Duct burners
Peter F. Barry and Stephen L. Somers†
Stephen B. Londerville1, Kenneth Ahn1 and Kevin Anderson1, 1John Zink Company, LLC, Hayward, CA, United States
Abstract
Linear and in-duct burners were used for many years to heat air in drying operations before their general use in cogeneration and combined cycle systems. Some of the earliest systems premixed fuel and air in an often-complicated configuration that fired into a recirculating process airstream. The first use was in high-temperature, depleted oxygen streams downstream of gas turbines, in the early 1960s, to provide additional steam for process use in industrial applications and for electrical peaking plants operating steam turbines. As gas turbines have become larger and more efficient, duct burner supplemental heat input has increased correspondingly.
Keywords
Duct burners; gas turbines; turbine exhaust gas; unburned hydrocarbons; gas ports
7.1 Introduction
Linear and in-duct burners were used for many years to heat air in drying operations before their general use in cogeneration and combined cycle systems. Some of the earliest systems premixed fuel and air in an often-complicated configuration that fired into a recirculating process airstream. The first use was in high-temperature, depleted oxygen streams downstream of gas turbines, in the early 1960s, to provide additional steam for process use in industrial applications and for electrical peaking plants operating steam turbines. As gas turbines have become larger and more efficient, duct burner supplemental heat input has increased correspondingly.
Linear burners are applied where it is desired to spread heat uniformly across a duct, whether in ambient air or oxygen-depleted streams. In-duct designs are more commonly used in fluidized bed boilers and small cogeneration systems.
7.2 Applications
7.2.1 Cogeneration
Cogeneration implies simultaneous production of two or more forms of energy, most commonly electrical (electric power), thermal (steam, heat transfer fluid, or hot water), and pressure (compressor). The basic process involves combustion of fossil fuel in an engine (reciprocating or turbine) that drives an electric generator, coupled with a recovery device that converts heat from the engine exhaust into a usable energy form. Production of recovered energy can be increased independently of the engine through supplementary firing provided by a special type of burner known as a duct burner. Most modern systems will also include flue gas emission control devices.
Reciprocating engines (typically diesel cycle) are used in smaller systems (10 MW=34×106 Btu/h and lower) and offer the advantage of lower capital and maintenance costs, but produce relatively high levels of pollutants. Turbine engines are used in both small and large systems (3 MW=10×106 Btu/h and above) and, although more expensive, generally emit lower levels of air pollutants.
Fossil fuels used in cogeneration systems can consist of almost any liquid or gaseous hydrocarbon, although natural gas and various commercial-grade fuel oils are most commonly used. Mixtures of hydrocarbon gases and hydrogen found in plant fuel systems are often used in refining and petrochemical applications. Duct burners are capable of firing all fuels suitable for the engine/turbine, as well as many that are not, including heavy oils and waste gases.
Supplementary firing is often incorporated into the boiler/heat recovery steam generator (HRSG) design as it allows increased production of steam as demanded by the process. The device that provides the supplementary firing is a duct burner, so called because it is installed in the duct connecting the engine/turbine exhaust to the heat recovery device, or just downstream of a section of the HRSG superheater. Oxygen required for the combustion process is provided by the turbine exhaust gas (TEG).
7.2.2 Combined cycle
Combined cycle systems incorporate all components of the simple cycle configuration with the addition of a steam turbine/generator set powered by the HRSG. This arrangement is attractive when the plant cannot be located near an economically viable steam user. Also, when used in conjunction with a duct burner, the steam turbine/generator can provide additional power during periods of high or “peak” demand.
7.2.3 Air heating
Duct burners are suitable for a wide variety of direct-fired air heating applications where the physical arrangement requires mounting inside a duct, and particularly for processes where the combustion air is at an elevated temperature and/or contains less than 21% oxygen. Examples include
• Fluidized bed boilers (see Fig. 7.1): where burners are installed in combustion air ducts and used only to provide heat to the bed during startup. At cold conditions, the burner is fired at maximum capacity with fresh ambient air; but as combustion develops in the bed, cross exchange with hot stack gas increases the air temperature and velocity. Burners are shut off when the desired air preheat is reached and the bed can sustain combustion unaided.
• Combustion air blower inlet preheaters: where burners are mounted upstream of a blower inlet to protect against thermal shock caused by ambient air in extremely cold climates (−40°F/°C and below). This arrangement is only suitable when the air will be used in a combustion process as it will contain combustion products from the duct burner.
• Drying applications: where isolation of combustion products from the work material is not required, such as certain paper and wallboard manufacturing operations.
7.2.4 Fume incineration
Burners are mounted inside ducts or stacks carrying exhaust streams primarily composed of air with varying concentrations of organic contaminants. Undesirable components are destroyed, both by an increase in the gas stream bulk temperature and through contact with localized high temperatures created in the flame envelope. Particular advantages of the duct burner include higher thermal efficiency as no outside air is used, lower operating cost as no blower is required, and improved destruction efficiency resulting from distribution of the flame across the duct section with grid-type design.
7.2.5 Stack gas reheat
Mounted at or near the base of a stack, heat added by a duct burner will increase natural draft, possibly eliminating the need for induced draft or eductor fans. In streams containing a large concentration of water vapor, the additional heat can also reduce or eliminate potentially corrosive condensation inside the stack. A source of ambient augmenting combustion air is often added if the stack gas oxygen concentration is low. This arrangement may also provide a corollary emissions reduction benefit (see Section 7.7). A discussion on testing duct burner performance is given in Ref. [1].
