Nanocomposites for food and beverage packaging
Abstract:
The use of polymer nanocomposite materials in food and beverage packaging offers many possibilities for improvements in barrier, mechanical and thermal properties and other important characteristics. As a result, there are already a number of commercial applications and, of particular note, an increasing interest in packaging from renewable materials based on biopolymer nanocomposites. This chapter provides a review of developments in petroleum-based polymer nanocomposites and summarizes the state-of-the-art in terms of R&D on biopolymer nanocomposites, including thermoplastic starch, polylactides, polyhydroxyalkanoates, chitosan, proteins or hemicelluloses in combination with various inorganic or organic nanofillers.
13.1 Introduction
By any measure, global food and beverage packaging is a large-scale business. According to the Helmut Kaiser Consultancy (http://www.hkc22.com), the industry has a value of US $100 billion worldwide and the packaging sector as a whole typically represents about 2% of the gross national product (GNP) in developed countries (Robertson, 2006). The research firm Euromonitor International (http://www.euromonitor.com) reported that the global retail beverage packaging market for 2009 comprised 943 billion containers, of which 34% were PET bottles, 28% glass containers and 26% metal cans, with the remaining 12% comprising other formats including aseptic cartons. Food and beverage packaging is said to represent 55-65% of the total US $130 billion packaging industry in the US (Brody, 2008). In April 2010, Beverage Industry magazine (http://www.bevindustry.com) credited the market research firm Nielsen with statistics showing that US carbonated soft drink sales had grown by 2.6% to US $18.8 billion amidst a global market of US $40 billion, although volumes had not increased. Figures from Plastics Europe (http://www.plasticseurope.org) show that 38% of all plastic production is destined for the packaging market, with roughly 40% of this total dedicated to food packaging. In this sector, plastic is used to package a very wide variety of foods including fruits and vegetables, grains, seeds and nuts, cereals, baked goods, confectionery, dairy products, meat, poultry and seafood. Packaging and plastic packaging in particular is, therefore, at the very heart of the worldwide food and beverage industry.
The convergence of nanocomposite technology and food packaging has been developing for some years and is now picking up pace, stimulated by the numerous packaging material improvements which are potentially available. These improvements include potential enhancements in the weight, strength, barrier and thermal attributes of plastic packaging films, while retaining film clarity and processability, thereby also providing opportunities for prolonged food packaging shelf life. Beyond these advances, smart nanocomposite technology may with further development offer counterfeit protection, antimicrobial protection or sensors which can signal food contamination or spoilage. According to a study from iRAP, Inc. (http://www.innoresearch.net), the total nano-enabled food and beverage packaging market was worth US $4.13 billion in 2008, was expected to reach US $4.21 billion in 2009 and to grow to US $7.3 billion by 2014. Active packaging, in the sense of oxygen scavenging, moisture absorption control and barrier enhancement, appears to be an essential component of the nanotechnology-based packaging market. Japan is said to be the leader with 45% of the global market and with most of the applications based on conventional plastics such as polypropylene (PP), polyethylene (PE), polystyrene (PS), polyamide (PA) and thermoplastic polyolefin (TPO). The Helmut Kaiser Consultancy has examined the impact of nanotechnology in the food and beverage packaging industry and has reported figures showing a rapid rise in nanorelated-packaging products worldwide from US $150 million in 2002 to US $860 million in 2004 and there are forecasts that nanotechnology could change 25% of the food packaging business over the next decade. Consequently, nano-enabled packaging has been attracting the attention of industry leaders, governments, universities and research institutes. Furthermore, global brand name companies such as Kraft, Henkel, Bayer, Kodak, Bud-weiser and Pepsi, to name just a few, are reported to have their own R&D projects targeting use of nanotechnology in food and beverage packaging.
13.2 Nanofillers and nanocomposites
In the context of food and beverage packaging, the use of nanotechnology to introduce material property improvements largely refers to the introduction of nanofillers to generate polymer nanocomposites. Nanofillers can be defined as particulate or fiber additives in which one or more dimensions are of the order of 100 nm or less. Similarly, nanocomposites can be defined as polymeric materials containing well-dispersed nanofillers in the form of particles, fibers or other elements, which exhibit dramatic improvement in a range of characteristics, including mechanical properties, barrier properties, thermal stability, and flame retardancy amongst others. Although, mineral nanofillers such as montmorillonite (MMT) clays have been of primary interest in terms of commercial adoption, other nanofillers such as layered double hydroxides (LDHs), nanocellulose, chitin whiskers and carbon nanotubes (CNTs) have also been widely investigated for general nanocomposite applications. A summary of nanofiller types and their characteristics is presented in Table 13.1.
Table 13.1
Nanofiller types and their characteristics
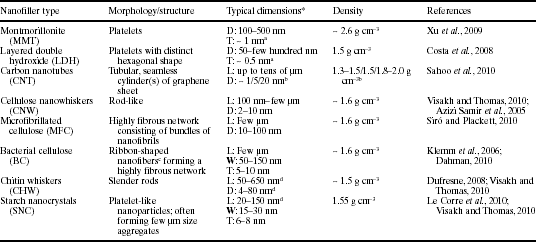
*L: length; W: width; T: thickness; D: diameter.
aThickness of a single platelet.
bFor single-, double- and multi-walled CNTs, respectively.
cAggregates of individual nanofibrils.
dDepending on raw material’s origin.
MMT clays for use in manufacturing polymer nanocomposites are available either in unmodified form or as various organomodified MMTs (OMMTs). Through modification using ion exchange processes, organic cations (e.g., from quaternary ammonium salts) are introduced in the clay galleries and replace the inorganic cations that are naturally present. The result is a modified clay with more hydrophobic character and a larger interlayer spacing, properties which are desirable for formation of well-dispersed clay nanocomposites based on commodity plastics. The nanos-tructure of an MMT clay is shown in Fig.13.1.The production,characterization and properties of polymer-clay nanocomposites have been covered extensively in previous publications (Utracki, 2004; Gupta et al., 2009; Pinnavaia and Beall, 2001; Usuki et al., 2005; Okamoto and Ray, 2004). The benefits of these materials rest largely upon processing into intercalated and/or exfoliated structures as illustrated in Fig. 13.2. Many authors argue that the best property enhancements are obtained in fully exfoliated products and, for example, the tortuous path theory has been used to explain increases in gas and water vapor barrier properties in nanocomposites (Fig. 13.3). Various models have been developed to allow prediction of polymer-clay nanocomposite properties as a function of clay content and distribution (Neilson, 1967; Fornes and Paul, 2003; Valavala and Odegard, 2005).
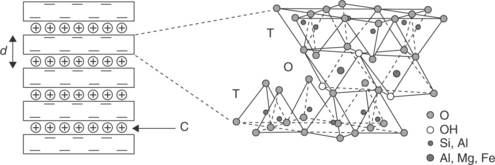
Fig. 13.1 Structure of a montmorillonite clay (C = cations, T = tetrahedra, O = octahedra, d = d-spacing).
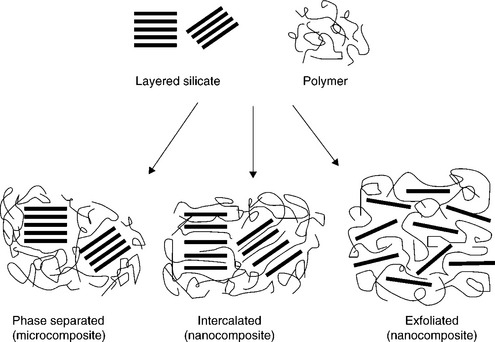
Fig. 13.2 Schematic showing the different morphologies for nanoclay dispersed in a polymer matrix. Reprinted with kind permission from Springer Science and Business Media: Polymer-silicate nanocomposites: Advances in Polymer Science, Model systems for confined polymers and polymer brushes. Vol. 138, 1999, page 108, Gianellis, E.P., Krishnamoorti, R. and Manias, E., Figure 1.
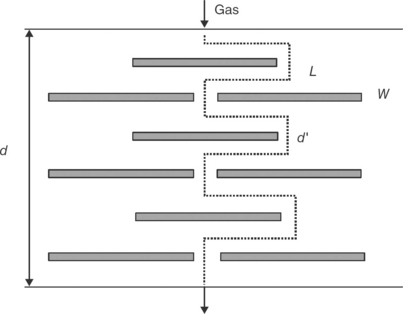
Fig. 13.3 Schematic of the tortuous path theory explaining how nanoparticles can influence gas transport through polymer membranes (d = membrane thickness, d' = length of gas diffusion pathway in the presence of clay nanoplatelets, L = clay nanoplatelet thickness, W = clay nanoplatelet width).
In addition to use of MMTs, layered double hydroxides (LDHs) are synthetic clays which have also been investigated for their potential use in nanocomposites. LDHs are composed of metal hydroxide layers based on M2 + and M3 + ions, typically containing Mg2 + and Al3 + or Mg2 + and Fe3 +. The best-known LDH compound is hydrotalcite, a double hydroxide of Mg and Al (Aisawa et al., 2002). The interlayer spaces in LDHs are typically occupied by inorganic anions such as CO32–, PO43– and NO3–, and organomodi-fied LDHs suitable for combining with hydrophobic plastics can be produced by one of several different mechanisms (e.g., direct synthesis, coprecipita-tion at constant pH, or calcination followed by mixing with an organomodi-fier in aqueous suspension). The crystal structure of an LDH is depicted in Fig. 13.4 and compared with that of brucite, a mineral form of magnesium hydroxide.
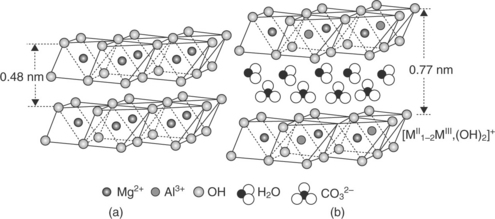
Fig. 13.4 Schematic representation comparing the structure of brucite (a), a common mineral form of magnesium hydroxide, and an LDH (b). Reprinted with kind permission from Springer Science and Business Media: Advances in Polymer Science, Layered double hydroxide based polymer nanocomposites, Vol. 210, 2007, page 105, Costa F.R., Saphiannikova, M. and Wagenknecht, U., Figure 1.
In addition to the use of inorganic clay nanofillers, there has been interest in nanocellulose as a bioderived reinforcing element in polymer films, especially in biopolymer matrices. Nanocellulose can take one of three main forms:
1. microfibrillated cellulose (MFC) – produced through high-shear homogenization of wood pulp,
2. cellulose whiskers–generated by acid hydrolysis of cellulose, and
3. bacterial cellulose–produced by certain strains of bacteria under specific culturing conditions.
A FEG-SEM (field emission gun–scanning electron microscopy) image of MFC, for example, is shown in Fig. 13.5. There has been considerable interest in nanocellulose as a material in new polymer nanocomposite packaging films with some promising results, but this technology awaits the availability of nanocellulose in larger than lab-scale quantities. Steps in the direction of nanocellulose production scale-up are presently in hand with an MFC pilot plant capable of producing a targeted 100 kg wet MFC/day (equivalent to 2000 litres/day at 2% concentration) now in operation at Innventia AB in Stockholm (Fig. 13.6) and a facility for manufacturing nanocrystalline cellulose being built by FP Innovations in Canada. Other nanofillers which have been studied for use in new forms of food packaging include CNTs and chitin nanowhiskers.
