for corrosion protection.18,68–71 Several studies have shown limited to moderate corrosion protection while other studies have shown evidence of poor adhesion limiting its corrosion-inhibiting properties.72,73
As was described in Section 3.4.1.1, CCCs inhibit corrosion by dissociation of chromium ions into solution especially in a chloride environment. This dissolution of soluble species can migrate to exposed metal, resulting in the inhibition of corrosion via a “smart release” of chromated species. Similar investigations into the precipitation and dissolution of cerium-based conversion coatings have been investigated by several groups as nonchromium alternatives possessing the same “smart release” mechanism as found for CCCs.18,74 These initial studies have shown evidence that the lanthanide ions form insoluble hydroxides, which can then provide corrosion protection in the form of cathodic inhibitors. The work based on Scully et al. showed evidence that coatings composed of Al, Co, and Ce did provide self-healing capabilities via a release of Ce ions at pH of 2.75 Additional work by Heller et al. demonstrated corrosion protection via the formation of an interfacial active layer between the aluminum substrate and the cerium conversion coating posttreated with 2.5 wt.% NaH2PO4 solutions after salt spray exposure.76–78 However, the as-deposited cerium conversion coatings did not show any interfacial active layers after exposure to salt spray. Further work by Joshi et al. provided additional evidence for the dissolution of Ce species at pH 2, although at pH ≥ 3 no dissolution of Ce species was detected, and these results showed that the as-deposited coatings were composed of insoluble species that hindered the dissolution and migration of Ce species.79 The mechanism proposed to account for these results was not determined, but rather dissolution and migration of Ce species were ruled out as possible scenarios. Additional studies using rare earth elements other than Ce such as La, Sm, and Y have been investigated by several research groups.80–82 Their results showed that like Ce species these compounds are capable of inhibiting corrosion via a conversion film formation.
3.4.1.4 Miscellaneous-based conversion coatings
Additional pretreatment coatings that are non-Cr(VI) have been investigated for several decades now, which include the following systems: pretreatments acting as passivating agents based on molybdates (Mo), permanganates (Mn), vanadates (V), and tungstate (W)83,84; fluotitante and fluozirconate conversion coatings85,86 and trivalent Cr(III) conversion pretreatment (TCP) coatings.87,88
Phosphate-permanganate and acidic—(HNO3 or HF)—potassium-permanganate conversion coatings have been studied by several groups.89–91 Their results in NaCl solutions showed similar corrosion protection to CCC, however, if Mn is reduced to the divalent oxidation state resulting in the formation of the more soluble Mn(II) oxide species, its corrosion-inhibiting properties are reduced.92
The molybdates have been the most studied metal oxyanion compounds to date and their corrosion-inhibiting properties are due to the formation of Mo films that are passivating at anodic sites on a metal surface.93 Vanadates, molybdates, and tungstates can inhibit corrosion on aluminum when used with other compounds such as chromic species,94 sulfuric acid,95,96 and tetraborate solutions.97 These combinations showed improved corrosion resistance containing the chromate acid species. The chromate anions had exhibited a passivating effect on the aluminum alloy forming stable Al2O3 films with further formation of molybdate layers preventing attack of Cl− ions. The tetraborate solutions showed improved corrosion resistance with thicker anodic films contaminated with higher concentration of molybdate ions.97
Conversion coatings based on the group IVB metals, such as titanium (Ti), zirconium (Zr), and hafnium (Hf), have been investigated.87,98–100 Group IV metal coatings require shorter immersion times (≤ 10 min) and are a potentially green alternative to CCCs.101
In addition, trivalent chromium pretreatment (TCP) coatings have also been investigated and commercialized as a nontoxic, environmentally green alternative to CCCs.102–105 TCP coatings are formed by solution immersion in a treatment bath composed of hexafluorozirconates, trivalent chromium oxides, chromium sulfate, and fluorborate salts. The solution is maintained in acidic medium (pH = 3.8-4.0) using either hydrofluoric (HF) or sulfuric acids (H2SO4). The color and thickness of the TCP coating can be modified depending on the solution composition, temperature, pH, and immersion time.106 TCP has been shown to inhibit corrosion on various metals/alloys and has been accepted by the U.S. military as an environmentally friendly, nontoxic alternative to CCCs. Recent work by Swain et al. has investigated the mechanism(s) by which TCP can inhibit corrosion on aerospace aluminum, specifically AA2024-T3.107 Their work using Raman spectroscopy suggests that mobile CrO4− 2 species are present in the TCP film and can diffuse to corroding sites inhibiting corrosion via a passivation mechanism.
