Emerging coating technologies for food and beverage packaging materials
Abstract:
Coatings are increasingly recognized as a powerful tool to improve many of the properties of food and beverage packaging materials. The goal of this chapter is to provide an in-depth overview of the latest innovations in this area. Following a section reviewing coatings for food packaging materials from the past to the present, attention is then focused on the strategies recently adopted to design high-performance coatings, with special emphasis on the impact of nanotechnology. Applications of new types of coatings to improve food quality are then considered. Future trends in the field are discussed in the final section.
14.1 Introduction
The term 'coating' refers to a general covering that is applied to the surface of an object, defined as a substrate (see Fig. 14.1). In the field of food and beverage packaging, coatings include (but are not limited to) thin layers that may be either in direct contact with the surroundings or sandwiched between two substrates. Although 'thin' is a relative term, it can be said that the thicknesses of such layers normally ranges from tenths of a nanometer to a few micrometers, and that they are generally much thinner than the substrate beneath. Nowadays, coatings used for food and beverage packaging purposes may afford the substrate several benefits in terms of:
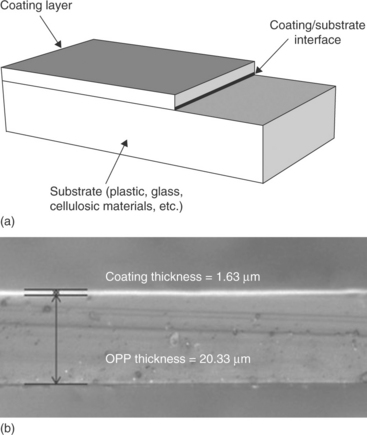
Fig. 14.1 Illustrative representation of a coating on a generic substrate (a) and image of a coating onto a plastic (oriented polypropylene, OPP) substrate (b, adapted from Farris et al., 2009a).
• barrier performance, e.g. against gases (O2, CO2), water vapor, VOC (volatile organic compounds), aromas, potential unwanted migrants, light (UV radiation in particular), oils, and fats;
• optical properties, especially haze and gloss;
• mechanical properties, such as friction, tensile strength, and scratch resistance;
• surface characteristics, particularly wettability, adhesion, and sealability;
• thermal attributes, such as flame resistance; and
• new functions (for example, in the case of active coatings with antimicrobial and antioxidant properties).
These thin layers of coating can be applied to a substrate using different techniques falling into four main categories, which are outlined below:
• roll-to-roll (e.g., gravure coating, curtain coating, wire-wound rod coating, slot die coating);
• chemical vapor deposition (CVD);
The choice of coating method depends on both the substrate and the physical form of the coating before it is applied (i.e., solid or liquid). Specific information about these techniques can easily be found in many books and book chapters, such as those listed in Section 14.10. In this chapter, the discussion will be devoted exclusively to coatings intended for food and beverage packaging materials (especially plastic flexible films) in the form of solutions, dispersions, and/or emulsions, generally deposited by roll-to-roll techniques, spraying, or dipping. In particular, as the use of waterborne coatings has increased significantly over the last few years, special emphasis will be given to coatings in the forms listed above that are based on water as a solvent. Furthermore, due to the today's industrial trends, barrier coatings will be the major topic of the chapter. Following this introduction, after a brief section reviewing coatings from the past to the present, an in-depth discussion of the more recent strategies adopted to obtain high-performance coatings will be delivered in Sections (14.3-14.7), while in Section 14.8 the focus will be on the more relevant recent applications in the food and beverage packaging sector. The closing section of the chapter will be dedicated to the expected trends in future years. A detailed description of the various deposition techniques has purposely been omitted, as it is beyond the scope of this chapter.