13.3 Current commercial application of nanocomposites in food and beverage packaging
It is fair to say that the extent of research on nanocomposites for food packaging still far outweighs the commercial adoption of these materials. However, there are some examples of materials already in the marketplace. Bayer Polymers (Pittsburgh, PA, USA) has introduced Durethan®, a polyamide containing layered silicate as nanofiller to provide gas and moisture barrier properties and enhanced food product shelf life (Anon, 2009). Nanocor (Arlington Heights, IL, USA) advertises the supply of nanocrys-tals which can be used to improve the barrier properties of plastic beer bottles and enhance the beverage shelf life. US Army research has led to scaled up production of ready-to-eat meal bags based on low-density polyethylene (LDPE) incorporating 7.5% layered silicate MMT nanofiller. At 6 mil (152 μm) thickness, this product has significantly better thermal, mechanical and barrier properties than either neat LDPE film of the same thickness or a currently produced meal bag based on 11 mil (280 μm) thick LDPE (National Nanotechnology Initiative, 2009).
Honeywell offers three grades of nanoclay-nylon 6 resins under the Aegis® trade name. These products have been used by the Anchor Brewing Company of San Francisco in polyethylene terephthalate (PET) beer bottles and specialty films. Nanocor's Nano-PA-6 was reported to be the first commercial plastic nanocomposite and has found use in flexible packaging such as multi-layer polyolefin/PA-6 cast and blown films, as well as in extrusion coating of paperboard to provide a barrier against moisture and oxygen (e.g., in packaging cartons for milk and juice). As well as barrier improvement, Nano-PA-6 provides improved stiffness and heat resistance. Other nanocomposite resins from Nanocor have been used in PET/multilayer bottles and in bottles for oxygen- and carbon dioxide-sensitive food products. In December 2009, the US Food and Drug Administration (FDA) approved a Nanocor MMT product surface treated with quaternary ammonium compounds (hydrogenated tallow alkyl)bis(hydroxyethyl)methyl chlorides or 1-octadecanaminium,N,N-bis(2-hydroxyethyl)-A-methyl chloride) for use as a gas barrier in mono-layer PET bottles. The products were approved for use at levels not to exceed 5 wt% in a blend with nylon MXD6 (a crystalline polyamide resin containing meta-xylylene groups and supplied by Mistsubishi Gas Chemical Co., Inc.). Finished articles may contact aqueous, acidic, and low and high alcoholic foods under certain specified conditions. The FDA database specifies cumulative estimated daily intake (CEDI) of MMT surface treated with (hydrogenated tallow alkyl) bis(hydroxyethyl)methyl chlorides as 0.000029 mg/kg/body weight/day. Nanocor has also developed Nanomer® I.35 L for compounding with EVOH which reduces the oxygen permeability by 66-80% within the 50-80% RH range. Nanomer® I.28MC is also available for compounding with nylon MXD6, which is potentially useful for manufacturing packaging for moisture- and oxygen-sensitive foods. Other suppliers of nanoclays for use in food and beverage packaging include Southern Clay Products, a subsidiary of Rockwood Specialties Inc., NanoBioMatters S.L., a Spanish company specializing in nanoclay-based additives, Laviosa Chimica Mineraria in Italy and FCC Inc. in China, amongst others.
In terms of manufacturing, although many literature reports are based on solution-cast materials, polymer nanocomposites can generally be manufactured by one of two commercial methods; first, by direct addition of the nanofiller to the polymer in a melt extrusion process and, second, by an in-situ polymerization technique in which the appropriate monomer and nanofiller are mixed and then the polymer is synthesized. In the direct mixing approach, optimum nanofiller dispersion is obtained when it is introduced to the polymer melt in the extruder barrel; however, the in-situ technique is said to provide better nanofiller dispersion. Where possible, the production of polymer nanocomposite masterbatches in dedicated facilities could have significant advantages in terms of handling and safety, especially considering materials for food and beverage packaging.
13.4 State-of-the-art nanocomposites
The state of development of nanocomposites in food and beverage packaging has been discussed in a number of recent papers, some of which have been written in the context of nanotechnology as a whole (Brody, 2006; Graveland-Bikkerand de Kruif, 2006; Chaudhry et al., 2008; de Azeredo, 2009; Sozer and Kokini, 2009; Arora and Padua, 2010; Sekhon, 2010). In this section, research on polymer-clay nanocomposites for packaging uses is discussed by polymer type; first, by looking at synthetic petroleum-derived polymers and, second, by considering bio-derived polymer-based nanocomposites.
13.4.1 Synthetic polymer-clay nanocomposites
As discussed already, the production of polymer-clay nanocomposites can provide materials with better mechanical, thermal and barrier properties. Developments in this direction since the 1990s have led to new packaging products based on PA or PET. These were logical choices since OMMT/PA compatibility has long been established and PET has been in widespread use in packaging films and bottles for some decades. PET-based nanocomposites, for instance, are designed to improve the shortcomings of PET such as low thermal distortion temperature and low elastic modulus, as well as to improve the barrier properties. A common feature of nanocomposite technology is that significant property improvements are obtained at levels of filler addition much lower than could be expected on the basis of traditional micro- or macrocomposite theory, typically in the 2–8 wt% range. For example, the Young's modulus and tensile strength of Nylon-6 were increased by 103% and 49% respectively at these low loading levels (Kojima et al., 1993). Similarly, barrier property improvements in Nylon-6 have been reported by Akkapeddi et al. (2003) who used the in-situ polymerization method.
With regard to PET/OMMT nanocomposites, oxygen permeability reductions of 30-50% with 3-5% nanofiller loadings have been reported (Ke and Yongping, 2005; Sanchez-Garcia et al. 2007; Frounchi and Dour-bash, 2009). However, a 94% reduction in oxygen permeability has been achieved in PET/5% OMMT nanocomposites prepared by in-situ polymerization (Choi et al. 2006) and a reduction in water vapor permeability by up to 80% was recently demonstrated (Greco et al., 2010).
Research on PE, one of the most common synthetic polymers used for food packaging, has confirmed the benefits of nanofiller addition in respect to improvements in mechanical, thermal and barrier properties. For example, a systematic study on blown film PE nanocomposites by Golebiewski et al. (2008) showed that numerous factors, including the type of MMT and the type of compatibilizer, as well as the extrusion and film blowing process parameters, could significantly influence final product properties. In particular, the oxygen permeability of blown PE films clearly depended on the orientation of the nanoclay platelets and their degree of dispersion. These authors found that, for the best PE nanocomposite films, the oxygen permeability was a factor of 2.5 times less than that of unfilled LDPE. Similarly, the addition of 3 wt% organoclay into a LDPE/LLDPE blend gave a 50% reduction in oxygen permeability (Dadbin et al., 2008). In contrast, in another study a reduction of only 20% in oxygen permeability was achieved in HDPE/clay nanocomposites, despite the use of an ultrasonic treatment to enhance intercalation (Swain and Isayev, 2007). Reports on the use of compatibilizers in combination with clay additives provide varying accounts in terms of oxygen permeability results, including limited or no reduction (Zhong et al., 2007; Picard et al., 2007) or even an in increase in oxygen permeability compared to the neat polymer (Zhong et al., 2007; Marini et al., 2010).
Mechanical properties may be significantly influenced by nanofiller addition. For instance, Guo et al. (2009) prepared LLDPE/MMT nanocomposites via ethylene copolymerization with in situ-generated α-olefins at high pressures. All of the as-prepared PE/MMT nanocomposites showed a significant enhancement in tensile strength and Young's modulus while elongation at break decreased. Other researchers have shown that addition of nanoclays can result in increased photo-oxidative degradation of LDPE (Reddy et al., 2009; Kumanayaka et al., 2010), which has been linked to the presence of Fe in MMT. The decomposition of alkyl ammonium ions in OMMT may create olefinic and acidic sites on the clay surface which could in turn accelerate radical formation in the PE matrix. In research on poly-vinyl chloride nanocomposites, Yoo et al. (2004) observed that thermal stability and dimensional stability of PVC could be improved by addition of organically modified clays.
In addition to the use of MMTs, the application of other nanofillers such as CNTs (Noroozi and Zebarjad, 2010; Pucciariello et al., 2011), cellulose nanowhiskers (CNWs) (Junior de Menezes et al., 2009) and MFC (Wang and Sain, 2007) has been explored as a way of obtaining improved thermo-mechanical properties in PE and hence opening up other food packaging applications.
In summary, although the properties of synthetic polymers are generally adequate for current types of food and beverage packaging, improvements through nanocomposite technology have the potential to upgrade product performance and may broaden the areas of application.
13.4.2 Biodegradable polymer nanocomposites
In recent years there has been increasing interest in and commercial development of bio-based plastics for use in the food and beverage packaging industry. Most of the plastics that fall into this category are also biodegradable, although this is not the case for sugar-cane derived bio-PE, a pilot plant for which was started up recently in Brazil (Morschbacker, 2009, 2010) and on which Tetra Pak (Lund, Sweden) and other international brand name companies are currently running trials.
There are multiple reasons for the interest in bio-based packaging and these include: reduced dependence on fossil fuel resources, completion of the carbon cycle, fit with the cradle-to-cradle (C2C) concept, reduced greenhouse gas emissions and, providing suitable collection systems are in place, reduction in plastics waste disposal. Governments are introducing regulations which are driving the industry towards more sustainable packaging and there is also the element of consumer demand. The bioplastics industry continues to grow on the back of increased need for such products in packaging and also in textiles, clothing, composite materials, electronics and other consumer goods.
Although bioplastics already have a significant profile in packaging, further uptake would be stimulated if film properties could be improved (e.g., mechanical, barrier, thermal) and hence there has been much interest in producing and evaluating bioplastic nanocomposites. In this section we look at the status in terms of individual bioplastic types. A number of review articles in this field have recently been published (Ray et al., 2006; Rhim and Ng, 2007; Sorrentino et al., 2007; Brody et al., 2008; Nanda et al., 2009; Lagarón and López-Rubio, 2010).
Starch/clay nanocomposites
Starch has a number of attractive features as a substrate for bioplastics. It is readily available in large quantities from a wide variety of plant sources, inexpensive, completely degradable in soil and water and, although not suitable itself as a material for film formation, may be plasticized by addition of glycols (e.g., glycerol, sorbitol), polyethers, urea or water. The application of heat and mechanical energy in an extruder then allows the production of thermoplastic starch (TPS). The development of starch plastics and opportunities for their use in packaging have been discussed in a number of articles (Bastioli, 2001; Roper and Koch, 1990; Lorcks, 1998; Petersen et al., 1999; Kim and Pomietto III, 1994). Examples include starch-based absorbent pads for meat exudation, films or bags for packaging of fresh fruits and vegetables and packages for dry products such as nuts and grains. However, TPS suffers from poor mechanical properties as well as sensitivity to moisture and therefore ways to improve these and other properties have been sought, including the formation of starch nanocomposites (Avella et al., 2005; Sorrentino et al., 2007). Numerous authors have reported specific studies on starch-nanoclay combinations for packaging applications (de Carvalho et al., 2001; Park et al., 2002; McGlashan and Halley, 2003; Wilhelm et al., 2003; Chen and Evans, 2005; Yoon and Deng, 2006).