3.5 Organic Pretreatment Coatings
We have discussed in detail the various “smart” inorganic pretreatment coatings that are available commercially and that are under investigation. We shall now turn our attention to organic-based “smart coatings.” There are various types of organic “smart coatings” that have been commercialized and are under development such as sol-gel and hybrid sol-gel coatings,108 conductive polymers (CPs),109 self-assembled films,110 polyelectrolyte multilayers,111 controlled release coatings,112 and biofilms,113 which all will be presented in the following sections.
3.5.1 Hybrid sol-gel coatings
Over the past two decades sol-gel-based thin films have been investigated by several groups for corrosion inhibition of metal alloys. This research has been spurred by the need for environmentally friendly and nontoxic alternatives to CCCs.28,46–48,114–116 Significant efforts have been employed to develop sol-gel coatings that can act as adhesion promoters between metal alloys and organic primers/topcoats as well as provide corrosion protection.117
There are two methods available to prepare sol-gel coatings via either a hydrolytic method in aqueous media or a nonhydrolytic process in organic media. Either method can produce various sol-gel coatings with different properties. These properties can be controlled via adjusting the composition of the reactive species, organic functionalization, temperature, time, and pH, affecting their potential applications for corrosion protection.118 Sol-gel based pretreatment coatings have been studied on various metals/alloys, such as copper,119,120 aluminum,121 magnesium,122 and carbon steel.123 These studies have shown that sol-gel pretreatment coatings exhibit corrosion- inhibiting properties.
The sol-gel process is based on the hydrolysis and condensation reactions of metal alkoxides [M(OR)n] where M = Si, Ti, Zr, or Al; and R = alkyl group (methyl, ethyl, butyl, and so on) (Figure 3.4).115,124 The sol-gel process involves two distinct and separate methods for the preparation of the “particles or films,” which consists of either an inorganic or organic approach, and the latter is the preferred method. The sol-gel process involves the initial formation of a colloidal suspension or solution referred to as the “sol,” which is followed by the formation of the integrated network referred to as the “gel” to give either discrete particles or network polymers.

The sol-gel process typically occurs in four stages:
■ condensation and polymerization of monomers to form chains or particles
■ growth of the particles or chains
■ finally, agglomeration of the networks, thickening and formation of the gel115,118,124
Inorganic sol-gel pretreatment coatings have been investigated for their corrosion-inhibiting properties, but have suffered from cracking for thick coatings ≥ 200 nm. Cracking of the sol-gel film is formed during the heating stage when shrinkage of the gel occurs during solvent evaporation.125 In order for crack-free sol-gel films to be formed, the thickness of the pretreatment film must be ≤ 100 nm, but their barrier properties are compromised due to presence of micropores in the film. Films produced by the sol-gel method show excellent adhesion between the alloy/metal substrate and primer/paint system.126
In order to produce crack-free films, researchers have developed hybrid sol-gel-derived coatings that combine the unique properties of both the inorganics/ceramics with organic polymeric materials.127 The inorganic/ceramic coatings provide scratch resistance, durability, and improved adhesion between the metal/alloy substrate and organic primer/paint system, whereas the organic components increase flexibility and functional compatibility with the organic primer/paint system.128,129 There are two classes of organically functionalized sol-gel coatings: (1) nonfunctional organoalkoxysilane and (2) organo-functional alkoxysilanes. Nonfunctional organoalkoxysilane sol-gel coatings are prepared using methyl groups as the organic part for the preparation of the hybrid coatings,130 whereas, organo-functional alkoxysilanes incorporate such functionalities as epoxy,131,132 methacrylic,133,134 acrylics,135 amino,136,137 allyl,138 pyridine,139,140 or vinyl/phenyl moieties 138,141,142 as the precursor groups for sol-gel formation. These additional functional groups are also available for further polymerization producing sol-gel films with high crosslink densities, improved mechanical properties, and tailored compatibility of the sol-gel pretreatment coating to the organic primer/paint system. Corrosion resistance using sol-gel films has been dependent on obtaining crack-free films. The hybrid sol-gel films offers several advantages, but still has limitations associated with reduced wear resistance and lower mechanical properties. Incorporation of nanoparticles into a sol-gel network can improve their mechanical properties while providing for corrosion resistance of metals/alloys.143–146 Further improvements in hybrid sol-gel networks’ corrosion-inhibiting properties have focused on introducing corrosion inhibitors with various degrees of success. Incorporation of inorganic inhibitors that are non-Cr(VI) such as phosphates,147 V,148 Ce,147,149–153 and Mo154,155 compounds as well as organic corrosion inhibitors such as phenylphosphonic acid,156 mecaptobenzothiazole,149,157 benzotriazole,158 and 8-hydroxyquinoline159 have been investigated for their corrosion-inhibiting properties.
