Advances in freshness and safety indicators in food and beverage packaging
Abstract:
This chapter begins with an overview of the various principles behind commercially released freshness and safety indicators. These include time-temperature integrators and leak detectors that simply indicate risk, and sensor labels that offer direct assessment of safety and freshness (including fruit ripeness). Since this arena has been thoroughly reviewed recently, just a couple of leading examples of each category are described to set the scene for a discussion of why most of these inventions have not proved commercially successful. The bulk of the chapter focuses on recent advances with potential to lead to successful new types of freshness and safety indicators.
10.1 Introduction
This review seeks to complement, rather than duplicate, material presented in several excellent recent reviews of freshness and safety indicators (Brody et al., 2008; Hogan and Kerry, 2008; O’Sullivan and Kerry, 2008; Pacquit et al., 2008; Poças et al., 2008; Smolander, 2008; Taoukis, 2008). A brief overview of the various principles behind currently available freshness and safety indicators, including one or two current representative examples of each type, is intended primarily to set the scene. The reader will be directed to recent reviews for a more complete coverage of the range of technologies that have been applied in indicator labels of the past and present. This will permit us to focus on some advances and ideas that have appeared over the past three years that potentially could give rise to some completely new types of freshness and safety indicator labels.
The food freshness and safety arena is a dynamic and challenging area of research and commercial endeavour. The stakes are high. Some big players have moved in to occupy niches that were originally carved out by very small research teams and spin-off companies. For example, in 2006 Finland’s VTT (the largest contract research organisation in Northern Europe) established its Centre for Printed Intelligence as a strategic initiative in response to market forecasts that ‘printed electronics’ will generate US$25–40 billion by 2015, rising to more than US$250 billion by 2025 (Kopola, 2008, 2009). Within three years, VTT doubled both its annual research effort (now exceeding 100 person years) and revenues from printed intelligence (Kopola, 2009). It has made some large-scale investments in machines and facilities to permit sensors and other printed electronics to be produced by roll-to-roll printing and coating in the most cost-effective manner. Other big companies that have recently entered the freshness indicator and sensor arena include Ciba (now part of BASF), Avery Dennison and 3 M (Taoukis, 2008).
A dynamic field of research and product development is inherently competitive and risky. One should not assume that many of the freshness sensors portrayed on the internet and discussed in reviews more than two years old will still be available. Several of their websites have not been updated for two or more years, and emails to their ‘contact us’ addresses either bounce or are never answered. Presumably the harsh realities of the commercial environment have hit hard. The real challenges of achieving adequate reliability, cost-effectiveness, and of complying with increasingly restrictive food contact safety regulations, will be discussed.
Commercial secrecy often restricts collaboration and communication of fresh ideas and advances. Most companies are reluctant to publicise why their products fail in the marketplace, so others are likely to fall into the same traps. Some products are never patented, remaining as trade secrets. These factors combine to make this a difficult area to review comprehensively, but the inevitable lingering elements of intrigue and uncertainty will, it is hoped, add interest and spark imagination.
10.2 Principles of freshness and safety indicators in food and beverage packaging
Many freshness and safety indicators actually monitor the storage environment, rather than the product itself or compounds released from it, and therefore can only offer predictive, indirect indications based on established correlations. In this category are numerous time-temperature integrators (TTIs), leak detectors and tamper-evidence devices, which have recently been thoroughly reviewed (Hogan and Kerry, 2008; O’Sullivan and Kerry, 2008; Pacquit et al., 2008; Poças et al., 2008; Taoukis, 2008). TTIs are designed to exhibit an irreversible response, usually a change or migration of colour, that cumulatively reflects the time-temperature exposure history of the product they accompany. A critical prerequisite for the successful application of a TTI-based monitoring system is prior determination and modelling of the temperature dependence of the shelf life of the particular product. Data collected by monitoring parameters such as the appearance of off-flavours or loss of a particular nutrient in relation to time and temperature can be used to calculate the activation energy of the chemical reactions related to spoilage of food (Taoukis, 2008). Ideally the TTI sensor should be selected or pre-adjusted so that the activation energy required to produce a colour change is similar to that required to initiate significant deterioration in the food or beverage being monitored. If there is a microbial component involved in the deterioration, TTIs and indicators of leakage or tampering rely on assumptions regarding the likely level of microbial contamination in the product initially or in the surrounding environment.
Freshness sensors, also referred to as food quality indicators (FQIs), directly monitor the products of biochemical processes or microbial growth that bring about changes in freshness and safety. Their indications should therefore be less ambiguous than those of the indirect environment monitoring devices. FQIs can be either dosimeters (i.e. cumulative, irreversible) or current-status sensors (i.e. non-cumulative, reversible). Dosimeters tend to be more sensitive and are generally more appropriate, provided the particular compound being monitored is initially at a very low concentration that then increases many-fold during ripening or rotting. If the target indicator compound exhibits a significant baseline concentration that undergoes a much smaller increase (e.g. 2–3-fold), a dosimeter will eventually produce a false positive if simply left in the presence of unripe or sound produce for long enough. In such a case, a current-status sensor that clearly indicates the altered rate of release of the target compound may be more appropriate. Such a sensor has the potential to revert towards its starting colour, which can be problematic if the pack is opened, or once the fruit passes through its climacteric peak and the rate of indicator volatile release begins to decline.
FQIs are typically more expensive than TTIs to manufacture, may suffer from instability in storage, and normally need to be placed inside the packaging or attached directly to the food, making them subject to increasingly stringent food contact regulations (Heckman, 2007; Rijk, 2008). In a recent comprehensive review of TTIs and FQIs in the smart packaging of fish and seafood products (Pacquit et al., 2008), it was concluded that these two fundamentally different approaches should be viewed as complementary; TTIs to monitor cold-chain compliance and FQIs to monitor quality. However, few products will bear the extra cost of displaying both types of indicator.
10.3 Current technologies and their limitations
10.3.1 Time-temperature integrators
TTIs have gained a commercial foothold on commodities for which cold-chain compliance is critical for safety, such as fish and poultry. The types of TTIs commercially available, and validation studies establishing their usefulness, have recently been reviewed (Hogan and Kerry, 2008; Pacquit et al., 2008; Poças et al., 2008; Taoukis, 2008). Two of the current front runners are described briefly below.
OnVu™
OnVu™ (www.onvu.com) is one of the leading TTIs for a number of reasons. It can be activated easily at the point of application by exposure to UV light from a special activator supplied by the manufacturer. A UV blocking filter should then be permanently applied over the label to prevent subsequent UV exposure from causing reversion to the starting colour. The sensitivity of the indicator colour change can be controlled by the length of the UV activation exposure period. It utilises a photochromic ink that loses its blue colour in a time-temperature dependent spontaneous solid state crystal phase reaction following activation by UV light. This means that it can be printed directly onto packaging if required, in a very cost-effective manner. The fading of the active colour zone can be assessed visually, relative to a stable reference surrounding colour (www.onvu.com/content/en/retailer#2).