Recently, Ren et al. (2009) examined carbamide-ethanolamine-plasticized starch/OMMT combinations which were compounded using a twin-screw extruder and then hot-pressed into 2 mm thick sheets. Film tensile strength and Young's modulus increased and elongation at break decreased with increasing OMMT content in the range 2–8 wt%. Good dispersion of OMMT was observed using x-ray diffraction (XRD) and scanning electron microscopy (SEM). The use of chitosan-modified MMT has been investigated by mixing this additive with an aqueous suspension of gelatinized starch granules, subsequent mixing with plasticizer and hot pressing. In this case, strength and modulus increased without a decrease in elongation at break (Chung et al., 2010). These researchers concluded that the formation of clay nanocomposites could improve the stability of starch-based products in transportation and storage. Avella et al. (2005) produced potato starch-clay nanocomposite material using a twin-screw extruder and then cast films. As in other studies, good intercalation of the polymer into the clay galleries and enhanced mechanical properties were observed. Migration tests and tests using direct contact with vegetables showed that the samples conformed with regulations and European directives on biodegradable materials (Avella et al., 2005). In an investigation by Park et al. (2003) a comparison was made between two melt-intercalated starch-clay nanocomposites containing either a commercial OMMT or an unmodified MMT, both from Southern Clay Products (Gonzales, TX, USA). TPS was prepared from potato starch after gelatiniza-tion and plasticization with glycerol and water and then injection molded into test material after compounding on a roller mixer. The starch/unmodified MMT material gave the best mechanical strength, thermal stability and barrier to water vapor, which was attributed to better nano-scale dispersion of the nanoclay in this case.
Starch nanocomposites with other nanofillers
Researchers have sought to develop new starch-based nanomaterials through the use of fully bio-derived nanofillers, especially those based on cellulose, starch nanocrystals and chitin. As indicated earlier, nanocellulose is available in three forms and each one of these has been studied in terms of starch reinforcement. For example, Plackett et al. (2010) used two types of MFC in combination with amylopectin and showed that the resulting changes in film mechanical properties depended on MFC type. Of particular note, pure MFC films exhibited rather low oxygen permeability which was comparable with that of high barrier films such as ethylene vinyl alcohol (EVOH). However, such films are also very brittle and, without modification, are not directly suitable for use in packaging. In a recent fundamental research investigation, the feasibility of producing nanocellulose-reinforced starch materials in situ was examined by culturing bacterial cellulose in the presence of partially gelatinized corn or potato starch. The resulting gels were then hot pressed into sheets containing up to 90 wt% bacterial cellulose. As shown by XRD and Fourier Transform Infra-red (FTIR) spec-troscopy, cellulose crystallinity was maintained and hence the mechanical properties of the nanocomposites were similar to those of pure bacterial cellulose (Grande et al., 2009). Svagan et al. (2009) prepared starch films containing up to 70 wt% MFC. At the highest loading, moisture uptake was half that of pure plasticized starch films and moisture diffusivity was found to decrease with increasing nanofiber content. This latter effect could be modeled on the basis of a dense composite structure oriented in the plane of the film, but effects such as reduced amylopectin molecular mobility due to cellulose-starch molecular interactions and constraints on swelling imposed by the high modulus/hydrogen-bonded cellulose network were also suggested as factors reducing moisture diffusivity.
Cellulose whiskers have also been extracted from tunicates and examined as potential bio-derived nanofiller in plastics. Tunicates are oceanic filter feeders and are thought to be the only animal species which produces cellulose. In this case, cellulose microfibrils are large in width and highly crystalline (Kimura and Itoh, 2007). Cellulose from tunicates has been combined with glycerol-plasticized starch by a simple mixing procedure and the resulting films then analyzed (Angles and Dufresne, 2000). The added plasticizers, glycerol and water were found to migrate towards the cellulose surfaces. The result was an enhanced tendency for amylopectin to crystallize, leading to the formation of a transcrystalline zone around the whiskers. The authors assigned reduced water uptake in the cellulose-starch nano-composites, as a function of increasing cellulose content, to the increasingly restricted mobility of amylopectin chains. The interesting possibility of a synergistic effect between MFC and an organo-modified MMT in starch films has been evaluated by Nordqvist et al. (2009). A specific combination of clay, organomodifier and mixing temperature, as well as heat treatment, gave a good combination of high stiffness, strength and extensibility. However, in terms of mechanical properties, no synergistic effect was found between MFC and MMT as nanoadditives.
The concept of an all-starch nanocomposite was investigated by Garcia et al. (2009) who reinforced cassava starch with waxy starch nanocrystals. Transmission electron microscopy (TEM) was used to visualize the nanoc-rystals and SEM was employed to examine film fracture surfaces. Films containing the starch nanocrystals took up more water after conditioning at 98% RH and 25 °C. Thermal analysis pointed to greater association between glycerol plasticizer and OH groups on the starch nanocrystals, leading to a greater availability for interaction with water. In contrast, water vapor permeability was reduced by about 40% when nanocrystals were present at 2.5 wt%, which was attributed to a longer tortuous path for water molecules moving across the films.
Chang et al. (2010) investigated chitin nanoparticle reinforcement of solvent-cast glycerol-plasticized starch films and found good dispersion and interaction between filler and matrix at loadings of 5 wt% or less. Improvements in tensile strength, storage modulus, glass transition temperature (Tg) and water vapor barrier were achieved.
Polylactide-clay nanocomposites
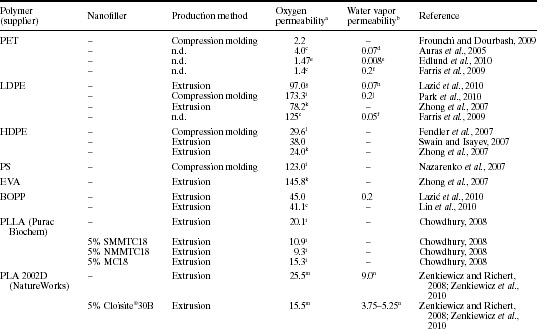
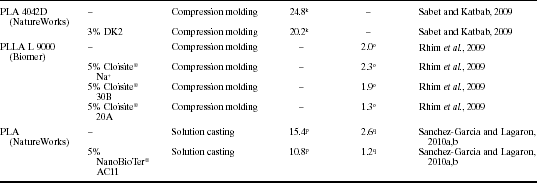
Polylactide (PLA) has received considerable attention as a sustainable, compostable bioplastic with good mechanical and optical properties. Commercially available PLA is also now in use in some areas of the food packaging industry; however, wider use is hampered by cost and a lack of certain technical properties. As noted by others, it should be possible to produce PLA with barrier properties for food packaging applications (Sinclair, 1996; Thelen et al., 2005). There has therefore been a significant history of research on PLA-clay nanocomposites, which is continuing to this day. These investigations have covered the full spectrum of preparation methods (e.g., solvent casting, melt compounding, melt extrusion), characterization methods (e.g., XRD, SEM, TEM, FTIR) and properties (e.g., mechanical, thermal, barrier) (Maiti et al., 2002; Pluta et al., 2002; Ray et al., 2002; 2003a; 2003b; 2003c; 2003d; 2003e; Ray and Okamoto, 2003; Chow and Lok, 2009; Fukushima et al., 2009; Ozkoc and Kemaloglu, 2009). The use of organo-modified clays to improve barrier properties has been a particular focus since, as illustrated in Table 13.2, significant reductions in oxygen and water vapor permeability would be desirable in order for PLA to compete with synthetic plastics in a range of food packaging uses. With good nanoclay dispersion, an increase in barrier properties of ~ 50% or more is feasible (Cabedo et al., 2006).
Table 13.2
Oxygen and water vapor permeability of PLA, PLA-based nanocomposites and selected petroleum-based polymers. Values reported in the original publications have been transferred into common units
amL mm m1– 2 day− 1 atm− 1.
bg mm m− 2 day− 1.
cat 23 °C and 0% RH.
dat 37.8 °C and 100% RH.
eat 23 °C and 50% RH.
fat 23 °C and 90% RH.
gat 23 °C.
hASTM E-96-67.
iat 25 °C and 0% RH.
jat 25 °C and 100% RH.
kASTM D 1434-82.
lat 25 °C and 80% RH.
mStandard PN-EN ISO 2556.
nStandard PN-EN ISO 15106-1.
o25 °C and 50% RH.
p24 °C and 80% RH.
q24 °C and 40% RH.
As a general rule, changes in mechanical properties, increased heat distortion temperature and higher barrier properties can be expected in PLA-organoclay composite materials. Researchers have also reported increased rates of PLA crystallization and biodegradation in the presence of incorporated nanoclays (Ray et al., 2003d). However, the results obtained in any particular case are very dependent on clay type, organomodifier chemistry and processing parameters (e.g., extruder screw geometry and barrel length/ diameter ratio). XRD and TEM are widely used to characterize PLA-clay nanocomposites, as seen, for example, by the TEM illustration of nanoclay dispersion in a PLA matrix shown in Fig.13.7.
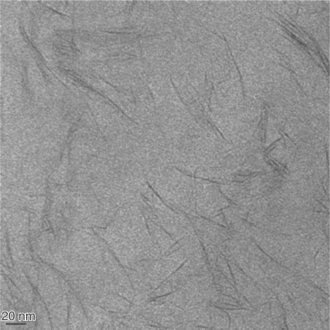
Fig.13.7 A transmission electron microscopy (TEM) image of Cloisite® 30B organomodified montmorillonite platelets dispersed in a melt-processed PLA film. The scale bar at lower left in the image represents 20 nm and gives an indication of the dimensions of the platelets. The photograph was obtained by Dr Vimal Katiyar of DTU using a Tecnai 12 TEM (FEI Company, Hillsboro, OR, USA) located at the Centre for Electron Nanoscopy, Technical University of Denmark (CEN-DTU).
As well as studies on MMT and other clays for fabrication of PLA nano-composites, LDHs have also been investigated. These synthetic clays offer the advantage that they can be custom-synthesized and therefore the trace metals that are present in MMTs may be avoided. More uniform platelet size may also be an advantage in terms of processing and ultimate properties. LDHs can be organomodified in a number of ways to increase inter-layer spacing and introduce hydrophobicity for better compatibility with bioplastics such as PLA. In a recent example of research on PLA-LDH combinations, Mahboobeh et al. (2010) used stearate-modified LDH to introduce flexibility improvements. In this work, the organomodified LDH was produced by a co-precipitation method from solutions of magnesium and aluminum nitrates and then PLA nanocomposite films were produced by mixing and solvent casting. Evidence for LDH organomodification was found from XRD analysis, which showed that the basal spacing of the synthetic clay was increased from 8.72 to 31.68 A. When incorporated at 1 wt%, the introduction of the stearate-modified LDH dramatically improved PLA elongation at break, while tensile strength and modulus were similar to those of unfilled PLA. Other researchers have explored preparation of PLA-organomodified LDH masterbatches and subsequent melt processing; however, the results were complicated by significant degradation of PLA during processing (Katiyar et al., 2010, 2011).