3.5.2 Conductive polymer coatings
Over the past three decades, evidence that CPs such as polyaniline (PANI) and polypyrrole (PPy) could inhibit corrosion has come from the pioneering work of Mengoli,160 DeBerry,161 and MacDiarmid.162,163 Mengoli showed that electrochemically deposited CP coatings on iron (Fe) anodes resulted in an adherent and corrosion-inhibiting film.160 Further proof was obtained by DeBerry in 1985161 who showed that PANI electrochemically deposited onto stainless steel showed a change in its corrosion behavior in a sulfuric acid solution, which provided concrete evidence that the PANI films provided anodic protection, thus maintaining a native passive film on the steel metal. There are numerous studies using CPs deposited onto metals/alloys under a variety of conditions such as electropolymerization, solvent-casting, water-dispersive formulations, spray, and dip-coating. These CPs are new “smart coatings” for corrosion protection of ferrous and nonferrous alloys. CPs can function by an anodic protection mechanism for ferrous alloys and therefore can potentially replace such corrosion-inhibiting compounds as Cr(VI) and cadmium (Cd), which are known carcinogenic and environmentally hazardous materials.42–48,164–166
The corrosion mechanism for ferrous alloys as mentioned in the preceding paragraph is via anodic protection for steel alloys, passivation for stainless steels,167–169 and released dopant ions for mild steels.170,171 The corrosion protection mechanism for aluminum alloys is still under intense investigation. There are several proposed mechanism(s) by which CPs can potentially inhibit corrosion of aluminum alloys in corrosive aqueous environments.172 These proposed mechanism(s) are as follows: (1) ennobling of the aluminum alloy,173 (2) anodic protection/passivation,174 (3) galvanic and ion-exchange release of oxygen reduction inhibitors,175 and (4) barrier protection by the reduced form of the CP following an initial galvanic coupling.176
Most of the investigations into the corrosion-inhibiting properties of CPs have contained either a nonconductive primer and/or topcoat for enhanced corrosion protection.177 Additional work has focused on the corrosion mechanism of CPs via electrochemical and spectroscopic techniques that was also described in the preceding paragraph. There are fewer reports in the literature that focus exclusively on the corrosion-inhibiting properties of CPs as thin films with direct comparisons to the corrosion-inhibiting properties and performance of CCCs. Several recent reports highlight the corrosion-inhibiting properties of PANI, PPy, and PEDOT thin films (< 1.0-2.5 μm) deposited on steel alloys with and without topcoats.178–181
Researchers at the Naval Air Warfare Center Weapons Division, China Lake, CA synthesized,182–184 coated, and evaluated a CPs performance both in laboratory103,185,186 and field testing environments,187 which was directly compared to CCC. This compound, poly(2,5-bis(N-methyl-N-hexylamino)phenylene vinylene) (BAM-PPV), (Figure 3.5) was shown to have promise as a viable nontoxic, environmentally friendly alternative to CCCs as reported by Zarras et al.182–188 The neutral salt fog laboratory results showed that BAM-PPV coated on aluminum alloy could meet the military requirement as a replacement for CCCs. Electrochemical noise method analysis of BAM-PPV coatings showed evidence of a surface passivation mechanism similar to other CPs.186

However, when BAM-PPV was coated on aluminum alloy with a non-Cr(VI) primer and topcoat, the neutral salt fog survivability did not match a fully Cr(VI) military coating system [CCC + Cr(VI) epoxy primer + polyurethane topcoat]. A 1-year Air Force field test of BAM-PPV incorporated into a full military coating [BAM-PPV + non-Cr(VI) epoxy primer + polyurethane topcoat] on the C-5 cargo plane’s rear hatch door showed equal performance to the fully Cr(VI) military coating.187 There are several recent reviews available to the reader to get a more comprehensive understanding of CP coatings and their ability to act as “smart coatings” in a variety of corrosive environments.109,189–191
3.5.3 Self-assembling pretreatment coatings
Self-assembling (SA) molecules are compounds that provide surface protection for the underlying metal or alloy from corrosive environments via the formation of thin films. These SA films can be very thin (~ 100 Ǻ) and produce flexible, densely packed, stable films capable of blocking electron transfer or inhibiting the transport of corrosive species to the underlying metal or alloy.192
