Edible chitosan coatings for fresh and minimally processed foods
M. Vargas, L. Sánchez-González, M. Cháfer, A. Chiralt and C. González-Martínez, Universitat Politècnica de València, Spain
Abstract:
Chitosan is a biopolymer that shows great potential as an ingredient for the preparation of new edible films and coatings for different food applications. Chitosan has antimicrobial, antioxidant and emulsifying properties. Moreover, it is quite compatible with other biopolymers and lipids and thus can be used to formulate different film-forming dispersions adapted to specific target applications. This chapter reviews some of the most recent studies into the properties of chitosan-based film-forming dispersions, either containing chitosan alone or in combination with other food ingredients. The most relevant results of current research on the properties of chitosan-based composite coatings are also presented. Some examples of recent applications of chitosan-based coatings are reviewed and discussed as a foundation for the development of new chitosan-based coatings with improved functionality and performance.
5.1 Introduction
Chitosan is a natural polysaccharide that has become of great interest for both the scientific community and the food industry due to its multiple possible applications, which include the formation of biodegradable films and coatings. Chitosan is obtained by N-deacetylation of chitin (poly-β-(1→4)-N-acetyl-d-glucosamine), which is a major component of the shells of crustaceans and insects. Chitosan can be obtained from the wastes of the seafood processing industry (shells of crabs, shrimps, lobsters and krill) by using a concentrated basic solution combined with high temperatures (Tharanathan and Kittur, 2003). Chitosan can also be obtained from the cell wall of some fungi, thus there is an alternative method of production that does not depend on environmentally harmful chemicals or the variable abundance of crustaceans. This naturally occurring chitosan is produced in the fungal cells through enzymatic deacetylation subsequent to the formation of the polymer chain (Synowiecki and Al-Khateeb, 2003).
Chitosan is non-toxic, biocompatible and biodegradable and it is a member of a type of biopolymer called hydrocolloids, but it shows atypical properties. For instance, while most hydrocolloids are neutral or negatively charged at biological pH values, chitosan is positively charged. Moreover, its cationic nature in acid solution means that the positively charged chitosan molecules are attracted to negatively charged surfaces (Marudova et al., 2005).
5.2 Antimicrobial activity of chitosan
Chitosan shows antibacterial and antifungal activity (Shaidi et al, 1999), which has promoted its use as a new natural biopolymer for the development of edible coatings that improve food preservation and reduce the use of chemical preservatives. The exact mechanism of the antimicrobial action of chitosan is not well known, although it is believed to be related to the amino groups of chitosan that are positive charged and can interact with the cell wall. In this way, the permeability of the cell wall changes and the cytoplasma flows out leading to the extinction of the cell (Cuero, 1999).
The antimicrobial activity of chitosan depends not only on the external conditions (target microorganism, nature of the medium, pH temperature, etc.), but also on different intrinsic factors such as its molecular weight, and degree of polymerization and deacetylation. Chitosan with a high degree of deacetylation (DD) is more effective in inhibiting bacterial growth than chitosan with lower DDs (Jung and Kim, 1999; Synowiecki and Al-Khateeb, 2003). The effect of the molecular weight of chitosan on its antibacterial activity has been also investigated. No et al. (2002) showed that chitosan markedly inhibited the growth of Gram-positive and Gram-negative bacteria, although the inhibitory effects differed with molecular weight and the particular bacterium. Chitosan showed stronger bactericidal effects against Gram-positive bacteria than Gram-negative bacteria. Moreover, the minimum inhibitory concentration (MIC) of chitosan also differed with individual bacteria and the molecular weight of chitosan. Kim et al. (2010) observed that molecular weight and viscosity of chitosan are highly correlated with the antimicrobial properties of chitosan-based films.
5.3 Antioxidant properties of chitosan
Chitosan is considered a secondary antioxidant since it has the ability to chelate the metal ions involved in the catalysis of an oxidative reaction (Tharanathan and Kittur, 2003). Chitosan of different N-deacetylation degrees obtained from crab shells showed antioxidant activity, scavenging ability on hydroxyl radicals and chelating ability on ferrous ions, being more effective as an antioxidant agent as the DD increased (Yen et al., 2008). The origin of the scavenging ability of chitosan is related to the presence of active hydroxyl and amino groups in the polymer chains. The hydroxyl groups in the polysaccharide units can react with free radicals and, according to free radical theory, the amino groups of chitosan can react with free radicals to form additional stable macroradicals (Yen et al., 2007). As regards the effect of the molecular weight of chitosan on its antioxidant properties, Xing et al. (2007) showed that low molecular weight chitosan had a stronger scavenging activity effect on oxygen and hydroxyl groups than high molecular weight chitosan in an in vitro study. The same effect was observed by Feng et al. (2007) by reducing chitosan molecular weight by means of irradiation treatments.
5.4 Emulsification properties of chitosan
Chitosan has emulsifying properties (Rodriguez et al., 2002). It yields stable water-in-oil emulsions and it is considered as a polymeric surfactant with amphiphilic properties, since it promotes both electrosteric and thickening stabilization mechanisms (Del Blanco et al., 1999). The ability of chitosan to either destabilize or stabilize emulsions depends on many factors such as dispersed phase concentration, droplet charge and particle size distribution, chitosan concentration, molecular weight and degree of deacetylation, mixing conditions, pH, and ionic strength of the solvent.
Chitosan has been used to prepare oil-in-water emulsions by means of the layer-by-layer deposition technique (Thanasukarn et al., 2006). These emulsions, which are stabilized by multiple layers of surfactants and/or polyelectrolytes, were not affected by changes in pH and ionic strength of the solvent, thermal treatments, freezing or lipid oxidation (Ogawa et al., 2003a, 2003b, 2004). However, the application of chitosan as a stabilizer is so far limited if its molecular weight is too high, which can result in low solubility in acid-free aqueous media, or if the pH of the solvent is close to neutral. Therefore, surfactants are usually incorporated to promote emulsion formation and interfacial stabilization in chitosan-based dispersions. Following this approach, stable sunflower oil emulsions have been prepared by combining low and medium molecular weight chitosan and Tween 20 (Mun et al., 2005, 2006).
5.5 Characterization of chitosan-based film-forming dispersions
The physicochemical properties of chitosan-based film-forming dispersions (FFD) play an important role in the properties of the coatings. For instance, when a lipid is incorporated in chitosan-based dispersions, the lipid particle size greatly affects the development of the system during the film drying process (Morillon et al., 2002). Water evaporation leads to flocculation, coalescence and the creaming of lipid droplets, depending on droplet size and distribution, among other factors, such as viscosity of the continuous phase, interactions between droplets and rheological properties of the O/W interface. The stability of the FFD greatly affects the final microstructure of the film matrix, which defines to a greater extent its functional properties. In this sense, the characterization of some stability factors of the FFD could contribute to an understanding of the differences in functional properties of the final coatings (Vargas et al., 2009a). In particular, the analysis of the surface charge of the particles and their size distribution allows us to obtain criteria of stability (McClements, 2007). Moreover, the analysis of rheological behaviour is useful not only to establish stability criteria, but also to define the application technique when the FFD are applied to a particular product.
5.5.1 Effect of solvent properties
The conformation of chitosan in solution greatly depends not only on structural parameters like the degree of acetylation and chain length but also on solution parameters, such as ionic strength, solvent, temperature, pH, dielectric constant of the solvent, etc. (Sorlier et al., 2002). The properties of the solvent (ionic concentration, pH) have a very important role in the properties of chitosan-based FFD. Table 5.1 shows the effect of both the pH and ionic strength of the solvent (sodium acetate buffer 175 mM) on the particle size, expressed as volume-length diameter (d43) and area-volume mean diameter (d32), and ζ-potential of FFD prepared with 1% high molecular weight chitosan and 2% oleic acid and homogenized by means of a rotor-stator. As the pH and the ionic strength of the solvent increased (solvent II), ζ-potential values became less positive, as a result of the depro-tonation of the -NH3 + groups of chitosan (pKa = 6.2) and electrostatic screening effects, which make chitosan molecules lose their positive surface charge. As a consequence, the electrostatic interactions between chitosan and oleic acid (pKa = 4.8) would be expected to weaken. The latter can also explain the smaller particle size (lower d43 and d32 values) measured in the FFD at solvent II conditions as compared to solvent I.
Table 5.1
Particle size and surface charge (ζ-potential) of 1% chitosan-2% oleic acid film-forming dispersions as affected by pH and ionic strength (I). Mean values and standard deviations (in parentheses)

a,bDifferent letters indicate 95% signifi cant differences.