7.3 Burner technology
7.3.1 In-duct or inline configuration
Register or axial flow burner designs are adapted for installation inside a duct. The burner head is oriented such that the flame will be parallel to and coflow with the air or TEG stream, and the fuel supply piping is fed through the duct sidewall, turning 90 degrees as it enters the burner (see Fig. 7.2). Depending on the total firing rate and duct size, one burner may be sufficient, or several may be arrayed across the duct cross section. Inline burners typically require more air/TEG pressure drop, produce longer flames, and offer a less uniform heat distribution than grid-type. On the other hand, they are more flexible in burning liquid fuels, can be more easily modified to incorporate augmenting air, and sometimes represent a less expensive option for high firing rates in small ducts without sufficient room for grid elements.
7.3.2 Grid configuration (gas firing)
A series of linear burner elements that span the duct width are spaced at vertical intervals to form a grid. Each element is comprised of a fuel manifold pipe fitted with a series of flame holders (or wings) along its length. Fuel is fed into one end of the manifold pipe and discharged through discrete multiport tips attached at intervals along its length, or through holes drilled directly into the pipe. Gas ports are positioned such that fuel is injected in coflow with the TEG. The wings meter the TEG or airflow into the flame zone, thus developing eddy currents that anchor ignition. They also shield the flame in order to maintain suitably high flame temperatures, thereby preventing excessive flame cooling that might cause high emissions. Parts exposed to TEG and the flame zone are typically of high-temperature alloy construction (see Figs. 7.3 and 7.4).
7.3.3 Grid configuration (liquid firing)
As with the gas-fired arrangement, a series of linear burner elements comprised of a pipe and flame holders (wings) span the duct width. However, instead of multiple discharge points along the pipe length, liquid fuel is injected downstream of the element through the duct sidewall, and directed parallel to the flame holders (cross flow to the TEG). This configuration utilizes the duct cross section for containment of the flame length, thus allowing a shorter distance between the burner and downstream boiler tubes (see Fig. 7.5). The injection device, referred to as a side-fired oil gun, utilizes a mechanical nozzle supplemented by low-pressure air (2–8 psi) (14–55 kPa) to break the liquid fuel into small droplets (atomization) that will vaporize and readily burn. Although most commonly used for light fuels, this arrangement is also suitable for some heavier fuels, where the viscosity can be lowered by heating. In some cases, high-pressure steam may be required, instead of low-pressure air, for adequate atomization of heavy fuels.
7.4 Fuels
7.4.1 Natural gas
Natural gas is, by far, the most commonly used fuel because it is readily available in large volumes throughout much of the industrialized world. Because of its ubiquity, its combustion characteristics are well understood, and most burner designs are developed for this fuel.
7.4.1.1 Refinery/chemical plant fuels
Refineries and chemical plants are large consumers of both electrical and steam power, which makes them ideal candidates for cogeneration. In addition, these plants maintain extensive fuel systems to supply the various direct and indirect-fired processes as well as to make the most economical use of residual products. This latter purpose presents special challenges for duct burners because the available fuels often contain high concentrations of unsaturated hydrocarbons with a tendency to condense and/or decompose inside burner piping. The location of burner elements inside the TEG duct, surrounded by high-temperature gases, exacerbates the problem. Plugging and failure of injection nozzles can occur, with a corresponding decrease in online availability and an increase in maintenance costs.
With appropriate modifications, however, duct burners can function reliably with most hydrocarbon-based gaseous fuels. Design techniques include insulation of burner element manifolds, insulation and heat tracing of external headers and pipe trains, and fuel/steam blending. Steam can also be used to periodically purge the burner elements of solid deposits before plugging occurs.
7.4.1.2 Low heating value
Byproduct gases produced in various industrial processes, such as blast furnaces, coke ovens, and flexicokers, or from mature landfills, contain combustible compounds along with significant concentrations of inert components, thus resulting in relatively low heating values (range of 50–500 Btu/scf=1.9–19 MJ/m3). These fuels burn more slowly and at lower temperatures than conventional fuels, and thus require special design considerations. Fuel pressure is reduced to match its velocity to flame speed, and some form of shield or “canister” is employed to provide a protected flame zone with sufficient residence time to promote complete combustion before the flame is exposed to the quenching effects of TEG.
Other considerations that must be taken into account are moisture content and particulate loading. High moisture concentration results in condensation within the fuel supply system, which in turn produces corrosion and plugging. Pilots and igniters are particularly susceptible to the effects of moisture because of small fuel port sizes, small igniter gap tolerance, and the insulation integrity required to prevent “shorting” of electrical components. A well-designed system might include a knockout drum to remove liquids and solids, insulation and heat tracing of piping to prevent or minimize condensation, and low-point drains to remove condensed liquids. Problems are usually most evident after a prolonged period of shutdown.
Solid particulates can cause plugging in gas tip ports or other fuel system components and should therefore be removed to the maximum practical extent. In general, particle size should be no greater than 25% of the smallest port, and overall loading should be no greater than 5 ppm by volume (weight).
7.4.1.3 Liquid fuels
In cogeneration applications, duct burners are commonly fired with the same fuel as the turbine, which is typically limited to light oils such as No. 2 or naphtha. For other applications, specially modified side-fired guns or an inline design can be employed to burn heavier oils such as No. 6 and some waste fuels.