14.2 Coatings for food and beverage packaging applications: from the past to the present
14.2.1 Barrier coatings
Barrier properties are certainly a never-ending concern for any scientist or technologist working with food and beverage packaging materials. There is an increasing demand by industries for high-barrier packaging for shelf-life extension purposes, especially in terms of gas (O2 and CO2) and water vapor transmission (i.e., penetration and loss) across the package. Historically, early barrier materials used included glass and tinplate, followed by aluminum to produce, for example, cans. All of these provide a total barrier to gases and water vapor, whilst tinplate and aluminum also provide a barrier to light. By contrast, glass allows the consumer to see through the package, which is a highly desired property for marketing purposes. Although these materials have served the food and beverage packaging industry pretty well, and still continue to do so, some drawbacks linked to their inherent nature have led to new convenient solutions. For example, the heaviness of glass and tinplate represents a logistical limit at both the economic and environmental levels, whereas aluminum-based laminated packages are difficult to recycle, which is one reason why landfill sites and incineration are still the most common means of disposal. Currently, the alternatives to glass, tinplate, and aluminum available on the market are limited, especially considering that most applications require transparent packaging, thus excluding forms such as metalized films. Manufacturers of the most widely employed polymers for food and beverage packaging applications (i.e., polyethylene terephthalate (PET), oriented polypropylene (OPP), polyethylene (PE), and oriented polyamide (OPA)), have over the last 60 years, developed coatings with outstanding performance in terms of barrier properties and, to some extent, sealing, mechanical resistance, and optical features. Here, the most common barrier coatings that have penetrated the food and beverage packaging market are discussed briefly.
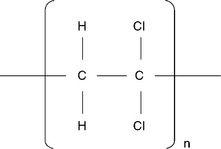
Polyvinylidene chloride (PVdC)
PVdC (see Fig. 14.2) was developed at Dow Chemical by Ralph Wiley and collaborators in the period from 1932–1939, and it was initially commercialized under the trade name of Saran®. PVdC is currently used by both film manufacturers and converters, in forms that include organic solvent and aqueous dispersions. The reason for the long-lasting success of PVdC relies on its undisputable performance, that makes its derivatives unique and still inimitable even today. Indeed, PVdC coatings are the only organic polymers able to provide a water vapor and oxygen barrier simultaneously. The high-barrier PVdC coating aqueous dispersions for food packaging applications, which are available on the market today, allow oxygen transmission rete (OTR) values of ~ 6 ml m− 2 day− 1 (23 °C; 50% RH) and water vapor transmission rate (WVTR) values of ~ 5 g m− 2 day− 1 (38 °C; 90% RH) (3 g m− 2 PVdC coating on PET film of 12 μm thick). Sealability, gloss, transparency, and flexibility are additional sought-after features for any coating application. However, despite its valuable barrier performance, PVdC is gradually being used less in many countries around the world due to environmental and health concerns that have arisen in the last few years. Like many other chlorinated polymers, PVdC is increasingly being excluded from many packaging structures, mainly because of the problems linked to its incineration, specifically the dioxins dumped into the air. Moreover, as a consequence of the market driving forces for continuously higher barrier performance for extended shelf life, PVdC is constantly being replaced by new coatings deriving from alternative polymer technologies (Leonard, 2009).
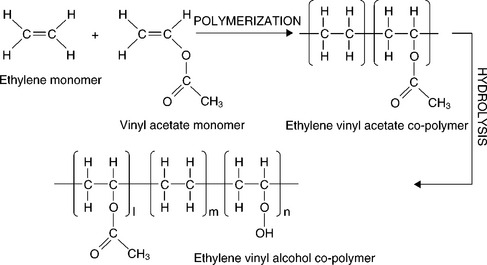
Ethylene vinyl alcohol (EVOH)
EVOH is currently used as a coating for food and beverage packaging applications, due to its outstanding barrier properties against oxygen (OTR ~ 1 ml m− 2 day− 1 at 23 °C; 50% RH; 2 g m− 2 EVOH coating on PET film of 12 μm thick). This is linked to the coating's innate chemical structure. EVOH is an ethylene and vinyl alcohol copolymer, produced by the hydrolysis of ethylene vinyl acetate (EVA), which is, in turn, obtained from the polymerization of ethylene and vinyl acetate monomers (see Fig. 14.3). Different EVOH grades can be obtained depending on both the polymerization and hydrolysis processes; i.e. depending on the vinyl content (%) and the degree of hydrolysis (%). Nowadays EVOH grades are controlled with a hydrophobic-hydrophilic balance, with a degree of hydrolysis from partial to full. This coating provides excellent barrier properties against gases, good water resistance, sufficient viscosity stability of the aqueous solutions, and water solubility. Generally, solubility in water of EVOH polymers decreases as both the ethylene content and the degree of hydrolysis increase due to the hydrophobic nature of the ethylene content and the high degree of crystallinity, owing to the hydrogen bonds between hydroxyl groups. In addition, the higher the ethylene content, the lower the barrier against oxygen and the higher the ease of extrusion. Conversely, the barrier against oxygen increases for the highest degree of hydrolysis. EVOH copolymers were put on the market in Japan starting in 1972, although worldwide commercialization did not begin until 10 years later. Nowadays, EVOH coatings used in food and beverage packaging are produced from copo-lymers with 25-30% ethylene. The final coatings provide excellent oxygen barrier performance, representing a valuable alternative to PVdC coatings, especially from an environmental perspective. However, the barrier performance of EVOH coatings is lost at high relative humidity values (~ 70%), which hinders the applicability of EVOH coatings under certain conditions (e.g., tropical) (Leonard, 2009).