Polylactide nanocomposites with other nanofillers
Researchers have sought to produce fully bio-based PLA nanocomposites incorporating renewable nanofillers such as cellulose as a means of obtaining improved PLA plastics for food packaging. In this case, there is an additional challenge due to the semi-hydrophobic nature of PLA and therefore an expected lack of compatibility with nanocellulose. However, this has not inhibited researchers from pursuing PLA/unmodified nanocellulose combinations, either by solvent-casting or by melt extrusion processes. For example, Nakagaito et al. (2009) formed papers from a PLA fiber suspension, added MFC in a papermaking-like process and then hot-pressed the products into thick films. Excellent reinforcement was achieved with significant increases in tensile strength, modulus and elongation at break as a function of MFC loading in the 10–90 wt% range. Good reinforcement of PLA has also been obtained by dispersing MFC in excess acetone, adding dissolved PLA, evaporating the solvent and then kneading the residue in a twin rotary roll mixer, followed by compounding at 140 °C (Iwatake et al., 2008). Mathew et al. (2006) produced cellulose microfibers by a refining and cryo-crushing process and then combined the resulting material with PLA in a co-rotating extruder. Although the problem of removing steam during melt processing was solved, there was no positive effect on mechanical properties, which was attributed to poor dispersion of the cellulose microfi-bers. In a recent investigation, a chloroform solvent-casting method was employed to generate PLA films containing 1-5% CNWs. The CNWs were prepared by acid hydrolysis of highly purified α-cellulose microfibers and were found to have lengths of 60–160 nm and thicknesses of 10–20 nm. The addition of CNWs had a number of positive effects on film properties, most notably through reducing water vapor permeability by up to 82% and oxygen permeability by up to 90% (Sanchez-Garcia and Lagaron, 2010a). Optimum barrier enhancement was found with a CNW addition level of 3 wt% and the researchers concluded that CNWs show significant potential for use in coatings, membranes and food agro-based packaging applications.
Some researchers have investigated the idea of using modified nanocel-lulose to obtain better interfacial compatibility with PLA. For example, Tingaut et al. (2010) were able to produce bionanocomposites with tunable properties by conducting acetylation of MFC, which resulted in a dramatic improvement in the dispersability of the powdered nanofibers in chloroform, a solvent for PLA with low polarity. Improved compatibility at the interface between PLA and the chemically modified MFC was illustrated by DSC results which showed a Tg increase when incorporating 8.5% acetylated MFC at 17 wt% in PLA.
In another study, Bondeson and Oksman (2007) explored the use of polyvinyl alcohol (PVOH) as an additive to improve the dispersion of cellulose nanocrystals in a PLA matrix. Two methods were examined for combining the materials, one in which cellulose nanocrystals and PVOH were dry-blended with PLA before extrusion and a second, in which the additives were pumped as a suspension into the extruder. However, phase separation was observed and the cellulose nanocrystals were mostly located in the discontinuous PVOH phase. Consequently, thermal properties were not improved and mechanical properties were improved only slightly, probably because cellulose reinforced the PVOH phase and not the PLA matrix. In a recent study, Pei et al. (2010) explored the use of cellulose nanocrystals, derived from cotton by acid hydrolysis, as a route to bio-based nucleation and accelerated crystallization in PLA. In this work, cellulose nanocrystals were solvent-exchanged into acetone and then into dry toluene, from which they were precipitated, and then reacted with n-dodecyldimethylchlorosilane in the presence of imidazole as a trapping agent for hydrochloric acid by-product. The grafting reaction was carried out at room temperature for 12 hours before washing the reaction product to remove excess silylating agent. Solvent-cast PLA films containing the modified cellulose nanocrystals were then characterized by FTIR, DSC, polarized optical microscopy and mechanical testing. The silylated cellulose nanocrystals were well-dispersed in the PLA matrix and, as a result, the tensile strength and tensile modulus were improved by more than 20% relative to unfilled PLA.
As well as investigations on cellulose-based nanofillers, researchers have also examined combinations of PLA with CNTs or chitin nanowhiskers. The effect of functionalized CNTs on the mechanical properties of PLA has been highlighted in recent reports (Pillai et al., 2010; Yoon et al., 2010). Although these studies have not been directed at packaging applications per se, they do indicate another possibility using nanotechnology to tune mechanical properties according to requirements. Another approach which, like the use of nanocellulose, is based on the fully bio-derived nanofiller concept, was explored by Rizvi et al. (2009) and involved melt compounding of PLA with chitin whiskers produced by an acid hydrolysis process. In this case, the addition of chitin was found to increase PLA crystallinity but decreased its thermal stability.
Polyhydroxyalkanoate-clay nanocomposites
Polyhydroxyalkanoates (PHAs) generated in situ by bacteria under controlled growth conditions have been at the center of considerable attention for several decades as biopolymers which might be commercialized for food packaging applications. Although originally observed in the laboratory as early as the 1920s, it was not until the 1950s that the chemical nature of PHAs was really understood and the 1960s before commercial developments started. The interest in using PHAs as food packaging materials has continued to the present day (Plackett and Siró, 2011). The commercial use of PHAs such as polyhydroxybutyrate (PHB) or polyhydroxybutyrate-co-valerate (PHBV) in commodities such as food packaging is now being stimulated by the establishment of scaled-up production facilities by Metab-olix and Archer Daniels Midland (ADM) in the US and by other companies elsewhere. In order to produce PHA films with better mechanical and barrier properties, a number of studies have been conducted on PHA-OMMT or PHA-LDH nanocomposites, either by solution casting or by melt processing (Lim et al., 2003; Bruzaud and Bourmaud, 2007; Hsu et al., 2007; Maiti et al., 2007; Parulekar et al., 2007; Bordes et al., 2008; 2009a; Bruno et al., 2008; Hablot et al., 2008; Sanchez-Garcia et al., 2008; Wu et al., 2008; Cabedo et al., 2009; Ohashi et al., 2009; Botana et al., 2010; Erceg et al., 2010). Full nanofiller exfoliation has not been reported, although the beginning of clay exfoliation has been seen in a few studies (Bordes et al., 2008).
One of the drawbacks of PHB as a candidate material for food packaging is its lack of flexibility. For this reason, there has been particular interest in copolymers (e.g., PHBV, Nodax™ copolymers from Meredian, Inc.) which display better properties in this respect. The properties and applications of Nodax™ copolymers have recently been summarized by Noda et al. (2010). The addition of clay nanofillers, which generally leads to an increase in polymer stiffness, has therefore to be carefully considered. Another disadvantage of PHAs is their relative heat sensitivity and some attention has consequently been paid to this aspect when processing PHA nanocomposites (Cabedo et al., 2009; Hablot et al., 2008; Bordes et al., 2009b). PHAs generally have relatively good gas and water vapor barrier properties (Miguel and Iruin, 1999; Kuusipalo 2000a; 2000b; Cyras et al., 2007) and probably as a result there have been a rather limited number of studies on PHA nanocomposites for packaging uses. Sanchez-Garcia et al. (2008) compared the thermal and barrier properties of organically modified kaolinite and OMMT in PHB-based nanocomposites, using polycaprolactone (PCL) as a plasticizing additive. Reduction in oxygen permeability under dry conditions by up to 43% was reported when 4 wt% nanoclay was used. In an earlier report from the same laboratory, 5% OMMT addition resulted in a 20-27% reduction in oxygen permeability (Sanchez-Garcia et al., 2007).
Polyhydroxyalkanoate nanocomposites with other nanofillers
There have been relatively few reports on the exploration of organic nano-fillers in PHAs. Dufresne et al. (1999) used hydrolyzed tunicin whiskers to reinforce PHAs and observed formation of a transcrystalline network at the interface between the cellulose whiskers and the polymer matrix, as well as significant enhancement in mechanical properties. Jiang et al. (2008) prepared CNW/PHBV nanocomposites by solution casting and also by extrusion blending, followed by injection molding of PHBV with freeze-dried nanowhiskers. Improvements in mechanical properties were found in solvent-cast composites but the formation of agglomerates during freeze drying resulted in a strength reduction in the melt-processed materials.
There have been a number of other studies on mixing of PHAs with nanocellulose or CNTs to generate nanocomposites. For example, Dufresne and Samain (1998) used a latex of poly(3-hydroxyoctanoate) (PHO) and combined it with hydrolyzed cellulose to obtain PHO/cellulose whisker nanocomposites with enhanced mechanical properties. Sanchez-Garcia et al. (2009) prepared and characterized solvent-cast PHBV films containing either CNTs or carbon nanofibers and found reductions in oxygen permeability at 80% RH of 33% and 50% respectively at 10% nanofiller loadings. Interestingly, the reduction in oxygen permeability was ~ 63% when the CNT loading was 1 wt%, presumably due to agglomeration of the nano-tubes at higher loadings. The authors concluded that these findings, combined with significant increases in Young's modulus and yield stress, indicated future possible applications in food and beverage packaging as well as in medicine, public transportation and engineering uses.
Chitosan-based nanocomposites
Chitosan derived from chitin, an abundant polysaccharide obtained commercially from the shells of crustaceans such as shrimps, possesses a unique cationic nature when compared to other neutral or negatively charged polysaccharides (Ravi Kumar, 2000; Xu et al., 2006). Commercial chitosan products have been used in dietary products, biomedical applications, cosmetics, and wastewater treatment, to name but a few uses, and food packaging has also been explored due to the excellent film-forming capacity, biocompatibility, biodegradability and non-toxicity of this biopolymer. Chi-tosan also has the additional benefit of antimicrobial activity (Ravi Kumar, 2000; Li et al., 2009). However, crucially for food packaging considerations, chitosan films exhibit poor mechanical properties, low thermal stability and poor water resistance. Numerous studies have therefore been carried out with the aim of improving the properties of chitosan film using nanoclays or organic nanofillers and elucidating the structural properties of chitosan nanocomposites. As with other nanocomposite materials, successful improvements significantly depend upon the preparation method and the state of nanofiller dispersion.
In a study by Rhim et al. (2006), four different types of chitosan-based nanocomposite films were prepared using a solvent-casting method and incorporating an unmodified montmorillonite (Na-MMT), an OMMT (Cloisite® 30B), a nano-silver and an Ag-zeolite (Ag-Ion®). A certain degree of intercalation was observed, with the best results obtained when using Na-MMT, followed by Cloisite® 30B and Ag-Ion®. Film tensile strength increased by 7-16% and water vapor permeability decreased by 25-30%, depending on the particular nanofiller. In addition, chitosan-based nanocomposite films showed a promising range of antimicrobial activity.
Xu et al. (2006) reported both exfoliated and intercalated structures when Na-MMT was added to a chitosan matrix at loadings up to 5 wt%. A micro-scale composite material containing tactoids was, however, formed when the organomodified Cloisite® 30B was added (Xu et al., 2006), which is perhaps not surprising given that this commercial MMT is designed for improved compatibility with hydrophobic or partly hydrophobic polymer systems.
Chitosan/MMT nanocomposite materials with improved mechanical properties have been produced by a two-step dispersion procedure including mechanical stirring and ultrasonic agitation (Paluszkiewicz et al., 2010). In this work, an FTIR technique was useful for explaining local changes in the chemical structure of the chitosan/MMT nanocomposites.