SA films are generated when molecules with specific chemical groups are deposited onto a surface have strong affinities for the surface and undergo ordering due to chemical interactions between substrate and SA molecules. In addition to the chemical or physical affinities necessary to form strong interactions on the substrate surface, the type and strength of the intermolecular interactions between the SA molecules that hold the assembly together are equally as important.193 The SA molecules that are deposited onto substrates (metal or alloy surfaces) are either physically or chemically adsorbed. A significant portion of the research investigating SA films have focused primarily on the self-assembly of n-alkanethiolates and related structures on gold substrates. These well-ordered SA films are easily formed under ambient conditions due to the chemical inertness of the gold substrate and the affinity of thiol compounds to chemisorb onto gold.194
SA molecules have also been studied for their corrosion-inhibiting properties on various alloys. These SA molecules are specific for certain metals such as alkanethiols for gold,192–195 silver,196 and copper197; alcohols; amines for platinum198; and phosphonate compounds for aluminum.199 The fabrication of SA films onto either a metal or alloy consists of finding suitable functional groups sandwiched between an aliphatic spacer unit capable of adhering onto the surface of the metal or alloy. The opposite functional group can then react further with the primer layer to form a more robust and durable film (Figure 3.6).
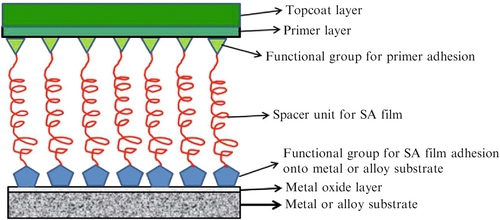
SA films can provide effective barriers against the diffusion of oxygen,200 and/or water.201 Cross-linking reactions can further enhance the durability and corrosion- inhibiting properties of the SA film.202 There are several studies of SA films for corrosion inhibition of copper metal using sodium 1-octadecyl-1H-benzimidazole,203 diethydithiocarbamate,204 which provided cathodic inhibitor protection, and hexane-1,6-diamine and 2-mercapto-ethanol, which provided evidence for enhanced corrosion inhibition on copper metals.205 Corrosion inhibition of aluminum metal using octadecylphosphonic acid monolayers was investigated by Grundmeier et al.206 The 1-tetradecylphosphonic acid SA films deposited on Al 2024 and 1060 showed corrosion inhibition during initial corrosion monitoring,207 and alkane diphosphonate monolayers on 1050 aluminum showed improved corrosion resistance when the aluminum surface was exposed to oxide formation treatments.208 Recent work on SA films coated onto iron and steel substrates has been investigated by several groups.209,210 Adipic acid SA molecules formed compact films onto carbon steel exhibiting reduced electrochemistry on the steel surface209; compact and superior seawater stability were found with SA polydopamine/dodecanethiol films on 304 stainless steel211; and triazinethiol SA monolayers coated onto zinc treated steel substrates showed improved corrosion resistance but poor water repellency.212 Brass alloys and magnesium alloys have also been investigated using SA films. In the case of brass alloys several silane SA films inhibited corrosion in 0.2 M NaCl,213 and SA nanophase particle films provided corrosion protection for AZ31B magnesium alloy in 0.005 M NaCl solution for 354 h.214
3.5.4 Polyelectrolyte multilayer films
Polyelectrolyte (PE) films are fabricated by a layer-by-layer (LbL) approach using alternating assemblies of polyelectrolyte pairs consisting of a polyanion and polycation (Figure 3.7).215 The adsorption of the alternating PE multiple layers is driven by electrostatic interactions, and assemblies of hundreds of alternating polyanions and polycations are possible.216 The LbL approach for multilayer PE films allows for the precise control over the physical properties of the film (e.g., film thickness and morphology).217
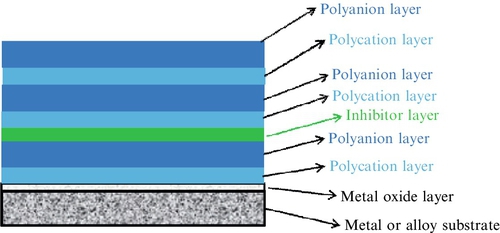