7.5 Combustion air and turbine exhaust gas
7.5.1 Temperature and composition
When used for supplementary firing in HRSG cogeneration applications, the oxygen required for the combustion reaction is provided by the residual in the TEG instead of a new, external source of air. Because this gas is already at an elevated temperature, duct burner thermal efficiency can exceed 90% as very little heat is required to raise the combustion products’ temperature to the final fired temperature. TEG contains less oxygen than fresh air, typically between 11% and 16% by volume, which, in conjunction with the TEG temperature, will have a significant effect on the combustion process. As the oxygen concentration and TEG temperature become lower, emissions of CO and unburned hydrocarbons (UHCs) occur more readily, eventually progressing to combustion instability. The effect of low oxygen concentration can be partially offset by higher temperatures; conversely, higher oxygen concentrations will partially offset the detrimental effects of low TEG temperatures. This relationship is depicted graphically in Fig. 7.6. Duct burner emissions are discussed in more detail elsewhere in this chapter.
7.5.2 Turbine power augmentation
During periods of high electrical demand, various techniques are employed to increase power output, and most will increase the concentration of water vapor in TEG. The corresponding effect is a reduction in TEG oxygen concentration and temperature with consequent effects on duct burner combustion. Depending on the amount of water vapor used, CO emissions may simply rise, or in extreme cases the flame may become unstable. The former effect can be addressed with an allowance in the facility operating permit or by increasing the amount of CO catalyst in systems so equipped. The latter requires air augmentation, a process whereby fresh air is injected at a rate sufficient to raise the TEG oxygen concentration to a suitable level.
7.5.3 Velocity and distribution
Regardless of whether TEG or fresh air is used, velocity across flame stabilizers must be sufficient to promote mixing of the fuel and oxygen, but not so great as to prevent the flame from anchoring to the burner. Grid-type configurations can generally operate at velocities ranging from 20 to 90 ft/s or 6 to 27 m/s and pressure drops of less than 0.5 in. water column. Inline or register burners typically require velocities of 100–150 ft/s (31–46 m/s) with a pressure drop of 2–6 in. water column (5–15 mbar).
Grid burners are designed to distribute heat uniformly across the HRSG or boiler tube bank, and thus require a reasonably uniform distribution of TEG or air to supply the fuel with oxygen. Inadequate distribution causes localized areas of low velocity, resulting in poor flame definition along with high emissions of CO and UHCs. Turbine exhaust flow patterns, combined with rapidly diverging downstream duct geometry, will almost always produce an unsatisfactory result that must be corrected by means of a straightening device. Likewise, the manner in which ambient air is introduced into a duct can also result in flow maldistribution, requiring some level of correction. Selection and design of flow-straightening devices are discussed elsewhere in this chapter (see Fig. 7.7).

In instances where bulk TEG or air velocity is lower than required for proper burner operation, flow straightening alone is not sufficient and it becomes necessary to restrict a portion of the duct cross section at or near the plane of the burner elements, thereby increasing the “local” velocity across flame holders. This restriction, also referred to as blockage, commonly consists of unfired runners or similar shapes uniformly distributed between the firing runners to reduce the open flow area.
Inline, or register, burners inject fuel in only a few positions (or possibly only one position) inside the duct, and can therefore be positioned in an area of favorable flow conditions, assuming the flow profile is known. On the other hand, downstream heat distribution is less uniform than with grid designs, and flames may be longer.
As with grid-type burners, in some cases, it may be necessary to block portions of the duct at or just upstream of the burners to force a sufficient quantity of TEG or air through the burner.
7.5.4 Ambient air firing (air-only systems and HRSG backup)
Velocity and distribution requirements for air systems are similar to those for TEG, although inlet temperature is not a concern because of the relatively higher oxygen concentration. As with TEG applications, the burner elements are exposed to the products of combustion, so material selection must take into account the maximum expected fired temperature.
Ambient (or fresh) air backup for HRSGs presents special design challenges. Because of the temperature difference between ambient air and TEG, designing for the same mass flow and fired temperature will result in velocity across the burner approximately one-third that of the TEG case. If the cold condition velocity is outside the acceptable range, it will be necessary to add blockage, as described earlier. Fuel input capacity must also be increased to provide heat required to raise the air from ambient to the design firing temperature. By far, the most difficult challenge is related to flow distribution. Regardless of the manner in which backup air is fed into the duct, a flow profile different from that produced by the TEG is virtually certain. Flow-straightening devices can therefore not be optimized for either case, but instead require a compromise design that provides acceptable results for both. If the two flow patterns are radically different, it may ultimately be necessary to alter the air injection arrangement independently of the TEG duct-straightening device.
7.5.5 Augmenting air
As turbines have become more efficient and more work is extracted in the form of, for example, electricity, the oxygen level available in the TEG continues to get lower. To some extent, a correspondingly higher TEG temperature provides some relief for duct burner operation.
In some applications, however, an additional oxygen source may be required to augment that available in the TEG when the oxygen content in the TEG is not sufficient for combustion at the available TEG temperature. If the mixture adiabatic flame temperature is not high enough to sustain a robust flame in the highly turbulent stream, the flame may become unstable.
The problem can be exacerbated when the turbine manufacturer adds large quantities of steam or water for NOx control and power augmentation. A corresponding drop in the TEG temperature and oxygen concentration occurs because of dilution. The TEG temperature is also reduced in installations where the HRSG manufacturer splits the steam superheater and places tubes upstream of the duct burner.
With their research and development facilities, manufacturers have defined the oxygen requirement with respect to TEG temperature and fuel composition, and are able to quantify the amount of augmenting air required under most conditions likely to be encountered. It is usually not practical to add enough air to the turbine exhaust to increase the oxygen content to an adequate level. Specially designed runners are therefore used to increase the local oxygen concentration. In cases where augmenting air is required, the flow may be substantial: from 30% to 100% of the theoretical air required for the supplemental fuel.
