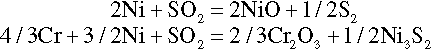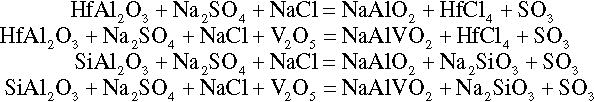The Importance of Corrosion and the Necessity of Applying Intelligent Coatings for Its Control
I. Gurrappa; I.V.S. Yashwanth Defence Metallurgical Research Laboratory, Kanchanbagh PO, Hyderabad, India
Abstract
Corrosion of metals is a vital concern for the materials scientist. This natural phenomenon causes substantial financial losses in various sectors and requires extensive efforts to limit its impact. Therefore, there is a need to innovate an effective and economically viable technique for producing and also applying intelligent/smart coatings to minimize corrosion. This chapter explains the significance of corrosion and the economics involved followed by the developments in producing intelligent coatings. There are different types of intelligent coatings, such as organic, inorganic, or nanostructured materials used at ambient temperatures for various applications; a review has been presented. Then emphasis is given to elevated temperature corrosion failures reported during service and mechanisms causing components to degrade under various environmental conditions. Subsequently, the necessity of development of Intelligent Coating to combat different types of hot corrosion and oxidation, the concepts involved, preparation techniques, related microstructure, and the performance of developed intelligent coatings are explained.
2.1 Introduction
Corrosion is a significant challenge to most of the industries in the world because it causes disasters and massive financial loss.1 It is essential to remember that corrosion knows no national boundaries and takes place both at ambient and high temperatures. The primary forms of corrosion that have been observed on various materials are given below:
(b) Crevice corrosion
(c) Pitting corrosion
(d) Galvanic corrosion
(e) Intergranular corrosion
(f) Dealloying
(g) Erosion-corrosion
(h) Stress corrosion cracking (SCC)
(i) Corrosion fatigue
(j) Microbiologically Influenced Corrosion (MIC)
The susceptibility to degradation by any of these forms of corrosion depends on the nature of materials used, applications, and the surrounding environment. The latest surveys show that the total worldwide, direct annual estimated cost of corrosion (essentially materials, equipment, and services involved with repair, maintenance, and replacement) is approximately US$4 trillion, that is, about 4% of the nation’s gross domestic product (GDP) (Figure 2.1). This figure does not include the environmental damage, waste of resources, loss of production, or personal injury resulting from corrosion. Different sectors and related industries are mentioned in Table 2.1. According to corrosion experts, it is possible to save a net of 25% of that annual cost by applying currently available corrosion control technologies. Moreover, it is possible to extend the savings (up to 35%) by innovating and applying intelligent coatings. Therefore, there is a need to innovate an effective, environmentally friendly and economically viable technique for producing and also applying intelligent coatings to minimize corrosion.

Table 2.1
Category and Related Industrial Sectors
| Category | Industry Sector |
| Infrastructure | Highway bridges Gas and liquid transmission pipelines Waterways and ports Hazardous materials storage Airports Railway tracks |
| Utilities | Gas distribution Drinking water and sewer systems Electrical utilities Telecommunications |
| Transportation | Motor vehicles Ships Aircraft Trains Hazardous materials transport |
| Production and manufacturing | Oil and gas exploration and production Mining Petroleum refining Chemical, petrochemical, and pharmaceutical Pulp and paper Agricultural Food processing Electronics Home appliances |
| Government | Defense Nuclear waste storage |
Intelligent coatings, also known as smart/self-healing coatings, are multifunctional and contain compositionally optimized inhibiting elements/compounds to respond to the service conditions appropriately. Application of intelligent/smart/self-healing coatings is a cost-effective method of improving the corrosion protection and thereby the durability of metallic structures. A wide range of engineering structures, from cars to aircraft, from chemical factories to household equipment, can be effectively protected by these coating systems. Intelligent coatings are based on epoxy with polymer and nanoparticulates applied on the structures used at ambient temperatures. However, elevated temperature applications like gas turbine engines used in aero and industrial applications need metallic and ceramic intelligent coatings to enhance their efficiency. Further, the coatings should be multilayered with different compositions applied by varied surface engineering techniques. Here, intelligent coatings have to respond suitably to the surrounding environments and temperatures and enhance the component’s life significantly by forming appropriate corrosion resistant films such as chromia or alumina. Hence, the selection of materials and intelligent coatings for a particular application is a challenging task for scientists and engineers. Therefore, intelligent coatings can be divided into low/ambient temperature intelligent coatings and elevated temperature intelligent coatings. The details will be explained in subsequent sections.
2.2 Low Temperature Intelligent Coatings
Traditional coatings like zinc and tin have been utilized for more than one and half centuries as protective coatings providing galvanic protection of defects and as organic or inorganic coatings to protect the substrate (metal/alloy) by acting as a barrier between the substrate surface and the environment. Other types of coatings contain basic pigments that are based on releasing inhibiting compounds such as strontium, calcium, and zinc chromates. The advanced coating contains a small amount of functional additives that enable the coating to provide enhanced functionality. Other coatings have some functionality incorporated into the resin itself. The functionality of the coating depends primarily on the composition or formulation of the coating.
The intelligent coatings provide the best protection by sensing a change in the environment and respond appropriately. Therefore, the intelligent coatings offer multifunctional and multidimensional benefits. With recent developments in nanotechnology, the inherent function of conducting polymers and composite materials can be precisely engineered in such a way that the coating provides intelligent characteristics. The intelligent coatings also overcome the historic usage of hazardous and carcinogenic materials such as hexavalent chromium and cadmium. The drug release concept that has been successfully developed and used for treating various ailments (including heart attacks) provides considerable information for the potential development of intelligent coatings that release corrosion inhibitors.
Development of intelligent coatings that can be produced cost effectively is one of the main issues. Further, the intelligent coatings must possess exceptional performance attributes that justify the additional cost for their manufacture. The developed intelligent coatings must be brought out of the laboratories into reality in the form of proven applications that are commercially viable. The development of newer intelligent coatings will involve multidisciplinary research by materials scientists of varied expertise such as in chemistry, physics, polymers, biology, medicine, and engineering.
Often microbial growths on the surface of materials accelerate corrosion and cause their faster degradation. Under such conditions, intelligent coatings should contain pigments that release toxic compounds so that bacterial growth can be arrested and provide reasonable protection to the substrate material. Another important property of an intelligent coating is its ability to respond to mechanical damage or environmental stresses during service conditions by releasing inhibitors.
Low temperature intelligent coatings can be broadly categorized in many different ways—based on their functional ingredients, applications, synthesis techniques, and so on. The coatings that respond to changes in heat, light, or pressure are sensors; bioactive coatings including antifouling, biodecontamination, and biocatalysis; color shifting coatings; corrosion control coatings; and command destructive coatings. There are intelligent coatings that are difficult to classify including self-lubricating coatings, superinsulating coatings, self-repair and self-healing coatings, electrically conducting coatings, self-assembling coatings, superhydrophobic coatings, optical coatings, and so on. The intelligent coatings primarily contain functional ingredients within, as mentioned above, and these can be resin itself or a variety of additives such as pigments, nanoparticles, nanotubes, microelectromechanical devices (MEMS), radio frequency identification devices (RFIDs), microencapsulated ingredients, enzymes, antimicrobial agents, and so on, depending upon the application.
Considerable research has been carried out in the area of conducting polymers to develop intelligent coatings because the conducting polymer can form a dense, adherent, and low porosity, passive film on the metal surface and thus prevent corrosion. Some intelligent coatings were developed by employing conducting polymers like polyaniline (PANI) and polypyrrole.2,3 These materials have a few unique characteristics: first, they are highly stable; second, they are conducting; and third, they hold and release ionic species depending on their state of charge. It is possible to synthesize intelligent conducting polymer coatings by doping ions that release when a corrosion process is sensed.
The other way is to use a combination of inorganic and organic hybrids, which are named as creamers. They are partly organic polymer coupled with an inorganic ceramic. More specifically, they are nanophase-separated metal-oxo clusters connected via a phase coupling agent. The creamer coatings can self-assemble on metallic surfaces to create a passivating preceramic phase, which has been shown to inhibit corrosion even on surfaces in which corrosion has already initiated. These coatings provide mechanical stiffness coupled with the ability to self-heal, deflect high-energy particles protection against deep ultra violet rays, and remain optically transparent.4
Tiwari et al. have studied different combinations of coatings, such as a pure epoxy polymer coating, a hybrid coating consisting of epoxy and silicone, a ceramer coating consisting of organo-silicone and a quasiceramic coating consisting of specialty silicone composition. Authors have observed that the hybrid coating exhibits superior nanomechanical properties compared to the pure polymer coating, and the coating containing high silicone levels displayed superior hardness.5 The hybrid coating had a rough surface that was damaged and partially recovered after the scratch test. The creamer and quasiceramic coatings displayed brittle failure. They have also reported the use of silicone creamer coatings for protection of metals against corrosion.6
Two technology enterprises offer energy-efficient, environmentally sound, and attractively priced plant technology (Aquence™) for lean corrosion protection.7 Aquence is a chemical autodeposition process that draws together decisive commercial and ecological advantages. Aquence is an innovative chemical autodeposition process that provides high-quality corrosion protection without utilizing heavy metals in just a few process stages. The aqueous organic solution builds up a coating film wherever the chemicals come into contact with ferrous metal (Figure 2.2). The Aquence process produces a coating only where it interacts with ferrous metal; other materials, such as rubber and plastics, remain untreated and are not damaged because low oven temperatures can be selected. Complex parts, like assemblies with prefitted plastic components, can be coated evenly in a single process stage. Uniform contact with the chemicals ensures that even complex assemblies of closely arranged parts as well as cavities can be protected against corrosion. The enhanced hardness and direct bonding of the coating with metal substrate increases durability of the parts.7