5.5.2 Effect of the incorporation of lipids
The incorporation of a lipid dispersed phase (such as essential oils) leads to significant changes in the properties of chitosan FFD and films. Table 5.2 shows the ζ-potential, particle size (d43 and d32), and apparent viscosity values (at a shear rate of 100 s– 1) of FFD prepared with chitosan and different concentrations of essential oils (EO): bergamot (B) and tea tree essential oil (TTO). The increase in EO content increased the mean particle size and decreased the ζ-potential of the particles, when compared to the values of the chitosan dispersion. Thus, the increase in EO content led to bigger droplets with lower electrical net charge. The reduction in the electrical net charge of chitosan-EO particles (decrease in ζ-potential) when EO content was increased could be explained by the presence of electrostatic interactions between chitosan and EO components. At the pH of the FFD (around 4.3), the amino group of chitosan (pKa NH3+/NH2 ≈ 6.5) is positively charged and could be partially neutralized through the interactions at the O/W interface with some polar groups of essential oils, which in the absence of chitosan showed negative charge particles with lower mean particle size. Thus, chitosan chains are adsorbed on the EO droplets, leading to bigger positively charged particles. Similar results were found when using oleic acid as the dispersed phase in the FFD at pH 5.2. At this pH, the amino group of chitosan is partially neutralized through the interactions with the carboxylate function of oleic acid (pKa COOH/COO– ≈ 4.8), which is negatively charged (Vargas et al., 2009a).
Table 5.2
ζ-potential, particle size (d43 and d32) and apparent viscosity (ηap) at 100– 1 and 25°C of chitosan (CH) and chitosan-essential oils (CH-EO) film forming dispersions (FFD) containing bergamot (B) or tea tree (TTO) essential oils. Mean values and standard deviations

a,b,c,dDifferent letters indicate signifi cant differences (p < 0.05).
The steric stabilization promoted by the chitosan interfacial adsorption and the high values of particle ζ-potential (significantly higher than + 30 mV) ensure the stability of these kinds of emulsified systems (Roland et al., 2003) and, as has been proved in additional experiments (data not shown), chitosan-essential oil and chitosan-oleic acid particles remained well dispersed for a long time, without evidence of flocculation or creaming.
The apparent viscosity values decreased when the EO content increased, but this effect was not significant. This is not how oil-in-water emulsions are expected to behave, as viscosity usually increases when the concentration of the dispersed phase increases. As deduced from droplet size and ζ-potential values, chitosan molecules are adsorbed on EO droplets, thus reducing their viscous contribution in the continuous phase. In this sense, the increase in the EO ratio led to a greater reduction not only in the amount of chitosan available in the aqueous phase but also in the particle charge, while increasing the mean particle size. All these effects can explain the viscosity decrease when EO ratio increased in the emulsion. Similar results were found when incorporating 2% (v/w) oleic acid in chitosan FFD (Vargas et al., 2009a) and when chitosan film-forming dispersions were enriched with 1% basil or thyme essential oils (Bonilla et al., 2012).
As seen in Table 5.2, the nature of the EO (bergamot or tea tree oil) also promoted changes in the particle size, surface charge and apparent viscosity values of the FFD. In this sense, FFD prepared with tea tree essential oils were more viscous and presented particles with lower size and slightly more charged. These effects could be due to the different oil composition, thus affecting the relationship with the dispersed and the continuous phase.
5.5.3 Effect of homogenization conditions
The combination of chitosan with lipids to formulate coatings with improved barrier properties requires the use of homogenization treatments. The stability of such film-forming dispersions depends on complex mechanisms, but it is well known that some of the key aspects are the average particle size and their distribution pattern which, in turn, affect the stability, colour and rheological properties of the FFD, among other factors (Becher, 2001). Rotor-stator systems have been used to homogenize chitosan-based FFD incorporated with lipids, reaching particle sizes in the range of 1 (Vargas et al., 2009a; Sánchez-González et al., 2010a, 2010b). To reduce the size of the lipid particles dispersed in chitosan-based FFD, a Microfluidizer® that can provide homogenization pressures up to 200 MPa has been also used (Vargas et al., 2011a; Bonilla et al., 2012). Microfluidization can provide dispersions and emulsions with narrower particle size distributions due to the high shear stresses developed in the microchannels of the interaction chamber. Table 5.3 shows the average particle size of 0.5% chitosan-0.5% oleic acid FFD as affected by different homogenization conditions: rotor-stator or rotor-stator homogenization combined with microfluidization in a single step at two different pressures. The particles were smaller and the particle size distributions tend to be narrower as the homogenization pressure increased. Moreover, the increase in homogenization pressure led to a decrease in the ζ-potential of the particles, which was significant at 160 MPa. The latter can be explained by the promotion of electrostatic interactions between the oleic acid micelles and chitosan with the subsequent decrease in the net surface electrical charge of chitosan-oleic acid particles due to microfluidization.
Table 5.3
Average particle size (d43, d32) of 0.5% chitosan–0.5% oleic acid fi lmforming dispersions as affected by homogenization conditions. Mean values and standard deviations
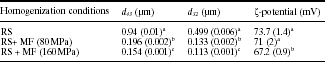
a,b,cDifferent letters indicate significant differences (p < 0.05). RS: rotor-stator; MF: Microfl uidizer®.
On the other hand, some studies have also pointed out that high homog-enization pressures can affect the properties of chitosan in solution. In fact, apparent viscosity of chitosan-based FFD significantly decreased as the homogenization pressure increased (Vargas et al., 2011a; Bonilla et al., 2012). The latter could be explained not only by changes in the degree of polymer aggregation but also by the fragmentation of the polymer and the subsequent decrease in its molecular weight due to high pressure, as it was observed in chitosan-acetic acid solutions (Kasaai et al., 2003) and xanthan gum systems (Paquin, 1999) submitted to microfluidization.
5.6 Physicochemical characteristics of chitosan-based coatings
Chitosan coatings have a selective permeability to gases (CO2 and O2) and good mechanical properties. However, they are highly permeable to water vapour, which is an important drawback since effective control of moisture transfer is a desirable property for most food applications. In order to improve the water barrier properties of chitosan coatings, lipid compounds such as fatty acids and vitamin E, and/or less hydrophilic hydrocolloids like methylcellulose can be incorporated into chitosan-based matrices.
5.6.1 Effect of the incorporation of lipids
Lipids are incorporated in the formulation of chitosan-based films and coatings in order to improve their water vapour barrier properties, which in turn can have an effect in the microstructural, mechanical and optical properties of the coatings. Table 5.4 shows the mechanical characteristics of chitosan essential oils composite coatings, in terms of percentage of elongation at break (E%), tensile strength (TS) and elastic modulus (EM). Moreover, the water vapour permeability (WVP) and the gloss at an incidence angle value of 60° of the pure and composite chitosan coatings equilibrated at 54.4% and 20 °C are also reported. The incorporation of the EO dispersed phase led to coatings that were softer, less resistant to break and less stretchable. This could be explained by discontinuities in the polymer matrix introduced by the lipid incorporation and by changes in the polymer chain interactions when oil components are present, which led to a weak mechanical response. These results coincide with those reported by Vargas et al. (2009a) when adding an unsaturated fatty acid (oleic acid) at a chitosan : oleic acid ratio higher than 1 : 1 into the chitosan matrix.
Table 5.4
Percentage of elongation at break (E%), tensile strength (TS) and elastic modulus (EM), water vapour permeability (WVP) at 20°C, RH gradient of 100%/54% and gloss values of chitosan and chitosan-essential oils composite coatings. Mean values and standard deviations
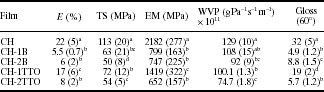
a,b,cDifferent letters in the same column indicate signifi cant differences (p < 0.05). CH: chitosan; B: bergamot essential oil; TTO: tea tree essential oil.
The effect of the increment in the EO concentration depended on type of EO incorporated, which can be explained by the different particle size (Table 5.2) and the nature of interactions established with the polymer matrix.
Lipid compounds can enhance the water barrier properties of polymer-based films due to their hydrophobic nature. In this way, WVP values of chitosan-EO composite films showed a decrease in line with the increase in EO concentration. This reduction in WVP values (around 30-40% with regard to pure chitosan films) was not notably affected by the nature of the EO. The decrease in WVP shown in composite films is coherent with the reduction of the hydrophilic phase (polysaccharide) ratio where water molecules diffuse preferentially. A similar trend was found when incorporating an unsaturated fatty acid, such as oleic acid into the chitosan matrix (Vargas et al., 2009a).