A strong correlation was reported between the shelf life of fresh domestic marinated salmon trout and the end-point of an OnVu™ indicator activated by exposure to UV light for 2 seconds (Smolander, 2009). A similar validation study was carried out using OnVu™ to predict the quality of seabream fillets (Taoukis et al., 2008). Both studies were part of the EU FRESHLABEL project aiming to develop tailor-made TTIs for specific fish and meat products.
A variant of OnVu™ in the advanced stages of development (CoolVu Active Barcode) initially obscures part of a two-stage barcode. If the product has been subjected to temperature abuse, the pigment coating the barcode becomes transparent so that the previously obscured portion of the barcode can then be read (http://www.freshpoint-tti.com/product/CoolVu-Active-Barcode.apx).
TT Sensor™
TT Sensor™ (Avery Dennison Corp, USA) is another TTI that can be distributed and stored in an inactive form, which is an important feature. It is supplied as two separate components; an indicator label and a transparent activator overlay. These are brought together automatically by a special dual-spindle applicator to activate the sensor at the time of application to the package to be monitored (Pacquit et al., 2008). Unlike some TTIs, TT Sensor™ labels do not need to be refrigerated prior to application, and offer a six-month shelf life. Once the two layers are brought together, diffusion of an acidic species from one polymer layer to the other results in a progressive, irreversible colour change in a pH indicator from fluorescent yellow to bright pink at a rate dependent on time and temperature (Anon., 2008, 2009). Different sensitivities are available to suit particular applications, and TT Sensor™ tags can be decorated, printed and cut to suit specific design requirements. They are intended for application to the outside of product packaging, not to the product itself. The Avery Dennison online listing of important things to know about the sensor (Anon., 2008) emphasises that ‘TT Sensor™ monitors temperature exposure over time – not product quality’.
Limitations of TTIs
Whereas TTIs are generally used to reveal exposure to undesirably high temperatures, most fruit and vegetables may also be damaged by storage below their safe minimum temperatures. Detection of such treatment is not a strength of most TTIs. Soon-to-be-released OnVu Ice™ is intended to demonstrate whether cold-sensitive produce has been exposed to sub-zero temperatures. However, it is difficult to use TTIs to demonstrate that tropical fruits like mango and banana have been subjected to sub-optimal temperatures in the 1–10 °C range. This task of unequivocal monitoring would be better performed by a downloadable temperature logger, such as an iButton™, or a disposable temperature sensor connected to an RFID that can permit automatic, regular downloading of temperature data to the internet. Systems like X-Sense™ (http://www.coldchainquality.com/how-xsense-works) and X_Tract™ (Anon, 2004; ****Anon, 2004, 2008) permit remote, real-time monitoring of RFIDs connected to disposable temperature sensors that could be distributed amongst packages at various representative points in a truck or container in transit. Such active RFID-linked temperature sensors cost about $US1 each, 10–50 times more than typical TTI labels, but they offer a more complete, unambiguous temperature record, revealing the time spent above or below the optimum transit or storage temperature.
10.3.2 Freshness sensors
Struggle for survival
FQIs offer a more direct indication of food freshness and safety than do correlative indicators such as TTIs and leak detectors. However, despite this apparent advantage, FQIs have generally been far less successful than TTIs in gaining a market foothold. Dermot Diamond, a prominent research leader with many years of involvement in the field, recently observed that the food quality indicator arena ‘is littered with abandoned ideas and technologies; probably because food related research is not as heavily funded as other areas, and research can be subject to stop-start-stop patterns that disrupt bringing technologies to the market; also the margins are very tight in the food industry, and it is often the case that a great idea cannot be properly developed at the right price’.
For most products that conform to the adage ‘freshest is best’, a colour change in a freshness sensor is generally bad news from the perspective of a wholesaler or retailer who at that point owns the product and wishes to sell it for the best price. Retailers are therefore typically unwilling to absorb the additional cost that such a sensor adds to a product line. Instead, they simply pass it on to consumers, who also often baulk at paying the extra price premium necessary to make a sensor profitable. Added to these problems are the unnecessary wastage or discounting that result from false positives, and, more seriously, possible liability claims that might result from false negatives if customers believe they can depend on the sensor as a guarantee of safety.
In view of the above maze of hurdles and obstacles, it is hardly surprising that very few freshness sensors for use on perishable foods have survived for more than a year or two as commercial products. Of the seven that were listed as commercially available in a review of the topic two years ago (Smolander, 2008), only a couple are still represented by up-to-date websites, and even they do not respond to invited inquiries via ‘contact us’, suggesting that they are no longer actively seeking sales.
One means of survival might be to target niche markets of socio-economic groups or supermarket chains that place particular emphasis and value on food freshness and safety. For example, It’s Fresh! Inc. has taken the novel approach of selling its Food Freshness Indicator mounted on a card, or pre-attached inside empty storage containers (http://www.foodfreshnesstechnology.com/researchanddevelopment.html). These are intended to be purchased by consumers for detecting signs of microbial spoilage in stored meats and meat-containing products after they have purchased them.
Fruit ripeness sensors open up fresh opportunities
Fruit marketing opportunities offer a ray of hope in an otherwise bleak outlook for freshness sensor developers. Many fruits are picked in a mature but unripe condition. Their eating qualities improve and ideally become optimal while on display, or shortly after a consumer has purchased them. Fruit ripeness sensors are therefore, in principle at least, viewed positively by all concerned; wholesalers, retailers and consumers alike. Occasional failure to give perfectly accurate information about the state of ripeness is unlikely to lead to a costly liability lawsuit. Anything that generally distinguishes ripe from unripe fruit is better than nothing for fruits that exhibit little or no significant changes in skin colour as they ripen.
Since fruit normally needs to be packed and distributed at a pre-optimal level of ripeness to permit damage-free handling and shipping, a ripeness indicator should ideally undergo a progressive change, making it useful at its different stages to inventory managers, produce managers and finally the consumer. Alternatively, the differing requirements of inventory managers and consumers might be better served by two distinct indicator systems. A sensor intended for inventory management use might preferably be machine readable (e.g. by optical or wireless scanning) and not meaningful to consumers, whereas sensors for consumer use ideally should be colorimetric and self-evident.
ripeSense®
ripeSense® for pears (Fig. 10.1) was released onto the market in 2004 in the US and New Zealand. Being the first commercially available fruit ripeness sensor, it attracted considerable attention from the world’s press, and featured in Time Magazine’s collection of 37 ‘Most amazing inventions of 2004’. In 2005, ripeSense® won the World Label Association’s Excellence for Technical Achievement Award. Since then it has been marketed for use on pears in the US, Canada, Australia, New Zealand and a number of European countries. ripeSense® for avocado (Fig. 10.1) was released commercially in 2007, having received the runner-up Innovation Award at Fruit Logistica in 2006. Both sensors change progressively and irreversibly from red through orange to yellow as a cumulative response to volatiles that accumulate in the headspace of customised packaging as the fruit ripen. Pear sensors have refrigerated shelf lives of more than 12 months and are supplied in a range of sensitivities to suit different varieties, pre-treatments and pack designs. For avocado, the sensor shelf life is about 9 months.