The effect of shear rate during homogenization on the mechanical, barrier, and structural properties of Na-MMT/chitosan films (1–11 wt% Na-MMT loading) was examined by Hong et al. (2011). These authors reported the coexistence of intercalation, exfoliation and tactoids, which was supported by XRD and TEM observations. Oxygen permeability and water vapor permeability of the chitosan films decreased, while tensile strength and elongation at break increased with the addition of Na-MMT. A filler content of 5 wt% and a homogenization shear rate of 16000 rpm were optimal in terms of property enhancement. Recently, a new type of layered zirconium phosphonate (zirconium glycine-N,N-methylphospho-nate) modified by n-butylamine was used to prepare chitosan nanocomposite films. When compared to neat chitosan, the composites prepared by solvent casting exhibited improved tensile strength, elongation and water resistance with a filler content of 1–2 wt% (Wu et al., 2010; Liu et al., 2011).
The effect of cellulose whiskers on the structure, morphology and properties of chitosan/CNW nanocomposite films has also been investigated (Li et al., 2009). Increasing the CNW content from 0 to 15–20 wt% resulted in tensile strength increases in the dry and wet states from 85 to 120 MPa and 9.9 to 17.3 MPa, respectively. Furthermore, in this case the chitosan nano-composite films containing CNWs displayed excellent thermal stability and water resistance.
Protein-based nanocomposites
Proteins of animal or plant origin such as soy protein, whey protein, casein, collagen, corn zein, gelatin, and wheat gluten possess good film-forming capability. However, protein-based films often suffer from high brittleness and high water absorption, and may not form particularly good gas or water vapor barriers, thereby limiting their application in food packaging. In order to overcome brittleness, plasticizers can be used, albeit with a reduction in barrier properties. Consequently, efforts have been made to improve protein film properties through the addition of nanofillers such as MMTs or nanocellulose.
Soy protein isolate (SPI) is made from defatted soy meal by removing most of the fats and carbohydrates and contains more than 90% protein. A few studies have demonstrated reduced water vapor permeability, improved mechanical properties and enhanced thermal stability in SPI nanocompos-ite films. Chen and Zhang (2006), for instance, prepared highly exfoliated and intercalated SPI-MMT nanocomposites by using the solution exfoliation method in a neutral aqueous medium and investigated the correlation between the microstructure and mechanical properties. These authors proposed that the surface electrostatic interaction between the positively charged soy protein and the negative charges on the MMT layers, as well as hydrogen bonding between -NH and Si-O groups, were the main reasons for improved mechanical strength. Highly exfoliated or intercalated structures were observed when the MMT content was below or above 12 wt% respectively. Lee and Kim (2010) also found that 3–12 wt% filler contents were optimal for improving the mechanical and barrier properties of SPI-MMT nanocomposite films. The effect of the pH of film casting solutions, MMT content, and melt extrusion processing parameters (screw speed and barrel temperature distribution) on the structure and properties of SPI-MMT nanocomposite films have been investigated (Kumar et al., 2010). The arrangement of MMT in the SPI matrix ranged from exfoliated at lower MMT content (5%) to intercalated at higher MMT content (15%). A reduction in the water vapor permeability (WVP) of SPI-MMT nanocomposite films of ~ 50% relative to unfilled SPI films has been reported (Kumar et al., 2010; Lee and Kim, 2010). However, it is important to note that the WVP for SPI-MMT films is still higher than that of synthetic plastics such as LDPE, PP or polyvinylidene chloride (PVDC).
Wheat gluten (WG), a by-product of the wheat starch industry, is commercially available at low cost, and displays unique viscoelastic properties and low water solubility. As a unique characteristic, WG-based films exhibit selective barrier properties for O2 and CO2, which potentially makes them ideal candidates for the packaging of fresh commodities (Mujica Paz and Gontard, 1997). However, due to their low mechanical resistance and high water sensitivity their utilization would be restricted to dry and intermediate aw products. The addition of MMT to WG films has demonstrably improved the mechanical properties as well as the barrier to water vapor and aroma compounds (Tunc et al., 2007; Angellier-Coussy et al., 2008).
Cho et al. (2011) prepared WG-MMT nanocomposites by injection molding, which is a versatile and widely applied technique for plastics processing. These authors reported that unmodified MMT (Cloisite® Na+) had a lubricating effect and facilitated injection molding. Injection-molded plates also had increased strength and stiffness as a result of nanofiller addition.
In a recent study by Mauricio-Iglesias et al. (2010), the safety of WG-MMT was assessed by testing the migration of MMT and the protein into four standard food-simulating liquids, as well as into solid food simulants. The effect of high-pressure treatment on mass transfer was investigated. In this case, the lack of a proper marker for MMT meant that the release of MMT from the packaging material could not be clarified.
Zein is a relatively hydrophobic protein derived from corn kernels and has good film-forming ability. Zein products, although less water sensitive than other biopolymers, show high water vapor permeability and low tensile strength when compared with synthetic polymers. Arora and Padua (2010) referred to research on zein nanocomposites in their review on nanocomposites in food packaging and mentioned the effect of adding kaolinite clay to zein-coated paper. The result was a reduction in water vapor permeability by about 50% as well as decreases in water absorption and oil permeation rate. These findings were attributed to possible layering of nano-scale kaolin platelets within the zein coating. According to these researchers, further research on the properties of zein-nanoclay film properties is needed.
Hemicellulose nanocomposites
Hemicelluloses are branched polysaccharides which are widely distributed in wood and in other plants and which, compared with cellulose and lignin, have received much less attention as a source of new bio-materials. Research in this direction has, however, been discussed in papers by Hansen and Plackett (2008) and Cunha and Gandini (2010). Research efforts have largely been directed towards chemically or physically modifying hemicel-luloses in order to obtain more practically useful biomaterials; however, there have also been a few investigations on the preparation of hemicel-lulose nanocomposites.
The production of hemicellulose-clay combinations has been the subject of two published investigations. Ünlü et al. (2009) investigated various composites and focused on improvements in thermal and rheological properties. In contrast, Viota et al. (2010) prepared hemicellulose-nanoclay films by combining an OMMT clay or an inulin non-ionic surfactant-coated OMMT with an oat spelt xylan. Film formation was influenced by pH and xylan type and the films were notably less hydrophilic when inulin-coated OMMT was used as the additive. Soler (2008), in a Master's thesis report, discussed research on sorbitol-plasticized arabinoxylan-clay films and the beneficial effects of adding MMT clays in terms of improving oxygen barrier properties.
Dammstrom et al. (2005) prepared highly transparent nanocomposite films of glucuronoxylan reinforced with bacterial cellulose. The potential of reinforcing xylan films with sulfonated cellulosic whiskers (0–10 wt%) has also been explored (Saxena and Ragauskas, 2009; Saxena et al. 2009). This research showed that the addition of cellulose whiskers to xylan films resulted in improvements in tensile strength and water vapor barrier in particular when sulfonated whiskers were applied.
13.5 Conclusions
Nanocomposite technology as a path to plastic-based food and beverage packaging materials with better properties has been widely studied, and a few products, particularly those based on petroleum-derived plastics including PA, PET and PE, have already entered the market or are at an advanced stage of development. Generally speaking, progress in new nanocomposites hinges upon obtaining the best possible dispersion of nanofiller in each polymer matrix and this exercise in itself is still an art under development, influenced by a wide range of processing parameters in each particular case. As reflected in the scientific literature and also in trade magazines and websites, attention has now turned particularly towards combinations of bio-derived polymers and nanofillers. The need for bio-nanocomposite materials in packaging is stimulated by heightened interest in bioplastics across the sector, and the realization that the use of nanofillers should provide a way to bring mechanical, thermal and barrier properties more into line with broad market requirements. Much of the research discussed in this chapter has addressed packaging of foods, but it is acknowledged that there may be big future opportunities to utilize bio-based nanocom-posites in the packaging of beverages, especially for carbonated drinks where barrier requirements are critical.
The literature discussed here covers a broad swathe of polymer/nano-filler combinations from commercial bioplastics such as PLA in combination with clay nanofillers through to polymers such as chitosan and the hemicelluloses which, while not melt processable, may have future promise in respect to bio-based barrier coatings for plastic films or paperboard. The positive technical attributes of nanocomposites for food and beverage packaging are expressed in many of the publications in this field, but it is also worth pointing out the challenges which lie ahead.
As demonstrated in many reports, although property enhancements can be shown in the laboratory or pilot plant, it is another thing altogether to convert such findings into commercial products, in which all of the characteristics needed for specific types of packaging are met. This is particularly true for more demanding foods such as meat, fish and cheeses, in which case the use of enhanced bioplastic packaging would open up entirely new markets for nanocomposite materials. The addition of nanofillers, even at low addition levels, may also raise cost concerns which would add to the existing cost of bioplastics, the prices for which are usually higher than those of commodity plastics.
As well as optimizing nanocomposite processes and products for particular market segments, there are two other issues which have so far received relatively little attention but which, it can be argued, are now likely to come under increased scrutiny. These are the topics of nanoparticle migration and toxicity. In commercial organomodified clays, for example, examination of these material characteristics must take into consideration the chemical organomodifier as well as the clay itself. To date, there is no strong body of scientific evidence to suggest that there is cause for concern; however, since it is only logical that new food and beverage packaging materials should be thoroughly assessed and, in fact, necessary in order to meet the requirements of the European Food Safety Authority (EFSA) and other regulatory bodies, it is likely that research on migration properties and toxicity of nanocomposite films for food and beverage packaging will expand in the future, especially as and when these materials come closer to the marketplace.
13.6 References
Aisawa, S., Hirahara, H., Uchiyama, H., Takahashi, S., Narita, E. Synthesis and thermal decomposition of Mn-Al layered double hydroxides. J Solid State Chem. 2002; 167:152–159.
Akkapeddi, K., Socci, E., Kraft, T., Facinelli, J., Worley, D. 'A new family of barrier nylons based on nanocomposites and oxygen scavengers', Proceedings of the 61st. Annual Technical Conference – Society of Plastics Engineers. 2003; 3:3845–3848.
Angellier-Coussy, H., Torres-Giner, S., Morel, M.H., Gontard, N., Gastaldi, E. Functional properties of thermoformed wheat gluten/montmorillonite materials with respect to formulation and processing conditions. J Appl Polym Sci. 2008; 107:487–496.
Angles, M.N., Dufresne, A. Plasticized starch/tunicin whiskers nanocomposites. 1. Structural analysis. Macromol. 2000; 33:8344–8353.
Anon. Nanotechnology can enhance packaging. Smartech Global Solutions Ltd, 2009. http://www.plastemart.com/upload/Literature/Nanotechnol-ogy-enhance-improve-food%20packaging.asp [Available from: (accessed 31 August 2009).].
Arora, A., Padua, G.W. Review: Nanocomposites in food packaging. J Food Sci. 2010; 75(1):R43–R49.
Auras, R.A., Singh, S.P., Singh, J.J. Evaluation of oriented poly(lactide) polymers vs. existing PET and oriented PS for fresh food service containers. Packag Technol Sci. 2005; 18:207–216.
Avella, M., De Vlieger, J.J., Errico, M.E., Fischer, S., Vacca, P., Volpe, M.G. Biodegradable starch/clay nanocomposite films for food packaging applications. Food Chem. 2005; 93:467–474.
Azizi Samir, M.A.S., Alloin, F., Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromol. 2005; 6:612–626.
Bastioli, C. Global status of the production of biobased packaging materials. Starch. 2001; 53:351–355.
Bondeson, D., Oksman, K. Polylactic acid/cellulose whisker nanocomposites modified by polyvinyl alcohol. Composites: Part A. 2007; 28:2486–2492.