The augmenting air runner of one manufacturer consists of a graduated air delivery tube parallel to and upstream of the burner runner. It is designed to ensure a constant velocity of the augmenting air along the length of the tube. Equal distribution of augmenting air across the face of the tube is imperative. The augmenting air is discharged from the tube into a plenum and passes through a second distribution grid to further equalize flow. The air passes through perforations in the flame holder, where it is intimately mixed with the fuel in the primary combustion zone. This intimate mixing ensures corresponding low CO and UHC emissions under most conditions likely to be encountered. Once the decision has been made to supply augmenting air to a burner, it is an inevitable result of the design that the augmenting air will be part of the normal operating regime of the combustion runner.
7.5.6 Equipment configuration and TEG/combustion airflow straightening
The TEG/combustion air velocity profile at the duct burner plane must be within certain limits to ensure good combustion efficiency; in cogeneration applications, this is rarely achieved without flow-straightening devices. Even in nonfired configurations, it may be necessary to alter the velocity distribution to make efficient use of the boiler heat transfer surface. Fig. 7.8 shows a comparison of flow variation with and without flow straightening.

Duct burners are commonly mounted in the TEG duct upstream of the first bank of heat transfer tubes, or they may be nested in the boiler superheater between banks of tubes. In the former case, a straightening device would be mounted just upstream of the burner, while in the latter it is mounted either upstream of the first tube bank or between the first tube bank and (upstream of) the burner. Although not very common, some HRSG design configurations utilize two stages of duct burners with heat transfer tube banks in between, and a flow-straightening device upstream of the first burner. Such an arrangement is, however, problematic because the TEG downstream of the first-stage burner may not have the required combination of oxygen and temperature properties required for proper operation of the second-stage burner.
Perforated plates that extend across the entire duct cross section are most commonly used for flow straightening because experience has shown that they are less prone to mechanical failure than vane-type devices, even though they require a relatively high pressure drop. The pattern and size of perforations can be varied to achieve the desired distribution. Vanes can produce comparable results with significantly less pressure loss but require substantial structural reinforcement to withstand the high velocities, turbulence and flow-induced vibration inherent in HRSG systems. Regardless of the method used, flow pattern complexity—particularly in TEG applications—usually dictates the use of either physical or computational fluid dynamic (CFD) modeling for design optimization.
7.6 Physical modeling
TEG/airflow patterns are determined by inlet flow characteristics and duct geometry, and are subject to both position and time variation. Design of an efficient (low pressure loss) flow-straightening device is therefore not a trivial exercise, and manual computational methods are impractical. For this reason, physical models, commonly 1:6 or 1:10 scale, are constructed, and flow characteristics are analyzed by flowing air with smoke tracers or water with polymer beads through the model (see Fig. 7.9).

Although this method produces reliable results, tests conducted at ambient conditions (known as “cold flow”) are not capable of simulating the buoyant effects that may occur at elevated temperatures.
7.6.1 CFD modeling
Flow modeling with CFD, using a computer-generated drawing of the inlet duct geometry, is capable of predicting flow pattern and pressure drop in the turbine exhaust flow path. The model can account for swirl flow in three dimensions, accurately predict pressure drop, and subsequently help design a suitable device to provide uniform flow. The CFD model must be quite detailed to calculate flow patterns incident and through a perforated grid or tube bank while also keeping the overall model solution within reasonable computation time. Combustion effects can be included in the calculations at the cost of increased computation time. The biggest obstacle to obtaining a good CFD solution is the difficulty in obtaining good velocity and temperature profiles of the flow exiting the gas turbine.
CFD simulation has the capability to provide complete information, provided the aforementioned is true. The issue of validity has been a hot topic for years. A Department of Energy report [2] has cited CFD to be capable of
1. predicting catastrophic failure
2. qualitative trends and parametric analysis
4. predicting nonreacting gaseous flows
5. quantitative analysis of gas velocity and temperature patterns
6. qualitative analysis of radiation heat transfer
9. models of temperature and heat release patterns and qualitative trends associated with major species
10. integration of detailed burner codes with heating process
For combustion systems, CFD is the only general-purpose simulation model capable of modeling reacting flows in order to predict emissions, heat transfer, and other furnace parameters. Fig. 7.10 shows a sample result of CFD modeling performed on a HRSG inlet duct.
7.6.1.1 Wing geometry: variations
Flame holders
Design of the flame stabilizer, or flame holder, is critical to the success of supplementary firing. Effective emission control requires that the TEG be metered into the flame zone in the required ratio to create a combustible mixture and ensure that the combustion products do not escape before the reactions are complete. In response to new turbine and HRSG design requirements, each duct burner manufacturer has proprietary designs developed to provide the desired results.
Basic flame holder
In its basic form, a fuel injection system and a zone for mixing with oxidant are all that is required for combustion. For application to supplemental firing, the simple design shown in Fig. 7.11 consists of an internal manifold or “runner,” usually an alloy pipe with fuel injection orifices spaced along the length. A bluff body plate, with or without perforations, is attached to the pipe to protect the flame zone from the turbulence in the exhaust gas duct. The low-pressure zone pulls the flame back onto the manifold. This low-cost runner may overheat the manifold, causing distortion of the metallic parts. Emissions are unpredictable with changing geometry and CO is usually much higher than the current typically permitted levels of under 0.1 lb/MMBtu.