Fig. 10.1 ripeSense® for pears (a) and avocado (b). In each case, the sensor is attached on the inside of the package and changes colour progressively from red through orange to yellow in response to ripening-related volatiles that accumulate in the headspace. Images used with permission of Ripesense Ltd (www.ripesense.com).
ripeSense® packs were welcomed by both consumers and produce managers alike, proving particularly useful on varieties that undergo little visible change in skin colour as they ripen (e.g. d’Anjou and Conference pears, predominant winter pear varieties in the US and Europe, respectively). Wholesalers and retailers found them to be a useful inventory management tool, helping them reduce wastage through more informed placement of fruit on display, and the sturdy, tamper-evident sealed clamshell protects the softening pears from compression and handling damage by customers. After purchasing a ripeSense® pack, customers are advised to leave it unopened at room temperature until the fruit reach their preferred ripeness, as indicated by the sensor. Then either the fruit should be eaten immediately, or the pack can be transferred to the refrigerator, which will maintain the fruit at that preferred ripeness for several days.
RediRipe®
Six years after the commercial release of ripeSense®, two other companies are reportedly close to marketing their own alternative fruit ripeness sensors. RediRipe® LLC (USA) is planning to release a sticker that will change from white to blue in response to ethylene (Klein et al., 2005). Currently they are working on a direct-to-fruit label for pears (R. Klein, pers. comm.), although inclusion of toxic salts of palladium and molybdenum, as described in their patent application (Klein et al., 2005) would appear incompatible with direct-to-fruit applications. These compounds, however, should not prevent their use in package labelling provided there is no chance of accidental human consumption of the label or of migration of its toxic components into the fruit (Rijk, 2008).
Fresh’o’meter
Intelligent Plastic Ltd (www.intelligentplastic.com.au) is a new Australian company planning to soon begin marketing fruit ripeness sensors as part of its proposed ‘fresh’o’meter’ range of sensor labels for monitoring food and beverages. A point of difference from other sensor labels that change colour uniformly will be their utilisation of progressive diffusion of the analyte to produce a moving front of colour change, the position of which relates to the prevailing concentration or exposure history of the analyte. This seems similar in principle to Kitagawa tubes, widely used to detect various gases but not previously miniaturised into a food label. Prototype examples of two sensor labels employing this ‘migrating front’ concept are shown in Fig. 10.2. Only food-grade, compostable materials will be used in their construction. These and other design variations utilising this ‘migrating front’ concept (e.g. a circular sensor label exhibiting progressive concentric colour changes from the centre to the edges, or vice versa) form the basis of a recent broad patent application (Brockwell and Holland, 2008).
Fig. 10.2 Sensor labels employing progressive colour band migration. (a) Reaction front moving with increasing generation of the scavenged quality analyte shows current state of freshness as a cumulative measure. (b) Masking layers make words appear/disappear along a graduated scale as the reaction front migrates. Images used with permission of Intelligent Plastic Pty Ltd (www.intelligentplastic.com.au)
Other targets and challenges for fruit quality sensors
Although they have proven popular with consumers and produce managers alike, ripeness sensors and their associated packaging remain a challenge to produce at a cost that will permit widespread use on commodity fruits. Sensors for use in inventory management may prove easier to produce cost-effectively since a single sensor may then be used to monitor whole cartons, rather than small packs typically containing just 4–8 fruit. It should be possible to detect rots and other abnormal fruit based on their volatile signatures (Li et al., 2009, 2010; Moalemiyan et al., 2007). This type of behind-the-scenes application also opens up the attractive possibility of employing machine-readable transduction interfaces for monitoring the fruit quality without requiring a vivid associated colour change.
10.4 Recent advances in freshness and safety indicators and their potential applications in food and beverage packaging
Several new approaches and technologies have appeared in recent years with the potential to lead to new types of sensors suitable to be incorporated into packaging that monitors freshness and safety of food and beverages.
10.4.1 Novel methods of leak detection
A problem with most oxygen indicating inks is their poor heat resistance, making them poorly suited for use on packaging of sterilisable products. A recently developed package leakage indicator system is not only heat stable, but is actually activated by heating (e.g. 121 °C), or by pre-treatment with a volatile reducing agent, such as ammonia. The indicator dye (indigo carmine or similar), when incorporated with a suitable food grade polymeric binder, pH adjuster and volatile solvent, can be printed onto the inner surface of the package or onto internally mounted stickers (Hurme and Rajamaki, 2007; Sipilainen-Malm, 2009). Once activated, the printed sensor undergoes a marked, irreversible colour change (yellow to blue) in response to oxygen, indicating leakage of the package, or to change of pH, indicating deterioration of the product being monitored. The components and fabrication of the indicator are inexpensive, and the amounts of reactants required to fabricate the oxygen indicator are very small.
When the indicator is used in connection with a barcode identifier or other optical identification code in a package, any oxygen brought into the package through ageing and/or breaking of the package induces a colour change. This will eventually inactivate the identifier, making it impossible to read and/or identify it and automatically preventing the sale of an expired and/or broken package.
Moisture detection-based leak indicators are typically colorimetric. Often the colour change is reversible, which is normally undesirable. An irreversible moisture detector based on an optical grating formed from a deliquescent or hygroscopic salt combined with a binder has recently been described (Hurme and Sipilainen-Malm, 2009). The indicator material of the optical grating structure reacts to moisture by variation of volume, causing changes in the grating effect that may be detected visually (as opacification or complete fading of the pattern) or scanned automatically without breaking the package. Production of such a grating, which can be fabricated directly onto the package material, is technically demanding, involving hot pressing, laser or UV lithography, meaning that the sensor may also double as a mark of authenticity.
10.4.2 Radio frequency interrogation of passive sensors based on complex impedance changes and capacitive coupling
Wireless sensors employing printable nanoparticle inks
Printable metallic nanoparticle inks have opened up new, cheaper ways to produce printed circuits on packaging. Once printed, sintering is necessary to partially melt and fuse adjacent nanoparticles together to form a conductive structure. A new method of electrical sintering of printed conductive silver nanoparticles to produce printed conductor structures such as antennas, circuits and sensors on substrates offers a number of significant advantages over thermal oven sintering (Allen et al., 2008). This approach has been used to produce a prototype wirelessly readable spoilage sensor for poultry that is based around the reaction of silver with hydrogen sulphide (Alastalo, 2008). The resulting impedance change in the printed pattern of the sintered silver nanoparticles comprising the sensor within the package was wirelessly read through the package utilising capacitive coupling between sensor and an external reading device (Alastalo, 2008). Unlike most other RFID-linked sensors, which require a battery to power the sensor and the active RFID that transmits the sensor data, the above type of printed sensor is passive, meaning that the only powered component in the system is the reader.