The oil phase introduces an increase in the tortuosity factor for water transfer in the matrix, thus increasing the distance travelled by water molecules diffusing through the coating. The tortuosity factor is higher when the oil phase ratio increases or when the oil particle size is reduced (Morillon et al., 2002). As we have commented on above, the use of micro-fluidization led to a significant reduction in the particle size of chitosan-oleic acid FFD (higher number of dispersed particles in the chitosan matrix) as compared with rotor-stator (RS) homogenization, thus increasing the tortuosity factor and promoting a significant decrease in the WVP (Vargas et al., 2011a).
The gloss of the films is linked to the morphology of their surface. In general, the smoother the surface, the glossier they are (Ward and Nussinovitch, 1996). Gloss values of chitosan-EO composite films measured at incidence angle values of 60° are shown in Table 5.4. The addition of EO to the chitosan matrix led to a decrease in gloss, especially for chitosan-bergamot essential oil composite coatings, regardless of EO concentration. For chitosan-tea tree essential oil composite coatings, a smaller gloss reduction was observed, which, in this case, was dependent on the EO concentration. In this sense, the decrease in gloss with the incorporation of EO could be explained by an increase in the surface roughness of the composite coatings. This roughness appears as a consequence of the migration of droplets or aggregates to the top of the coating during drying, which leads to surface irregularities. Since essential oils are highly volatile, plenty of voids appeared in the coating surface which contributes to the gloss reduction. Differences found between bergamot and tea tree essential oils could be related with the higher stability of chitosan-tea tree essential oil FFD, which is reflected in the smaller droplets and narrower particle size distribution deduced from the smaller difference between d43 and d32 values (Table 5.2), leading to a different quantity and size of voids in the film surface. On the other hand, the gloss of chitosan films can be increased as a consequence of the incorporation of a high amount of unsaturated fatty acids such as oleic acid. In this case, the reported increase in gloss in line with the increase in oleic acid content could be explained by the coalescence and creaming of the oleic acid droplets during drying. This leads to a decrease in surface roughness and an increase in specular reflectance in the air-film interface, since oleic acid is in a liquid state and can fill the small surface voids generated during coating formation (Vargas et al., 2009a).
5.6.2 Effect of the interaction with other polymer matrices
The water barrier properties of chitosan-based films can also be improved by combining chitosan with other biopolymers that soften its hydrophilic character (Park et al., 2002; Xu et al., 2004). To this end, Hoagland and Parris (1996) developed chitosan/pectin laminated films by interacting cationic groups of chitosan with the anionic groups of pectin. Xu et al. (2004) observed a decrease in water vapour transmission rates by combining chi-tosan with two thermally gelatinized corn starches. Pinotti et al. (2007) studied the mechanical properties and the microstructure of chitosan-based films as affected by methylcellulose content and García et al. (2004) evaluated the water vapour permeability of chitosan-methylcellulose composite films, which had intermediate permeability values as compared to the films prepared with the pure components.
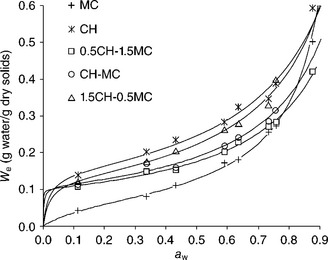
The water sorption isotherms (WSI) of pure high molecular weight chitosan and methylcellulose films together with the composite films prepared by mixing both polymers at different ratios are shown in Fig. 5.1. The WSI were sigmoid in shape, increasing slowly in line with aw up to values around 0.45, beyond which a steep rise in moisture content was observed, owing to the solubilization phenomenon. The highest water sorption capacity was observed for pure chitosan coatings in agreement with their greater hygroscopic nature. According to Fernández Cervera et al. (2004), chitosan has three predominant adsorption sites: hydroxypropyl group, amine group and polymer chain end (composed of a hydroxyl group or aldehyde group). Experimental sorption data were fitted to the Guggenheim-Andserson-De Beer (GAB) model and the results are reported in Table 5.5 together with the water vapour permeability values. For chitosan-methylcellulose composite films, monolayer moisture content (W0) increased when the chitosan content increased, due to the greater hygroscopic nature of this polymer and to the stronger interactions established between water molecules and the substrate at low aw values, where no solubilization phenomena occur. The values of WVP of pure and composite films at 5 °C and a RH gradient of 100/59% are also shown in Table 5.5. Composite films have WVP values lower than chitosan or methylcellulose pure films. For composite films, the increase chitosan content led to a significant increase in WVP values, in agreement with the higher sorption capacity of chitosan. As the chitosan ratio in the composite coating increases, the equilibrium moisture content of the coatings increases (Fig. 5.1), which plasticizes the coating structure and promotes water mobility and diffusion, thus contributing to an increase in WVP (Vargas et al., 2011b).
Table 5.5
GAB parameters at 5°C and water vapour permeability (WVP) at 5°C and a RH gradient of (100%/59%) of pure chitosan (CH) or methylcellulose (MC) and CH-MC composite coatings. Mean values and standard deviations (in parentheses)
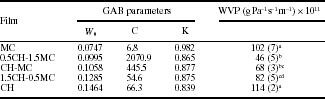
a,b,c,dDifferent letters indicate signifi cant differences (p < 0.05). W0: (g water/g dry solids); C, K: constants related to the heat of sorption of the monolayer and the multilayer, respectively; GAB: Guggenheim-Anderson-De Boer
5.7 Antimicrobial activity of chitosan-based coatings
Numerous studies emphasize the wide spectrum of antimicrobial action of chitosan, which is effective against fungi, yeasts and bacteria. Several hypotheses about the mechanism of action of chitosan have been suggested, for when this compound is added both in liquid or in solid media. However, the mechanisms of bacterial and fungal inhibition have not been clearly explained for chitosan-based coatings. In this case, other external factors such as anaerobic conditions should be considered. For instance, the addition of other polysaccharides may potentiate the antimicrobial effectiveness of chitosan coatings. In this sense, Rao et al. (2010) reported that the incorporation of guar gum to chitosan at 15% (v/v) led to further decrease in Escherichia coli counts, although no significant differences were observed in terms of Staphylococcus aureus inhibition. Nevertheless, for most of the concentrations of guar gum tested, the antimicrobial effectiveness of chito-san coatings decreased. This phenomenon has been explained by a reduction in the availability of the active groups of chitosan since intermolecular hydrogen bondings were formed between NH3+ and OH– groups of chitosan and guar gum, respectively.
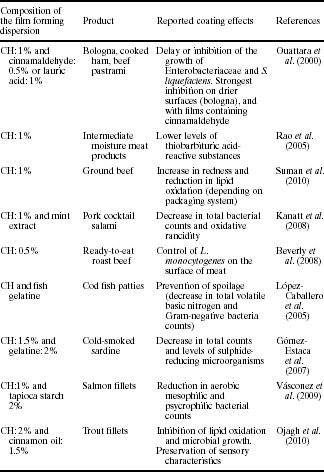
The incorporation of antimicrobial compounds such as peptides, metals or essential oils onto the polymeric matrix can also enhance the antimicrobial action of chitosan coatings. Some examples reported in the literature are shown in Table 5.6. The most recent approaches deal with the addition of nanoparticles to the chitosan matrix. Silver nanoparticle-loaded chitosan-starch based coatings exhibited bactericidal performance against E. coli, S. aureus and B. cereus (Yoksan and Chirachanchai, 2010). Moreover, the combination of silver nanoparticles with zinc oxide further improved these positive results (L. Li et al., 2010).