2.3 Encapsulation for Self-Healing Coatings
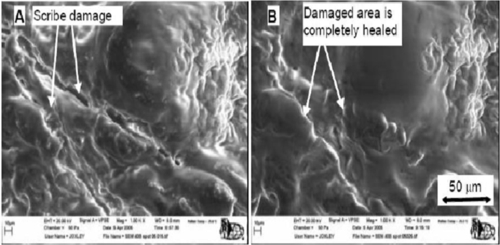
Developments in self-healing technology have opened a new area of multifunctional coatings with the potential to increase the lifetime and reduce the enormous costs associated with maintenance of protective coatings. There has been significant research in this area, particularly for the past decade.8,9 Synthetic methods are being developed to produce materials with desirable healing properties while maintaining high performance as protective coatings.10 Accelerated aging testing conducted in this research has shown that corrosion is considerably delayed and reduced (Figures 2.3 and 2.4).

It is essential to note that the use of protective coatings over various substrates is based upon natural systems such as human skin. All protect the underlying substrates. The main difference with natural protective systems and man-made analogues is their ability to repair themselves after damage. The self-healing of the skin occurs naturally to reproduce an identical surface. Certain plants continually renew the surfaces of their leaves with waxy residue to prevent waterborne contaminant growth such as fungus. Thus, the research in the area of self-healing coatings to add functionality to protective surfaces was inspired by the natural systems.
The main self-healing coatings were prepared by incorporating an encapsulated “healing” agent, which is subsequently released upon damage of the coating. These encapsulation methods have been demonstrated to successfully heal materials after selected events of cuts, scribes, impacts, and deep scuffs on the surface of the coatings (Figure 2.3). These healing materials will re-form the coating over the damaged area, resulting in a protective surface comparable to that of the original undamaged surface. In these coatings, the only initiation source to heal the material is the initial damage event. The use of encapsulated self-healing materials was originally developed by White et al. from University of Illinois. The idea is to embed an encapsulated monomer, dicyclopentadiene (DCPD), into a thermoset composite system. Once cracks were formed in the composite, the capsules rupture, and the DCPD flows into the crack plane via capillary action. The monomer then comes in contact with a catalyst present in the resin, which enables the polymerization into a solid. This innovative concept has been described to be similar to the “self-healing” of cracks in bones (a composite of rigid inorganic hydroxyapatite, collagen, and other flexible organic components).
U.S. Army researchers Kumar and Stephenson demonstrated a self-healing, corrosion-inhibiting coating system for use on outdoor steel structures.8 Similar research in the area of encapsulated self-healing coatings were performed at Luna Innovations. This concept is similar to the self-healing composites with encapsulated monomers, except that their use will be for protective coating applications. The capsules are broken under stress like a scratch in the coating. Monomers flow out of ruptured capsules into damaged areas to repair coatings via autooxidation processes. For their effective function, the capsules must possess significant structural and chemical integrity in order to withstand normal stress of application and use in a coating.10 Further, in the case of corrosion prevention, the coencapsulation of corrosion inhibitors have been included to ensure that the resultant healed coating possesses the same inhibition to corrosion as the original coating.10
There are several techniques to synthesize the microcapsules like interfacial polymerization,11 coacervation,12 in-situ polymerization,13 extrusion, and sol-gel techniques. However, among different techniques, the simple and appropriate technique is in-situ polymerization. The comparison among the encapsulation techniques is provided in Figure 2.5. Hence, the majority of researchers have used in-situ polymerization as a major technique for fabrication of micro/nanocapsules. The common healant material phase is liquid, because of free flow through the crack plane. An excellent review on encapsulation, in particular in-situ polymerization, was published by Samazdeh et al.14

Nature uses the energy inherent in active base metal surfaces in oxidizing corrosive environment to form passive films. When passive films cannot be formed, for example, for Al in chloride environments, chemical or nanostructures built into the protective coating can be imagined such that the corrosion reaction drives the release of a suitable corrosion inhibitor. This is likely accomplished for suitably doped PANI coatings on Al 2024-T3 exposed to a salt fog environment. Anionic organic ORR inhibitors appear to be suitable dopants. Hamdy et al. have attempted to design a chromate-free anticorrosion intelligent coating for aluminum- and magnesium-based materials for marine, automotive and aerospace applications (Figure 2.6).15 They reported that a simple vanadia-based chemical conversion coating improves the corrosion resistance of high strength AA2024 alloy.