Low-emissions design
Modifications to the design for lower emission performance generally have a larger cross section in the plane normal to the exhaust flow. The increased blocked area protects the fuel injection zone and increases residence time. The NOx is reduced by the oxygen-depleted TEG and the CO/UHC is reduced by the delayed quenching. The correct flow rate of TEG is metered through the orifices in the flame holder, and the fuel injection velocity and direction are designed to enhance combustion efficiency. The flame zone is pushed away from the internal manifold (“runner” pipe), creating space for cooling TEG to bathe the runner and flame holder and enhance equipment life.
Each manufacturer approaches the geometry somewhat differently. One manufacturer uses cast alloy pieces welded together to provide the required blockage. These standard pieces often add significant weight and are difficult to customize to specific applications. Hot burning fuels, such as hydrogen, may not receive the cooling needed to protect the metal from oxidation. Alternately, fuels subject to cracking, such as propylene, may not have the oxygen needed to minimize coke buildup.
Another manufacturer supplies custom designs to accommodate velocity extremes, while maintaining low emissions. In the design shown in Fig. 7.12, the flame holder is optimized with CFD and research experimentation to enhance mixing and recirculation rate. Special construction materials are easily accommodated. This supplier also uses removable fuel tips with multiple orifices, which can be customized to counteract any unexpected TEG flow distribution discovered after commercial operation. Fig. 7.13 depicts the flow patterns of air/TEG and fuel in relation to the duct burner flame holder.
7.7 Emissions
Duct burner systems can either increase or reduce emissions from the generally large volume of mass flow at the input. Generally this flow includes particulates, NOx, CO, and a variety of HCs including a subset of HCs defined as VOCs (volatile organic compounds). VOCs are defined by the EPA (40 CFR 51.100, February 3, 1992) as “any compound of carbon, excluding carbon monoxide, carbon dioxide, carbonic acid, metallic carbides or ammonium carbonate, which participates in atmospheric chemical reaction.” Other compounds are also exempt such as methane, ethane, methylene chloride, methyl chloroform, and other minor chemicals.
To accurately predict emission, kinetic equations are created using first-order equations for oxidation in the general form of
(7.1)
where
(7.2)
A is the preexponential factor/frequency factor in appropriate units
R is the universal gas constant in appropriate units
7.7.1 Visible plumes
Stack plumes are caused by moisture and impurities in the exhaust. Emitted NO is colorless and odorless, and NO2 is brownish in color. If the NO2 level in the flue gas exceeds about 15–20 ppm, the plume will take on a brownish haze. NOx also reacts with water vapor to form nitrous and nitric acids. Sulfur in the fuel may oxidize to SO3 and condense in the stack effluent, causing a more persistent white plume.
7.7.2 NOx and NO versus NO2
Formation of NO and NO2 is the subject of ongoing research to understand the complex reactions. Potentially, several oxides of nitrogen (NOx) can be formed during the combustion process, but only nitric oxide (NO) and nitrogen dioxide (NO2) occur in significant quantities.
In the elevated temperatures found in the flame zone in a typical HRSG turbine exhaust duct, NO formation is favored almost exclusively over NO2 formation. Turbine exhaust NOx is typically 95% NO and 5% NO2. In the high-temperature zone, NO2 dissociates to NO by the mechanism of
However, after the TEG exits the hot zone and enters the cooling zone at the boiler tubes, reaction slows down and the NO2 is essentially fixed. At the stack outlet, the entrained NO is slowly oxidized to NO2 through a complex photochemical reaction with atmospheric oxygen. The plume will be colorless unless the NO2 increases to about 15 ppm, at which time a yellowish tint is visible. Care must be taken in duct burner design because NO can also be oxidized to NO2 in the immediate post-flame region by reactions with hydroperoxyl radicals:
if the flame is rapidly quenched. This quenching can occur because of the large quantity of excess TEG commonly present in duct burner applications. Conversion to NO2 may be even higher at fuel turndown conditions where the flame is smaller and colder. NO2 formed in this manner can contribute to “brown plume” problems and may even convert some of the turbine exhaust NO to NO2.
There are two principal mechanisms through which nitrogen oxides are formed:
1. Thermal NOx: The primary method is thermal oxidation of atmospheric nitrogen in the TEG. NOx formed in this way is called thermal NOx. As the temperature increases in the combustion zone and surrounding environment, increased amounts of N2 from the TEG are converted to NO. Thermal NOx formation is most predominant in the peak temperature zones of the flame.
2. Fuel-bound nitrogen NOx: The secondary method utilized to form NOx is the reaction of oxygen with chemically bound nitrogen compounds contained in the fuel. NOx formed in this manner is called fuel NOx. Large amounts of NOx can be formed by fuels that contain molecularly bound nitrogen (e.g., amines and mercaptans). If a gaseous fuel such as natural gas contains diluent N2, it simply behaves as atmospheric nitrogen and will form NOx only if it disassociates in the high-temperature areas. However, if the gaseous fuel contains, for example, ammonia (NH3), this nitrogen is considered bound. In the low concentrations typically found in gaseous fuels, the conversion to NOx is close to 100% and can have a major impact on NOx emissions.
Bound nitrogen in liquid fuel is contained in the long carbon chain molecules. Distillate oil is the most common oil fired in duct burners as a liquid fuel. The fuel-bound nitrogen content is usually low, in the range of 0.05 weight percent. Conversion to NOx is believed to be 80%–90%. For No. 6 oil, containing 0.30 weight percent nitrogen, the conversion rate to NOx would be about 50%. Other heavy waste oils or waste gases with high concentrations of various nitrogen compounds may add relatively high emissions. Consequently, fuel NOx can be a major source of nitrogen oxides and may predominate over thermal NOx.