Table 5.6
Chitosan-based films with reported antimicrobial activity
| Coating composition | Target microorganisms | Reference |
| Chitosan | Aspergillus niger | Martínez-Camacho et al. (2010) |
| Chitosan | Listeria monocytogenes Escherichia coli Salmonella typhimurium |
Kim et al. (2010) |
| Chitosan/poly (jV-vinyl-2-pyrrolidone), polyethylene oxide | Escherichia coli | J Li et al. (2010) |
| Sweet potato starch/chitosan | Escherichia coli Staphylococcus aureus |
Shen et al. (2010) |
| Chitosan/guar gum | Escherichia coli Staphylococcus aureus |
Rao et al. (2010) |
| Chitosan/nisin | Escherichia coli Staphylococcus aureus Listeria monocytogenes Bacillus cereus |
Li et al. (2006) |
| Chitosan/starch/Ag nanoparticle | Escherichia coli Staphylococcus aureus Bacillus cereus |
Yoksan and Chirachanchai (2010) |
| Chitosan/Ag/ZnO | Staphylococcus aureus Escherichia coli Bacillus subtilis Penicillium Aspergillus Rhizopus Yeast |
L Li et al. (2010) |
| Chitosan/galangal extract Chitosan/garlic oil, potassium sorbate, nisin | Staphylococcus aureus Escherichia coli Listeria monocytogenes Staphylococcus aureus Bacillus cereus Salmonella typhimurium |
Mayachiew et al. (2010) Pranoto et al. (2005) |
| Chitosan/oregano oil | Escherichia coli Listeria monocytogenes |
Zivanovic et al. (2005) |
| Chitosan/cinnamon oil | Escherichia coli Listeria monocytogenes Lactobacillus plantarum Lactobacillus sakei Pseudomonas fluorescens |
Ojagh et al. (2010) |
| Chitosan/clove oil | Escherichia coli Pseudomonas fluorescens Listeria innocua Lactobacillus acidophilus |
Gómez-Estaca et al. (2010) |
| Chitosan/tea tree oil | Penicillium italicum Listeria monocytogenes |
Sánchez-González et al. (2010a) |
| Chitosan/bergamot oil | Penicillium italicum | Sánchez-González et al. (2010b) |
In line with the increasing consumer demand for healthier and safer natural preservatives, natural plant extracts (mainly essential oils or pure substances derived from them) have also been extensively studied as antimicrobial compounds that can be potentially incorporated into edible films and coatings (Sánchez-González et al., 2011a). Several studies have pointed out the antimicrobial effectiveness of chitosan-essential oils composite coatings (Zivanovic et al., 2005; Ojagh et al., 2010; Sánchez-González et al., 2010a, 2010b). The extent of the antimicrobial efficacy of the chitosan-essential oil film depends on the target microorganisms. The effect of pure chitosan and chitosan-essential oil composite coatings on the in vitro growth and survival of E. coli at 10 °C is shown in Fig. 5.2(a). Pure chitosan films presented a significant antimicrobial activity against E. coli, showing a complete inhibition of the microbial growth during the whole storage period. However, this high antimicrobial effectiveness was not observed in the composite films, possibly because of the dilution effect of chitosan when bergamot oil or tea tree essential oils are present, thus being less available for microorganisms. However, as shown in Fig. 5.2(b), this behaviour is different from that observed for Penicillium italicum. Pure chitosan films were not effective against this fungus but chitosan-essential oils composite films presented significant antifungal properties.
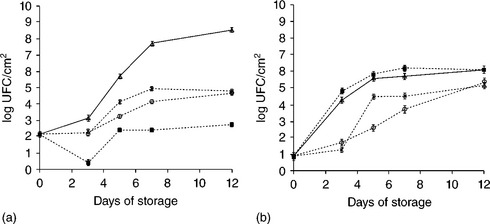
Fig. 5.2 Effect of chitosan (CH) enriched with bergamot (B) or tea tree (TTO) essential oils coatings on the growth and survival of (a) Escherichia coli and (b) Penicillium italicum. Mean values and 95% LSD intervals (![]() control, • CH, O CH-B, * CH-TTO).
control, • CH, O CH-B, * CH-TTO).
Another issue that arises when incorporating other antimicrobial compounds into chitosan-based coatings is the the control of the release of antimicrobial compounds, which is a complex phenomenon. The diffusion and release of the antimicrobials is dependent on numerous factors such as film microstructure, food characteristics and storage conditions (Cagri et al., 2004). In this sense, Sánchez-González et al. (2011b) evaluated the antimicrobial properties of chitosan films enriched with essential oils against three foodborne pathogens (E. coli, L. monocytogenes and S. aureus) and concluded that the microstructure of the coating and the possible interactions existing between chitosan and the constituents of essential oils must be considered in order to understand the antimicrobial effectiveness of such coatings.
5.8 Application of chitosan coatings to fresh and minimally processed foods
The use of chitosan edible films and coatings to extend the shelf life of fresh and processed fruits and vegetables, as well as meat products and seafood has been extensively examined and will be discussed in the following sections.
5.8.1 Application of chitosan coatings to fresh fruits and vegetables
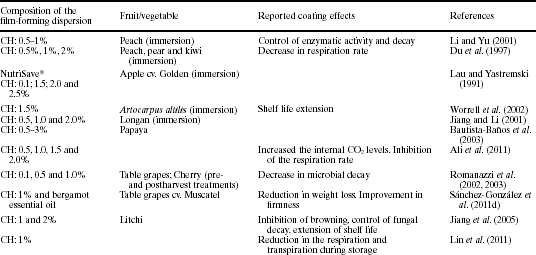
Chitosan-based coatings have been applied to a great variety of fresh fruits and vegetables (Table 5.7). The reported effects of coatings include, among others, a reduction in respiration rate, a preservation of fruit colour and firmness, and in general an extension of product shelf life. The incorporation of minor constituents, such as fatty acids and essential oils, into the chitosan matrix can improve the water vapour barrier of coatings, thus diminishing the product's weight loss. Fig. 5.3 shows the changes in weight loss for non-coated grapes and those coated with chitosan or chitosan-bergamot essential oil film-forming dispersions throughout cold storage. Weight loss occurred mainly during the first days of storage and was more pronounced for non-coated samples and those coated with pure chitosan, which did not provide an effective water barrier. The incorporation of bergamot essential oil to the chitosan matrix improved the water barrier properties of the coatings since the lower weight loss was detected for samples coated with chitosan-bergamot essential oil throughout all the storage time. This is in agreement with the hydrophobic nature of bergamot oil and coincides with the reported trend for the water vapour permeability (WVP) of the standalone films (Table 5.4). Moreover, weight loss changes were in agreement with the evolution detected in fruit firmness. The greater the water loss the lower fruit turgor and thus the lower values of firmness (Sánchez-González et al., 2011c). Figure 5.4 shows the respiration rate of non-coated and coated grapes during cold storage. Chitosan-based coatings enriched with berga-mot essential oil (CH-B) inhibited both O2 consumption and CO2 generation throughout all the storage time. The latter can be associated with the low oxygen permeability values of chitosan-based coatings. Moreover, the highly charged chitosan chains seemed to develop strong interactions with bergamot essential oil compounds, which can modify the gas barrier properties of coatings to a great extent (Sanchez-Gonzalez et al., 2011c).
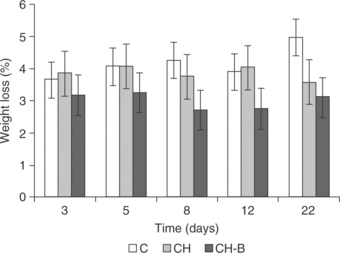
Fig. 5.3 Weight loss of grapes cv. Muscatel as affected by chitosan-based coatings during cold storage. Mean values and 95% LSD intervals. CH: chitosan; B: bergamot essential oil; C: non-coated.
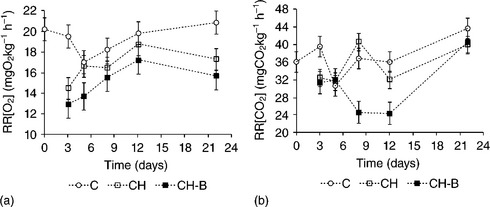
Fig. 5.4 Respiration rate in terms of oxygen consumption (a) and carbon dioxide generation (b) of cold-stored grapes cv. Muscatel as affected by chitosan-based coatings. Mean values and 95% LSD intervals. CH: chitosan; B: bergamot essential oil; C: non-coated.
Other reported effects of chitosan-based coatings are related with changes in the colour and appearance of the coated product. Fig. 5.5(a) shows the changes in luminosity (L*) of strawberries cv. Camarosa during cold storage. A decrease in the luminosity after coating application was detected in samples coated with the formulation containing oleic acid (CH-OA). The latter was explained by changes in the surface reflection properties when the fruit was coated (Vargas et al., 2006b). Changes in luminosity values of non-coated and coated samples throughout storage are plotted in Fig. 5.5(b). Colour was not measured on the tenth day of storage in non-coated strawberries because of fungal decay. Luminosity decreased both in non-coated samples and in those coated with pure chitosan (CH), which showed a darker colour due to surface dehydration. Moreover, the incorporation of oleic acid into the coating led to the lowest luminosity changes in agreement with the lower WVP of these coatings (Vargas et al., 2009a). On the other hand, Sanchez-Gonzalez et al. (2011c) observed that pure chitosan coatings applied to white grapes promoted an increase in fruit luminosity and softened the colour development during cold storage, thus improving the product's appearance.