The deposition by the LbL approach affords nanometer-scale precision with multifunctional capabilities for the PE multilayer coating, which can exhibit excellent adhesion to the metal or alloy and can “self-heal” to seal surface defects.218 The PE multilayer films exhibit pH-buffering activity and can stabilize the pH between 5 and 7.5 at the metal surface in a corrosive environment. The PE multiple layer coatings are relatively mobile and also have the ability to seal and eliminate mechanical cracks in the coating via a “self-healing” mechanism.219 Additionally, active inhibitors can be trapped in the PE multilayer films. The inhibitor is trapped to the layers closest to the substrate for release of inhibitors while maintaining effective substrate barrier properties for maximum corrosion efficiency.220–223
3.5.5 Controlled release coatings containing inhibitor-loaded nanocontainers
Controlled release and encapsulation of various materials within an inert host containing the active ingredient, such as drugs, oils, perfumes, and so on, have been studied for several decades.224–226 Recent efforts have focused on developing encapsulated inhibitors via LbL, polyelectrolytes, copolymer vesicles and interfacial polymerization methods for corrosion inhibition on various metals and alloys.227 These inhibitor microcapsules can be formulated into coating systems for controlled release of the inhibitor triggered by corrosion to provide a “self-healing” mechanism.228,229 The size of the microencapsulated inhibitor containers are normally too large (≥ 1 μm) to be effective in pretreatment, conversion, or multilayered coatings.
The development of “smart nanocontainers” offers a method by, which nanostructured materials can store the inhibitors, providing a new corrosion-inhibiting system based on a “passive matrix/active container structure” for use in pretreatment, conversion, or multilayered coating systems.230 Corrosion processes are normally accompanied by local pH changes on the surface of the metal or alloy. Nanocontainers placed near these surfaces can be activated via pH changes or mechanical damage to release the encapsulated inhibitor effectively inhibiting corrosion via a self-healing mechanism. There are numerous literature reports on nanocontainers based on cyclodextrins,231 mesoporous silica,232,233 halloysite clays,234 carbon nanotubes,235 layered double hydroxides,236and titanium dioxide,237 which have been synthesized and tested for their corrosion-inhibiting properties.112
3.5.6 Biofilms as pretreatment coatings
Corrosion control using beneficial biofilms has recently gained interest as a corrosion-inhibiting strategy that is promoted as an environmentally green and nontoxic alternative to current corrosion control methods.238 Biofilm formation is a natural process, which consists of a highly organized bacterial community with cells entrapped in a matrix formed by extracellular polymer.239 The formation of biofilms may impede or accelerate the corrosion process on metals or alloys.240 The factor that promotes corrosion is the bacteria colonization process on metal substrates. This colonization is nonuniform, producing colonies that are thinner or thicker resulting in anodic and cathodic regions promoting corrosion on the substrate. Additionally corrosion can be impeded if corrosion products formed by the biofilm produce passive layers.241 The interaction between the metal substrate and biofilm formation depends solely on the type of metal and the degree of microbial activity. The inhibition of corrosion on various metals and alloys using biofilms can be accomplished via several strategies: (1) biofilms secreting antimicrobials,113,242,243 (2) formation of protective layers,244,245 (3) biofilms secreting corrosion inhibitors,246,247 and (4) beneficial biofilms.248,249
3.6 Conclusions
A comprehensive review of corrosion inhibition on various metals and alloys using either inorganic or organic pretreatment methods has been presented. Surface pretreatment coatings,whether they are inorganic or organic, serve as the first line of defense in inhibiting corrosion. The selection of the proper pretreatment coating is essential in providing basic corrosion protection for the underlying metal or alloy and promoting adhesion and compatibility between the pretreatment and primer coating. There are a number of pretreatment coatings that are commercially available and are being investigated not only to better understand the complex relationship between metals/alloys and corrosion, but to design and commercialize new pretreatment coatings that are economically viable, environmentally friendly and nontoxic to both humans and ecosystems.