Bordes, P., Pollet, E., Bourbigot, S., Avérous, L. Structure and properties of PHA/clay nano-biocomposites prepared by melt intercalation. Macromol Chem Phys. 2008; 209:1473–1484.
Bordes, P., Pollet, E., Avérous, L. Nano-biocomposites: biodegradable polyester/nanoclay systems. Prog Polym Sci. 2009; 34:125–155.
Bordes, P., Hablot, E., Pollet, E., Avérous, L. Effect of clay organomodifiers on degradation of polyhydroxyalkanoates. Polym Degrad Stab. 2009; 94:789–796.
Botana, A., Mollo, M., Eisenberg, P., Torres Sanchez, R.M. Effect of modified montmorillonite on biodegradable PHB nanocomposites. Appl Clay Sci. 2010; 47:263–270.
Brody, A.L. Nano and food packaging technologies converge. Food Technol. 2006; 60(3):92–94.
Brody, A.L. Packaging by the numbers. Food Technol. 2008; 62(2):89–91.
Brody, A.L., Bugusu, B., Han, J.H., Sand, C.K., Mchugh, T.H. Innovative food packaging solutions. J Food Sci. 2008; 73(8):R107–R116.
Bruno, M., Tavares, M.I.B., Motta, L.M., Miguez, E., Preto, M., Fernandez, A.O.R. Evaluation of PHB/clay nanocomposite by spin–lattice relaxation time. Mater Res. 2008; 11:483–485.
Bruzaud, S., Bourmaud, A. Thermal degradation and (nano)mechanical behavior of layered silicate reinforced poly(3-hydroxybutyrate-co-3-hydroxyval-erate) nanocomposites. Polym Test. 2007; 26:652–659.
Cabedo, L., Feijoo, J.L., Villanueva, M.P., Lagarón, J.M., Giménez, E. Optimization of biodegradable nanocomposites based on PLA/PCL blends for food packaging applications. Macromol Symp. 2006; 233(1):191–197.
Cabedo, L., Plackett, D., Giménez, E., Lagarón, J.M. Studying the degradation of polyhydroxybutyrate-co-valerate during processing with clay-based nanofillers. J Appl Polym Sci. 2009; 112:3669–3676.
Chang, P.R., Jian, R., Yu, J., Ma, X. Starch-based composites reinforced with novel chitin nanoparticles. Carbohyd Polym. 2010; 80:420–425.
Chaudhry, Q., Scotter, M., Blackburn, J., Ross, B., Boxall, A., Castle, L., Aitken, R., Watkins, R. Applications and implications of nanotechnologies for the food sector. Food Addit Contam. 2008; 25(3):241–258.
Chen, B., Evans, J.R.G. Thermoplastic starch-clay nanocomposites and their characteristics. Carbohyd Polym. 2005; 61(4):455–463.
Chen, P., Zhang, L. Interaction and properties of highly exfoliated soy protein/montmorillonite nanocomposites. Biomacromol. 2006; 7:1700–1706.
Cho, S.-W., Gällstedt, M., Johansson, E., Hedenqvist, M.S. Injection-molded nanocomposites and materials based on wheat gluten. Int J Bio Macromol. 2011; 48:146–152.
Choi, W.J., Kim, H.-J., Yoon, K.H., Kwon, O.H., Hwang, C.I. Preparation and barrier property of poly(ethylene terephthalate)/clay nanocomposite using clay-supported catalyst. J Appl Polym Sci. 2006; 100(6):4875–4879.
Chow, W.S., Lok, S.K. Thermal properties of poly(lactic acid)/organo-montmorillonite nanocomposites. J Therm Anal Cal. 2009; 95(2):627–632.
Chowdhury, S.R. Some important aspects in designing high molecular weight poly(L-lactic acid)-clay nanocomposites with desired properties. Polym Int. 2008; 57(12):1326–1332.
Chung, Y.-L., Ansari, S., Estevez, L., Hayrapetyan, S., Giannelis, E.P., Lai, H.-M. Preparation and properties of biodegradable starch-clay nanocomposites. Carbohyd Polym. 2010; 79:391–396.
Costa, F.R., Saphiannikova, M., Wagenknecht, U., Heinrich, G. Layered double hydroxide based polymer nanocomposites. Adv Polym Sci. 2008; 210:101–168.
Cunha, A.G., Gandini, A. Turning polysaccharides into hydrophobic materials: a critical review. Part 2. Hemicelluloses, chitin/chitosan, starch, pectin and alginates. Cellulose. 2010; 17:1045–1065.
Cyras, V.P., Commisso, M.S., Mauri, A.N., Vázquez, A. Biodegradable double-layer films based on biological resources: polyhydroxybutyrate and cellulose. J Appl Polym Sci. 2007; 106:749–756.
Dadbin, S., Noferesti, M., Frounchi, M. Oxygen barrier LDPE/LLDPE/ organoclays nano-composite films for food packaging. Macromol Symp. 2008; 274:22–27.
Dahman, Y. Nanostructured biomaterials and biocomposites from bacterial cellulose nanofibers. J Nanosci Nanotech. 2010; 9:5105–5122.
Dammström, S., Salmén, L., Gatenholm, P. The effect of moisture on the dynamical mechanical properties of bacterial cellulose/glucuronoxylan nanocomposites. Polymer. 2005; 46(23):10364–10371.
De Azeredo, H.M.C. Nanocomposites for food packaging applications. Food Res Int. 2009; 42(9):1240–1253.
De Carvalho, A.J.F., Cúrvelo, A.A.S., Agnelli, J.A.M. A first insight on composites of thermoplastic starch and kaolin. Carbohyd Polym. 2001; 45:189–194.
Dufresne, A. Polysaccharide nanocrystal reinforced nanocomposites. Can J Chem. 2008; 86:484–494.
Dufresne, A., Samain, E. Preparation and characterization of a poly(ß-hydroxyalkanoate) latex produced by Pseudomonas oleovorans. Macromol. 1998; 31:6426–6433.
Dufresne, A., Kellerhals, M.B., Witholt, B. Transcrystallization in Mcl-PHAs/cellulose whiskers composites. Macromol. 1999; 32:7396–7401.
Edlund, U., Ryberg, Y.Z., Albertsson, A.-C. Barrier films from renewable forestry waste. Biomacromol. 2010; 11:2532–2538.
Erceg, M., Kova![]() i
i![]() t, Perinovic, S. Isothermal degradation of poly(3-hydroxybutyrate)/organically modified montmorillonite nanocomposites. Polym Compos. 2010; 31:272–278.
t, Perinovic, S. Isothermal degradation of poly(3-hydroxybutyrate)/organically modified montmorillonite nanocomposites. Polym Compos. 2010; 31:272–278.
Farris, S., Introzzi, L., Piergiovanni, L. Evaluation of a bio-coating as a solution to improve barrier, friction and optical properties of plastic films. Packag Technol Sci. 2009; 22:69–83.
Fendler, A., Villanueva, M.P., Gimenez, E., Lagarón, J.M. Characterization of the barrier properties of composites of HDPE and purified cellulose fibers. Cellulose. 2007; 14:427–438.
Fornes, T.R., Paúl, D.R. Modeling properties of nylon 6/clay nanocomposites using composite theories. Polymer. 2003; 17:4993–5013.
Frounchi, M., Dourbash, A. Oxygen barrier properties of poly(ethylene terephthalate) nanocomposite films. Macromol Mater Eng. 2009; 294:68–74.
Fukushima, K., Abbate, C., Tabuani, D., Gennari, M., Camino, G. Biodegradation of poly(lactic acid) and its nanocomposites. Poly Degr Stab. 2009; 94(10):1646–1655.
Garcia, N.L., Ribba, L., Dufresne, A., Aranguren, M.I., Goyanes, S. Physico-mechanical properties of biodegradable starch nanocomposites. Macromol Mater Eng. 2009; 294:169–177.
Golebiewski, J., Rozanski, A., Dzwonkowski, J., Galeski, A. Low density polyethylene – montmorillonite nanocomposites for film blowing. Eur Pol J. 2008; 44:270–286.
Grande, C.J., Torres, F.G., Gomez, C.M., Troncoso, O.P., Canet-Ferrer, J., Martínez-Pastor, J. Development of self-assembled bacterial cellulose-starch nanocomposites. Mater Sci Eng: C. 2009; 29:1098–1104.
Graveland-Bikkerand, J.F., De Kruif, C.G. Food nanotechnology. Trends Food Sci Technol. 2006; 27:82–89.
Greco, A., Corcione, C.E., Strafella, A., Maffezzoli, A. Analysis of the structure and mass transport properties of clay nanocomposites based on amorphous PET. J Appl Polym Sci. 2010; 118:3666–3672.
Guo, C.-Y., Ke, Y., Liu, Y., Mi, X., Zhang, M., Hu, Y. Preparation and properties of polyethylene/montmorillonite nanocomposites formed via ethylene copolymerization. Polym Int. 2009; 58:1319–1325.
Gupta, R.K., Kennel, E., Kwang-Jea, K. Polymer Nanocomposites Handbook. Boca Raton, FL: CRC Press; 2009.
Hablot, E., Bordes, P., Pollet, E., Avérous, L. Thermal and thermo-mechanical degradation of poly(3-hydroxybutyrate)-based multiphase systems. Polym Degrad Stab. 2008; 93:413–421.
Hansen, N.M.L., Plackett, D. Sustainable films and coatings from hemicel-luloses: a review. Biomacromol. 2008; 9:1493–1505.
Hong, S.I., Lee, J.H., Bae, H.J., Koo, S.Y., Lee, H.S., Choi, J.H., Kim, D.H., Park, S.-H., Park, H.J. Effect of shear rate on structural, mechanical, and barrier properties of chitosan/montmorillonite nanocomposite film. J Appl Polym Sci. 2011; 119:2742–2749.
Hsu, S., Wu, T., Liao, C. Nonisothermal crystallization behavior and crystalline structure of poly(3-hydroxybutyrate)/layered double hydroxide nanocom-posites. J Polym Sci Part B. 2007; 45:995–1002.
Iwatake, A., Nogi, M., Yano, H. Cellulose nanofiber reinforced polylactic acid. Compos Sci Technol. 2008; 68:2103–2106.
Jiang, L., Morelius, E., Zhang, J., Wolcott, M., Holbery, J. Study of the poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/cellulose nanowhisker composites prepared by solution casting and melt processing. J Compos Mater. 2008; 42:2629–2645.
Junior De Menezes, A., Siqueira, G., Curvelo, A.A.S., Dufresne, A. Extrusion and characterization of functionalized cellulose whiskers reinforced polyethylene nanocomposites. Polym. 2009; 50:4552–4563.
Katiyar, V., Gerds, N., Koch, C.B., Risbo, J., Hansen, C.B.H., Plackett, D. Poly L-lactide-layered double hydroxide nanocomposites via in situ polymerization of L-lactide. Polym Degr Stab. 2010; 95:2563–2573.
Katiyar, V., Gerds, N., Koch, C.B., Risbo, J., Hansen, C.B.H., Plackett, D. Melt processing of poly (L-lactic acid) in the presence of organomodified anionic and cationic clays. J Appl Polym Sci. 2011; 122(1):112–125.
Ke, Z., Yongping, B. Improve the gas barrier property of PET film with montmorillonite by in situ interlayer polymerization. Mater Lett. 2005; 59:3348–3351.