Nanostructured materials engineering extends the possibility of engineering “intelligent” coatings that can release corrosion inhibitors on demand when, for example, the coating is breached, stressed (mechanically or chemically), or an electrical or mechanical control signal is applied to the coating. Ideally, an “intelligent” corrosion inhibiting coating will generate or release an inhibitor only when demanded by the initiation of corrosion. In an aerated electrolyte, aluminum metal provides about 1.7 V to power a chemical or physical nanomachine to deliver a corrosion inhibitor on demand. Natural passivity, as occurs for steel in nitric acid, makes use of the naturally available driving force to synthesize a “corrosion inhibitor,” namely a compact oxide film. In principle, nanoengineered functional coatings can lead to a “smart” active coating that uses the energy inherent in the surface to be protected to produce an inhibiting material when called upon.16
Nanocrystalline cobalt (Co) and cobalt/multiwalled carbon nanotube (Co/MWCNT) coatings were prepared by direct current (DC) and pulse reverse current (PRC) electrodeposition from aqueous bath-containing cobalt sulfate, MWCNTs, and so on. Effect of the functionalization of MWCNTs and electrodeposition techniques, that is, DC and PRC, on the microstructure and properties of these coatings revealed that the incorporation of MWCNTs, particularly the functionalized MWCNTs, substantially improve the hardness and resistance to wear and corrosion of the deposited coatings.17 PRC electrodeposition produces Co and Co/MWCNT coatings that are featured by small grain size, low surface roughness, and more uniform distribution of MWCNTs in the matrix, and consequently display the higher hardness and better resistance to wear and corrosion. The functionalization of MWCNTs favors the codeposition of MWCNTs with Co ions, and then improves the hardness and corrosion and wear resistance of the produced composite coatings. Among all samples, the composite coating incorporated with functionalized MWCNTs produced by PRC electrodeposition exhibited the highest hardness around 1180 kgf × mm− 2 and the best resistance to wear and corrosion. The differences in friction and wear behavior of these nanocrystalline Co and Co/MWCNT coatings as a function of treatment of MWCNTs or electrodeposition techniques are attributed to their different hardness, microstructures, and the corresponding wear mechanisms (Figures 2.7 and 2.8).


The successful application of spiro[1H-isoindole-1,9-[9H]xanthen]-3(2H)-one, 3,6-bis(diethylamino)-2-[(1-methylethylidene)amino](FDI) as a “turn-on” early aluminum corrosion detector in epoxy coatings was reported.18 Electrospray ionization mass spectrometry (ESI-MS) and 1H NMR investigations revealed that the nonfluorescent FD1 is sensitive to low pH due to its acid-catalyzed hydrolysis to Rhodamine B hydrazide (RBH) that subsequently becomes protonated to its fluorescent ring-opened form. Both clear and filled FD1-containing smart epoxy coatings were capable of sensing acidic pH produced at the anodic site of localized aluminum corrosion at a low indicator concentration (0.5 wt.%). It was demonstrated by the observation of fluorescent, bright-orange areas corresponding to localized pitting corrosion of the aluminum substrate illuminated by a handheld UV lamp.18 Therefore, early corrosion of aluminum can be easily and nondestructively detected via “turn-on” fluorescence strategy.
The major structures, such as offshore platforms, ships, submarines, pipes carrying oil, gas, and water, are manufactured from carbon steel and protected from corrosion primarily by paint coatings. During service, the paint coating deteriorates and anodic areas are formed. Particularly the ship hulls have complex structures and paint damage is more likely either due to mechanical damage or during sailing.19–22 In addition, the underwater hull of the ships have more corrosion sensitive areas like propellers and rudders that are noble to the hull material and are in contact with each other and thus lead to accelerated corrosion. Similarly, the pipelines thousands of kilometers long experience varied environmental conditions that cause disasters. Therefore, it is highly essential to apply an efficient and economic intelligent coating in association with an appropriately designed cathodic protection system in order to protect the structures and pipelines under all operating conditions. It is important to note that the intelligent coatings alone will not be sufficient for these structures, and the installation of cathodic protection system is a must. However, complementary to a cathodic protection system, the intelligent coatings facilitate and enhance the material life significantly.
2.4 Cathodic Protection
Cathodic protection can be obtained either by using sacrificial anodes or by using an impressed current cathodic protection (ICCP) system. It has been established through experience that the corrosion of structural steel stops when its potential is − 800 mV against silver/silver chloride or + 250 mV against zinc reference electrode or − 850 mV against copper/copper sulfate electrode.
2.4.1 Sacrificial anodes
The principle involved in the development of sacrificial anodes for cathodic protection purposes is that galvanic current flows when two dissimilar metals are electrically connected in the conducting environment. The noble metals are mostly protected by consumption of the less noble metals. Magnesium, zinc, and aluminum alloys are base materials versus mild steel, which is the structural material for the marine structures and, therefore, these alloys produce galvanic current when coupled with mild steel in the seawater, resulting in their sacrificial dissolution for protecting the mild steel.
Sacrificial anodes are often used in preference to ICCP systems when the current requirements are low and when in relatively high conducting environments. Capital investment will generally be lower, and it is often been the most economical method for a short-term protection of marine structures.
2.4.1.1 Advantages of sacrificial anodes
1. Operate independent of electric supply
2. Relatively simple to install; additional anodes can always be fitted if adequate protection is not achieved
3. No electrical hazard to the divers
4. No control to be exercised
5. Eliminate incorrect fitting problems
6. Minimum maintenance costs
7. Low installation costs
However, there are limitations to sacrificial anodes, as well:
2.4.1.2 Disadvantages of sacrificial anodes
(a) Periodic replacement is essential as the life of anodes is limited
(b) Current output cannot be regulated according to the demand (pollution, paint damage, etc.)
(c) They are not economically viable for protecting large and poorly coated pipelines
(d) They require a large number of anodes that results in an increase of frictional drag and weight
2.4.2 ICCP system
This is the most ideal and rugged system that overcomes the above limitations and has been in use in most of the pipelines for protection of ship hulls, offshore structures, and submarines all over the world. In this system, the protection current is drawn through the power supply and is impressed on the pipes/ship hulls/submarines/offshore structures that act as cathodes via inert anodes.
2.4.2.1 Advantages of ICCP system
(ii) Higher current output
(iii) Flexibility of current output control
(iv) Applicable in almost any resistivity soil environment
(v) Applicable for poorly coated pipelines
(vi) Protects larger and more expensive pipes
Gurrappa has published a significant number of books and research articles on sacrificial anodes and designing smart cathodic protection systems for cooling water pipelines and ship hulls.23,24
2.5 High Temperature Intelligent Coatings
As mentioned earlier, the corrosion takes place both at low and high temperatures depending on the service conditions. The desire for ever greater efficiency and increased performance have driven the developments in modern gas turbine engines. These engines require high performance materials to exhibit maximum efficiency by increasing their operating temperatures. Higher operating temperatures lead to high temperature corrosion of the components and thereby reduce their life significantly. Oxidation and hot corrosion are the two overriding factors (under high temperature corrosion) that determine the life of gas turbine engine components. The rate of degradation is slow under oxidation conditions, while it is significantly fast under hot corrosion conditions, and catastrophic failures can result if proper materials in association with appropriate coatings are not used (Figure 2.9).25,26 Assessment of the current status on hot corrosion problems in gas turbine engines is imperative in order to improve the life of the engines by selecting advanced hot corrosion resistant materials and coatings. This process may help in highlighting the issues that need to be addressed not only to enhance the efficiency of gas turbine engines, but also to avoid failures during service.

A section of a typical gas turbine engine in which superalloys and titanium-based alloys are used is shown in Figure 2.10. As shown, the hot sections of the engine are dominated by Ni-based superalloys and compressor sections with titanium-based alloys. Advances about three decades ago in processing of Ni-based superalloys allowed evolution of microstructures from equiaxed structures to directionally solidified (DS) multigrain and single crystal (SC) components today. With added capability from compositional flexibility coupled with advances in processing over that time period, high-pressure turbine blade temperatures have increased to about 1250 °C, and metal surface temperature at the hottest locations approach 1150 °C in state-of-the-art gas turbine engines.