The impact of temperature on NOx production in duct burners is not as pronounced as in, for example, fired heaters or package boilers. One reason is that both the bulk fired temperature and the adiabatic flame temperature are lower than in fired process equipment.
In the formation of NOx, the equations are similar to formation of thermal NOx and are presented as follows:
(7.3)
 (7.3)
(7.3)and
(7.4)
One generally accepted practice is to assume (O2) in equilibrium with (O) and (O2) concentration using the Westenberg [3] results for k0 for (O2) equilibrium and Zeldovich constants, A, E, as measured by Bowman [4].
When used to provide supplementary firing of turbine exhaust, duct burners are generally considered to be “low NOx” burners. Because the turbine exhaust contains reduced oxygen, the peak flame temperature is reduced and the reaction speed for O2 and N+ to form NOx is thus lowered. The burners also fire into much lower average bulk temperatures—usually less than 1600°F (870°C)—than process burners or fired boilers. The high-temperature zones in the duct burner flames are smaller due to large amounts of flame quenching by the excess TEG. Finally, mixing is rapid and therefore retention time in the high-temperature zone is very brief.
The same duct burner, when used to heat atmospheric air, is no longer considered “low NOx,” because the peak flame temperature approaches the adiabatic flame temperature in air.
Clearly, operating conditions have a major impact on NO formation during combustion. To properly assess NOx production levels, the overall operating regime must be considered, including TEG composition, fuel composition, duct firing temperature, and TEG flow distribution.
7.7.3 CO, UBHC, SOx, and particulates
7.7.3.1 Carbon monoxide
Carbon monoxide (CO), a product of incomplete combustion, has become a major permitting concern in gas turbine–based combined cycle and cogeneration plants. Generally, CO emissions from modern industrial and aeroderivative gas turbines are very low, in the range of a few parts per million (ppm). There are occasional situations in which CO emissions from the turbine increase due to high rates of water injection for NOx control or operation at partial load, but the primary concern is the sometimes-large CO contribution from supplementary firing. The same low-temperature combustion environment that suppresses NOx formation is obviously unfavorable for complete oxidation of CO to CO2. Increased CO is produced when fuels are combusted under fuel-rich conditions or when a flame is quenched before complete burnout. These conditions (see Fig. 7.14) can occur if there is poor distribution of TEG to the duct burner, which causes some burner elements to fire fuel-rich and others to fire fuel-lean, depending on the efficiency of the TEG distribution device. The factors affecting CO emissions include
• low TEG approach temperature
• flame quench on “cold” screen tubes
• improperly designed flame holders that allow flame quench by relatively cold TEG

For utilization, and performance prediction, kinetic data can be utilized from the literature. For instance, for CO destruction, several kinetic data are available such as [5]
(7.5)
Most published CO rates involve H2O because CO destruction requires the (OH)−1 radical to produce the reaction.
7.7.3.2 Unburned hydrocarbons
In the same fashion as carbon monoxide generation, UHCs are formed in the exhaust gas when fuel is burned without sufficient oxygen, or if the flame is quenched before combustion is complete. UHCs can consist of hydrocarbons (defined as any carbon–hydrogen molecule) of one carbon or multiple carbon atoms. The multiple carbon molecules are often referred to as long-chain hydrocarbons. UHCs are generally classified in two groups:
The reason for the distinction and greater concern for VOCs is that longer chain hydrocarbons play a greater role in the formation of photochemical smog. VOCs are usually defined as molecules of two carbons or greater, and are sometimes considered to be three carbons or greater. These definitions are set by local air quality control boards and vary across the United States.
UHCs can only be eliminated by correct combustion of the fuel. However, hydrocarbon compounds will always be present in trace quantities, regardless of how the HRSG system is operated.
For HC and VOC incineration several sources are available such as Barnes [6].
In general,
(7.6)
7.7.3.3 Sulfur dioxide
Sulfur dioxide (SO2) is a colorless gas that has a characteristic smell in concentrations as low as 1 ppm. SO2 is formed when sulfur (S) in the fuel combines with oxygen (O2) in the TEG. If oxygen is present (from excess of combustion) and the temperature is correct, the sulfur will further combine and be converted to sulfur trioxide (SO3). These oxides of sulfur are collectively known as SOx.
Except for sulfur compounds present in the incoming particulate matter (PM), all of the sulfur contained in the fuel is converted to SO2 or SO3. Sulfur dioxide will pass through the boiler system to eventually form the familiar “acid rain” unless a gas-side scrubbing plant is installed. Sulfur trioxide can, in the cooler stages of the gas path, combine with moisture in the exhaust gas to form sulfuric acid (H2SO4), which is highly corrosive and will be deposited in ducts and the economizer if the metal or exhaust gas is below condensing temperatures. Natural gas fuels are fortunately very low in sulfur and do not usually cause a problem. However, some oil fuels and plant gases can be troublesome in this respect.
7.7.3.4 Particulate matter
Particulate emissions are formed from three main sources: ash contained in liquid fuels, unburned carbon in gas or oil, and SO3. The total amount of particulate is often called TSP (total suspended particulate). There is concern for the smaller sized portion of the TSP, as this stays suspended in air for a longer period of time. The PM-10 is the portion of the total PM that is less than 10 μm (1×10−6 m) in size. Particles smaller than PM-10 are on the order of smoke. Typical NOx and CO emissions for various fuels are shown in Table 7.1.