Kim, M., Pomietto, A.L., III. Food packaging potential of some novel degradable starch-polyethylene plastics. J Food Prot. 1994; 57:1007–1012.
Kimura, A., Itoh, T. 'Biogenesis and function of cellulose in the tunicates', in Brown Jr R M and Saxena I M, Cellulose: Molecular and Structural Biology. New York: Springer; 2007. [217–236].
Klemm, D., Schumann, D., Kramer, F., Hebler, N., Nornung, M., Schmauder, H.-P., Marsch, S. Nanocelluloses as innovative polymers in research and application. Adv Polym Sci. 2006; 205:49–96.
Kojima, Y., Usuki, A., Kawasumi, M., Okada, A., Kurauchi, T., Kamigaito, O. One-pot synthesis of nylon-6 clay hybrid. J Polym Sci, Part A: Polym Chem. 1993; 31:1755–1758.
Kumanayaka, T.O., Parthasarathy, R., Jollands, M. Accelerating effect of montmorillonite on oxidative degradation of polyethylene nanocomposites. Polym Degrad Stab. 2010; 95:672–676.
Kumar, P., Sandeep, K.P., Alavi, S., Truong, V.D., Gorga, R.E. Preparation and characterization of bio-nanocomposite films based on soy protein isolate and montmorillonite using melt extrusion. J Food Eng. 2010; 100:480–489.
Kuusipalo, J. PHB/V in extrusion coating of paper and paperboard: Part I: Study of functional properties. J Polym Environ. 2000; 8:39–47.
Kuusipalo, J. PHB/V in extrusion coating of paper and paperboard – Study of functional properties. Part II. J Polym Environ. 2000; 8:49–58.
Lagarón, J.M., López-Rubio, A. 'Latest developments and future trends in food packaging and biopackaging', in Passos L M and Ribeiro C P, Innovations in Food Engineering. Boca Raton, FL: CRC Press; 2010. [485–508].
Lazic, V.L., Budinski-Simendic, J., Gvozdenovic, J.J., Simendic, B. Barrier properties of coated and laminated polyolefin films for food packaging. Acta Physica Polonica. 2010; 117:855–858.
Le Corre, D., Bras, J., Dufresne, A. Starch nanoparticles: a review. Biomacromol. 2010; 11:1139–1153.
Lee, J.-E., Kim, K.M. Characteristics of soy protein isolate-montmorillonite composite films. J Appl Polym Sci. 2010; 118:2257–2263.
Li, Q., Zhou, J., Zhang, L. Structure and properties of the nanocomposite films of chitosan reinforced with cellulose whiskers. J Polym Sci, Part B: Polym Physics. 2009; 47:1069–1077.
Lim, S.T., Hyun, Y.H., Lee, C.H., Choi, H.J. Preparation and characterization of microbial biodegradable poly(3-hydroxybutyrate)/organoclay nanocomposite. J Mater Sci Lett. 2003; 22:299–302.
Lin, Y., Hiltner, A., Baer, E. Nanolayer enhancement of biaxially oriented polypropylene film for increased gas barrier. Polym. 2010; 51:5807–5814.
Liu, C., Wu, H., Yang, Y., Zhu, L., Teng, Y. The structure and properties of a novel nanocomposite films from chitosan and layered zirconium phosphonate. J Appl Polym Sci. 2011; 120:1106–1113.
Lörcks, J. Properties and applications of compostable starch-based plastic material. Polym Degr Stab. 1998; 59:245–249.
Mahboobeh, E., Yunus, W.M.D., Hussein, Z., Ahmad, M., Ibrahim, N.A. Flexibility improvement of poly(lactic acid) by stearate-modified layered double hydroxide. J Appl Polym Sci. 2010; 118(2):1077–1083.
Maiti, P., Yamada, K., Okamoto, M., Ueda, K., Okamoto, K. New polylactide/layered silicate nanocomposites: role of organoclays. Chem Mater. 2002; 14(119):4654–4661.
Maiti, P., Batt, C.A., Giannelis, E.P. New biodegradable polyhydroxybutyrate/ layered silicate nanocomposites. Biomacromol. 2007; 8:3393–3400.
Marini, J., Branciforti, M.C., Alves, R.M.V., Bretas, R.E.S. Effect of EVA as compatibilizer on the mechanical properties, permeability characteristics, lamellae orientation, and long period of blown films of HDPE/clay nanocomposites. J Appl Polym Sci. 2010; 118:3340–3350.
Mathew, A.P., Chakraborty, A., Oksman, K., Sain, M. The structure and mechanical properties of cellulose nanocomposites prepared by twin screw extrusion. ACS Symposium Series (Cellulose Nanocomposites). 2006; 938:114–131.
Mauricio-Iglesias, M., Peyron, S., Guillard, V., Gontard, N. Wheat gluten nanocomposite films as food-contact materials: migration tests and impact of a novel food stabilization technology (high pressure). J Appl Polym Sci. 2010; 116:2526–2535.
Mcglashan, S.A., Halley, P.J. Preparation and characterization of biodegradable starch-based nanocomposite materials. Polym Int. 2003; 52:1767–1773.
Miguel, O., Iruin, J.J. Water transport properties in poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) biopolymers. J Appl Polym Sci. 1999; 73:455–468.
Morschbacker, A. Bio-ethanol based ethylene. J Macromol Sci Part C: Polym Rev. 2009; 49:79–84.
Morschbacker, A. Basics of bio-polyolefins. Bioplastics Magazine. 2010; 5:52–55.
Mujica Paz, H., Gontard, N. Oxygen and carbon dioxide permeability of wheat gluten film: effect of relative humidity and temperature. J Agric Food Chem. 1997; 45:4101–4105.
Nakagaito, A.N., Fujimura, A., Sakai, T., Hama, Y., Yano, H. Production of microfibrillated cellulose (MFC)-reinforced polylactic acid (PLA) nanocompos-ites from sheets obtained by a papermaking-like process. Compos Sci Technol. 2009; 69:1293–1297.
Nanda, R., Sasmal, A., Sasmal, S., Nayak, P.L. Biobased nanocomposites for food packaging applications: opportunities and challenges. Pop Plast & Pack. 2009; 54(2):50–57.
National Nanotechnology Initiative. Nanocomposites for Military Food Packaging, U.S. Army NSRDEC – NNI Scientific Accomplishments 2009, 2009. http://www.nano.gov/html/research/Achievements_pdf/08-Food-Agricultural/NanocompositesforMilitaryFoodPackaging-ArmyNSRDEC.pdf [Available from: (accessed 13 November 2010).].
Nazarenko, S., Meneghetti, P., Julmon, P., Olson, B.G., Qutubuddin, S. Gas barrier of polystyrene montmorillonite clay nanocomposites: effect of mineral layer aggregation. J Polym Sci, Part B: Polym Phys. 2007; 45:1733–1753.
Neilson, L.E. Models for the permeability of filled polymer systems. J Macromol Sci (Chem). 1967; A1(5):929–942.
Noda, I., Lindsey, S.B., Caraway, D., 'Nodax™ class PHA copolymers: their properties and applications'Chen G.G.-Q., ed. Plastics from Bacteria. Microbiology Monographs 14. Springer, New York, 2010:237–255.
Nordqvist, D., Sico, G., Hedenqvist, M.S. Starch nanocomposites with platelet and fibrillar fillers. J Biomater Bioen. 2009; 3:139–146.
Noroozi, M., Zebarjad, S.M. Effects of multiwall carbon nanotubes on the thermal and mechanical properties of medium density polyethylene matrix nano-composites produced by a mechanical milling method. J Vinyl Add Tech. 2010; 16:147–151.
Ohashi, E., Drumond, W.S., Zane, N.P., Faria Barros, P.W., Lachtermacher, M.G., Wiebeck, H., Wang, S.H. Biodegradable poly(3-hydroxybutyrate) nanocomposite. Macromol Symp. 2009; 279:138–144.
Okamoto, M., Ray, S.S. Polymer/clay nanocomposites. In: Nalwa H.S., ed. Encyclopedia of Nanoscience and Nanotechnology. Valencia, CA: American Scientific Publishers; 2004:1–52.
Ozkoc, G., Kemaloglu, S. Morphology, biodegradability, mechanical and thermal properties of nanocomposite films based on PLA and plasticized PLA. J Appl Polym Sci. 2009; 114(4):2481–2487.
Paluszkiewicz, C., Stodolak, E., Hasik, M., Blazewicz, M. FT-IR study of montmorillonite – chitosan nanocomposite materials. Spectrochim Acta A: Mol Biomol Spectrosc. 2010; 79(4):784–788.
Park, H.-M., Li, X., Jin, C.-Z., Park, C.Y., Cho, W.J., Ha, C.K. Preparation and properties of biodegradable thermoplastic starch/clay hybrids. Macromol Mater Eng. 2002; 287(8):553–558.
Park, H.-M., Lee, W.-K., Park, C.-Y., Cho, W.-J., Ha, C.-S. Environmentally friendly polymer hybrids. Part 1. Mechanical, thermal and barrier properties of thermoplastic starch/clay nanocomposites. J Mater Sci. 2003; 38:909–915.
Park, S.-I., Marsh, K.S., Dawson, P. Application of chitosan-incorporated LDPE film to sliced fresh red meats for shelf life extension. Meat Sci. 2010; 85:493–499.
Parulekar, Y., Mohanty, A.K., Imam, S.H. Biodegradable nanocomposites from toughened polyhydroxybutyrate and titanate-modified montmorillonite clay. J Nanosci Nanotechnol. 2007; 7:3580–3589.
Pei, A., Zhou, Q., Berglund, L.A. Functionalized cellulose nanocrystals as biobased nucleation agents in poly(L-lactide) (PLLA) – 'crystallization and mechanical property effects. Compos Sci Technol. 2010; 70:815–821.
Petersen, K., Veggemose Nielsen, P., Bertelsen, G., Lawther, M., Olsen, M.B., Nilsson, N.H., Mortensen, G. Potential of biobased materials for food packaging. Trends Food Sci Techn. 1999; 10:52–68.
Picard, E., Gauthier, H., Gérard, J.-F., Espuche, E. Influence of the intercalated cations on the surface energy of montmorillonites: consequences for the morphology and gas barrier properties of polyethylene/montmorillonite nanocomposites. J Coll Int Sci. 2007; 307:364–376.
Pillai, S.K., Ramontja, J., Ray, S.S. Controlled two-step amine functionaliza-tion of multi-walled carbon nanotubes for the preparation of polylactide/carbon nanotube composites. Adv Sci Lett. 2010; 3(2):117–122.
Pinnavaia, T.J., Beall, G.W. Polymer-Clay Nanocomposites. New York: John Wiley & Sons; 2001.
Plackett, D., Siró, I. Polyhydroxyalkanoates (PHAs) for food packaging. In: Lagarón J.M., ed. Multifunctional and Nanoreinforced Polymers for Food Packaging. Cambridge: Woodhead Publishing, 2011.
Plackett, D., Anturi, H., Hedenqvist, M., Ankerfors, M., Gällstedt, M., Lindström, T., Siró, I. Physical properties and morphology of films prepared from microfibrillated cellulose and microfibrillated cellulose in combination with amylopec-tin. J Appl Polym Sci. 2010; 117(6):3601–3609.