One of the most critical components in the engine is the gas turbine blade. The high-pressure turbine blade operates under more arduous conditions of temperature and stress than any component in the engine. Not only does the blade experience high temperature and direct stress, it also experiences rapid temperature transients at various points during the engine cycle. The hot gases surrounding the blade are highly oxidizing and contain high levels of contaminants like sulfur and chlorine if low-grade fuels are used. An ideal superalloy/protective coating should be able to survive this harsh corrosive environment for a designed period.
As mentioned earlier, the efficiency of a gas turbine is proportional to firing temperature. The increase in engine operating temperature meant that the traditional corrosion resistant turbine blade alloys such as IN 738 and IN 939 are no longer strong enough to last the expected 25,000 h of minimum life. It implies that higher strength alloys and single crystal alloys are required for creep strength. Progress in aero and industrial gas turbine blade materials has revealed the fact that in the last decade, dramatic competition in the power equipment industry has boosted the technology to a level that has been achieved recently in aviation turbines. Latest industrial gas turbines use single crystal, rhenium containing Ni-based superalloys and directionally solidified blades and vanes.
The majority of Ni-based superalloy development efforts have been directed towards improving the alloy high temperature strength with relatively minor concern being shown to its hot corrosion resistance. Further, it is not always possible to achieve both high temperature strength and hot corrosion resistance simultaneously because some alloying elements help to improve hot corrosion resistance while some may help to improve high temperature strength. It is rare that an alloying element leads to enhancement both in high temperature strength and in hot corrosion resistance. This is further complicated for marine applications by the aggressivity of the environment, which includes sulfur and sodium from the fuel and various halides contained in seawater. These features could drastically reduce the superalloy component life and reliability by consuming the material at an unpredictably rapid rate, thereby reducing the load-carrying capacity and potentially leading to catastrophic failure of components.25,26 Thus, the hot corrosion resistance of superalloys is as crucial as its high temperature strength in gas turbine engine applications. Recent studies have shown that the high temperature strength materials are most susceptible to hot corrosion, and the protective coating plays a key role in effectively combating the hot corrosion problem.26–31
2.6 Hot Corrosion
Break down of protective oxide layers by chemical interaction with certain aggressive species contained in the combustion environment can produce accelerated attack on the underlying metal. This process is known as hot corrosion. Hot corrosion takes place mainly because of high concentrations of sulfur, vanadium, and sodium in the fuels, which may be as high as 4%, 0.05%, and 0.01% (all are in wt.%), respectively. Chlorides and sulfates enter the engine with the air; sulfur, vanadium, and sodium oxidize, during combustion, and mostly volatile compounds such as SO2, SO3, NaOH, NaO, Na2O, VO (OH)3, V2 O5, and V2 O4 are formed. These compounds condense at 500-900 °C and build up deposits depending on the fuel. Na2SO4 is the main component of deposits in engines running on high sulfur and low vanadium fuel. Table 2.2 summarizes the main contaminants that are likely to form in a working environment for three types of gas turbine engines, that is, aero, marine, and industrial gas turbines.
Table 2.2
Contaminants in Gas Turbine Fuel and Air
| Engine Application | Contaminants |
| Aero | Na, Cl, S, Ca (all low) |
| Marine | Na, Cl, S, Mg (all high) |
| Industrial | Na, V, S, Pb, Cl |
2.6.1 Types of hot corrosion
Two different forms of attack have been identified, type-II hot corrosion, and type-I, which occur over, different temperature ranges; 600-750 °C and 800-950 °C respectively. Type-I results from a fluxing process where modification of the sodium sulfate deposit chemistry permits ingress of sulfur into the underlying metal; this produces localized depletion of protective elements, and progressive internal attack occurs. The overall process of type-I hot corrosion produces a characteristic of attack which includes a porous oxide scale, an irregular metal/scale interface, and internal attack with preceding metal sulfides.26,29
On the other hand, type-II hot corrosion requires sodium sulfate and sufficient sulfur trioxide to maintain low melting deposit, which readily fluxes the surface oxide. Thermodynamics favor sulfur trioxide formation in the lower temperature range. The associated attack is normally localized, producing pits with a lamellar scale rich in sulfur through progressive fluxing action of the deposits. Here, sulfur generally does not enter the alloy to form internal sulfide with this type of attack.26
2.6.2 Mechanism of hot corrosion
Several mechanisms have been proposed for hot corrosion. All the mechanisms involve deterioration of the reaction product barrier that forms on the alloys when the deposits are not present. Basically, the hot corrosion process proceeds in Four stages:
(a) An incubation stage during which the reaction proceeds at a rate essentially similar to that of normal oxidation
(b) An initiation step during which the corrosion is accelerated
(c) A propagation stage during which rapid corrosion takes place and
(d) Ultimate failure of a component
2.6.2.1 Incubation period
During this period, the alloy undergoes normal oxidation similar to that observed in the absence of salt deposit. Initially, the oxides of most of the alloying elements are formed as given below:
The rapid weight gain of the alloy takes place due to the reaction with oxygen in the initial stages. At the end of incubation stage, thermodynamically stable oxides such as Cr2O3 and Al2O3 are formed as a dense oxide scale on the surface of alloys. This oxide scale acts as a diffusion barrier for the ingress of deleterious species such as oxygen and sulfur.
2.6.2.2 Initiation stage
Cracking or spalling of oxide scale occurs in this stage due to the stresses developed during oxide growth. Thus, fresh alloy surface, which is depleted of scale forming alloying elements, is exposed to the action of deposit as shown below:
SO2 will be in the form of dissolved gas.
2.6.2.3 Propagation stage
The propagation stage during hot corrosion is substantially different from the behavior of the alloy in the absence of a deposit. It is accompanied by an exceptionally severe attack of the alloy as a result of fluxing of oxides as shown below:
The dissolved sulfur dioxide formed during the initiation period can react with the alloying elements to produce oxides, sulfides, and sulfur.
If the deposits contain chloride ions, it can selectively remove certain elements such as chromium or aluminum from the alloy. This process involves the formation of highly volatile gaseous chlorides inside the pores of the alloy, and thereby metal chlorides diffuse out from the alloy. As a result, mechanical properties of the alloy reduce significantly as the cracks are formed on the alloy surface. The metallic components of these chlorides convert to oxides eventually, but these oxides form as particles and not as continuous layers. Hence, severe attack of the alloy can take place.26
The mechanisms have been explained based on acidic and basic fluxing of protective oxide scales depending upon the conditions. The protective oxide scale, for example, Cr2O3, is fluxed either as acidic solutes, such as Cr2 (SO4)3 or CrS, or as basic solutes, such as Na2CrO4 and NaCrO2. As a result, the oxide scale can become nonprotective. Such nonprotective scales can be formed by dissolution of the oxide near the alloy surface and reprecipitation as discontinuous particles from the molten deposit. Rapp and his team32–34 carried out extensive studies pertaining to hot corrosion fluxing mechanisms, different oxide solubilities, and electrochemical evaluation methods for hot corrosion resistance. Natesan et al.35 have studied the high temperature corrosion of Ni-based superalloys in coal conversion environments.
As fluxing of the protective scale is crucial in hot corrosion attack, the selection of alloys or intelligent coatings for hot corrosion resistance should be based on the solubility of protective oxides. For a given deposit and environmental conditions, the most favored oxide is the one that has the least solubility and can form a protective scale in the presence of salt film. Figure 2.11 presents the measured solubilities of various oxides in fused Na2SO4 at 1200 K.32 The difference of six orders of magnitude in basicity between minima for the most basic Co3O4 to the acidic SiO2, Cr2O3 and Al2O3 is striking and is consistent with relevant known alloy systems and coatings under hot corrosion conditions. Cobalt-based alloys and coatings are more vulnerable to acidic fluxing than Ni-based alloys and coatings. Cr2O3 is resistant to acidic fluxing because the minimum in its solubility curve corresponds approximately to the acidity of gas turbine environments.