Polyvinyl alcohol (PVOH)
PVOH is obtained from the hydrolysis of polyvinyl acetate (PVAc) (see Fig. 14.4). Therefore, lacking the ethylene unit, PVOH polymer grades differ only as far as the degree of hydrolysis is concerned, since the highly hydro-lyzed PVOHs are the most suited to act as a barrier against oxygen. However, a higher hydroxyl group content in the coating leads to a greater affinity to water, making PVOH coatings extremely sensitive to the surrounding relative humidity.
For this reason, despite a higher oxygen barrier performance compared to EVOH coatings under dry conditions (OTR ~ 0.4 ml m− 2 day− 1 at 23 °C; 0% RH; 2 g m− 2 PVOH coating on PET film of 12 μm thick), the practical use of PVOH coatings for food and beverage packaging structures is restricted with regard to any application anticipating a relative humidity above 50%, even if sandwiched between two water vapor barrier layers (e.g., polyolefins). PVOH coatings are not suited for packages requiring pasteurization or autoclave steam cooking either (Leonard, 2009).
14.2.2 Other coatings
Phenolic resin-based coatings
Synthetic phenolic resins have been developed since the early 1900s, when Leo Baekland, a Belgian-born American chemist, invented Bakelite from the reaction between phenol and formaldehyde, starting the so-called 'Age of Plastics'. Phenolic resins have applications in the formulation of coatings intended for food cans, protecting the food from direct contact with the metal surface. In order to avoid damage possibly arising from external impacts, these coatings are required to exhibit quite a high degree of flexibility, which can be accomplished by blending phenol and cresols in different ratios. Bisphenol A is still being used as a starting monomer within the synthesis of epoxy resins as a coating on the inside of many food and beverage cans, despite many health concerns raised by the scientific community since 2008. Further issues with this coating concern sulfur staining related to food can formulations. More specifically, for all foods rich in proteins (e.g., meat and fish), sulfur, as H2S generated by the sulfur aminoacids, may permeate to the substrate, causing discoloration when reacting with the tinplate. Phenolic-based coatings afford noticeable resistance to sulfur-containing food due to their high degree of crosslinking (Bourlier, 2006).
Acrylic polymers
Acrylic polymers for coating formulation were first developed in the 1950s. They immediately showed improved flexibility and adhesion compared to polyvinyl acetate emulsions and phenolics resins. Today's acrylic coatings are available in various forms, though the emulsion form is the most widely used. The main application of acrylic emulsions in the food and beverage packaging field relies on the excellent adhesive properties of acrylic polymers. The adhesive properties may be varied and tailored according to the specific purposes by selecting a different starting monomer. Nowadays, acrylic coatings fall into three main types of adhesives, as listed below:
1. Heat-sealable adhesives (lidding-type adhesives e.g., for coffee creamers and jams).
2. Laminating adhesives, which normally require lower temperatures (~ 90 °C) to activate the adhesive, compared to the heat-sealable ones (~ 120 °C). In the flexible food packaging area, low-temperature curing type acrylic coatings are extensively used.
3. Pressure-sensitive adhesives (or cold seals), which only require pressure in order to be activated. In the food packaging industry, these coatings can be applied where high temperatures may be detrimental to both the structure and sensorial attributes of the packaged item. Cold seals are typical for chocolate-covered snacks and ice-creams, where they are laid on the perimeter of the package to avoid direct contact with food.
Resistance to yellowing from UV ray exposure is another widely desirable feature of acrylic coatings (Lombardi and Gasper, 2006).