Pluta, M., Galeski, A., Alexandre, M., Paul, M.-A., Dubois, P. Polylactide/mont-morillonite nanocomposites and microcomposites prepared by melt blending: structure and some physical properties. J Appl Polym Sci. 2002; 86(6):1497–1506.
Pucciariello, R., Villani, V., Giammarino, G. Thermal behaviour of nano-composites based on linear-low-density poly(ethylene) and carbon nanotubes prepared by high energy ball milling. J Polym Res. 2011; 18(5):949–956.
Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React Funct Polym. 2000; 46:1–27.
Ray, S.S., Okamoto, M. New polylactide/layered silicate nanocomposites. 6. Melt rheology and foam processing. Macromol Mater Eng. 2003; 288(12):936–944.
Ray, S.S., Maiti, P., Okamoto, M., Yamada, K., Ueda, K. New polylactide/layered silicate nanocomposites. 1. Preparation, characterization and properties. Macromol. 2002; 35(8):3104–3111.
Ray, S.S., Yamada, K., Okamoto, M., Ueda, K. Biodegradable polylactide/montmorillonite nanocomposites. J Nanosci Nanotech. 2003; 3(6):503–510.
Ray, S.S., Yamada, K., Okamoto, M., Ueda, K. New polylactide/layered silicate nanocomposites. 2. Concurrent improvement of material properties, biodegradability and melt rheology. Polym. 2003; 44(3):857–866.
Ray, S.S., Yamada, K., Okamoto, M., Ogami, A., Ueda, K. New polylactide/layered silicate nanocomposites. 3. High-performance biodegradable materials. Chem Mater. 2003; 15(7):1456–1465.
Ray, S.S., Yamada, K., Okamoto, M., Ogami, A., Ueda, K. New polylactide/layered silicate nanocomposites. 4. Structure, properties and biodegradability. Comp Interf. 2003; 10(4–5):435–450.
Ray, S.S., Yamada, K., Okamoto, M., Fujimoto, Y., Ogami, A., Ueda, K. New poly-lactide/layered silicate nanocomposites. 5. Designing of materials with desired properties. Polymer. 2003; 44(21):6633–6646.
Ray, S., Quek, S.Y., Easteal, A., Chen, X.D. The potential use of polymer clay nanocomposites in food packaging. Int J Food Eng. 2006; 2(4):1–11.
Reddy, M.M., Deighton, M., Bhattacharya, S., Parthasarathy, R. Biodegradation of montmorillonite filled oxo-biodegradable polyethylene. J Appl Polym Sci. 2009; 113:2826–2832.
Ren, P., Shen, T., Wang, F., Wang, X., Zhang, Z. Study on biodegradable starch/ OMMT nanocomposites for packaging applications. J Polym Environ. 2009; 17:203–207.
Rhim, J.W., Ng, P.K. Natural biopolymer-based nanocomposite films for food packaging applications. Crit Rev Food Sci Nutr. 2007; 47(4):411–433.
Rhim, J.-W., Hong, S.-I., Park, H.-M., Ng, P.K.W. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J Agric Food Chem. 2006; 54:5814–5822.
Rhim, J.-W., Hong, S.-I., Ha, C.-S. Tensile, water vapor barrier and antimicrobial properties of PLA/nanoclay composite films. LWT – Food Sci Tech. 2009; 42:612–617.
Rizvi, R., Cochrane, B., Naguib, H., 'Foaming behavior of melt-blended poly-lactide-chitin composites'. 67th Annual Technical Conference – Society of Plastics Engineers. 2009:932–938.
Robertson, G.L. Food Packaging – Principles and Practice. Boca Raton, FL: CRC Press; 2006.
Röper, H., Koch, H. The role of starch in biodegradable thermoplastic materials. Starch. 1990; 42:123–130.
Sabet, S.S., Katbab, A.A. Interfacially compatibilized poly(lactic acid) and poly(lactic acid)/polycaprolactone/organoclays nanocomposites with improved biodegradability and barrier properties: effects of the compatibilizer structural parameters and feeding route. J Appl Polym Sci. 2009; 111(4):1954–1963.
Sahoo, N.G., Rana, S., Cho, J.W., Li, L., Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog Polym Sci. 2010; 35:837–867.
Sanchez-Garcia, M.D., Lagarón, J.M. On the use of plant cellulose nano-whiskers to enhance the barrier properties of polylactic acid. Cellulose. 2010; 17(5):987–1004.
Sanchez-Garcia, M.D., Lagarón, J.M. Novel clay-based nanobiocompos-ites of biopolyesters with synergistic barrier to UV light, gas, and vapor. J Appl Polym Sci. 2010; 118:188–199.
Sanchez-Garcia, M.D., Giménez, E., Lagarón, J.M. Novel PET nanocompos-ites of interest in food packaging applications and comparative barrier performance with biopolyester nanocomposites. J Plast Film Sheet. 2007; 23:133–148.
Sanchez-Garcia, M.D., Giménez, E., Lagarón, J.M. Morphology and barrier properties of nanobiocomposites of poly(3-hydroxybutyrate) and layered silicates. J Appl Polym Sci. 2008; 108:2787–2801.
Sanchez-Garcia, M.D., Lagarón, J.M., Hoa, S.V. Effect of addition of carbon nanofibers and carbon nanotubes on properties of thermoplastic biopolymers. Compos Sci Technol. 2009; 70:1095–1105.
Saxena, A., Ragauskas, A.J. Water transmission barrier properties of biodegradable films based on cellulosic whiskers and xylan. Carbohyd Polym. 2009; 78:357–360.
Saxena, A., Elder, T.J., Pan, S., Ragauskas, A.J. Novel nanocellulosic xylan composite film. Composites: Part B. 2009; 40:727–730.
Sekhon, B.S. Food nanotechnology – an overview. Nanotech Sci Appl. 2010; 3:1–15.
Sinclair, R.G. The case for poly(lactic acid) as a commodity packaging plastic. J Mass Spec – Pure Appl Chem. 1996; 33(5):585–597.
Siró, I., Plackett, D. Microfibrillated cellulose and new nanocomposite materials – a review. Cellulose. 2010; 17:459–494.
Soler, C.A.B. Preparation and characterization of arabinoxylan-montmoril-lonite nanocomposites – greening plastics in food packaging. Master's Thesis in the Master's Programme in Advanced Materials, Department of Chemical and Biological Engineering (Polymer Technology), Chalmers University of Technology, Gothenburg, Sweden, 2008.
Sorrentino, A., Gorassi, G., Vittoria, V. Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci Tech. 2007; 18:84–95.
Sozer, N., Kokini, J.L. Nanotechnology and its applications in the food sector. Trends Biotechnol. 2009; 27:82–89.
Svagan, A.J., Hedenqvist, M.S., Berglund, L. Reduced water vapor sorption in cellulose nanocomposites with starch matrix. Comp Sci Technol. 2009; 69:500–506.
Swain, S.K., Isayev, A.I. Effect of ultrasound on HDPE/clay nanocompos-ites: rheology, structure and properties. Polymer. 2007; 48:281–289.
Thelen, C., Orroth, C., Froio, D., Ziegler, D., Lucciarini, J., Farrell, R., D'souza, N.A., Ratto, J.A. Influence of montmorillonite layered silicate on plasticized poly(L-lactide) blown films. Polymer. 2005; 46(25):11716–11727.
Tingaut, P., Zimmermann, T., Lopez-Suevos, F. Synthesis and characterization of bionanocomposites with tunable properties from poly(lactic acid) and acetylated microfibrillated cellulose. Biomacromol. 2010; 11(2):454–464.
Tunc, S., Angellier, H., Cahyana, Y., Chalier, P., Gontard, N., Gastaldi, E. Functional properties of wheat gluten/montmorillonite nanocomposite films processed by casting. J Membrane Sci. 2007; 289:159–168.
Ünlü, C.H., Günister, E., Atici, O. Synthesis and characterization of NaMt biocomposites with corn cob xylan in aqueous media. Carbohydr Polym. 2009; 76:585–592.
Usuki, A., Hasegawa, N., Kato, M. Polymer-clay nanocomposites. Adv Polym Sci. 2005; 79:135–195.
Utracki, L.A. Clay-containing Polymeric Nanocomposites. Shrewsbury, Shropshire: Rapra Technology Ltd.; 2004.
Valavala, P.K., Odegard, G.M. Modeling techniques for determination of mechanical properties of polymer nanocomposites. Rev Adv Mater Sci. 2005; 9:34–44.
Viota, J.L., Lopez-Viota, M., Saake, B., Stana-Kleinschek, K., Delgado, A.V. Organoclay particles as reinforcing agents in polysaccharide films. J Coll Int Sci. 2010; 347:74–78.
Visakh, P.M., Thomas, S. Preparation of bionanomaterials and their polymer nanocomposites from waste and biomass. Waste Biomass Valor. 2010; 1:121–134.
Wang, B., Sain, M. Isolation of nanofibers from soybean source and their reinforcing capability on synthetic polymers. Compos Sci Technol. 2007; 67:521–527.
Wilhelm, H.M., Sierakowski, M.R., Souza, G.P., Wypych, F. Starch film reinforced with mineral clay. Carbohyd Polym. 2003; 52:101–110.
Wu, H., Liu, C., Chen, J., Yanga, Y., Chen, Y. Preparation and characterization of chitosan/α-zirconium phosphate nanocomposite films. Polym Int. 2010; 59:923–930.
Wu, T.-M., Hsu, S.-F., Shih, Y.-F., Liao, C.-S. Thermal degradation kinetics of biodegradable poly(3-hydroxybutyrate)/layered double hydroxide nanocompos-ites. J Polym Sci Part B. 2008; 46:1207–1213.
Xu, B., Leisen, J., Beckham, H.W. Evolution of clay morphology in polypro-pylene/montmorillonite nanocomposites upon equibiaxial stretching: a solid state NMR and TEM approach. Macromol. 2009; 42:8959–8968.
Xu, Y., Ren, X., Hanna, M.A. Chitosan/clay nanocomposite film preparation and characterization. J Appl Polym Sci. 2006; 99:1684–1691.
Yoo, Y., Kim, S.S., Won, J.C., Choi, K.-Y., Lee, J.H. Enhancement of the thermal stability, mechanical properties and morphologies of recycled PVC/clay nanocom-posites. Polym Bulletin. 2004; 52(5):373–380.
Yoon, S.-Y., Deng, Y. Clay-starch composites and their application in paper-making. J Appl Polym Sci. 2006; 100(2):1032–1038.
Yoon, J.T., Lee, S.C., Jeong, Y.G. Effects of grafted chain length on mechanical and electrical properties of nanocomposites containing polylactide-grafted carbon nanotubes. Compos Sci Technol. 2010; 70(5):776–782.
Zenkiewicz, M., Richert, J. Permeability of polylactide nanocomposite films for water vapor, oxygen and carbon dioxide. Polym Test. 2008; 27(7):835–840.
Zenkiewicz, M., Richert, J., Rozanski, A. Effect of blow moulding ratio on barrier properties of polylactide nanocomposite films. Polym Test. 2010; 29:251–257.
Zhong, Y., Janes, D., Zheng, Y., Hetzer, M., De Kee, D. Mechanical and oxygen barrier properties of organoclay-polyethylene nanocomposite films. Polym Eng Sci. 2007; 47:1101–1107.