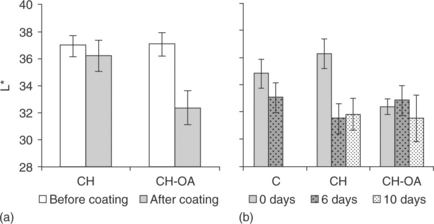
Fig. 5.5 Changes in luminosity of strawberries cv. Camarosa after coating application (a) and evolution of luminosity values during storage at 5 °C (b). Mean values and 95% LSD intervals. CH: chitosan; OA: oleic acid; C: non-coated.
Chitosan-based coatings have proved to be effective in the control of postharvest decay of different fruits and vegetables. The evolution of fungal decay in terms of percentage of fruits with visible signs of fungal decay is shown in Fig. 5.6. The application of chitosan-based coatings led to a decrease in the percentage of infected strawberries in comparison to non-coated strawberries, which started to decay from the beginning of storage. Moreover, the addition of oleic acid seems to enhance chitosan antifungal effect since strawberries coated with chitosan-oleic acid film-forming dispersions showed fewer signs of fungal infection during storage.
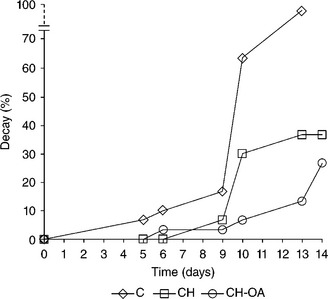
Fig. 5.6 Evolution of fungal decay (% of fruits with visible signs of fungal decay) in cold-stored strawberries coated with chitosan (CH) or chitosan-oleic acid edible coatings (CH-OA). C: non-coated.
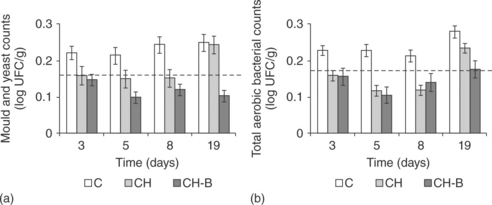
The antimicrobial effect of chitosan-based coatings has been also shown through the results of the microbiological counts performed with coated and non-coated grapes cv. Muscatel throughout storage at 5 °C (Fig. 5.7). The microbial counts at the beginning of the storage, indicated with a discontinuous line, were 0.167 ± 0.018 and 0.175 ± 0.013 log UFC/g for moulds and yeasts and total aerobic bacterial counts, respectively. It is remarkable that, for both non-coated and coated fruits, the microbial safety of grapes was maintained for 19 days of cold storage since the number of microorganisms remained below 0.4 log UFC/g, which is the maximum value allowed by legislation. The protective role of coatings was relatively low, although coatings enhanced the effect of initial washing and maintained the level of microorganisms at values lower than that of fresh grapes (commented on above). On the other hand, until 8 storage days the number of both mould and yeasts and mesophiles for coated samples remained lower than the respective uncoated samples. In both microbial counts, bergamot oil enhanced the antimicrobial activity of pure chitosan coatings.
5.8.2 Application of chitosan coatings to minimally processed fruits and vegetables
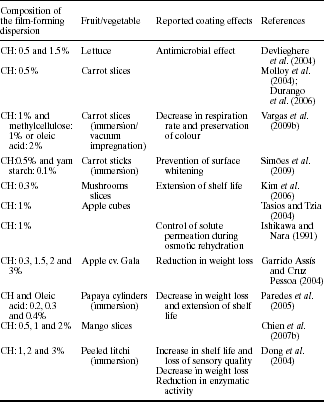
Chitosan-based coatings have been used as a method to extend the shelf life of different types of fresh-cut or minimally processed fruits and vegetables. As seen in Table 5.8, the reported effects are similar to that found for fresh fruits and vegetables, with a special emphasis on the inhibition of enzymatic browning that can occur after cutting vegetal tissues.
In some cases, chitosan-based coatings preserve or even enhance the initial colour of the coated fresh-cut samples. That is the case, for instance, with fresh-cut carrots, where chitosan coatings prevented or delayed the occurrence of white-blush during storage. White blush in fresh-cut carrots is caused, among other factors, by surface dehydration, and the latter may be delayed by maintaining an excess of moisture on the carrot's surface. In this sense, the use of highly hydrophilic coatings, such as chitosan-based coatings, led to the existence of a wet film that covers the cut surface of fresh-cut carrot thus reducing the whiteness index of coated samples (Vargas et al., 2009b).
5.8.3 Application of chitosan coatings to meat and fish products
Chitosan-based films have also been used to extend the shelf life of fish and meat products, alone or in combination with other antimicrobials and anti-oxidant agents (Table 5.9). Meat and fish products are very prone to lipid oxidation and microbial spoilage, thus the application of a natural compound with both antimicrobial and antioxidant effect such as chitosan shows a great potential in terms of meat safety and preservation.
Microbial contamination of meat and fish products occurs mainly at the surface. The use of chitosan-based coatings containing antimicrobial agents could be an alternative to extend meat shelf life, by maintaining high concentrations of antibacterial ingredients where they are required throughout the whole storage period. In this sense, the antimicrobial nature of chitosan coatings and their ability to act as a carrier of other antimicrobial substances makes them very useful to prevent microbial growth in fresh meat. Fig. 5.8 shows coliform and total aerobic bacterial counts of pork meat hamburgers as affected by chitosan films as compared with non-coated samples during storage at 5 °C. The initial counts (indicated with a dashed line) were 1.1 ± 0.4 and 2.5 ± 0.1 log UFC/g, for coliforms and total aerobic bacteria counts, respectively. Chitosan-based films showed bacteriostatic or even, in some sense, a bactericide effect, these effects being more significant against coliform bacteria. This is in accordance with the significant antibacterial effect of chitosan against E. coli found in in vitro studies (Sanchez-Gonzalez et al., 2011b).
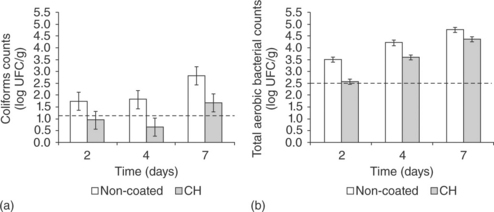
Fig. 5.8 Total viable and coliforms counts in pork meat hamburgers during storage at 5 °C as affected by the application of chitosan (CH) films. Initial counts are indicated with a dashed line. Mean values and 95% LSD intervals.
The antibacterial effect of chitosan films against L. monocytogenes has also been investigated in ready-to-eat roast beef. The results showed that bacterial counts were reduced by 2 to 3 log after 14 days of storage (Beverly et al., 2008). A similar effect against L. monocytogenes was obtained when the films were applied to processed hams (Ouattara et al., 2000).
Some studies have demonstrated the ability of chitosan to act as a preservative in fish. For instance, chitosan-fish oil coatings reduced lipid oxidation and reduced total aerobic bacteria and psychotropic microorganisms in fresh lingcod (Duan et al., 2010). In a similar way, chitosan-cinnamon essential oil coatings improved the quality of refrigerated rainbow trout (Ojagh et al., 2010).
Thus, the use of chitosan-based edible coatings seems to be a promising technique to preserve the quality and extend the shelf life of meat and fish products.
5.9 Future trends
The potential of chitosan, as shown by research and development efforts, is supported and enhanced by both the increasing consumer demand for natural and safer additives with functional properties, and increasing environmental concerns. It is likely that some of the future applications of chitosan in the food area will come from the medical and pharmaceutical sectors, where it has been extensively used because of its antioxidant and antimicrobial properties. Potentially, chitosan could be incorporated into recycled materials such as multilayer plastic packaging materials in which each layer of polymer would have a specific function: i.e., chitosan could act as antimicrobial/antioxidant agent and another material could act as a water vapour barrier. Moreover, since chitosan properties are much affected by the pH and the ionic strength of the surrounding medium, chitosan-based systems could also be used for the controlled release of active ingredients. Following this approach, composite polyelectrolyte blends combining negatively charged polymers such as pectins with chitosan have already been developed (Bernabé et al., 2005; Marudova et al., 2005).