Vinyl ether coatings
Although the method of converting vinyl ether into a resinous form has been used for more than a century, it only became widely available after 1930 thanks to Reppe's chemistry, thus attracting the interest of many industrial groups. Large-scale production finally got started in 1938 in the Ludwigshafen plants of the former IG-Farbenproduktion, currently the location of the main production site of BASF AG. Nowadays, the use of vinyl ether polymers in the food and beverage packaging field pertain mainly to the adhesive sector. Blends of polyvinyl methyl ether with starch or dextrin are used for label adhesives, whereas blends of vinyl ethyl ether polymers of various molecular weights are used as coatings with pressure-sensitive adhesive applications (Müller, 2006).
Polyurea-based coatings
Polyurea is a polymer which has recently been developed with the intention of it being applied to coatings. Early attempts to exploit polyureas date back to the 1970s, but the widespread use of such polymers has only taken place since the 1990s. This was because of advancements in polyurea chemistry, as well as improvements in equipment, which allowed the set time of the polymer-derived resins to increase from 3 seconds to 25 minutes. To date, polyureas tend to be a valid alternative to polyurethanes, which were developed a few decades earlier. As with polyurethanes, polyureas are a two-component system, one of which is isocyanate. The second polyurethane component is a polyol, whereas for polyureas, the second component is a polyamine. In addition, for polyurethanes, in order for the isocyanate and polyol to react in a reasonable time, a catalyst is required, whereas for polyureas, it is not. As a consequence, low temperatures inhibit the reaction yielding polyurethanes, whilst the reaction leading to polyureas takes place regardless of the temperature. Furthermore, the polyurethane chemistry is sensitive to moisture, whereas polyureas can still be obtained in the presence of moisture. However, polyurea systems are aromatic, which means that polyurea-based coatings will suffer from UV radiation exposure, although this does not affect performance of the coating's main properties. Polyurea coating systems contain 100% solids, so they do not involve any issues related to the release of solvent vapors. The potential of polyureas for coating applications in food and beverage packaging systems is linked to the noticeable adhesion and sealing properties, which can be exploited profitably to design high-performance sealable coatings (Baxter, 2006).
14.3 Driving forces for developments in coating technologies and materials
In the last ten years, the food and beverage packaging industry has increased research into new high-performance materials, including coatings. There are two major driving forces: consumer trends and technological trends (Lange and Wyser, 2003).
14.3.1 Consumers trends
For a long time, attention to the environmental aspects linked to the manufacturing of packaging materials has lagged behind interest in overall performance and functional properties. However, for approximately ten years now, this seems to have changed. Today's consumer awareness of environmental issues is now one of the most important factors dictating companies' market strategies. Chlorine-based packaging polymers (e.g., polyvinyl chloride (PVC); polyvinylidene chloride (PVdC)) as well as aluminum-based structures are increasingly perceived as environmentally unfriendly, which has urged many national markets to seek replacements. Besides the continuous demand for extended shelf life, a more recent consumer trend concerns the demand for very fresh products with shorter shelf lives. Consumers are more and more willing to eat foods that exhibit 'freshly-harvested' properties rather than deep-frozen and canned foods. This implies a development of materials (e.g., coatings) with tailor-made barrier properties (e.g., selective barrier properties for minimally processed fruits and vegetables) is required for chilled and modified atmosphere packaging systems. Another push for new materials is the demand for transparent packaging which can maintain the same level of performance as metalized film, although for certain applications, this requirement is in conflict with the necessity for light-protective packaging. The best sealing attributes (e.g., effective re-sealable packaging materials by finger pressure) are also required, especially for refrigerated items such as fresh cheeses and cold cuts. Finally, smart materials which are able to behave based upon a 'sense-and-respond' mechanism still represent an intriguing challenge for future developments.
14.3.2 Technological trends
At present, the main technical issue to face is the development of high oxygen barrier materials, which can perform even at high relative humidity. Research is especially focused on attempting to replace costly multi-layer structures (with the oxygen barrier placed between two water vapor barrier polymers) with lighter solutions (e.g., coatings), thus fulfilling the increasing demand for upstream reduction in packaging materials. An additional general trend foresees switching from glass and metal packages to plastic, particularly for beverages which are particularly sensitive to gas exchange. For this purpose, plastic bottles and jars require high gas barrier performance as well as mechanical, chemical, and heat resistance. These characteristics are currently being pursued through the development of coating technologies. Multi-functionality is another feature which is expected to be developed in the next generation of coatings. Thus, coating manufacturers are exerting significant efforts to develop new, thin layers, capable of providing simultaneously more than one technical advantage, such as barrier properties, sealability, scratch resistance, and so on. Finally, the economic thrust to hold down costs cannot be neglected. Although many approaches can be undertaken with this in mind, increasing line speeds seems to be the most appealing option. To do so, packaging materials will be required to withstand higher stresses and strains without losing overall performance. Rising to these trends, new technologies and novel approaches have paved the way for highly engineered structures, that show great potential for the next generation of coatings.