It is extremely essential to assess the intelligent coatings in the laboratory under simulated engine conditions for evaluating their performance since it is not feasible to test each coating directly in the engine. A satisfactory test should yield a prediction of service performance, with an estimation of component life as a desirable objective. Different techniques, which are used for evaluation of various superalloys, and intelligent coatings for hot corrosion resistance, are described later.
2.6.3 Hot corrosion of superalloys
Different superalloys of varied categories, namely, forged alloys such as Nimonic 75, Nimonic 105, and Inconel 718; conventional casting (CC) alloys such as Inconel 713 and Inconel 100; and directionally solidified alloys such as CM 247 LC, MAR-M200, and MAR-M247, were reported for their hot corrosion resistance in different environments. Single crystal superalloys of different generations have been developed. For instance, first generation CMSX-2, TMS-12, TMS-26, PWA1480, and Rene N4; second generation with up to 3 mass percentage rhenium (CMSX-4, Rene N5, TMS 82 + etc.); third generation with up to 6 mass percentage rhenium (CMSX-10, TMS-75, TMS 80 +, etc.); fourth generation with rhenium and ruthenium (TMS-138, etc.); and fifth generation superalloys with iridium, ruthenium, and rhenium have been developed.
Single crystal airfoils offer the potential to further improve component high temperature materials strength and by control crystal orientation, can provide an optimum balance of properties. In single crystal material, all grain boundaries are eliminated from the material structure, and a single crystal with controlled orientation is produced in an airfoil shape. By eliminating all grain boundaries and the associated grain boundary strengthening additives, a substantial increase in the melting point of the alloy can be achieved, thus providing a corresponding increase in high temperature strength. The single crystal alloys have been in use in gas turbine engines since 1995. Together with intelligent protective coatings, the new superalloys will provide enhanced growth capability for gas turbine engines in the future.
Figure 2.12 shows the hot corrosion behavior of a few superalloys such as IN 792, CMSX-4, and DMS-4 under type-I conditions. The hot corrosion resistance of CM 247 LC and Rene 80 superalloys under type-II and type-I conditions in chloride and vanadium environments are presented in Figures 2.13 and 2.14. Appreciable corrosion was observed for all the superalloys. It indicates that hot corrosion plays a significant role in causing faster degradation, thereby reducing the superalloy life considerably. Among the superalloys, CM 247 LC was corroded severely, indicating that the superalloy is highly susceptible to hot corrosion. The appreciable corrosion attack of CM 247 LC superalloy was clearly evidenced by observing large cracks (Figure 2.15), broken samples (Figure 2.13), and a large corrosion affected zone (due to appreciable diffusion of corrosive elements present in the environment). Sulfur diffusion and formation of metal sulfides, preferentially chromium and nickel sulfides, are reported to be the influential factor. When sulfide phases are formed in superalloys, Ni-based alloys are inferior to cobalt- and iron-based alloys, which are especially effective in destroying the corrosion resistance of alloys.26,36–42 In fact, the alloying elements play a significant role and decide the life of superalloys under hot corrosion conditions (Table 2.3).




2.6.4 Oxidation characteristics of DMS-4
Figure 2.16 shows an oxidized DMS-4 superalloy, and typical surface morphology of the oxidized superalloy is shown in Figure 2.17.43 As can be seen, the superalloy was oxidized to the maximum extent by forming thicker oxide scales and was subsequently spalled. It indicates that the rate of formation of oxide scales is considerably high on the newly developed high rhenium-containing and exceptionally low chromium-containing DMS-4 superalloy. This leads to the formation of thick oxide scales over a period of time and subsequently spalled because of adherence problems. The typical results clearly indicate high vulnerability of the newer generation superalloys to high temperature oxidation.