Chitosan shows a great potential to encapsulate bioactive compounds and protect them from the detrimental effect of oxygen, high temperatures and light during processing and storage of foods. Vitamin C has been successfully encapsulated with chitosan nanoparticles. These nanoparticles were incorporated in the diet of rainbow trout and the results showed that chitosan-vitamin C nanoparticles have a promising potential to increase shelf life and delivery of vitamin C in biological systems (Alishahi et al., 2011). The electrostatic extrusion technique has been applied to immobilize polypheno-lic extracts of six different medicinal herbs in alginate-chitosan copolymer microbeads. In order to increase the solubility of chitosan, ascorbic acid was used, which additionally increased the antioxidant potential of encapsulated herbal polyphenolic extracts (Belscak-Cvitanovic et al., 2011).
In order to design efficient antimicrobial packaging, it is important to determine the diffusion rates of the active compounds. However, release kinetics of antimicrobial substances from biodegradable films based on chitosan to food products has been little explored. Some attempts have been made by using food simulants. Sanchez-Gonzalez et al. (2011d) evaluated the release kinetics of limonene in chitosan films enriched with different concentrations of bergamot essential oil in different food simulating solvents (ethanol aqueous solutions and isooctane). The results indicated that the antimicrobial effectiveness of chitosan-bergamot essential oil composite films could be improved by promoting higher water content in the films since it favours limonene diffusion to the product surface. In contact with non-polar foods, such as fats, some active compounds will be released very slowly and their effectiveness could be limited, although the solubiliza-tion in the food of non-polar compounds like limonene is more feasible. However, more studies performed with real food systems are required since the antimicrobial release depends not only on film structure, solvent and migrant polarities and solubility of active compounds but also on the characteristics of the coated product.
Another important issue that needs to be addressed to achieve future developments is to standardize chitosan production to obtain a reproducible material. In fact, the batch-to-batch variations found in chitosan samples supplied under the same brand name with identical reported degree of acetylation and molecular weight leads to different properties in terms of viscosity, solubility, colour, antioxidant and antimicrobial activity and thus to non-reproducible measurements. These variations among, theoretically the same chitosan samples, are an important drawback when using chitosan which could be solved by improving the characterization procedures used by the manufacturers.
A further challenge is to reduce the cost of chitosan-based coatings, which is still relatively high. Thus, it seems essential to combine chitosan with other biodegradable ingredients with film-forming ability and thermoplastic properties such as starch or some proteins in order to allow chitosan to succeed as a real food packaging material.
5.10 Acknowledgements
The authors acknowledge the financial support provided by Universitat Politècnica de València (Project PAID-06-09-2834), Conselleria de Empresa, Universidad y Ciencia (GV/2010/082) and Ministerio de Ciencia e Innovación (AGL2010-20694).
5.11 References
Ali, A., Muhammad, M.T.M., Sijam, K., Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Caricapapaya L.) fruit during cold storage. Food Chem. 2011; 124:620–626.
Alishahi, A., Mirvaghefi, A., Tehrani, M.R., Farahmand, H., Shojaosadati, S.A., Dorkoosh, F.A., Elsabee, M.Z. Shelf life and delivery enhancement of vitamin C using chitosan nanoparticles. Food Chem. 2011; 126:935–940.
Bautista-Baños, S., Hernández-López, M., Bosquez-Molina, E., Wilson, C.L. Effects of chitosan and plant extracts on growth of Colletrichum gloesosperioides, anthracnose levels and quality of papaya fruit. Crop Prot. 2003; 22:1087–1092.
Becher, P. Emulsions: Theory and Practice. Oxford: Oxford University Press; 2001.
Belscak-Cvitanovic, A., Stojanovic, R., Manojlovic, V., Komes, D., Cindric, I.J., Nedovic, V., Bugarski, B. Encapsulation of polyphenolic antioxidants from medicinal plant extracts in alginate-chitosan system enhanced with ascorbic acid by electrostatic extrusion. Food Res Int. 2011; 44:1094–1101.
Bernabé, P., Peniche, C., Argüelles-Monal, W. Swelling behaviour of chitosan/ pectin polyelectrolyte complex membranes. effect of thermal cross-linking. Polym Bull. 2005; 55:367–375.
Beverly, R.L., Janes, M.E., Prinyawiwatkul, W., No, H.K. Edible chitosan films on ready-to-eat roast beef for the control of Listeria monocytogenes. Food Microbiol. 2008; 26:534–537.
Bonilla, J., Atarés, L., Vargas, M., Chiralt, A. Effect of essential oils and homog-enization conditions on properties of chitosan based films. Food Hydrocolloid. 2012; 26:9–16.
Cagri, A., Ustunol, Z., Ryser, E.T. Antimicrobial edible films and coatings. J Food Prot. 2004; 67:833–848.
Cheah, L.H., Page, B.B.C., Shepherd, R. Chitosan coating for inhibition of Sclerotinia rot of carrots. New Zeal J Crop Hort. 1997; 25:89–92.
Chien, P.Y., Sheu, F., Lin, H.R. Coating citrus (Murcott tangor) fruit with low molecular weight chitosan increases postharvest quality and shelf life. Food Chem. 2007; 100:1160–1164.
Chien, P.J., Sheu, F., Yang, F.H. Effects of edible chitosan coating on quality and shelf life of sliced mango fruit. J Food Eng. 2007; 78:225–229.
Cuero, R.G. Antimicrobial action of exogenus chitosan. In: Jollés P., Muzzarelli R.A.A., eds. Chitin and Chitinases. Basel, Switzerland: Birkhäuser Verlag; 1999:315–333.
Del Blanco, L.F., Rodríguez, M.S., Schulz, P.C., Agulló, E. Influence of the deacetylation degree on chitosan emulsification properties. Colloid Polym Sci. 1999; 277:1087–1092.
Devlieghere, F., Vermeulen, A., Debevere, J. Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004; 21:703–714.
Dong, H., Cheng, L., Tan, J., Zheng, K., Jiang, Y. Effects of chitosan coating on quality and shelf life of peeled litchi fruit. J Food Eng. 2004; 64:355–358.
Du, J., Gemma, H., Iwahori, S. Effects of chitosan on the storage of peach, Japanese pear and kiwifruit. J Jpn Soc of Hortic Sci. 1997; 66:15–22.
Duan, J., Cherian, G., Zhao, Y. Quality enhancement in fresh and frozen lingcod (Ophiodon elongates) fillets by employment of fins oil incorporated chitosan coatings. Food Chem. 2010; 119:524–532.
Durango, A., Soares, N.F.F., Andrade, N.J. Microbiological evaluation of an edible antimicrobial coating on minimally processed carrots. Food Control. 2006; 17:336–341.
El Ghaouth, A., Ponnampalam, R., Castaigne, F., Arul, J. Chitosan coating to extend the storage life of tomatoes. Hortscience. 1992; 27:1016–1018.
El Ghaouth, A., Arul, J., Wilson, C., Benhamou, N. Ultrastructural and cytochemi-cal aspects of the effect of chitosan on decay of bell pepper fruit. Physiol Mol Plant P. 1994; 44:417–432.
Feng, T., Yumin, D., Li, J., Hu, Y., Kennedy, J.F. Enhancement of antioxidant activity of chitosan by irradiation. Carbohydr Polym. 2007; 73:126–132.
Fernández Cervera, M., Karjalainen, M., Airakisan, S., Rantanen, J., Krogars, K., Heinäimäki, J., Iraizoz Colarte, A., Yliruusi, J. Physical stability and moisture sorption of aqueous chitosan-amylose starch films plasticized with polyols. Eur J Pharm Biopharm. 2004; 58:69–76.
Galed, G., Fernandez-Valle, M.E., Martinez, A., Heras, A. Application of MRI to monitor the process of ripening and decay in citrus treated with chitosan solutions. Magn Reson Imaging. 1994; 22:127–137.
García, M.A., Pinotti, A., Martino, N.M., Zaritzky, N.E. Characterization of composite hydrocolloid films. Carbohydr Polym. 2004; 56:339–345.
Garrido Assis, O.B., Cruz Pessoa, J.D. Scientific note: Preparation of thin films of chitosan for use as edible coating to inhibit fungal growth on sliced fruits. Braz J Food Technol. 2004; 7:17–22.
Gómez-Estaca, J., Montero, P., Giménez, B., Gómez-Guillén, M.C. Effect of functional edible films and high pressure processing on microbial and oxidative spoilage in cold-smoked sardine (Sardina pilchardus). Food Chem. 2007; 105:511–520.