Table 7.1
Typical NOx and CO emissions from duct burners
| Gas | NOx (lb/106 Btu fired) | CO (lb/106 Btu fired) |
| Natural gas | 0.1 | 0.08 |
| Hydrogen gas | 0.15 | 0.00 |
| Refinery gas | 0.1–0.15 | 0.03–0.08 |
| Plant gas | 0.11 | 0.04–0.01 |
| Flexicoker gas | 0.08 | 0.01 |
| Blast furnace gas | 0.03–0.05 | 0.12 |
| Producer gas | 0.05–0.1 | 0.08 |
| Syn fuels | 0.08–0.12 | 0.08 |
| Propane | 0.14 | 0.14 |
| Butane | 0.14 | 0.14 |
Note: NOx emissions from butane and propane can be modified by direct steam injection into a gas or burner flame. CO emissions are highly dependent on TEG approach temperature and HRSG fired temperature.
Source: Londerville, Stephen; Baukal Jr., Charles E. (Eds.), The Coen & Hamworthy Combustion Handbook: Fundamentals for Power, Marine & Industrial Applications, CRC Press, 2013.
For particulate oxidation, an equation can be developed from fundamental principles, utilizing a combination of diffusion of oxygen and surface reactivity as follows:
(7.7)
km is the diffusion coefficient of oxygen in nitrogen
kr is the reaction coefficient of the form Ae−E/RT,
where A is the frequency factor, E is the activation energy,
The equation can be integrated for constant density particles and using particle tracking in time steps with constant or varying oxygen and temperature. An excellent source of char rate data is available by Smith and Smoot [7].
Then, in all cases, one can postprocess thermal map data in some discrete volume form and/or insert into a CFD code using the Rayleigh flux theorem as follows:
(7.8)
where described in words, the formation of (n) through the volume surface is equal to the integrated rate of formation over the control volume.
It is a simple extrapolation to extend this concept for even coarse volumes as follows:
(7.9)
This method can be very useful for fully mixed downstream products even with coarse volumes. But one must be careful with coarse volumes to be sure that the temperature and concentrations are uniform.
7.8 Maintenance
1. Normal wear and tear: If nothing has been replaced in the past five years and the burner (or turbine/HRSG set) is operated fairly continuously, it is likely that some tips and wings may require replacement.
2. Damage due to misuse, system upsets, or poor maintenance practices: Older systems designed without sufficient safety interlocks (TEG trip, high temperature) sometimes expose parts to excessively high temperatures, which results in wing warpage and oxidation failure.
3. Fuel quality/composition: Some refinery fuels or waste fuels contain unsaturated components and/or liquid carryover. Eventually, these compounds will form solids in the runner pipes or directly in tips, which results in plugging.
The following are some items to consider when operational problems are encountered:
• Plugged gas ports: These are evidenced by gaps in the flame or high fuel pressure. Gas ports may simply consist of holes drilled into the element manifold pipe, or they may be located in individual removable tips. Designs of the former type may be redrilled or else the entire manifold pipe must be replaced. Discrete tips can be replaced individually as required.
• Warped flame holders (wings): Some warping is normal and will not affect flame quality, but excessive deformation such as “curling” around the gas ports will degrade the combustion and emission performance. Most grid-type burner designs permit replacement of individual flame holder segments.
• Oxidation of flame holders (wings) or portions of flame holders: If more than one-third of the flame holder is missing, it is a good candidate for replacement. Fabricated and cast designs are equally prone to oxidation over time. Most grid-type burner designs permit replacement of individual flame holder segments.
• Severe sagging of runner pipes (grid design only): If the manifold pipe is no longer supported at both ends, it should be replaced. Beyond that relatively extreme condition, sagging at midspan in excess of approximately 2–3 in. (5–7 cm) should be corrected by runner replacement and/or installation of an auxiliary support.
7.8.1 Accessories
7.8.1.1 Burner management system
All fuel-burning systems should incorporate controls that provide for safe manual light-off and shutdown, as well as automatic emergency shutdown upon detection of critical failures. Control logic may reside in a packaged flame safeguard module, a series of electromechanical relays, a programmable logic controller (PLC), or a distributed control system (DCS). At a minimum, the duct burner management system should include the following:
• flame supervision for each burner element
• proof of completed purge and TEG/combustion airflow before ignition can be initiated
• proof of pilot flame before main fuel can be activated
• automatic fuel cutoff upon detection of flame failure, loss of TEG/combustion air, and high or low fuel pressure
Other interlocks designed to protect downstream equipment can also be included, such as high boiler tube temperature or loss of feed water.