Inkjet printed single wall carbon nanotube patterns (‘electronic coding’) have also been found suitable for wireless capacitive read-out applications (Helisto, 2008). A single printed electronic code is estimated to cost less than US 0.1 cents and can be invisible to the naked eye. Initially commercialised as an anti-counterfeiting measure (http://www.nicanti.com/technology.html), such electronic codes appear to have the potential to form the basis of a very cheap, wireless sensor system. One or more of the several different conductive inks used in producing a unique code might be made inherently sensitive to the target analyte in a way that affects its conductivity and thus the complex impedance of the code pattern when interrogated by a wireless reader. Alternatively, the analyte-responsive component might comprise an overlay or bridge between elements of the coding pattern sufficient to alter the complex impedance of the code.
Wireless sensors based on standard passive RFID tags
Whereas the above wireless sensor systems require a component of the printed conductive pattern to be altered by interaction with the target analyte, a team at GE Global Research has shown that ubiquitous passive 13.56 MHz RFID tags can be used for diverse sensing applications with little or no modifications (Potyrailo and Morris, 2007; Potyrailo et al., 2009a, 2010). With the aid of a special reader, the complex impedance of the RFID resonant antenna is measured and correlated to physical, chemical or biological properties of interest (Fig. 10.3). By measuring several parameters from the resonant antenna and applying multivariate data analysis, it is possible to perform multianalyte sensing and rejection of environmental influences using a single sensor (Fig. 10.4).
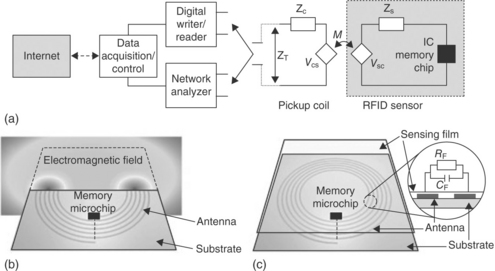
Fig. 10.3 Operating principle of physical, chemical and biological passive RFID sensors: (a) System schematic of writing and reading digital information into the sensor IC memory chip and measuring complex impedance of the sensor antenna. ZC and ZS are intrinsic impedance of the pickup coil and sensor, respectively; and ZT is total impedance; VCS and VSC are dependent voltage sources; M is mutual inductance coupling. (b) Origin of response of RFID sensors to physical parameters. (c) Origin of response of RFID sensors to chemical and biological parameters via a sensing film deposited onto the resonant antenna. Inset, analyte-induced changes in the film affect the complex impedance of the antenna circuit through the changes in film resistance RF and capacitance CF between the antenna turns. (Reproduced from Potyrailo et al., 2009a, with permission from R. Potyrailo and John Wiley & Sons, Ltd).
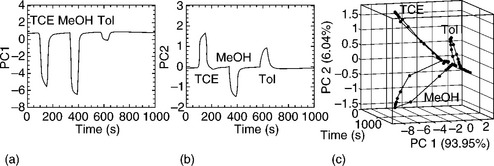
Fig. 10.4 Typical experimental data of diverse response of an individual RFID sensor: (a) sensor response as a principal component 1; (b) sensor response as a principal component 2; (c) three response planes in a 3D response plot of principal components 1 and 2 as a function of experimental time demonstrate a strong diversity in sensor response to trichloroethylene (TCE), methanol (MeOH) and toluene (Tol) vapours, all at a 0.04 P/Po concentration. (Reproduced from Potyrailo et al., 2009a, with permission from R. Potyrailo and John Wiley & Sons, Ltd.)
Generally, a sensing film is deposited onto the resonant antenna of the RFID tag to alter its impedance response in a manner that varies with the concentration of the analyte(s) of interest. Various overlay film compositions can be employed to give the best response and selectivity for the particular target analyte(s). Sensitivity varies between analytes. Alcohols can be detected at low ppm concentrations, while the lower limit for ammonia so far achieved was calculated to be 20 ppb (Potyrailo et al., 2009b). The sensitivity is also dependent on the power of the RF emission from the reader and the distance between the reader and the RFID sensor (Potyrailo et al., 2009b). These distances can be up to several centimetres, so a sensor RFID monitoring the headspace inside a sealed cardboard or plastic container should be readable from outside without the need for line of sight contact.
Additional bonuses of using industry standard passive RFID tags in sensors are (i) they are comparatively cheap (currently around US 5 cents each); and (ii) the memory chip can be used to store a product’s pedigree, along with sensor calibration and end-user data.
It is not always necessary to overlay a sensor film on the antenna in order to achieve sensor functionality from a passive RFID tag. Milk can be monitored non-invasively simply by attaching a standard disposable RFID tag to the outside of the milk carton (Fig. 10.5). Changes in the solution dielectric constant as a function of age of the milk can be monitored by its effects, through the wall of the container, on the electromagnetic field penetration depth out of the plane of the sensor (Potyrailo et al., 2009a). These initial studies need to be followed up by similar comparisons of milk with known differences in microbial contamination to determine whether the sensor is providing a measure of the extent of microbial growth or simply responding to biochemical changes associated with ageing of milk. It would also be interesting to discover whether this non-invasive system is more broadly applicable to the monitoring of other beverages.
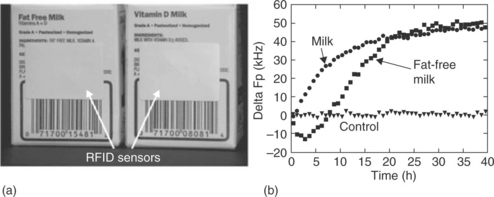
Fig. 10.5 Non-invasive monitoring of two types of milk using disposable RFID sensors: (a) Milk cartons with attached RFID sensors: (b) responses of RFID sensors to spoilage of two types of milk and to a control (water) sample. (Reproduced from Potyrailo et al., 2009a, with permission from R. Potyrailo and John Wiley & Sons, Ltd.)
10.4.3 Nanobiosensors
Nanotechnology has begun to play an increasingly important role in biosensor development (Chen et al., 2004; de Dios and Díaz-García, 2010; Lee et al., 2010; Smolander and Chaudhry, 2010). Such nanobiosensors often comprise a part of equipment that is far too elaborate and expensive to be considered for food package monitoring applications. Some applications of bioanalytical nanosensors involving food monitoring have been devised and include detection of pathogens, toxins, nutrients, allergens, etc. (Brody et al., 2008; Yezza, 2009). There are also some further examples of nanobiosensors (described below) that could be simplified to function possibly as sensor labels on packaging. However, growing public unease regarding possible safety risks associated with nanoparticles (Chaudhry et al., 2008) may slow development and hinder acceptance of such sensors for food contact use.
DNA nanobarcodes
A group at Cornell devised a system for specific pathogen detection utilising short Y-shaped strands of DNA linked together into a tree-like structure with many branching ends, to which antibodies or molecular probes and fluorescent dye molecules can be attached (Li et al., 2005). Since both dye type and dye number can be precisely controlled, multicolour fluorescence intensity-encoded ‘nanobarcodes’ could be fabricated, distinguished by the ratios of the different types of dye. Using just three fluorescent dyes that can be distinguished optically, up to 1,000 different coded structures can be created and linked to specific antibodies or molecular probes (Steele, 2005). Consequently, the resultant DNA nanobarcodes not only have coding capacity, but also contain molecular recognition elements that can be used for molecular detection.
Several methods of detection and evaluation of specific binding were tested, including fluorescence microscopy, dot blotting and flow cytometry. However, simple visual observation could distinguish four different pathogens based on colours of dot blots after each pathogen was applied to a separate spot on the membrane (Li et al., 2005). Although originally intended for rapid medical diagnostics, this technology certainly has the potential for wider applications (Lee et al., 2010), and it may be feasible to simplify and adapt it for use in quality and safety indicators on packaging.
Quantum dots in fluorescence resonance energy transfer systems
Quantum dots (QDs) are luminescent semiconductor nanocrystals in the size range 2–6 nm with unique electro-optical properties that confer on them better brightness and photostability than conventional fluorescent dyes and make them better suited for multicolour applications (Algar et al., 2010). They have shown great potential in the development of novel fluorescence resonance energy transfer (FRET) assays, for, among other things, chemical sensing (Snee et al., 2006). The narrow, size-tuneable emission spectrum of QDs enables them to be FRET donors that can be easily customised to match the acceptor absorption features of various environmentally sensitive dyes that can be conjugated to or otherwise co-immobilised with QDs (Algar et al., 2010). QDs entrapped in sol–gel matrices have been shown to have potential to constitute a sensitive new type of pH sensing label with a number of advantages over the use of conventional pH indicator dyes alone, including high stability, luminescence and robustness (Coto-Garcia et al., 2009). These authors concluded that ‘our experiments indicate that the co-immobilization of an acceptor dye with the QDs in the sol–gel matrix could provide a novel, simple, general and advantageous approach for the future development of a wide variety of FRET-based sol–gel sensors’. It remains to be seen whether sensors containing QDs will become practical and cost-effective in package label applications. If low concentrations of QDs are used to reduce cost, their photoluminescence may fluctuate with time, a troublesome phenomenon known as blinking (de Dios and Díaz-García, 2010). Also, the toxicity of cadmium selenide, commonly found in QDs, may preclude its use in food-contact situations.
QDs as an optical transduction system for detection of analyte binding on molecularly imprinted polymers (MIPs) offer a number of advantages over organic fluorescent dyes that have been used widely in this role (Lin et al., 2004). Binding of the analyte (or template) to the corresponding imprint in the MIP can result in quenching of photoluminescence, presumably due to FRET between QDs and adjacent template molecules. In spite of the potential functionality, there are few published studies on MIP materials in conjugation with QD as probes (de Dios and Díaz-García, 2010). Perhaps the most relevant of these to food labelling has been the recent demonstration of a MIP attached to photoluminescent gold-silver nano-clusters being used for Bacillus cereus spore detection (Gultekin et al., 2010). Binding of dipicolinic acid, a characteristic component of B. cereus spores used to imprint the MIP, caused significant decreases in fluorescence intensity because it induced photoluminescence emission from the adjacent Au–Ag nanoclusters in the crosslinked nanoshell polymer matrix. B. cereus can cause food poisoning, so novel sensor labels for food safety monitoring could conceivably exploit this approach.
10.4.4 Other novel or recently resurrected approaches to sensor label construction
Polydiacetylene
Polydiacetylene (PDA) is in the recently resurrected category, having been highlighted again recently as a polymer with unique optical properties that seem to make it ideal for use in sensors (Pires et al., 2010). Its potential for use in sensors has long been known. Diacetylenic lipid monomers are readily polymerised in monolayers by UV irradiation to form a conjugated PDA backbone that changes colour from deep blue to red in response to various stimuli (e.g. increasing temperature or changes in pH) because of conformational changes in the conjugated backbone (Tieke et al., 1977). More remarkably, Charych et al. (1993) demonstrated the potential of PDA to undergo a similar colour transition from blue to red solely due to recep-tor–ligand interactions when the PDA layer was functionalised with a receptor ligand for the influenza virus haemagglutinin.
The basis of the above sensor principle was a bilayer assembly containing both the receptor-binding ligand and the capability (inherent in the adjacent PDA layer) to signal the specific binding event through a colour transition readily visible to the naked eye. Since other ligands can be incorporated into the film, ‘affinitychromism’ was claimed to offer the possibility of a general method for the direct detection of receptor-ligand interactions (Charych et al., 1993). That same team at the Ernest Orlando Lawrence Berkeley National Laboratory made further headlines by demonstrating a new sensor that offered the first instant test for a toxic strain of E. coli (Kahn, 1996). The method provided an instant analysis, and proponents predicted it could be formulated into a very inexpensive sensor that could be placed on plastic, paper or glass. They suggested that it could, for example, be part of a bottle cap or container lid. A colour change from blue to red would indicate contamination. They went on to demonstrate that polydiacetylenic lipids incorporating gangliosides that specifically bound cholera toxin or botulinum neurotoxin would change colour from blue to red when exposed to the toxins (Charych et al., 1996). They concluded that ‘poly-diacetylenic lipid membranes offer a general “litmus test” for molecular recognition at the surface of a membrane. A concentration of 20 ppm of protein could be detected using polymerized thin films. The speed, sensitivity and simplicity of the design offer a new and general approach towards the direct colorimetric detection of a variety of different molecules.’
Several patents were submitted and the technology was expected to be licensed to private industry. However, recent enquiries revealed that this much publicised, innovative technology was in fact never commercialised and its inventors have long since left the laboratory. One is left to speculate as to the possible reasons for yet another apparently great idea failing to become a commercial product.
Despite this initial commercialisation failure, scientific interest in the potential of PDA for use in novel detection systems has continued. An Israeli group more recently demonstrated that by combining a PDA film with various phospholipid bilayers, it is possible to obtain colorimetric detection of bacteria without the need to include specific recognition elements (Scindia et al., 2007). This utilises the general affinity of bacteria and bacterially secreted amphiphilic compounds for thin films of lipids. Their binding can induce chromatic blue-red/fluorescence transformations of interspersed PDA domains that serve as built-in optical reporters. They found that the degrees of bacterially induced colour transformations depended both on the bacterial strains examined and the lipid compositions of the films. This permitted bacterial fingerprinting through pattern recognition obtained by recording the chromatic transformations in an array of lipid/PDA films with different lipid components. These authors envisaged utilisation of their colorimetric bacterial film sensor in high-throughput medical screening, but it would seem possible to use this concept in a package label to detect bacteria.
Poly(thiophene)
Poly(thiophene) as a single cross-reactive conjugated polymer has been shown to generate a multidimensional response capable of identifying and differentiating between 22 structurally similar amines with 97% accuracy in a competitive aqueous environment, as well as detecting these compounds in real life assays of fish samples (Maynor et al., 2007). The poly(thiophene) polymer was found to exhibit various analyte-induced aggregation patterns with different amines, each with its unique optical signature (the shape of its absorption spectrum). Entire absorption spectra were used in linear discrimination analysis (LDA), permitting a single polymer to distinguish between 22 different amines, each applied separately. It seems unlikely that analysis of a single spectrum could identify all the component biogenic amines if applied together as a mixture. It was shown to be possible to use a ratiometric approach to determine the amount of amine present in fish samples spiked with known amounts of histamine. If this approach were to be used to produce a freshness sensor label for fish packaging, it is likely that a scanner or hand-held spectrometer with capabilities to assess label absorption across the entire visible spectrum would be required.
Dye-labelled single-stranded DNA
Single-stranded (ss) DNA oligomers (20–24 bases) stained with a fluorescent dye and dried onto a polyethylene substrate have been shown recently to change in fluorescence intensity upon exposure to various organic volatiles in a manner that reflected the precise sequence of bases (White et al., 2008). Individual DNA oligomer-dye probes typically do not offer a specific response to a particular volatile compound. Rather, they each exhibit a different characteristic pattern of both positive and negative changes in fluorescence in response to a range of different volatiles. An array comprised of microdots of 29 ss-DNA sequences, each covalently attached to a fluorescent dye, produced a complex pattern of responses that amounted to a multivariate fingerprint of each volatile (White et al., 2008).
Because of the intrinsically combinatorial nature of DNA, an oligomer 21 bases long can yield 421 different sequences, each with the potential to be unique in some respects of its multivariable interactions with a range of organic volatiles. Since DNA oligomers can be readily engineered, amplified and replicated precisely, this discovery appears to open the door to a wide spectrum of new sensors for detecting volatile compounds in many applications. Oligomers can be selected that offer the most sensitive responses to particular target compounds and then replicated precisely. For example, one oligomer proved remarkably sensitive to 2,4-dinitrotoluene, permitting detection down to 6 ppb for this vapour characteristic of TNT-containing landmines (White et al., 2008). Generally, an array of fluorescent DNA oligomers will be needed to give non-ambiguous information about a product’s vapour profile. Tethering of the DNA oligomers to gold nano-particles may offer advantages, including improved sensitivity and selectivity, based on experience with other sensors employing linear DNA (Lee et al., 2010).
Practical applications of the selective odorant interactions of single-stranded DNA oligomers are likely to initially be in electronic odorant sensors, probably in combination with single-walled semiconducting carbon nanotubes (e.g. Johnson et al., 2010). For sensor label applications, it may be possible to print microarrays of different fluorescent DNA oligomers onto packaging to sensitively and specifically detect and quantify particular target analytes. Responses of dye-stained DNA oligomers reported so far have all been transient, and completely and rapidly reversible, so quantification would provide a current concentration rather than cumulative information about a particular volatile. The oligomer arrays could conceivably be cheaply produced and applied to packaging, but the reader would probably need to be a precise fluorometer, possibly hand-held. This could initially restrict this technology within food and beverage package labelling to applications in inventory management, rather than on the consumer interface. However, exciting advances are being made in adapting cell phones so that their cameras can be used as microscopes and colorimeters (Korhonen, 2009; Makinen, 2006). Such devices may one day allow consumers to interpret instantly a microscopic array of fluorescent DNA dots that might constitute a sensor label on packaging.
10.5 Future trends
In view of the established high attrition rate, very few of the indicator and sensor labels currently on the market for use primarily as freshness and safety indicators for food and beverages are likely to still be there in five years. Those that can also tap into the more lucrative margins available in monitoring medical and scientific goods are more likely to survive. TTIs and tampering/leak detectors generally have this advantage of broader applications, but they will never be able to fill adequately the niche that also needs to be occupied by sensors that actually monitor the freshness and safety of the product, based on direct evidence. As new entrants into that arena continue to appear, what factors and attributes are likely to determine whether they will survive commercially?
Sensors intended as inventory management tools, to discretely inform the warehouse manager or retailer about decreasing freshness and increasing safety risk, are more likely to succeed than those that can be interpreted by the consumer as giving bad news about a product. Increasing use of sensor labels by inventory managers can be expected as they become more reliable, particularly if they also offer other advantages. For example, RFID sensors can also offer the bonus of the same product pedigree information that is carried by normal RFIDs. The market for standard passive RFIDs is growing as their cost continues to fall, so acceptance of variants with bonus sensor properties for particular inventory management applications at minimal extra cost seems very likely. They also offer independence from the need for line-of-sight monitoring, being able to function through carton walls.
If food freshness sensor labels are to offer no bonus benefits through dual functionality, they will need to cost significantly less than a passive RFID tag (currently about US 5 cents). Ideally. they should either be printed directly onto packaging, or mass produced as labels in a roll-to-roll press to keep costs down. If intended as an aid for consumers to purchase and eat food in its preferred state, then the ‘freshest is best’ rule should not apply to the product being monitored. Otherwise, the moment the sensor begins to change colour, the value of the product and its attractiveness to customers begins to fall, and retailer support for the scheme can be expected to crumble rapidly. To survive in the market place, sensor labels as they change colour need to offer win-win opportunities for both retailer and customer alike. Fruit ripeness sensors are in this category, for reasons explained earlier. We can expect to see an increase in the range of fruits displaying ripeness sensors as new technologies are adapted to detect changes in specific volatile indicators of fruit ripeness.
10.6 Sources of further information and advice
In addition to the references listed at the end of this chapter, the following contacts can be expected to provide further information either online or in response to specific requests:
• Plant & Food Research Ltd, New Zealand: Sharrock, Active & Intelligent Packaging team leader. Tel.: + 64 7 959 4495; email: [email protected]
• General Electric Global Research, Niskayuna, NY, USA. Contact Radis-lav Potyrailo, Chem/Bio Screening and Measurement Laboratory. Email: [email protected]
• VTT’s Centre for Printed Intelligence: http://www.vtt.fi/proj/cpi/index.jsp
• Intelligent Plastic Pty Ltd: http://www.intelligentplastic.com.au/pages/index.html
• RediRipe LLC: Contact Robert Klein, PhD, CEO, RediRipe® LLC, Albuquerque, NM 87104, USA. Tel.: + 1 505 247 4665; e-mail: [email protected]
• Ripesense Ltd http://www.ripesense.com and http://www.ripesense.com/ripesense_retailers.html
10.7 Acknowledgement
I would like to thank John Mitchell for his helpful criticism of this manuscript.
10.8 References
Alastalo, A., Technologies and applications for printing conductors. Research and Development Activities in Printed Intelligence 2008 2008; 26–27. http://www.vtt.fi/proj/cpi/files/cpi_vsk_08.pdf [available at: (accessed 19 January 2012)].
Algar, W.R., Tavares, A.J., Krull, U.J. Beyond labels: a review of the application of quantum dots as integrated components of assays, bioprobes, and biosensors utilizing optical transduction. Anal Chim Acta. 2010; 673:1–25.
Allen, M.L., Aronniemi, M., Mattila, T., Alastalo, A., Ojanpera, K., Suhonen, M., Seppa, H. Electrical sintering of nanoparticle structures. Nanotechnology. 2008; 19:175–201.
Anon, RFID-enabled cold chain monitoring to cut costs. Food Production Daily.com 2004;. http://www.foodproductiondaily.com/Supply-Chain/RFID-enabled-cold-chain-monitoring-to-cut-costs [available at: (accessed 19 January 2012)].
Anon, TT Sensor®. Avery Dennison 2008;. http://www.iapd.averydennison.com/iapd/iapdsite.nsf/0d0c0d41b2da5639882569de00078a22/6a1eb2d843ea0fe4852575bd004f6121/$FILE/Avery%20Time%20Temp%20Sensor%20Technology.pdf [Available at: (accessed 19 January 2012)].
Anon, TT Sensor™ smart label technology. Avery Dennison 2009;. http://www.iapd.averydennison.com/iapd/iapdsite.nsf/5688fbde61e3772f88256a3000710800/8c73e649fcf735378525725f00697161?OpenDocument [Available at: (accessed 19 January 2012).].
Brockwell, P.N., Holland, R.V. Indicator system for determining analyte concentration 2008; [Patent No. WO 2008/006152 A1].
Brody, A.L., Bugusu, B., Han, J.H., Koelsch Sand, C., Mchugh, T.H. Innovative food packaging solutions. J Food Sci. 2008; 73:R107–R116.
Charych, D.H., Nagy, J.O., Spevak, W., Bednarski, M.D. Direct colorimetric detection of a receptor-ligand interaction by a polymerized bilayer assembly. Science. 1993; 261:585–588.
Charych, D.H., Cheng, Q., Reichert, A., Kuziemko, G., Stroh, M., Nagy, J.O., Spevak, W., Stevens, R.C. A “litmus test” for molecular recognition using artificial membranes. Chem Biol. 1996; 3:113–120.
Chaudhry, Q., Scotter, M., Blackburn, J., Ross, B., Boxall, A., Castle, L., Aitken, R., Watkins, R. Applications and implications of nanotechnologies for the food sector. Food Additives and Contaminants. 2008; 25:241–258.
Chen, J.R., Miao, Y.Q., He, N.Y., Wu, X.H., Li, S.J. Nanotechnology and biosensors. Biotechnol Adv. 2004; 22:505–518.
Coto-Garcia, A.M., Fernandez-Arguelles, M.T., Costa-Fernandez, J.M., Sanz-Medel, A. Entrapment of quantum dots in sol–gel matrices to develop sensing material based on fluorescence resonance energy transfer. Chem Commun. 2009; 36:5454–5456.
De Dios, A.S., Díaz-García, M.E. Multifunctional nanoparticles: analytical prospects. Anal Chim Acta. 2010; 666:1–22.
Gultekin, A., Ersoz, A., Sariozlu, N.Y., Denizli, A., Say, R. Nanosensors having dipicolinic acid imprinted nanoshell for Bacillus cereus spores detection. J Nanopart Res. 2010; 12:2069–2079.
Heckman, J. Active and intelligent packaging: a European anomaly. In: Wilson C.L., ed. Intelligent and Active Packaging for Fruits and Vegetables. Boca Raton, FL: CRC Press; 2007:307–313.
Helisto, P., Printed electric tag technology. Research and Development Activities in Printed Intelligence 2008. 2008:46–47. http://www.vtt.fi/proj/cpi/files/cpi_vsk_08.pdf [available at: (accessed 19 January 2012)].
Hogan, S.A., Kerry, J.P. Smart packaging of meat and poultry products. In: Kerry J., Butler P., eds. Smart Packaging Technologies for Fast Moving Consumer Goods. Chichester: John Wiley & Sons; 2008:33–59.
Hurme, E., Rajamaki, T. Ink composition, oxygen and/or pH indicator and package 2007; [(VALTION TEKNILLINENE TUTKIMUSKESKUS), Patent No. WO 2007/017555 A1].
Hurme, E., Sipilainen-Malm, T. Indicator 2009; [(VALTION TEKNILLINEN TUTKIMUSKESKUS), Patent No. WO 2009/153406 A1].
Johnson, A.T.C., Khamis, S.M., Preti, G., Kwak, J., Gelperin, A. DNA-coated nanosensors for breath analysis. IEEE Sensors Journal. 2010; 10:159–166.
Kahn, J., New sensor provides first instant test for toxic E. coli organism. Berkeley Lab Research News 1996;. http://www.lbl.gov/Science-Articles/Archive/E-coli-sensor.html [available at: (accessed 19 January 2012).].
Klein, R.A., Riley, M.R., Decianne, D.M., Srinavakul, N. Non-invasive colorimetric ripeness indicator 2005; [(THE UNIVERSITY OF ARIZONA), US Patent 2006/0127543 A1 (15 June 2006).].
Kopola, H., Preface. Research and Development Activities in Printed Intelligence 2008. 2008:4–5. http://www.vtt.fi/proj/cpi/files/cpi_vsk_08.pdf [available at: (accessed 19 January 2012)].
Kopola, H., Towards the commercialisation of research efforts. Research and Development Activities in Printed Intelligence 2009. 2009:4–6. http://www.vtt.fi/files/download/scientific_reports/cpi_09_review.pdf [available at: (accessed 19 January 2012)].
Korhonen, R., Mobile phone microscope. Research and Development Activities in Printed Intelligence 2009. 2009:37–39. http://www.vtt.fi/files/down-load/scientific_reports/cpi_09_review.pdf [available at: (accessed 19 January 2012)].
Lee, J.B., Campolongo, M.J., Kahn, J.S., Roh, Y.H., Hartman, M.R., Luo, D. DNA-based nanostructures for molecular sensing. Nanoscale. 2010; 2:188–197.
Li, C.Y., Krewer, G.W., Ji, P.S., Scherm, H., Kays, S.J. Gas sensor array for blueberry fruit disease detection and classification. Postharvest Biol Technol. 2010; 55:144–149.
Li, Y.G., Cu, Y.T.H., Luo, D. Multiplexed detection of pathogen DNA with DNA-based fluorescence nanobarcodes. Nat Biotechnol. 2005; 23:885–889.
Li, Z.F., Wang, N., Raghavan, G.S.V., Vigneault, C. Ripeness and rot evaluation of “Tommy Atkins” mango fruit through volatiles detection. J Food Eng. 2009; 91:319–324.
Lin, C.I., Joseph, A.K., Chang, C.K., Lee, Y.D. Molecularly imprinted polymeric film on semiconductor nanoparticles: analyte detection by quantum dot photoluminescence. J Chromatogr. 2004; 1027:259–262.
Makinen, J.-T., Portable microscope and laser reading device for a camera phone. Research and Development Activities in Printed Intelligence 2006. 2006:49–52. http://www.vtt.fi/liitetiedostot/cluster1_tieto-ja_viestintatekniikka_elektroniikka/printed_intelligence_06.pdf [available at: (accessed 19 January 2012)].
Maynor, M.S., Nelson, T.L., O’sullivan, C., Lavigne, J.J. A food freshness sensor using the multistate response from analyte-induced aggregation of a cross-reactive poly(thiophene). Org Lett. 2007; 9:3217–3220.
Moalemiyan, M., Vikram, A., Kushalappa, A.C. Detection and discrimination of two fungal diseases of mango (cv. Keitt) fruits based on volatile metabolite profiles using GC/MS. Postharvest Biol Technol. 2007; 45:117–125.
O’sullivan, M.G., Kerry, J.P. Smart packaging technologies for beverage products. In: Kerry J., Butler P., eds. Smart Packaging Technologies for Fast Moving Consumer Goods. Chichester: John Wiley & Sons; 2008:211–232.
Pacquit, A., Crowley, K., Diamond, D. Smart packaging technologies for fish and seafood products. In: Kerry J., Butler P., eds. Smart Packaging Technologies for Fast Moving Consumer Goods. Chichester: John Wiley & Sons; 2008:75–98.
Pires, A.C.D.S., Soares, N.D.F.F., Silva, L.H.M.D., Andrade, N.J.D., Silveira, M.F.A., Carv-Alho, A.F.D. Polydiacetylene as a biosensor: fundamentals and applications in the food industry. Food and Bioprocess Technology. 2010; 3:172–181.
Poças, M.F.F., Delgado, T.F., Oliveira, F.A.R. Smart packaging technologies for fruits and vegetables. In: Kerry J., Butler P., eds. Smart Packaging Technologies for Fast Moving Consumer Goods. Chichester: John Wiley & Sons; 2008:151–166.
Potyrailo, R.A., Morris, W.G. Multianalyte chemical identification and quantitation using a single radio frequency idenfication sensor. Analytical Chemistry. 2007; 79:45–51.
Potyrailo, R.A., Morris, W.G., Sivavec, T., Tomlinson, H.W., Klensmeden, S., Lindh, K. RFID sensors based on ubiquitous passive 13.56-MHz RFID tags and complex impedence detection. Wireless Communications and Mobile Computing. 2009; 9:1318–1330.
Potyrailo, R.A., Surman, C., Go, S., Lee, Y., Sivavec, T., Morris, W.G. Development of radio-frequency identification sensors based on organic electronic sensing materials for selective detection of toxic vapours. Journal of Applied Physics. 2009; 106:124–902.
Potyrailo, R.A., Surman, C., Morris, W.G., Go, S., Lee, Y., Cella, J., Chichak, K.S. Selective quantitation of vapors and their mixtures using individual passive multivariable RFID sensors. 2010 IEEE Int Conf on RFID. 2010; 22–28.
Rijk, R. Legislative issues relating to smart packaging. In: Kerry J., Butler P., eds. Smart Packaging Technologies for Fast Moving Consumer Goods. Chichester: John Wiley & Sons; 2008:305–323.
Scindia, Y., Silbert, L., Volinsky, R., Kolusheva, S., Jelinek, R. Colorimetric detection and fingerprinting of bacteria by glass-supported lipid/polydiacetylene films. Langmuir. 2007; 23:4682–4687.
Sipilainen-Malm, T., Producing devices using printing techniques to assess quality and add value to packages for consumers. Research and Development Activities in Printed Intelligence 2009. 2009:22–23. http://www.vtt.fi/files/download/scientific_reports/cpi_09_review.pdf [available at: (accessed 19 January 2012)].
Smolander, M. Freshness indicators for food packaging. In: Kerry J., Butler P., eds. Smart Packaging Technologies for Fast Moving Consumer Goods. Chichester: John Wiley & Sons; 2008:111–127.
Smolander, M., FRESHLABEL – Time-temperature indicators for chilled fish products. Research and Development Activities in Printed Intelligence 2009. 2009:28–31. http://www.vtt.fi/files/download/scientific_reports/cpi_09_review.pdf [available at: (accessed 19 January 2012)].
Smolander, M., Chaudhry, Q. Nanotechnologies in food packaging. In: Chaudhry Q., Castle L., Watkins R., eds. Nanotechnologies in Food. Cambridge: The Royal Society of Chemistry; 2010:86–101.
Snee, P.T., Somers, R.C., Nair, G., Zimmer, J.P., Bawendi, M.G., Nocera, D.G. A ratiometric CdSe/ZnS nanocrystal pH sensor. J Am Chem Soc. 2006; 128:13320–13321.
Steele, B., Researchers make synthetic DNA “barcodes” to tag pathogens, providing an inexpensive, off-the-shelf monitoring system. Cornell University News Service (Internet) 2005;. http://www.news.cornell.edu/stories/June05/Luo.barcodes.ws.html [available at:, (accessed 19 January 2012)].
Taoukis, P.S. Application of time-temperature integrators for monitoring and management of perishable product quality in the cold chain. In: Kerry J., Butler P., eds. Smart Packaging Technologies for Fast Moving Consumer Goods. Chichester: John Wiley & Sons; 2008:61–74.
Taoukis, P.S., Tsironi, T., Gogou, E., Giannoglou, M., Chill chain management and shelf life optimisation of MAP seabream fillets: a TTI based alternative to FIFO. 3rd International Workshop Cold Chain Management, 2–3 June, 2008, 2008. http://ccm.ytally.com/fileadmin/user_upload/downloads/Taoukis.pdf [available at:, (accessed 19 January 2012)].
Tieke, B., Graf, H.J., Wegner, G., Naegele, B., Ringsdorf, H., Banerjie, A., Day, D., Lando, J.B. Polymerization of mono- and multilayer forming diacetylenes. Colloid and Polymer Science, Kolloid-Zeitschrift & Zeitschrift für Polymere. 1977; 255:521–531.
White, J., Truesdell, K., Williams, L.B., Atkisson, M.S., Kauer, J.S. Solid-state, dye-labeled DNA detects volatile compounds in the vapor phase. PLoS Biol. 2008; 6:30–36.
Yezza, I.A., Printed intelligence in packaging: current and potential applications of nanotechnology. 2009 Symposium on Nanomaterials for Flexible Packaging 2009;. http://www.tappi.org/content/events/09PLACESY/Symp_Papers/yezza.pdf [available at:, (accessed 19 January 2012)].