Gómez-Estaca, J., López De Lacey, A., López-Caballero, M.E., Gómez-Guillén, M.C., Montero, P. Biodegradable gelatin-chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010; 27:889–896.
Han, C., Zhao, Y., Leonard, S.W., Traber, M.G. Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria x anan-assa) and raspberries (Rubus ideaus). Postharvest Bio Technol. 2004; 33:67–78.
Han, C., Lederer, C., Mcdaniel, M., Zhao, Y. Sensory evaluation of fresh strawberries (Fragaria ananassa) coated with chitosan-based edible coatings. J Food Sci. 2005; 70:172–180.
Hoagland, P., Parris, N. Chitosan/pectin laminated films. J Agric Food Chem. 1996; 44:1915–1919.
Ishikawa, M., Nara, H. Inhibition of solute permeation in osmotic dehydration of food by chitosan membrane coating. Bull Jap Soc Scientific Fisheries. 1991; 57:767.
Jiang, Y., Li, Y. Effects of chitosan coating on postharvest life and quality of Longan fruit. Food Chem. 2001; 73:139–143.
Jiang, Y., Li, J., Jiang, W. Effects of chitosan coating on shelf life of cold-stored litchi fruit at ambient temperature. LWT-Food Sci Technol. 2005; 38:757–761.
Jung, B., Kim, C. Preparation of amphiphilic chitosan and their antimicriobial activities. J Appl Polymer Sci. 1999; 72:1713–1719.
Kanatt, S.R., Ramesh, C., Sharama, A. Chitosan and mint mixture: a new preservative for meat and meat products. Food Chem. 2008; 107:845–852.
Kasaai, M.R., Charlet, G., Paquin, P., Arul, J. Fragmentation of chitosan by micro-fluidization process. Innov Food Sci Emerg Technol. 2003; 4:403–413.
Kim, K.W., Thomas, R.L., Lee, C., Park, H.J. Effect of modified atmosphere packaging on the shelf Iife of coated, whole and sliced mushrooms. Lebensm.-Wiss. u. -Technol. 2006; 39:365–372.
Kim, K.W., Min, B.J., Kim, Y.T., Kimmel, R.M., Cooksey, K., Park, S.I. AntimicrobiaI activity against foodborne pathogens of chitosan biopolymer films of different molecu-Iar weights. LWT-Food Sci Technol. 2010; 44:565–569.
Lau, O.L., Yastremski, R. Retention of quality of "Golden Delicious" apples by controIIed- and modified-atmosphere storage. HortScience. 1991; 26:564–566.
Li, B., Kennedy, J.F., Peng, J.L., Yie, X., Xie, B.J. Preparation and performance evalu-ation of glucomannan-chitosan-nisin ternary antimicrobiaI blend film. Carbohydr Polym. 2006; 65:488–494.
Li, H., Yu, T. Effect of chitosan on incidence of brown rot, quality and physio-IogicaI attributes of postharvest peach fruit. J Sci Food Agric. 2001; 81:269–274.
Li, J., Zivanovic, S., Davidson, P.M., Kit, K. Characterization and comparison of chitosan/PVP and chitosan/PEO blend films. Carbohydr Polym. 2010; 79:786–791.
Li, L.H., Deng, J.C., Deng, H.R., Liu, Z.L., Li, X.L. Preparation, characterization and antimicrobiaI activities of chitosan/Ag/ZnO blend films. Chem Eng J. 2010; 160:378–382.
Lin, B., Du, Y., Liang, X., Wang, X., Wang, X., Yang, J. Effect of chitosan coating on respiratory behavior and quality of stored Iitchi under ambient temperature. J Food Eng. 2011; 102:94–99.
López-Caballero, M.E., Gómez-Guillén, M.C., Pérez-Mateos, M., Montero, P. A chi-tosan-gelatin blend as a coating for fish patties. Food Hydrocolloid. 2005; 19:303–311.
Martínez-Camacho, A.P., Cortez-Rocha, M.O., Ezquerra-Brauer, J.M., Graciano-Verdugo, A.Z., Rodriguez-Félix, F., Castillo-Ortega, M.M., Yépiz-Gómez, M.S., Plascencia-Jato-Mea, M. Chitosan composite films: thermaI, structuraI, mechanicaI and anti-fungaI properties. Carbohydr Polym. 2010; 82:305–315.
Marudova, M., Lang, S., Brownsey, G.J., Ring, S.G. Pectin-chitosan multilayer formation. Carbohydr Res. 2005; 340:2144–2149.
Mayachiew, P., Devahastin, S., Mackey, B.M., Niranjan, K. Effects of drying methods and conditions on antimicrobiaI activity of edible chitosan films enriched with galangaI extract. Food Res Int. 2010; 43:125–132.
Mcclements, D.J. CriticaI review of techniques and methodologies for characterization of emulsion stability. Crit Rev Food Sci Nutr. 2007; 47:611–649.
Molloy, C., Cheah, L.H., Koolard, J.P. Induced resistance against Sclerotinia sclerotiorum in carrots treated with enzymaticaIIy hydrolised chitosan. Posthar-vest Bio Technol. 2004; 33:61–65.
Morillon, V., Debeaufort, F., Blond, G., Capelli, M., Volley, A. Factors affecting the moisture permeability of Iipid-based edible films: a review. Crit Rev Food Sci Nutr. 2002; 42:67–89.
Mun, S., Decker, E.A., Mcclements, D.J. Influence of droplet characteristics on the formation of oiI-in-water emulsions stabilized by surfactant-chitosan Iayers. Langmuir. 2005; 21:6228–6234.
Mun, S., Decker, E.A., Mcclements, D.J. Effect of molecular weight and degree of deacetylation of chitosan on the formation of oiI-in-water emulsions stabilized by surfactant-chitosan membranes. J Colloid Interface Sci. 2006; 296:581–590.
No, H.K., Park, N.Y., Lee, S.H., Meyers, S.P. AntibacteriaI activity of chitosan with different molecular weights. Int J Food Microbiol. 2002; 74:65–72.
Ogawa, S., Decker, E.A., Mcclements, D.J. Influence of environmentaI conditions on the stability of oiI in water emulsions containing droplets stabilized by Iecithin-chitosan membranes. J Agric Food Chem. 2003; 51:5522–5527.
Ogawa, S., Decker, E.A., Mcclements, D.J. Production and characterization of O/W emulsions containing cationic droplets stabilized by Iecithin-chitosan membranes. J Agric Food Chem. 2003; 51:2806–2812.
Ogawa, S., Decker, E.A., Mcclements, D.J. Production and characterization of o/w emulsions containing droplets stabilized by Iecithin-chitosan-pectin mutilayered membranes. J Agric Food Chem. 2004; 52:3595–3600.
Ojagh, S.M., Rezaei, M., Razavi, S.H., Hosseini, S.M.H. Development and evaluation of a noveI biodegradable film made from chitosan and cinnamon essentiaI oiI with Iow affinity toward water. Food Chem. 2010; 122:161–166.
Ouattara, B., Simard, R.E., Piette, G., Bégin, A., Holley, R.A. Inhibition of surface spoilage bacteria in processed meats by application of antimicrobiaI films prepared with chitosan. Int J Food Microbiol. 2000; 62:139–148.
Paquin, P. TechnologicaI properties of high pressure homogenizers: the effect of fat globules, milk proteins, and polysaccharides. Int Dairy J. 1999; 9:329–335.
Paredes, J.L., Sosa, M.E., Argaiz, A. Evaluación de Ia vida de anaqueI de papaya (Carica papaya) deshidratada osmóticamente con película de quitosano. Actas del CIBIA V; 2005. [Tomo II].
Park, S.Y., Marsh, K.S., Rhim, J.W. Characteristics of different molecular weight chitosan films affected by the type of organic solvents. J Food Sci. 2002; 67:194–197.
Perdones, A., Cháfer, M., González-Martínez, C., Chiralt, A., Vargas, M., Aplicación de recubrimientos a base de quitosano y aceite esenciaI de Iimón en fresones. de Ia Cátedra Fomesa, Jornadas, ed. Universidad Politécnica de Valencia, 2010.
Pinotti, A., García, M.A., Martino, M.N., Zaritzky, N.E. Study on microstructure and physicaI properties of composite films based on chitosan and methylceIIulose. Food Hydrocolloid. 2007; 21:66–72.
Pranoto, Y., Rakshit, S.K., Salokhe, V.M. Enhancing antimicrobiaI activity of chitosan films by incorporating garlic oiI, potassium sorbate and nisin. Lebensm.-Wiss. u.-Technol. 2005; 38:859–865.
Rao, M.S., Chander, R., Sharma, A. Development of shelf-stable intermediate moisture meat products using active edible chitosan coating and irradiation. J Food Sci. 2005; 70:M325–M331.
Rao, M.S., Kanatt, S.R., Chawla, S.P., Sharma, A. Chitosan and guar gum composite films: preparation, physicaI, mechanicaI and antimicrobiaI properties. Carbohydr Polym. 2010; 82:1243–1247.
Rodríguez, M.S., Albertengo, L.A., Agulló, E. Emulsification capacity of chitosan. Carbohydr Polym. 2002; 48:271–276.
Roland, I., Piel, G., Delattre, L., Evrard, B. Systematic characterization of oiI-in-water emulsions for formulation design. Int J Pharm. 2003; 263:85–94.
Romanazzi, G., Nigro, F., Ippolito, A., Di Venere, D., Salerno, M. Effects of pre- and postharvest chitosan treatments to controI storage grey mold of table grapes. J Food Sci. 2002; 67:1862–1867.
Romanazzi, G., Nigro, F., Ippolito, A. Short hypobaric treatments potentiate the effect of chitosan in reducing storage decay of sweet cherries. Postharvest Bio Technol. 2003; 29:73–80.
Sánchez-González, L., González-Martínez, C., Chiralt, A., Cháfer, M. PhysicaI and antimicrobiaI properties of chitosan-tea tree essentiaI oiI composite films. J Food Eng. 2010; 98:443–452.
Sánchez-González, L., Cháfer, M., Chiralt, A., González-Martínez, C. PhysicaI properties of chitosan films containing bergamot essentiaI oiI and their inhibitory action on Penicilium Italicum. Carbohydr Polym. 2010; 82:277–283.
Sanchez-Gonzalez, L., Vargas, M., González-Martínez, C., Chiralt, A., Cháfer, M. Use of essentiaI oils in bioactive edible coatings. Food Eng Rev. 2011; 3:1–16.
Sánchez-González, L., Cháfer, M., Hernández, M., Chiralt, A., González-Martínez, C. AntimicrobiaI activity of polysaccharide films containing essentiaI oils. Food Control. 2011; 22:1302–1310.
Sánchez-González, L., Pastor, C., Vargas, M., Chiralt, A., González-Martínez, C., Chafer, M. Effect of HPMC and chitosan coatings with and without bergamot essentiaI oiI on quality and safety of cold stored grapes. Postharvest Biol Technol. 2011; 60:57–63.
Sánchez-González, L., Chafer, M., González-Martínez, C., Chiralt, A., Desobry, S. Study of the release of Iimonene present in chitosan films enriched with bergamot oiI in food simulants. J Food Eng. 2011; 105:138–143.
Shaidi, F., Arachchi, J.K.V., Jeon, Y.J. Food applications of chitin and chitosan. Trends Food Sci Technol. 1999; 10:37–51.
Shen, X.L., Wu, J.M., Chen, Y., Zhao, G. AntimicrobiaI and physicaI properties of sweet potato starch films incorporated with potassium sorbate or chitosan. Food Hydrocolloid. 2010; 24:285–290.
Simöes, A.D.N., Tudela, J.A., Allende, A., Puschmanna, R., Gil, M.I. Edible coatings containing chitosan and moderate modified atmospheres maintain quality and enhance phytochemicals of carrot sticks. Postharvest Bio Technol. 2009; 51:364–370.
Sorlier, P., Viton, C., Domard, A. Relation between solution properties and degree of acetylation of chitosan: role of aging. Biomacromolecules. 2002; 3:1336–1342.
Suman, S.P., Mancini, R.A., Joseph, P., Ramanathan, R., Konda, M.K.R., Dady, G., Yin, S. Packaging-specific influence of chitosan on color stability and Iipid oxidation in refrigerated ground beef. Meat Sci. 2010; 86:994–998.
Synowiecki, J., Al-Khateeb, N. Production, properties, and some new applica-tions of chitin and its derivatives. Crit Rev Food Sci Nutr. 2003; 43:145–171.
Tasios, L., Tzia, C., Use of edible coatings and films in apple pieces cold preservation. Proceedings of International Conference Engineering and Food, ICEF 9, Montpellier, France, 2004.
Thanasukarn, P., Pongsawatmanit, R., Mcclements, D.J. Utilization of Iayer-by-Iayer interfaciaI deposition technique to improve freeze-thaw stability of oiI-in-water emulsions. Food Res Int. 2006; 39:721–729.
Tharanathan, R., Kittur, F. Chitin - the undisputed biomolecule of great potentiaI. Crit Rev Food Sci Nutr. 2003; 43:61–87.
Vargas, M., Albors, A., Chiralt, A., González-Martínez, C., Application of chito-san-methylceIIulose edible coatings to strawberry fruit. Proceedings of the IUFOST 2006, 13th World Congress of Food Science & Technology. Food is Life. 2006:389–390.
Vargas, M., Albors, A., Chiralt, A., González-Martínez, C. Quality of cold-stored strawberries as affected by chitosan-oleic acid edible coatings. Postharvest Biol Technol. 2006; 41:164–171.
Vargas, M., González-Martínez, C., Chiralt, A., Cháfer, M., Estudio preliminar deI uso de recubrimientos de quitosano y de microorganismos eficaces en eI controI postcosecha de Ia podredumbre azuI de Ias naranjas. V Congreso Iberoamericano de Tecnología Postcosecha y Agroexportaciones. 2007:1415–1423.
Vargas, M., Albors, A., Chiralt, A., González-Martínez, C. Characterization of chitosan-oleic acid composite films. Food Hydrocolloid. 2009; 23:536–547.
Vargas, M., Chiralt, A., Albors, A., González-Martínez, C. Effect of chitosan-based edible coatings applied by vacuum impregnation on quality preservation of fresh-cut carrot. Postharvest Biol Technol. 2009; 51:263–271.
Vargas, M., Perdones, A., Chiralt, A., Cháfer, M., González-Martínez, C. Water interactions and microstructure of chitosan-methylceIIulose composite films as affected by ionic concentration. LWT-Food Sci Technol. 2011; 44:2290–2295.
Vargas, M., Chiralt, A., Albors, A., González-Martínez, C. Effect of homogeniza-tion conditions on physicochemicaI properties of chitosan-based film-forming dispersions and films. Food Hydrocolloid. 2011; 25:1158–1164.
Vásconez, M.B., Flores, S.K., Campos, C.A., Alvarado, J., Gerschenson, L.N. Antimi-crobiaI activity and physicaI properties of chitosan-tapioca starch based edible films and coatings. Food Res Int. 2009; 42:762–769.
Ward, G., Nussinovitch, A. Gloss properties and surface morphology relationships of fruits. J Food Sci. 1996; 61:973–977.
Worrell, D., Carrington, C.M.S., Huber, D.J. The use of low temperatures and coatings to maintain storage quality of bread fruit, Artocarpus altilis (Parks.) Fosb. Postharvest Biol Technol. 2002; 25:33–40.
Xing, R., Liu, S., Guo, Z., Yu, H., Wang, P., Li, C., Li, Z., Li, P. Relevance of molecular weight of chitosan and its derivatives and their antioxidant activities in vitro. Bioorg Med Chem. 2007; 13:1573–1577.
Xing, Y., Li, X., Xu, Q., Yun, J., Lu, Y., Tang, Y. Effects of chitosan coating enriched with cinnamon oil on qualitative properties of sweet pepper (Capsicum annuum L.). Food Chem. 2011; 124:1443–1450.
Xu, Y.X., Kim, K.M., Hanna, M.A., Nag, D. Chitosan-starch composite film: preparation and characterization. Ind Crop Prod. 2004; 21:185–192.
Yen, M.T., Tseng, Y.H., Li, R.C., Mau, J.L. Antioxidant properties of fungal chitosan from shiitake stipes. LWT-Food Sci Technol. 2007; 40:255–261.
Yen, M.T., Yang, J.H., Mau, J.L. Antioxidant properties of chitosan from crab shells. Carbohydr Polym. 2008; 74:840–844.
Yoksan, R., Chirachanchai, S. Silver nanoparticle-loaded chitosan-starch based films: fabrication and evaluation of tensile, barrier and antimicrobial properties. Mater Sci and Eng C. 2010; 30:891–897.
Zivanovic, S., Chi, S., Draughon, F. Antimicrobial activity of chitosan films enriched with essential oils. J Food Sci. 2005; 70:45–51.