7.8.1.2 Fuel train
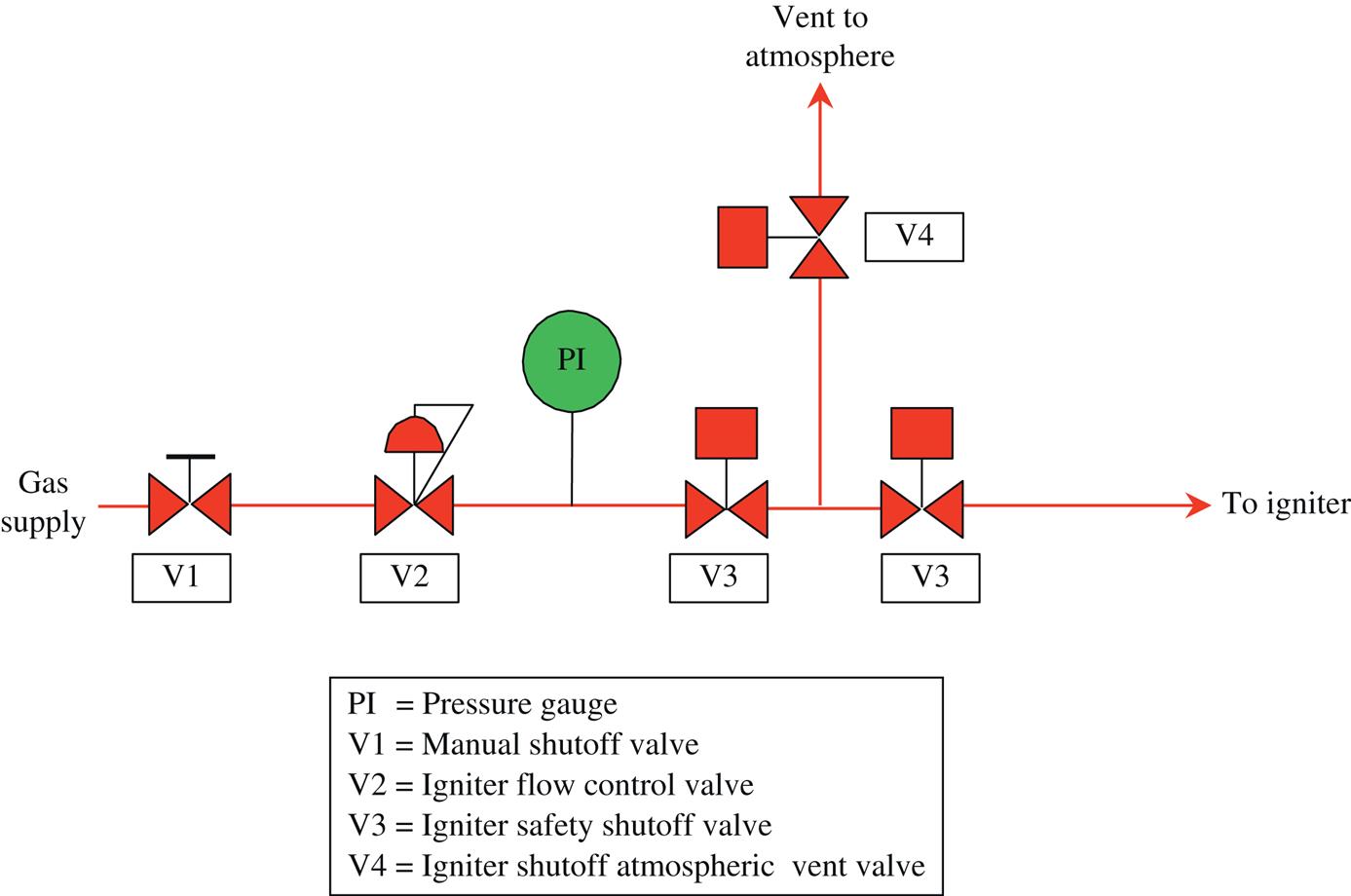
Fuel flow to the burners is controlled by a series of valves, safety devices, and interconnecting piping mounted on a structural steel rack or skid. A properly designed fuel train will include, at a minimum, the following:
• at least one manual block valve
• two automatic block valves in series
• one vent valve between the automatic block valves (gas firing only)
• high and low fuel pressure switches
• two pressure gauges, one each at the fuel inlet and outlet
Depending on the custom and operating requirements at a particular plant, pressure regulation, flow-measurement devices, and pressure transmitters can also be incorporated. See Figs. 7.15–7.22 for typical duct burner fuel system piping arrangements.





7.9 Design guidelines and codes
7.9.1 NFPA 8506 (National Fire Protection Association)
First issued in 1995, this standard has become the de facto guideline for HRSGs in the United States and many other countries that have not developed their own national standards. Specific requirements for burner safety systems are included, but as stated in the foreword, NFPA 8506 does not encompass specific hardware applications, nor should it be considered a “cookbook” for the design of a safe system. Prior to the issuance of NFPA 8506, designers often adapted NFPA boiler standards to HRSGs, which resulted in design inconsistencies.
7.9.2 Factory mutual
An insurance underwriter that publishes guidelines on combustion system design, Factory Mutual (FM) also “approves” specific components such as valves, pressure switches, and flame safeguard equipment that meet specific design and performance standards. Manufacturers are given permission to display the FM symbol on approved devices. Although FM approval may be required for an entire combustion control system, it is more common for designers to simply specify the use of FM-approved components.
7.9.3 Underwriters’ laboratories
Well known in the United States for its certification of a broad range of consumer and industrial electrical devices, Underwriters’ Laboratories (UL) authorizes manufacturers to display their label on specific items that have demonstrated compliance with UL standards. Combustion system designers will frequently require the use of UL-approved components in burner management systems and fuel trains. Approval can also be obtained for custom-designed control systems, although this requirement generally applies only to a few large cities and a few regions in the United States.
7.9.4 ANSI B31.1 and B31.3 (American National Standards Institute)
These codes address piping design and construction. B31.1 is incorporated in the NFPA 8506 guideline, while B31.3 is generally used only for refining/petrochemical applications.
7.9.5 Others
The following may also apply to duct burner system designs, depending on the country where equipment will be operated:












