Light-protective packaging materials for foods and beverages
Abstract:
Both ultraviolet and visible light energy affect food and beverage quality. Some food components are reactive to this light energy, causing degradation in nutrients and changes in sensory characteristics. Light barrier or interference properties of packaging materials should be considered when identifying food packaging options. This chapter focuses on the susceptibility of food components and products to light damage, which causes photo-oxidation, and packaging technologies that provide light protection. The influence of light barrier packaging on sensory quality of dairy foods, beverages, and other products is presented. Traditional and innovative technologies are described in the context of food quality protection.
15.1 Introduction
Foods and beverages are exposed to light during processing, storage, transportation, retail display, and consumer use. Both visible and ultraviolet light wavelengths can affect food quality by altering the chemical structure of light-reactive food components and/or initiating reactions that break down non-light reactive components of the food system. The effect of light on a food or beverage product depends on the intensity and spectrum of the light that reaches the product. This is affected by the light source, location of the light relative to the product, length of exposure time, and the optical properties (transmission, reflectivity) of the product package. Additional factors that affect the interaction of light and food and beverage products include storage temperatures, product composition, product pH, oxygen content in the product and package, and the oxygen permeability of the package. Light exposure can result in nutritional losses due to degradation of vitamins, lipids and proteins, and changes in color, flavor and odor (Duncan and Webster, 2009). Exposure to light has the ability to dramatically reduce the shelf life of a product.
Light damage can be reduced or eliminated by selecting packaging materials that afford a partial or complete light barrier. However, light protection often is considered a lower priority than other packaging factors, such as cost and consumer appeal. For example, there is a well-noted desire among customers to be able to see the product within the package. Food processors also express interest in package transparency so that the visual appearance of the product can serve to help market the product. Transparent packages, however, may play a role in reducing product quality due to light exposure. Finding the right packaging material that provides the desired product protection characteristics and meets other packaging requirements for package appearance, marketing, cost, and ease of use/ functionality is challenging. A variety of technologies exist and are being developed that aim to provide highly attractive packaging materials that meet consumer and processor desires for appearance but also afford light protection to the product within. The focus of this chapter is on the susceptibility of food components and products to light damage and packaging technologies that provide light protection.
15.2 Effect of ultraviolet and visible light wavelengths on sensory and nutritional quality of foods and beverages
15.2.1 Review of the light spectrum
Ultraviolet (UV) light includes light in the range of 10 nm to approximately 380 nm. The visible light spectrum spans from approximately 400 nm to 780 nm. UV and visible wavelengths relevant to food are illustrated in Fig. 15.1. UV light is higher in energy than visible light and has the ability to break chemical bonds.
The sun generates UV light of wavelengths in the range of 100–400 nm, but the majority of wavelengths emitted by the sun below 315 nm are blocked by the ozone layer. Sunlight produces light throughout the entire visible spectrum. Sun exposure typically is not the primary source of detrimental light energy on food systems, except perhaps during transport or if products are displayed in windows. Foods and beverages are primarily exposed to artificial incandescent and fluorescent lights present in storage facilities, processing plants, during transport, in retail venues, and in home and office settings. In commercial venues, fluorescent lighting is most common. Fluorescent lighting is increasing in popularity in consumer homes due to better energy efficiency compared to incandescent bulbs.
Fluorescent lights emit wavelengths in the UV as well as the visible spectrum. Fluorescent light bulbs rely on mercury vapor to produce light, but the spectrum, as well as the intensity, of a fluorescent light source varies depending on the phosphors used in their production. General purpose lighting, providing brightness in offices and warehouses, typically is focused in the green to yellow wavelength regions (480–580 nm). 'Cool white' fluorescent tubes, which emit all wavelengths of visible light and typically have higher emission spectra, are inappropriate for lighting display cases and storage of photosensitive foods (Bosset et al., 1994). 'Warm white' fluorescent tubes are rich in the upper visible spectrum (550–780 nm; yellow, orange, red) region and low in energy in the lower visible region (380550 nm; violet, blue, green). Light intensity in retail settings varies, ranging from 129–5,380 lux; 2,000 lux is a common level (Bosset et al., 1994).
15.2.2 Light-sensitive components of foods and beverages
There are many natural or added components of foods and beverages that react with light. Vitamins, pigments and colorants, lipids, proteins and sugars may respond directly to UV or visible light. Transfer of light energy to molecules in close proximity causes secondary reactions that contribute to loss of nutrients and degradation of sensory quality. Robertson (2006) provides equations for calculating the amount of light absorbed by a packaged food. Consideration is given to the intensity of the light absorbed by and reflected by the food, transmittance and reflectance of the packaging material, and intensity of incident light.
Photosensitizers
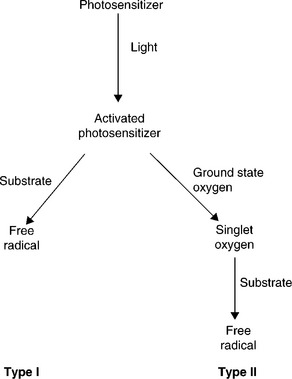
Some light-sensitive compounds in foods are photosensitizers - compounds that absorb light and subsequently induce secondary reactions. Photosensitizers absorb light of a specific wavelength depending on their chemical structure. As a result of absorbing light energy, photosensitizers are converted to an activated form. The activated photosensitizer can then cause subsequent reactions by two mechanisms (Fig. 15.2). Type I photochemical reactions occur when the activated photosensitizer reacts directly with a substrate molecule producing a free radical. Type II photochemical reactions occur when the activated photosensitizer reacts with oxygen, generating singlet oxygen. Singlet oxygen then reacts with substrate molecules such as lipids, thereby generating free radicals. Much of the light-induced damage in foods and beverages are a result of photosensitization. Although many different molecules may be photosensitizers, riboflavin (Vitamin B2) is most commonly described.
Vitamins
Many vitamins are damaged by light exposure. Light-sensitive vitamins include vitamin A, many of the B vitamins, vitamin C, D, E and K, as well as folic acid. An excellent review of the effects of lighting systems and packaging protection on vitamins in milk and dairy products is provided in Bosset et al. (1994). Light and oxygen transmission rates of packaging affect the degradation rate of vitamins. Loss of the yellow coloration contributed by riboflavin and β-carotene can cause discoloration of foods, such as yogurt and citrus and vegetable juices (Robertson, 2009). Many of the vitamins are affected by ultraviolet light but visible light regions also affect some of these essential molecules. Vitamin A has an absorption maximum at 326 nm in solvent whereas its precursor, β-carotene, has two absorption maxima at approximately 456 and 484 nm (Bosset et al., 1994). Retinol and retinyl palmitate, forms of vitamin A, have been shown to undergo isomerizaton from trans to cis form due to light exposure (Ball, 2006). Light-induced isomerization is also an issue for carotenoids, which are retinoid precursors (Ball, 2006).
Vitamin B1 (thiamin), vitamin B6 (pyridoxal phosphate), and vitamin B7 (biotin) are degraded by UV light in the 200–325 nm range and absorption maxima of vitamin B6 (pyridoxal phosphate) are affected by pH. Vitamin B12 (cobalamin) absorbs light at 278, 361, and 550 nm (Bosset et al., 1994). Light causes conversion of cyanocobalamin to hydroxocob(III)alamin, but the latter is still biologically active (Ball, 2006).
Riboflavin is a particularly well-known photosensitizer in foods. Absorption maxima of riboflavin are 223, 268, 359, 375, 446, and 475 nm (Bosset et al., 1994). Exposure of food products containing riboflavin to wavelengths in the range of 350–520 nm, and particularly, 415–455 nm causes excitation of this vitamin, with a resulting cascade of reactions causing significant sensory effects, especially in milk and dairy foods (Ball, 2006; Bosset et al., 1994). The numerous excitation maxima of riboflavin in the low ranges of the visible light spectrum increase the risk of photooxidation; cool white fluorescent bulbs, which have a broad spectrum of light emissions, therefore are not recommended for dairy cases. Degradation products of riboflavin are lumichrome and lumiflavin; solution pH plays a determining role in which degradation product is favored (Ball, 2006).
Riboflavin occurs in small quantities in most foods. Milk and other dairy products, liver and kidney, wheat bran, and eggs are some of the foods that naturally contain higher levels of riboflavin (Ball, 2006). Additionally, many foods including breads and cereals are enriched with riboflavin due to its important role in metabolism. Riboflavin is highly sensitive to light and, due to its role as a photosensitizer, reaction of riboflavin with light results in significant loss of vitamin activity (riboflavin as well as ascorbic acid, vitamin A, vitamin D), as well as subsequent effects on lipids and proteins through photooxidation. Degradation of lipids and proteins further reduces nutritional quality and produces off-odors and off-flavors.
Vitamin C (ascorbic acid) is highly sensitive to oxidative deterioration in the ultraviolet range (absorbance maximum at 245 nm and 365 nm in neutral solution) and therefore is susceptible to light damage through photosensitized oxidation. Vitamin D degrades during lipid oxidation; therefore, it can be considered light sensitive in foods, as photooxidation is a common mechanism for lipid oxidation (Ball, 2006). Vitamin E is damaged by light in the UV range (absorbance maximum 292 nm) and through light-induced oxidation (Ball, 2006). Vitamin K (phylloquinone and menaquni-nones) is degraded by ultraviolet wavelengths from fluorescent light and sunlight (Ball, 2006), with four absorbance maxima in the 240–270 nm range (Bosset et al., 1994).
Colorants
Colorants all absorb light in the visible range. Both naturally occurring and synthetic colorants are susceptible to light degradation and colorants from both groups have the potential to act as photosensitizers. The synthetic colorants rose bengal, erythrosine B (FD & C red no. 3), and phloxine B (FD & C red no. 28) act as photosensitizers in the oxidation of methylinole-ate (Pan et al., 2005). However, tartrazine (FD & C yellow no. 5), brilliant blue FCF (FD & C blue no. 1), new coccin, amaranth (FD & C red no. 2), indigo carmine (FD & C blue no. 2), acid red 52, fast green FCF (FD & C green no. 3), allura red AC (FD & C red no. 40) and sunset yellow FCF (FD & C yellow no. 6) do not (Pan et al., 2005). The authors believe that the xanthene skeleton and halogen substitution play a role in whether or not a colorant acts as a photosensitizer.
Porphy-ins, such as myoglobin, and chlorins, such as chlorophylls, are light-sensitive compounds that occur naturally in a range of foods. Chlorophyll is a green pigment that is naturally present in a wide range of vegetables, dairy foods, and food oils and also can be added to foods as a colorant. Chlorophyll absorbs light in the blue (chlorophyll a: about 420–430 nm; chlorophyll b: about 450 nm) and red regions (640–670 nm range) of the visible spectrum. Chlorophylls, as well as their degradation products, pheophytins, act as photosensitizers in foods (Thron et al., 2001). Hematoporphyrin, a component of myoglobin and therefore a pigment found in meat products, also acts as a photosensitizer (Fennema, 1996). Food products containing chlorophylls or porphyrins also must be shielded from the 600–700 nm range in order to protect product integrity (Wold et al., 2005; Larsen et al., 2009).
Non-nutritive functional food and other components
Consumer interest in health has increased the focus on food components that provide health benefits beyond those required for life and physiological maintenance (traditional vitamins, minerals and macronutrients). Food manufacturers are responding by adding these bioactive compounds to new products or increasing the amounts that are naturally present. Some of these bioactive compounds also are photosensitive and may function as photosensitizers. Many flavonoid compounds, found in fruits, vegetables, and other plant materials, are photoresponsive because of their biological function in protecting plants from ultraviolet light damage. They may function as photosensitizers, accepting light energy, or as quenchers, accepting energy from other molecules, depending on the system (Sisa et al. 2010).
The photoresponsive behavior of these molecules affects the bioactivity and potential health benefit reported for these molecules. Resveratrol, a polyphenol found in wine, berries, legumes and nuts, is reported to have health benefits but undergoes conversion from the trans configuration to the cis configuration due to exposure to UV light (Lopez-Hernandez et al., 2007). Daidzein and genistein were shown to degrade due to riboflavin photosensitization but not on their own (Yang et al., 2008). Lutein was degraded significantly by exposure to full spectrum light but UV wavelengths in the 200–380 nm range and in the low visible spectrum (463 nm) were most destructive (Kline et al., 2006; Kline, 2006). Omega-3 fatty acid rich oils, which are highly unsaturated and susceptible to oxidation, are derived from plants (flax seed, algae) or fish that consume algae, which is rich in chlorophyll and is a photoresponsive molecule. These alterations in chemical structure or conformation may alter the biological functionality of the molecule, negating the advantage of their addition into the food delivery system. There is only limited published information on the effects of specific light wavelengths on bioactive compounds in food or on how these compounds, when exposed to light, affect the chemical and sensory quality of food during shelf life (Osborn-Barnes and Akoh, 2003). There is an increasing need to be aware of food packaging needs that protect functional food ingredients from light (Deschênes, 2007). Protection of these valuable biomolecules in the food delivery system is important in order to provide the intended health value.
Other food components exhibit light-sensitive behavior as well. For example, the artificial sweetener aspartame has been shown to decompose upon exposure to fluorescent light in model beverage systems both with and without known photosensitizers (Kim, Jung and Kim, 1997). System pH significantly affected the degradation rate.
15.3 Improving the light barrier properties of food and beverage packaging materials
Foods and beverages are packaged most commonly in glass, plastic or paperboard materials. Presently, a majority of the plastic and paperboard packages are multi-component materials, e.g. plastic-coated paperboard, or plastic/foil laminate pouches. Glass was historically a popular packaging material and it is again popular for products such as high-end beverages. Packaging selection is based on product needs as well as marketing requirements. Factors that influence packaging selection include: containment needs, barrier properties (light, gas and water vapor), labeling requirements (nutritional and advertising), target market, shipping requirements, and cost. Light blocking properties often are not considered a main priority. Consumer desire to see the product within the package often takes a higher priority than protecting the product from light, despite the potential for reduced product quality and/or shortened shelf life as a result of high light transmission. Lack of manufacturer knowledge regarding the light sensitivity of their product also can be a factor in overlooking the need for light barrier properties. Packaging cost may prevent a manufacturer from choosing a light barrier package. As would be expected, the light barrier properties of packaging materials vary based on material composition and thickness.
15.3.1 Plastics
The term plastic includes a variety of materials with differing ranges of light transmission. The most commonly used plastics for food packaging applications are low density polyethylene (LDPE), and high density polyethylene (HDPE) (Robertson, 2006). Polypropylene (PP), polyethylene terephthalate (PET), and polystyrene (PS) also are used commonly. Barrier properties, including gas permeability and light barrier properties, as well as mechanical properties and cost vary based on the chemical composition, molecular weight, density, linearity, and crystallinity.
Without additives, these packaging materials are mostly transparent and transmit significant amounts of visible light. LDPE is the most commonly used polymer in food packaging. It is slightly translucent. HDPE has a higher level of crystallinity than LDPE and a whiter appearance. HDPE (1.7 mm thick) has a light transmittance of 57% (Mathlouthi, 1994) in contrast to clear polycarbonate (1.5 mm thick) with a 90% transmittance or clear flint glass (3.4 mm thick) with 91% light transmittance. Tailored crys-tallinity of plastics, by modifying chemical groups that absorb visible light, are usually translucent but this approach can modify the light transmission properties of the plastic (Robertson, 2009).
Although some shorter wavelengths of UV light are absorbed intrinsically by polymer materials, higher UV wavelengths generally are not absorbed. PET has slightly better UV blocking properties than PE and PP, the former of which has been reported to block UV light below 300 nm while PE and PP are reported to transmit all UV and visible wavelengths above 200 nm (Coltro et al., 2003). The presence of chromophoric impurities can reduce the UV transmittance of polymer packaging (Pospisil and Nespurek, 2000). The light barrier properties of plastic packaging can be improved by intentionally incorporating additives into the plastic itself (blending) or by applying a barrier material as a separate layer to the package through coating or laminating. Overwraps also can be used to reduce light exposure.
Blends
Blending is generally a lower cost option for reducing light transmittance than generating multilayer materials (Lange and Wyser, 2003). Carbon black and titanium dioxide are perhaps the most commonly used additives for reducing light transmission in plastic packages. Zinc oxide, chalk, talc, starch and clay also can be used (Lange and Wyser, 2003; Pospisil and Nespurek, 2000; Robertson, 2006; Singh and Singh, 2005). Particles that result in a white color, such as titanium dioxide, reduce exposure to visible light by scattering. Refractive index and particle size are key factors that determine the scattering efficiency of the particles. Titanium dioxide, which is a photoresponsive molecule, not only scatters light but absorbs wavelengths in the UV region, thus protecting UV-sensitive molecules. The rutile crystal pigment forms of titanium dioxide are more stable and more effective at light scattering and are less likely to catalyze photodegradation than the anatase form (Dupont, 2007). Particle size also influences light scattering and transmitted color and reflected color. Transmission spectra of a variety of plastic films, some of which include white and black pigments, are shown in Fig. 15.3.
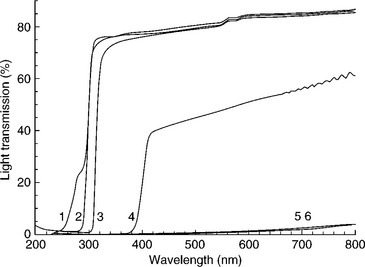
Fig. 15.3 Light transmission of polymer films incorporating white and black pigments. 1 = oriented polyamide (OPA)/linear low density polyethylene (LLDPE), 2 = OPA/PE, 3 = PET/polyvinyldine chloride(PVCD)/PE, 4 = OPA/PE with white pigment, 5 = OPA/PE with black pigment, 6 = OPA-barrier/PE with black pigment (Mortensen et al., 2002).
In addition to additives resulting in black and white colors, additives producing other colors also can be effective at offering light protection. A variety of pigments are used to color plastics including iron oxide, cadmium sulfide, molybdate orange, ultramarine blue, blue ferric ammonium ferro-cyanide, chrome green, blue copper phthalocyanines, and green copper phthalocyanines (Robertson, 2006). Natural compounds such as chlorophyll also are being investigated as dyes for plastics (Anonymous, 2002). Plastics prepared with these materials have reduced light transmission in the regions where the pigments absorb light. Colored plastics can prove very useful for specific products if they block a particularly damaging range of wavelengths. For example, yellow and green tinted packaging materials have been shown to protect the quality of milk because they block wavelengths that would result in photosensitization by riboflavin. In addition to a variety of coloring materials for aesthetic appeal, the company ColorMatrix (www.colorma-trix.com) offers an opaque colorant sold specifically for light blocking properties.
UV blockers/absorbers often are added to plastics to prevent transmission of UV light but maintain package transparency. UV absorbers are added as a means of protecting the polymer itself from UV degradation, but also act to protect the product. Commonly used UV blockers include benzophe-none, benzotriazoles, oxamide, and α-cyanoacrylate (Pospisil and Nespurek, 2000). UV transmission spectra of PET films containing the UV absorber 2-(2'-hydroxy-3'-tert-butyl-5'-methylphenyl)-5-chlorobenzotraizole (trade name Tinuvin 326TM) are shown in Fig. 15.4 (Coltro et al., 2003). Optical brighteners such as benzoxazole, phenylcumarine, and stillbene, which are added to polymer packaging materials in order to improve brightness, also absorb UV light (Pospisil and Nespurek, 2000). Packages including Eastman Heatwave and Voridian Vitiva (http://www.eastman.com/Pages/Home.aspx) are examples of transparent plastic containers containing a UV absorber (Fig. 15.4). ColorMatrix also offers a UV blocking additive in liquid form for PET for transparent container applications.
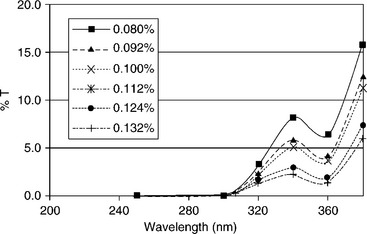
Fig. 15.4 Ultraviolet transmittance of PET films containing Tinuvin 326™ (Coltro et al, 2003).
Coating and lamination
A barrier layer can be added to plastic materials through a number of methods including adhesive lamination, extrusion coating, coextrusion, co-injection, and vacuum deposition. Coating and lamination are often employed to improve oxygen barrier properties of packages, but can also be effective at reducing light transmission depending on the materials used and manufacturing methods.
One of the best technologies for reducing light transmission of flexible packaging is metallization. Aluminum is the most commonly used material for metallization of films, although zinc, gold, silver, tin, copper and nickel also have been used (Robertson, 2006). Flexible polymer or paper packages containing metal layers can be generated using laminate technology or by vacuum deposition. An 'aluminum foil in laminate' film is typically 5–12 μm thick whereas a vacuum deposited aluminum film is typically between 10–40 nm (Piergiovanni and Limbo, 2004). Both types of films have been shown to afford good protection against visible light; however, thicker coatings seem necessary to provide complete protection against light in the UV wavelengths (Piergiovanni and Limbo, 2004). Ultimately the light barrier properties depend on coating composition, uniformity, and thickness as well as polymer type and thickness. Unmetallized oriented polypropylene (OPP) has 80-86% transmission whereas metallized OPP, at the same film thickness of 20 μm, has less than 1% transmission at 350, 450, 550, and 670 nm (Piergiovanni and Limbo, 2004). Metallization can be applied to a variety of polymer materials as well as paper, provided the paper can be treated to enable adhesion of the metal particles (Robertson, 2006). Metallized films have the advantage of being flexible, but can be expensive and present issues in terms of recyclability.
Colored inks for light blocking, such as those described above can be printed directly onto polymer films that are subsequently incorporated into multilayer laminate packages.
Overwrap describes a layer that is placed on the outside of the package. Overwraps are commonly used on plastic bottles for displaying product branding and nutritional information. Often overwraps are shrink sealed in order to conform to the package, but general labels also could be considered overwraps. Overwraps are usually made from polymeric materials that are not intrinsically light protective; however, printing and coloring can serve to reduce light transmission. In many cases overwraps do not cover the entire bottle and therefore even if they are opaque they do not provide complete protection to the product. Inks directly printed on polymer packaging also can afford light protection.
15.3.2 Paperboard
Paperboard is quite commonly used for packaging food and beverage products including juices, milk, and cereal products. Paperboard packaging comes in several grades. Solid bleached sulfate (SBS) paperboard contains at least 80% virgin bleached wood pulp. Most gable top and aseptic drink packages are composed of SBS paperboard. Coated unbleached kraft paperboard (CUK) is comprised of at least 80% virgin unbleached natural wood pulp. Non-gable top beverage cartons and frozen food packaging are often CUK paperboard. Coated recycled paperboard is used for dry food packaging, for example cereal and cracker boxes. Also, paperboard packages often are coated with various materials to improve printing and gas/ water vapor barrier, and strength properties. Coating materials include kaolin clay and polyethylene resins. Many of the same technologies used for adding additional layers to plastic materials also are employed with paperboard.
Paperboard generally outperforms plastic materials in terms of light blocking properties, although paperboard packages do not block all light. Unprinted fiberboard (0.7 mm thick) will still have about 4% transmittance. Bleaching of paper removes pigments that absorb light and therefore bleached paper has higher light transmission than unbleached paper (Singh and Singh, 2005).
15.3.3 Glass
Although plastic has replaced glass in a lot of food packaging applications, glass is still used for numerous thermally processed products and high-end beverages. Most of the glass in use for food packaging is non-colored (approximately 85%) (Robertson, 2006). Non-colored glass transmits approximately 90% of visible light (Singh and Singh, 2005). Glass can be colored with metal oxides, metal sulfides or metal selenides. The metallic compounds used absorb in UV and visible regions of the spectrum. Amber colored glass, which is produced using sodium sulfide, provides the greatest light protection of commonly colored glasses (Robertson, 2006). Celenium oxide, titanium dioxide, ferric oxide, and cerium oxide can be added to glass to reduce UV transmission without providing color for products that are sensitive to UV but not visible light (Robertson, 2006; Scheffler-Hudlet et al., 2008). General transmission properties of colored glass are shown in Fig. 15.5.
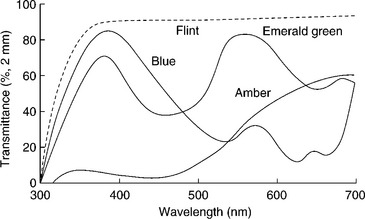
Fig. 15.5 Light transmission properties of glass (Robertson, 2009).
15.3.4 Environmental modifications
In addition to careful packaging selection, environmental modifications can help prevent damage to food and beverage products due to light. Any modification that reduces duration or intensity of light exposure could help prolong product quality. Filters, or special coatings applied to fluorescent lights or display case windows could be used to reduce light exposure across the entire spectrum or for specific wavelength ranges known to be detrimental for particular products. Recently retail venues have started to employ motion sensory systems to automatically turn lights on when a consumer is sensed in the vicinity of a case or aisle; when no consumer is sensed the lights power off. This not only saves energy but also reduces the time of exposure for food and beverage products.
15.4 Selecting light barrier properties of packaging to improve sensory and nutritive quality of foods and beverages
While it is well known that light can have a detrimental effect on the nutritive and sensory qualities of foods and beverages, there is no clear-cut guidance on the best packaging material for a specific product. Light barrier properties must be balanced with other needs. Furthermore, food manufacturers do not typically have the resources to thoroughly evaluate the interaction between light and packaging on their product. Different products have different requirements as well as differences in the severity of the consequences of light damage. Packaging material developers with knowledge of photosensitive food molecules can assist food processors with identifying the appropriate light barrier packaging that best protects the food and creates the best product image.
Dairy foods are by far the most widely studied products in terms of effects of light and the ability of different packaging materials to prevent light damage. Dairy foods contain the recognized photosensitizer riboflavin well as other photosensitizers such as chlorophyll. The energy from these molecules is readily transferred to oxygen in the system and to sensitive proteins, lipids and other vitamins. Dairy products, therefore, are very susceptible to light damage and several excellent reviews are available (Bekbolet, 1990; Bosset et al, 1994; Duncan and Webster, 2010). Due to the popularity of dairy products as well as their known sensitivity to light, and value as a model system, there has been substantial interest in evaluating different packaging materials for such products (Bosset et al., 1994; Duncan and Webster, 2009; Larsen et al., 2009; Webster et al., 2009, 2011; Dalsgaard et al., 2010; Intawiwat et al., 2010). Numerous studies have evaluated the effects of pigments in plastics in protecting milk from light. Only a few are described here and the reader is directed to the review articles for more information.
Saffert et al., (2008, 2009) found that increasing the level of pigment in PET bottles resulted in reduced loss of vitamins in milk. Vitamin A was retained to the same degree for all pigmented bottles evaluated compared to unpigmented PET. Vitamin B2 (riboflavin) also was retained in pig-mented bottles; however, in this case, higher levels of pigment proved more effective than lower levels. Bottles with the highest level of pigment contained both white and yellow pigments. Effectiveness of these bottles is likely a result of both the higher level of pigment and the inclusion of a yellow pigment, which blocks some of the riboflavin excitation wavelengths.
Larsen et al., (2009) evaluated the light-protective character of polystyrene thermoformed white cups (300 μm thick) with three different levels of light barrier: added titanium dioxide (white); a light barrier of aluminum oxide sandwiched in between white layers (medium light barrier); and, a light barrier of carbon black and aluminum oxide sandwiched in between white layers (high light barrier). The white barrier cup had seven large irradiance peaks at about 435 and 480 nm and large peaks at 550 nm, and between the 575 and 640 nm range, whereas the medium light barrier cup had two large peaks at 545 and 613 nm and smaller peaks at 588 and 713 nm. The cup with high light barrier had few very small peaks. Sour cream, packaged and stored in white and medium light barrier cups for 36 h at 5,610 lux at 4 °C showed significantly higher intensity of sunlight, odor and flavor and bitter and rancid flavors than did the sour cream in the high light barrier or a control product that was stored in the dark. The light barrier properties of the medium light barrier cup provided protection against riboflavin excitation but did not protect against photosensitization of the compounds in the longer wavelength region, such as proto- and hematoporphyrins and tetrapyrrols. High fat dairy products have a higher concentration of photosensitized molecules, increasing their susceptibility to photooxidation.
Vassila et al., (2002) evaluated milk stored in flexible single and multilayer LDPE pouches and multilayer LDPE/PA/LDPE pouches. Pouches without a light blocking additive as well as those containing differing levels of TiO2 and carbon black were evaluated. Paperboard cartons were used as control samples. The researchers did not see statistical differences in the degree of lipid oxidation (based on TBARS levels) after 7 days of storage. Differences in proteolysis and lipolysis also were small. Vitamin A and riboflavin were best preserved by control (paperboard) packages and multilayer LDPE packages containing both TiO2 and carbon black. Clear single-layer LDPE packages provided the least protection for vitamin A. Surprisingly, clear LDPE/PA/LDPE packages outperformed LDPE packages with TiO2 pigment for vitamin retention. The authors attribute this to differences in oxygen transmission of the pouches and possibly due to scattering by TiO2.
Pigmented PET bottles (green, amber) outperformed clear PET, LDPE pouches, and HDPE jugs for preserving milk sensory and nutritive quality. In comparing PET with UV blockers to PET with no blockers, the authors observed that the UV blockers helped prevent loss of vitamin A and slightly reduced lipid oxidation. Use of a label over the PET bottles also helped to preserve the product. The label was determined to reduce light exposure by 75% (Cladman et al., 1998). Amber pigmented PET was compared to clear PET, PET with a UV absorber and HDPE (van Aardt et al., 2001). Amber PET preserved milk quality the best, followed by PET containing the UV absorber.
Webster et al., (2009) found that iridescent polymer films (http://aurorasef.com/index.htm), layered as overwraps on glass bottles to provide less than 4% transmittance, were able to preserve milk quality to a degree, although they were not as effective as a complete light block. The tested films provided barriers at the major riboflavin excitation wavelengths. This study concluded that there were photosensitive compounds that responded to longer light wavelengths (> 550 nm) in milk, as had been reported previously (Wold et al., 2005), and that it is important that transmittance be less than 4% to provide adequate protection from light.
Growing interest in foods that provide health benefits beyond basic nutrition has generated a large increase in the amount and variety of 'functional food' products on the market. Such products often are formulated with added vitamins and natural compounds that are susceptible to light damage. The market for functional beverages has expanded dramatically. These products are typically packaged in transparent bottles but contain numerous light-sensitive components. There is a need for additional research into the effects of light on these types of products. Perez-Vicente et al.(2004) found that oxygen permeability was of greater importance than light barrier properties of packaging for pomegranate juice stability. Anthocyanin content decreased to the same extent for juice packaged in clear and green glass, and decreased to a greater extent for juice packed in a multilayer paperboard carton containing a foil layer. Color changes were more significant for juice packed in the paperboard carton than those in glass bottles.
Lu and Xu (2009) reported that packaging cookies in light barrier packaging materials extended shelf life, based on peroxide value. Cookies packaged in biaxially oriented polypropylene/vacuum aluminized cast polypropylene (BOPP/cmVPP, opaque, 45 μm) exhibited the longest shelf life, followed by those packaged in a polyethylene terephthalate/oriented nylon/ polyethylene film (PET/ONY/PE, semintransparent, 170 μm), then ONY/PE (transparent, 125 μm). The ONY/PE film extended the shelf life by 5 days compared to unpacked cookies, while the metallized BOPP film more than doubled the shelf life.
Stability of soybean oil in PET bottles during storage for 6 months under fluorescent lighting was improved for bottles containing the UV absorber Tinuvin 234 (2-(2-hydroxy-3,5-di(1,1-dimethylbenzyl)phenyl) benzo-traizole), relative to those without a UV absorber (de Azeredo et al., 2003). Amber glass effectively prevented light degradation of vitamin K in saf-flower oil and rapeseed oil when exposed to fluorescent light and sunlight (Ball, 2006).
Pierogiovanni and Limbo (2004) found that packaging lasagna in metallized films extended its shelf life by a factor of 2.5 and they were also able to establish a relationship between shelf life and transmission.
15.5 Future trends
There is growing interest in environmentally friendly technologies for packaging applications and recently much emphasis has been placed on replacing synthetic plastics with biologically derived polymers. Lipid-protein films prepared using pig-skin gelatin and acetylated monoglyceride reduced light transmission of synthetic plastic films when applied as a thin coating (Farris et al., 2009). As shown in Fig. 15.6, the coating successfully reduced the UV transmission of all three types of plastics coated, although the authors note that the reduction observed for LDPE and OPP is not particularly relevant as UV light below 280 nm is not commonly emitted from retail light sources. In this work the goal was to maintain optical clarity of the films for applications when transparent films were desirable. Coating did, however, reduce the transparency of the films (17% for OPP, 12% for PET, and 36% for LDPE) and increase the haze (85% for OPP, 70% for PET, and 78% for LDPE). Although considered undesirable for the proposed applications, these changes would reduce light exposure of products packaged within the materials.
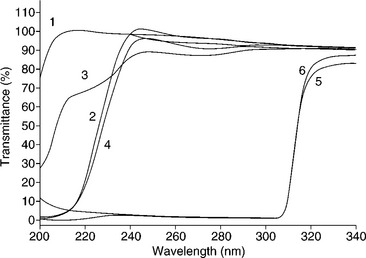
Fig. 15.6 UV transmission of bio-coated and uncoated plastic films. 1 = uncoated LDPE, 2 = coated LDPE, 3 = uncoated OPP, 4 = coated OPP, 5 = uncoated PET, 6 = coated PET (Farris et al., 2009).
Nanomaterials and nanocomposites increase the potential for photores-ponsive films and packaging materials that may be valuable in protecting product integrity. Tuning films to protect product against wavelength regions of interest is already possible (Webster et al., 2009) but is economically not feasible for most food products.
Challenges in selecting the most effective light barrier properties include the limits in understanding of product composition and response to light as well as economic barriers that limit the application of appropriate light barrier packaging materials. Novel materials and packaging technologies are most often adopted first in product areas with higher profit margin or value, such as military, automobiles, computing, medical, pharmaceuticals, and cosmetic applications. The breakthrough into applications in the food industry takes longer because of cost, primarily, but also the need for documenting safety as a food contact material and value in protecting food integrity.
15.6 Sources of further information and advice
For additional information, the reader is directed to the following reviews, books and book chapters:
• For more information on packaging of functional foods: the book chapter by Deschênes (2007).
• For more information on light transmittance of packaging materials in milk and dairy foods: reviews by Bekbolet (1990) and Bosset et al. (1994, 1995).
• For additional information on food packaging materials and shelf life: Robertson (2006, 2009).
• For information on the implications of interactions of food and packaging on sensory effects: Duncan and Webster (2009).
• For additional information on the role of packaging in protecting milk and dairy products from oxidation: Duncan and Webster (2010).
• For a good review of titanium dioxide and opacity: Dupont (2007).
The following websites also may be of interest for finding products that provide pigments, colorants, or novel materials. This list is not exhaustive and is not to be interpreted as endorsement of the products described within.
References
Anonymous, Light protection for food packaging. Innovations Report Forum for Science, Industry and Business. 2002. http://www.innovations-report.com/html/reports/materials_science/report-7442.thml [(accessed April 2, 2011).].
Ball, G. Vitamins in Foods: Analysis, Bioavailabilty, and Stability. Boca Raton, FL: CRC Press; 2006.
Bekbolet, M. Light effects on food. Journal of Food Protection. 1990; 53:430–440.
Bosset, J.O., Gallmann, P.U., Sieber, R. Influence of light transmittance of packaging materials on the shelf-life of milk and dairy products - a review. In: Mathlouthi M., ed. Food Packaging and Preservation. New York: Blackie Academic and Professional; 1994:222–268.
Bosset, J.O., Sieber, R., Gallman, P.U. Light transmittance: Influence on the shelf-life of milk and milk products. In: International Dairy Federation, ed. Technical Guide for the Packaging of Milk and Milk Products. 3rd edn. Brussels: International Dairy Federation; 1995:19–39. [N. 300].
Cladman, W., Scheffer, S., Goodrich, N., Griffiths, M.W. Shelf-life of milk packaged in plastic containers with and without treatment to reduce light transmission. International Dairy Journal. 1998; 8:629–636.
Coltro, L., Padula, M., Saraon, E.S., Borghetti, J., Buratin, A.E.P. Evaluation of a UV absorber added to PET bottles for edible oil packaging. Packaging Technology and Science. 2003; 16(1):15–20.
Dalsgaard, T.K., Sorensen, J., Bakman, M., Vognsen, L., Nebel, C., Albrechtsen, R., Nielsen, J.H. Light-induced protein and lipid oxidation in cheese: dependence on fat content and packaging conditions. Dairy Science and Technology. 2010; 90:565–577.
De Azeredo, H.M.C., Faria, J.A.F., Da Silva, M.A.A.P. The efficiency of TBHQ, beta-carotene, citric acid, and Tinuvin 234 on the sensory quality of soybean packaged in PET bottles. Journal of Food Science. 2003; 68(1):302–306.
Deschénes, L. Packaging technologies of functional foods. In: Shi J., ed. Functional Food Ingredients and Nutraceuticals: Processing Technologies. Boca Raton, FL: CRC Press; 2007:329–337.
Duncan, S.E., Webster, J.B. Sensory impacts of food-packaging interactions. Advances in Food and Nutrition Research. 2009; 56:18–63.
Duncan, S.E., Webster, J.B. Oxidation and protection of milk and dairy products. In: Decker E., Elias R., McClements D.J., eds. Oxidation in Foods and Beverages and Antioxidant Applications: Management in Different Industry Sectors, Volume 2. Cambridge: Woodhead Publishing, 2010.
Dupont Polymers, light and the science of TiO2. Dupont Titanium Technologies, 2007. www.titanium.dupont.com
Farris, S., Introzzi, L., Piergiovanni, L. Evaluation of a bio-coating as a solution to improve barrier, friction and optical properties of plastic films. Packaging Technology and Science. 2009; 22:69–83.
Fennema, O. Food Chemistry. New York: Marcel Dekker; 1996.
Intawiwat, N., Pettersen, M.K., Rukke, E.O., Meier, M.A., Vogt, M.A., Dahl, A.V., Skaret, J., Keller, D., Wold, J.P. Effect of different colored filters on photooxi-dation in pasteurized milk. Journal of Dairy Science. 2010; 93:1372–1382.
Kim, S.K., Jung, M.Y., Kim, S.Y. Photodecomposition of aspartame in aqueous solutions. Food Chemistry. 1997; 59(2):273–278.
Kline, M., The antioxidant function of lutein in controlling photo-oxidation of a colloidal beverage systemMaster's Thesis. Blacksburg, VA: Virginia Polytechnic Institute and State University, 2006.
Kline, M., Duncan, S., O'keefe, S. The role of lutein fortification on the protection of sensory quality and reduction of volatiles produced from the photo-oxidation of a model colloidal system. Annual Meeting of the Institute of Food Technologists Book of Abstracts. Abstract 018B-02. Chicago: IFT; 2006.
Lange, J., Wyser, Y. Recent innovations in barrier technologies for plastic packaging - a review. Packaging Technology and Science. 2003; 16(4):149–158.
Larsen, H., Tellefsen, S.B.G., Dahl, A.V., Quality of sour cream packaged in cups with different light barrier properties measured by fluorescence spectros-copy and sensory analysis. Journal of Food Science. 2009:S345–S350.
Lopez-Hernandez, J., Paseiro-Losada, P., Sanches-Silva, A., Lage-Yusty, M.A. Study of the changes of trans-resveratrol caused by ultraviolet light and determination of trans- and cis-resveratrol in Spanish white wines. European Food Research and Technology. 2007; 225:789–796.
Lu, L.X., Xu, F. Effect of light barrier property of packaging film on the photo-oxidation and shelf-life of cookies based on accelerated tests. Packaging Technology and Science. 2009; 22(2):107–113.
Mathlouthi, M. Food Packaging and Preservation. Gaithersburg, MD: Aspen Publishers; 1994.
Mortensen, G., Sorensen, J., Stapefeldt, H. Effect of light and oxygen transmission characteristics of packaging materials on photo-oxidative quality changes in semi-hard Havarti cheeses. Packaging Technology and Science. 2002; 15(3):121–127.
Osborn-barnes, H.T., Akoh, C.C. Effects of α-tocopherol, β-carotene, and soy isoflavones on lipid oxidation of structured lipid-based emulsions. Journal of Agriculture and Food Chemistry. 2003; 51(23):6856–6860.
Pan, S., Ushio, H., Ohshima, T. Effects of molecular configuration of food colorants on their efficacies as photosensitizers in lipid oxidation. Food Chemistry. 2005; 92:37–44.
Perez-Vicente, A., Serrano, P., Abellan, P., Garcia-Viguera, C. Influence of packaging material on pomegranate juice colour and bioactive compounds during storage. Journal of the Science of Food and Agriculture. 2004; 84(7):639–644.
Piergiovanni, L., Limbo, S. The protective effect of film metallization against oxidative deterioration and discoloration of sensitive foods. Packaging Technology and Science. 2004; 17(3):155–164.
Pospisil, J., Nespurek, S. Additives for plastics and their transformation products. In: Piringer O.-G., Baner A.L., eds. Plastic Materials for Food. New York: Wiley-VCH; 2000:47–77.
Robertson, G.L. Food Packaging Principles and Practice. Boca Raton, FL: CRC Taylor and Francis; 2006.
Robertson, G.L. Food Packaging and Shelf-Life: A Practical Guide. Boca Raton, FL: CRC Taylor and Francis; 2009.
Saffert, A., Pieper, G., Jetten, J. Effect of package light transmittance on vitamin content of milk: part 2. UHT whole milk. Packaging Technology and Science. 2008; 21:47–55.
Saffert, A., Pieper, G., Jetten, J. Effect of package light transmittance on vitamin content of milk: part 3. Fortified UHT low-fat milk. Packaging Technology and Science. 2009; 22:31–37.
Scheffler-Hudlet, H., Cid-Aquilar, J.G., Pinto-Negroe, R.E., Glass composition with high visible light transmission and low ultraviolet light transmission. Vidrio Plano De Mexico. 2008. [Patent no. 7,435,696].
Singh, R., Singh, N. Quality of packaged foods. In: Han J.H., ed. Innovations in Food Packaging. Boston, MA: Elsevier Academic Press, 2005.
Sisa, M., Bonnet, S.L., Ferreira, D., Van Der Westhuizen, J.H. Photochemistry of flavonoids. Molecules. 2010; 15:5196–5245.
Thron, M., Eichner, K., Ziegleder, G. The influence of light of different wavelengths on chlorophyll-containing foods. Lebensmittel-Wissenschaft und-Technologie - Food Science and Technology. 2001; 34:542–548.
Van Aardt, M., Duncan, S.E., Marcy, J.E., Long, T.E., Hackney, C.R. Effectiveness of poly(ethylene terephthalate) and high density polyethylene in protection of milk flavor. Journal of Dairy Science. 2001; 84(6):1341–1347.
Vassila, E., Badeka, A., Kondyli, D., Savvaidis, I., Kontominas, M.G. Chemical and microbial changes in fluid milk as affected by packaging conditions. International Dairy Journal. 2002; 12:715–722.
Vassila, E., Badeka, A., Kondyli, D., Savvaidis, I., Kontominas, M.G. Controlling light oxidation flavor in milk by blocking riboflavin excitation wavelengths by interference. Journal of Food Science. 2009; 74(9):S390–S398.
Webster, J.W., Duncan, S.E., Marcy, J.E., O'keefe, S.F. Effect of narrow wavelength bands of light on the production of volatile and aroma-active compounds in UHT milk. International Dairy Journal. 2011; 21(5):305–311.
Wold, J.P., Veberg, A., Nilsen, A., Iani, V., Juzenas, P., Moan, J. The role of naturally occurring chlorophyll and porphyrins in light-induced oxidation of dairy products. A study based on fluorescence spectroscopy and sensory analysis. International Dairy Journal. 2005; 15(4):343–353.
Yang, S., Lee, S., Chung, H., Lee, J. Stability of isoflavone daidzein and genistein in riboflavin, chlorophyll b, or methylene blue photosensitization. Journal of Food Science. 2008; 73(2):C100–C105.