It is generally accepted that the temperature capability increases with decreasing Cr content. Therefore, the chemistry of new superalloys was profoundly influenced by reducing Cr content and increasing rhenium (Re) concentration in association with small amounts of iridium, ruthenium with the objective to enhance the temperature capability. As a result, the new generation superalloys contain only 2-3% Cr, but also, instead, contain about 7% Re, 3% Ru, 2% Ir, which is in contrast to the earlier generation superalloys containing about 10% Cr and no Re, Ru, or Ir. It was reported that the rhenium and new alloying elements make the superalloys susceptible to high temperature oxidation and hot corrosion.44 Its effect is similar to Mo effect on oxidation (i.e., as the high vapor pressure of its oxide). It is because the superalloys cannot form either oxidation or hot corrosion resistant protective scale because of high Re content and other new alloying elements. It clearly stresses the need to apply intelligent protective coatings for their protection under high temperature conditions as the gas turbine blades experience high temperature oxidation and hot corrosion.
2.7 Surface Coating Technologies
The blade coatings are usually of either diffusion coatings (aluminides) or MCrAlY type (where M is Ni or NiCo) (overlay coatings). These coatings can provide protection against oxidation and hot corrosion and act as bond coatings for zirconia-based thermal barrier coating (TBC) systems. In both the cases, slow growth rates and optimum adherence of the alumina and chromia scales formed on the coatings during high temperature exposure are of significance for component life. These requirements can be fulfilled only by using coatings with sufficiently high aluminum and chromium contents. It will ensure protective alumina and chromia scale formation and rehealing, after oxide spallation/reaction with the environment. The life of a coating is mainly limited by aluminum and chromium depletion that occur upon their consumption as a result of alumina and chromia scale growth and also on the repeated spallation and rehealing during the oxidation process. On reaching a stage where the aluminum and chromium level in a bond coating falls below the level at which protective alumina and chromia scale cannot be formed preferentially, faster interaction between the corrosive species present in the environment and the nonprotective oxides of other constituents of the bond coating occurs. This phenomenon significantly affects the coating life under hot corrosion conditions. Further, the constituents of ceramic TBCs react easily with the corrosive species and shorten the coating life significantly. Therefore, the composition of coating is extremely valuable.
2.7.1 Diffusion coatings
In the diffusion coating application process, aluminum is made to react at the surface of substrate, forming a layer of mono-aluminide. For coatings applied over Ni-based superalloys, nickel aluminide is the resulting species. This coating and surface modification process is one of the most widely used for tailoring the surface properties of components.
The most notable improvement in the diffusion coatings has been the incorporation of platinum in aluminide coatings.45–47 This process involves the deposition of platinum by electrochemical method followed by aluminizing at the suitable temperature for the required period. These coatings became the accepted standard for turbine hot components to combat oxidation as well as hot corrosion. The principal reason for superior performance of these coatings is that the coefficient of thermal expansion of the coating is lower compared to that of underlying superalloy. In addition, platinum enhances the activity of aluminum and allows the formation of continuous alumina scale during high temperature exposure coupled with exceptional adherence, thereby prolonging the life of turbine blades considerably. Failure of coatings under turbine service conditions takes place due to interdiffusion between the coating and the substrate, with the loss of protective capability. Although diffusion coatings are well bonded to the substrate, they have limited compositional flexibility and their usefulness is strongly dependent on the substrate chemistry.
2.7.2 Overlay coatings
To develop coatings with compositions nominally independent of substrates and with capabilities for tailoring to a wide range of requirements for gas turbine applications, overlay coatings emerged. Coatings of this type are generally called MCrAlY (where M stands for Ni or NiCo) and essentially comprise a mono-aluminide component contained in a more ductile matrix of a solid solution. The supply of aluminum for the formation of protective alumina scale comes largely from the dispersed mono-aluminide phase during the useful life of such coatings. Overlay coatings are typically well bonded and have a wide compositional flexibility. Research and development on this type of coatings has led to a variety of compositions, with improved scale adherence. The function of all coatings is to provide a surface reservoir of critical elements that will form highly protective and adherent oxide layers, thus protecting the underlying superalloy from oxidation as well as hot corrosion. Further, MCrAlY based bond coatings play a significant role in providing rough surface for the application of TBCs and to provide protection for the alloy from oxidation and/or hot corrosion. The MCrAlY coatings have been studied extensively for over two decades due to their proven performance over varieties of superalloys in different applications.48–51 The effect of aluminum in forming a thermodynamically and chemically hot corrosion resistant layer and thereby improving the life of the components by reducing mass gain in MCrAlY coatings appears crucial. Further, optimum content of aluminum in the coatings is extremely necessary to enhance its lifetime. In fact, it only decides the lifetime of coatings and hence the essentiality of aluminum reservoir in the coatings.
Figure 2.18 illustrates the influence of aluminum on weight loss in the MCrAlY based coating model alloys. It is apparent that aluminum plays a vital role in affecting the hot corrosion resistance of MCrAlY alloys though the concentration of other alloying elements remains constant. The weight loss is maximum for the model alloy containing 6% aluminum, decreases with the increase in aluminum content to 9%, and minimum weight loss is observed for the alloy containing 12% aluminum. The behavior is same for all the model alloys irrespective of cobalt content, that is, whether the cobalt is 10% or 20% (Figure 2.18). Therefore, the minimum amount of aluminum required to be present in the MCrAlY based bond coatings is 9%. It is particularly essential to mention that the optimum content of aluminum required for providing exceptional hot corrosion resistance is 12%, although its effect is marginal when compared with 9% aluminum containing alloys.36
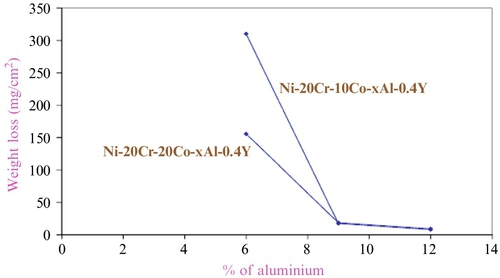
It is also necessary to mention that the combination of cobalt and aluminum contents is a must to exhibit appreciable hot corrosion resistance. The recent results clearly showed that cobalt plays a significant role in supporting aluminum to form a chemically and thermodynamically stable oxide scale. The optimum amounts of aluminum and cobalt are to be present in the MCrAlY bond coatings. Hence, the combination of cobalt and aluminum in association with chromium, yttrium, and nickel provides excellent hot corrosion resistance.38,39
Another crucial aspect is the selection of suitable surface engineering technique by which the coatings are applied. The life of a coating depends not only on coating composition, but also on the surface engineering technique employed for applying the coating. Therefore, selection of appropriate surface engineering technique as well as suitable coating composition (intelligent) becomes a challenging task.
2.7.3 Surface engineering techniques
The following are the most common surface engineering techniques available at present:
(b) Diffusion coating processes
(c) Thermal spray techniques
(d) Ion implantation
(e) Hardening and cladding
(f) Selective surface hardening by transformation of phase
(g) Vapor deposition
Electrodeposition, diffusion, and thermal spraying techniques are widely in use to improve the surface of components for hot corrosion resistance. Particularly, thermal spray and vapor deposition techniques are the most efficient techniques for prolonging the life of components significantly.
2.7.3.1 Thermal spraying processes
The coatings applied by these processes have advantage over other techniques. Thermal spraying processes can broadly be divided into the following techniques:
(ii) Arc Spraying
(iii) High Velocity Oxy-Fuel Spraying
(iv) Detonation Gun
(v) Air Plasma Spraying
(vi) Vacuum Plasma Spraying
Flame Spraying: In this process, an oxygen-acetylene mixture is passed through a nozzle and ignited to form a combustion flame. In order to form a deposit, the coating powder or wire is fed into the flame, accelerated, and projected onto the substrate. The combustion flame temperature is limited to 3000 °C, and gas particle velocities are relatively low.
Arc Spraying: This process involves the production of molten particles at the tips of two consumable wires via resistance heating. The coating material is subsequently atomized and projected onto the substrate by a compressed air jet. The process is limited to the spraying of conductive wires relatively cheaply and can achieve high deposition rates.
High Velocity Oxy-Fuel Spraying (HVOF): This process involves combustion of a fuel gas with oxygen at high pressure. This creates high velocity flame propelling powdered coating materials onto a substrate. This technique can produce high-quality coatings for gas turbine applications.
Detonation Gun Spraying: D-Gun and Super D-Gun are developed by Union Carbide. These guns use the energy released by a controlled series of oxygen-acetylene to heat and accelerate the coating powder so as to impart high velocity and propulsion to the powder onto the substrate. The resulting coatings are highly dense and of high quality with a low content of oxide and unprocessed particles.
Air Plasma Spraying (APS): The APS utilizes a DC arc struck between a central inert cathode and an annular copper anode. Moreover, an inert gas is fed into the arc to form high temperature plasma. Powder is fed into the plasma and is ejected at a high velocity towards the substrate.
Vacuum Plasma Spraying (VPS): This process has several advantages over APS. The problems associated with air contamination in the plasma jet are eliminated. Moreover, the plasma jet is longer than air and can achieve ~ 400-600 ms− 1 particle velocity that helps in depositing high purity and dense material. A further advantage is coating adhesion.
Kuroda et al. recently developed a novel coating technique called a “warm spray” process that incorporates the advantages of both HVOF and cold spray.52 Further developments may enable them to develop coatings with enhanced hot corrosion resistance.
2.7.3.2 Electron beam physical vapor deposition (EB-PVD) processes
The electron beam-PVD process has made the most significant impact over the PVD processes in respect of coating rotating blades. The microstructure of the coating is controlled extremely closely in this process, and coating process is slow compared to conventional PVDs. Thermal barrier oxide coatings obtained with this process have a unique columnar structure with high in-plane compliance, which tends to have higher thermal conductivity and has shown to survive a longer thermal cycle life as compared to plasma-sprayed TBC. Table 2.4 provides the comparison of different processes and their limitations.
Table 2.4
Comparison of Thermal Spray and EB-PVD Processes53
| Coating Type | Advantages | Disadvantages/Limitations |
| Diffusion (e.g., aluminides) | Simple to produce, proven ability < 900 °C, inexpensive | Thickness limited to 50 μm. Brittle at < 750 °C and degrade by interdiffusion. Available compositions are limited |
| Plasma overlays | High rates of coating and a wide range of compositions are available. Thickness not process limited (100 μm) | Rough surface and single line of sight coating versus EB coatings. Thin multilayers more difficult than EB. About 15% porosity and degrade by interdiffusion |
| EB-PVD overlays | Good control of microstructure, low contaminant level and control of composition within limits. Layered and graded coatings easily achieved, and multicomponent processing is possible | Low rates of deposition and expensive process. Degrade by interdiffusion |
| Plasma spray thermal barrier | High rate of coating and a wide range of compositions are available. Layered and graded coatings are possible. Porosity is an advantage and not limited by interdiffusion | Single line of sight coating and difficult to modify microstructure. High stresses generated in coatings |
| EB-PVD thermal barrier | Good control of microstructure and low contamination level. Layered and graded coatings are possible and not limited by interdiffusion | Low rate of deposition and multiple component processing are possible. Expensive process and generates high stresses, but relieved by structure control |
Figure 2.19 presents a hot corroded diamalloy coated IN 738 with HVOF technique. The surface morphologies of coated superalloy IN 738 (Figure 2.20) revealed poor performance of the coating, indicating that the composition of a coating plays a vital role.


2.8 Influence of Major and Trace Elements
Traces of silicon and hafnium make the MCrAlY coating highly susceptible to hot corrosion. Traces of silicon or hafnium modify oxide growth rate and the composition of oxide scale, and consequently reduce the coating life significantly. The fluxing of oxide scale also becomes easy when traces of silicon or hafnium is present in the scale. The underlying mechanism is that hafnium and silicon are present in the grain boundaries of alumina scale leach out selectively by readily reacting Si and Hf with chlorine, vanadium, sodium, and sulfur present in the environment to form corresponding compounds. This results in dislodging the grains of alumina scale and creates instability of the oxide scale and thereby reduces the life of coatings significantly. Further, the oxides of silicon and hafnium are soluble in molten basic sulfate, and the basic fluxing dominates in the high temperature hot corrosion region (850-950 °C). The reaction mechanisms leading to reducing the life of coatings containing silicon or hafnium are given below:
Kawagishi et al. have proposed an equivalent coating system for high temperature corrosion resistance of Ni-based superalloys.54 This coating composition is designed to be in thermodynamic equilibrium with the substrate so that no interdiffusion can occur. It is also reported that this coating technique helps in repair of turbine blades. However, hot corrosion resistance of this coating under varied gas turbine engine conditions is yet to be proved because the coating composition is similar to the superalloy.
2.9 Concept of Intelligent Coatings
A primary requirement for a coating to use in high temperature applications is slow growth rates and optimum adherence to the protective scales that form on the metallic coatings during high temperature exposure/service conditions. The gas turbine engines experience both types of hot corrosion as well as oxidation. There is a need to develop intelligent or smart coatings to effectively combat high temperature corrosion problems and to obtain maximum efficiency with reduced failures.
If a single coating can operate successfully over a range of temperatures with different forms of corrosion attack such as type-I and type-II hot corrosion and high temperature oxidation, the coating essentially responds to local temperature in such a way that it will form either an alumina or a chromia protective scale as appropriate. High purity alumina scales offer significant protection against high temperature oxidation and type-I hot corrosion as do chromia scales against type-II hot corrosion. Ideally a single coating should satisfy both the requirements. It Alumina and chromia scale formation is possible for a coating only if it contains chromium- and aluminum-rich graded coatings (Figure 2.21). The base coating should be a standard MCrAlY coating enriched with aluminum at its outer surface and a chromium-rich layer at its inner surface. Under high temperature oxidation and type-I hot corrosion conditions, the outer layer of the coating forms alumina scale, which provides protection and it, offers less protection under low temperature conditions. Under type-II hot corrosion conditions, a chromium-rich layer forms chromia scale at a faster rate and provides protection. Thus, the intelligent coating can provide optimum protection by responding suitably to the temperatures that are encountered under actual service conditions of gas turbine engines. This optimum protection is possible because of the formation of the most suitable protective oxide scale in each temperature range of operation envisaged. In this sense, the coating responds to its environment in a pseudo-intelligent manner and hence the name Intelligent Coating.

In essence, the development of intelligent coatings design involves the preparation of multilayered coating consisting of an appropriate MCrAlY base, enriched first in chromium and then aluminum to provide a chemically graded structure. The intermediate chromium-rich phase provides protection under low temperature hot corrosion conditions; while the aluminum-rich surface layer provides resistance to high temperature oxidation and type-I hot corrosion. Diffusion barrier coatings prior to chromium- and aluminum-rich layers help in preventing interdiffusion of coating and substrate elements. Thus, the intelligent coating permits operation of gas turbine engines over a wide range of temperatures successfully for more than the designed life and helps in enhancing the efficiency significantly by effectively preventing oxidation, type-I and type-II hot corrosion that are normally experienced in gas turbines.55–57
2.9.1 Preparation and selection of a suitable surface engineering technique
The development of intelligent coatings is a challenging task. Moreover, selection of suitable surface engineering techniques to produce a quality coating is extremely essential. Probably, selection of a single technique may not help, but a combination of techniques could be useful. Further, order of usage of selected techniques is also vital. Therefore, one has to be extremely careful in selection and utilizing the techniques for production of intelligent coatings in order to achieve maximum efficiency. The intelligent coatings can be task-prepared by using a combination of available surface engineering techniques. Among these, sputtering, thermal spraying, diffusion, electroplating, and laser treatments are more effective in producing intelligent coatings. Application of a suitable diffusion barrier on the superalloy followed by an optimized MCrAlY coating and chromized treatment to achieve about 70% Cr rich coating and then electroplating of platinum or palladium, followed by pack cementation, enables the desired graded structure. However, detailed research is needed to establish the process parameters of each surface engineering technique, coating thickness, suitable annealing treatments, and so on, to obtain a coating of desired characteristics.
The microstructures of coatings play a significant role in enhancing the life of components. Therefore, obtaining an appropriate microstructure by identifying the suitable surface engineering technique is of paramount importance. The selection of technique for coating preparation should be based on the parameters that are controllable to get the required microstructure. As mentioned earlier, the intelligent coating is a graded coating consisting of different zones (Figure 2.21). Each zone has its specific microstructure with definite composition. The top layer is diffusion-type aluminide, and second is chromium-rich layer followed by suitable MCrAlY coating and a diffusion barrier layer at the bottom. The composition of each zone requires detailed analysis to understand the effect of intelligent coating. The diffusion-type aluminide layer should contain a high amount of aluminum, the second layer should be about 70% rich in chromium, the third layer as that of MCrAlY coating composition, and the bottom diffusion layer should contain heavy elements. The microstructure of each zone varies depending on the coating composition and surface engineering technique used.
2.9.2 Techniques for assessment of intelligent coatings
The following laboratory techniques have been in use for assessing a variety of materials and intelligent coatings for gas turbine engine applications:
(ii) Furnace test
(iii) Crucible test
(iv) Thermogravimetric test
(v) Electrochemical methods
Burner rig test: This test simulates the operating conditions in gas turbine engines, and the gas composition, pressure, velocity, and temperature. In this test, rigs are composed of a combustion chamber from a small turbine, supplying air from compressors and burning fuel in the usual way. Salt can be sprayed into the combustion chamber. The hot exhaust gases are passed into the sample chamber, where several specimen coupons are placed and rotated in the gas stream. Here, the test variables (such as gas pressure, velocity, sample temperature, salt concentration, and fuel-to-air ratio) can be selected to simulate aero-, marine-, and land-based gas turbine engine’s operation.
This test was used extensively for evaluation of a variety of coated and uncoated materials for hot corrosion resistance. It was also established that the tested coatings displayed generally similar behavior during the service. Therefore, this test is more appropriate for evaluating different intelligent coatings to get comparable results with the service conditions of gas turbine engines.
Furnace test: This test employs a furnace with two zones whose temperatures can be controlled independently. The intelligent-coated specimen is placed in one zone, and a crucible containing the test salt is kept in the other zone. With the help of a carrier gas, the vaporized salt is transferred from one zone to other, where it deposits on the intelligent-coated specimen. In this manner, the deposition of salt per unit time can be strictly controlled. In a modified method known as “Dean Test,” the corrosion conditions can be controlled from exceptionally mild to extremely severe by simply altering the temperature differential within the two zones. This test was used mainly to study kinetics of various coated and uncoated materials in different environments.
Crucible test: This method involves direct immersion of the intelligent-coated sample to half its length in molten salt in air atmosphere and measurement of weight change to monitor the reaction rate. The gas could be bubbled through the melt or passed over the sample to simulate an oxidizing or reducing environment. This test was used for evaluation of a number of uncoated and coated Ni-based superalloys for hot corrosion resistance. The corrosion test conditions are more severe than that in normal operating conditions of the engines, but it is a simple and helpful test for screening the intelligent coatings.
Thermogravimetric test: It is also called “salt coated test.” In this test, an intelligent-coated sample is coated with saturated salt solution, then dried, weighed, and placed in a heated furnace. The sample weight is continuously recorded. In most cases, the weight increases as oxidation proceeds and the vaporization rate is small compared with the oxidation rate. This method can be used for determining the hot corrosion kinetics of different intelligent coatings because the experimental parameters such as gas composition, temperature, and salt loading can be controlled easily and precisely.
Electrochemical methods: This method involves measurement of corrosion current as a result of oxidation-reduction reaction continuously and provides instantaneous corrosion rates. The net reaction is the oxidation of intelligent coating to form oxide scales and the reduction of ![]() to a lower oxidation state. Generally, corrosion is accelerated when the salt film is in molten stage such that the intelligent coating and gas are physically separated by the ionically conducting fused salt. Fused sodium sulfate is a dominant ionic (Na+) conductor like in normal solutions. Thus, the reaction must involve electrochemical steps and hence this method is most ideal for assessing various intelligent coatings as well as other materials for hot corrosion resistance.
to a lower oxidation state. Generally, corrosion is accelerated when the salt film is in molten stage such that the intelligent coating and gas are physically separated by the ionically conducting fused salt. Fused sodium sulfate is a dominant ionic (Na+) conductor like in normal solutions. Thus, the reaction must involve electrochemical steps and hence this method is most ideal for assessing various intelligent coatings as well as other materials for hot corrosion resistance.
The electrochemical polarization of intelligent coatings in molten salts can cause corrosion attack, which is qualitatively similar to that found for a thin salt film in a combustion-product environment. In addition, the results from 600 h of a burner rig test are well comparable with a 4-h electrochemical test method. It proves that electrochemical techniques are extremely helpful in evaluating the intelligent coatings and other materials at a faster rate compared to other tests developed so far. The technique also helps to study reaction mechanisms and online monitoring of intelligent coatings in the actual plants. The merits and demerits of each technique are presented in Table 2.5.
Table 2.5
Comparison of Hot Corrosion Evaluation Techniques
| Technique | Advantages | Disadvantages/Limitations |
| Burner rig test | Simulates gas composition, pressure, velocity, and temperature of gas turbine engines | Complex process and requires long running time. Difficult to control all the parameters accurately |
| Furnace test | Corrosion conditions can be controlled depending upon the requirements, that is, severe or mild corrosion | Difficult to maintain salt deposition rate for longer times |
| Crucible test | Most simple and highly useful for preliminary screening of the coatings and materials | Corrosion is severe for alloys or coatings having low or intermediate resistance to hot corrosion |
| Thermogravimetric test | Precise weight gain measurement Possible under different test conditions, that is, gas composition, temperature, salt composition, etc. | Not useful for predicting the life of coatings and superalloys |
| Electrochemical test | Fast and useful for ranking the materials based on the oxidation-reduction phenomenon | Yet to be established fully |
2.9.3 Performance of a developed intelligent coating
Recently, an intelligent metallic coating has been developed, which is optimized compositionally and promotes an appropriate protective scale formation depending on the surrounding environmental conditions that enhance the durability of TBC, and, in turn, the life of CM 247 LC superalloy components. The performances of various compositions of MCrAlY bond coatings applied on CM 247 LC superalloy (without TBC) were evaluated systematically under type-I conditions in simulating gas turbine engine environments. Among the coatings, NiCoCrAlY coating exhibited a maximum life of more than 300 h (Figure 2.22). The high performance of this coating is due to its ability to form a continuous, adherent, and protective alumina scale on the surface of the bond coating during high temperature corrosion at 900 °C (Figure 2.23).


Table 2.6 presents the life of uncoated CM 247 LC superalloy, intelligent coating as well as different thicknesses of zirconia-based TBCs at 900 °C in simulating gas turbine engine environments. The results indicate that 100 μm thick TBC improved the superalloy life by 450 times to that of bare superalloy. It was also evident that 300 μm thick TBC in association with compositionally optimized 125 μm thick NiCoCrAlY bond coating (intelligent bond coating) enhanced the superalloy life by about 600 times, which was found to be optimum. Thick ceramic coatings could not help in improving the hot corrosion resistance of superalloys further due to adherence problems associated with spallation at higher temperatures. The maximum life of NiCoCrAlY (intelligent coating) + 300 μm thick TBC was attributed to the formation of protective alumina and chromia scales, not only on the surface of bond coating but also on the TBC surface (Figure 2.24). Furthermore, neither sulfur nor oxygen was diffused into the coating, indicating an excellent protection provided by 300 μm thick TBC in association with intelligent bond coating to the CM 247 LC superalloy. It is known that TBC reduces the temperature of bond coating/substrate by about 200 °C. It indicates that the substrate or bond coating temperature is about 700 °C only. Under such environmental conditions, that is, at 700 °C, the bond coating promoted chromia and alumina scale formation that is clearly observed in Figure 2.24. It was also observed that the bond coating promoted protective alumina scale formation at 900 °C in the same environments (Figure 2.23). It indicates that the NiCoCrAlY bond coating forms alumina scale at 900 °C, that is, without TBC and chromia and alumina scale in the presence of TBC at the same environmental conditions and provides maximum life to the TBC which in turn i.e. the superalloy components.
Table 2.6
Performance of Intelligent Coating on CM 247 LC Alloy + Different Thicknesses of Thermal Barrier Coatings in Vanadium Environments Under Type-I Hot Corrosion Conditions
| Type of Coating | Life (Hours) |
| Uncoated superalloy | < 2 |
| Intelligent coating | 300 |
| Intelligent coating + 100 μm TBC | 910 |
| Intelligent coating + 200 μm TBC | 975 |
| Intelligent coating + 300 μm TBC | 1175 |
| Intelligent coating + 400 μm TBC | 1170 |

Hence, the developed bond coating exhibited intelligence behavior both in the presence and absence of TBC and formed suitable protective scales and hence was named as intelligent bond coating. Other noteworthy observations are the formation of protective alumina and chromia scales on the surface of TBC as well as the prevention of diffusion of corrosive elements like chlorine, vanadium, sulfur, and oxygen. Thus, an intelligent bond coating was successfully developed for TBCs that promote appropriate protective scales preferentially depending on the environmental conditions and enhance the life of superalloy components significantly, which is essential for advanced gas turbines for their increased efficiency. Therefore, it is a potential bond coating for advanced gas turbines of different types, that is, marine and industrial, for their protection against high temperature oxidation, type-I and type-II hot corrosion.
2.10 Conclusion and Outlook
Corrosion prevention over the world, both at ambient and high temperatures, is extremely essential as the financial losses are enormous. It is possible only by designing and developing economically viable and environmentally friendly intelligent coatings over the existing coatings. Research in this direction paved the way for the development of intelligent coatings for ambient corrosion prevention. Further research is needed to understand and develop newer and alternative intelligent coating systems. The design and development of intelligent coatings to combat type-I and type-II hot corrosion and high temperature oxidation in gas turbines are challenging problems for scientists and corrosion engineers. The developmental work in this area has successfully resulted an intelligent coating, which provided an excellent protection to the superalloys against all the concerns that are being experienced by all types of gas turbines. Dedicated research is required both at the laboratory level and field to optimize coating composition, thickness, and microstructure. Additionally, identification of appropriate surface engineering techniques and their priority of use are needed to prove their performance. Finally, advanced processes and intelligent coatings development efforts are extremely necessary to manufacture modern gas turbine engines that could exhibit ever greater efficiency.