14.4 New coating materials
Environmental concerns, legislation constraints and economic events have forced coating manufacturers, as well as researchers in academia, to look with renewed interest at the development of new materials. Besides the aforementioned tendency to reduce the use of chlorinated polymers, aluminum-based structures are also being replaced by new materials. Indeed, metalized films have been in use for around 30 years, in place of aluminum foil, which represents an already significant step with regard to packaging optimization. However, recent trends have highlighted the decline in the use of metalized structures and the subsequent necessity to find new alternatives; this is especially due to the difficult task of recycling aluminum-coated films, as well as the opaque nature of metalized films. The high energy consumption associated with aluminum production is a further issue making metalized films for food and beverage packaging applications environmentally unacceptable.
A first attempt to replace aluminum foil and metalized coatings has been the development of transparent ultrathin (thickness < 100 nm) inorganic layers, the most promising of which are aluminum oxide and silicon oxide. Such ceramic-like coatings can be deposited by plasma-enhanced chemical vapor deposition (PECVD), which allows the production of coated structures with equivalent or improved barrier performance compared to the commonly used aluminum-metalized structures. The major drawback associated with these metal oxide coatings is their brittleness, which can dramatically affect the barrier performance of the coatings, as it is possible for them to break during the converting operations or during the normal handling of the final packaging. Furthermore, the costs and complexity linked to the vapor deposition processes limit the use of this technique to market niches. Wider exploitation lies strictly in setting up the same process at atmospheric pressure, taking advantage of cheaper surface activation technologies such as flame treaters or atmospheric plasma generators.
Hand in hand with the increasing attention to environmental matters is the growing interest in biomass-derived polymers and bio-macromolecules for food and beverage packaging applications. The aim of this research is to decrease plastic waste, thus improving environmental standing. With respect to coating technologies, developers are focusing on the versatile nature of proteins, polysaccharides, and lipids in order to find new opportunities to design efficient biopolymer coatings with desirable properties. Many macromolecules within these three main categories have been claimed to possess excellent features that position them as a suitable alternative to synthetic coatings. Both proteins and polysaccharides have been suggested as valid replacements for high oxygen barrier synthetic polymers such as PVOH and EVOH. Hong and Krochta (2003, 2004, 2006) have thoroughly investigated the oxygen barrier properties of whey protein isolate (WPI) on various flexible plastic films. They concluded that smooth and transparent oxygen barrier coatings based on whey proteins provide excellent oxygen barrier properties at low to intermediate relative humidity, when compared with synthetic oxygen barriers. Compression-moulded wheat gluten/paper or paperboard, as well as curtain-coated chitosan paperboard laminates, showed oxygen barrier properties similar to those of paper and paperboard coated with commercial barrier materials (Gällstedt et al., 2005). Kim and Ustunol (2001) tested WPI-lipid layers, plasticized with glycerol as potential sealing coatings, finding optimum seals (323 N m− 1) at approximately 110 °C. More recently (Farris et al., 2009a), it has been demonstrated that blending molecules with different characteristics (gelatin and acetic acid esters produced from monoglycerides) can be a valuable route to producing coatings with improved final properties (friction, oxygen barrier, and UV barrier properties) as a result of the synergies between components. Recent developments, still ongoing, have shown completely bio-based coatings with barrier properties throughout the whole UV region. The layers of the coatings are fully formed from bio-macromolecules, affording tailor-made wettability properties to the coated substrate, as well high-gloss appearance with improved adhesive and abrasion resistance properties.
14.5 Physico-chemical approaches for the development of coating materials
The physico-chemical approach for the development of high-performance materials is becoming increasingly important in the field of coatings development. Both physics and chemistry afford a wide array of different tools which can help researchers to control and manipulate systems at both the nano- and micro-scales, thus allowing the development of new materials with deliberately induced architectures. This approach offers the opportunity to produce materials (films and coatings) with previously unachievable properties. The need for new materials will force (and is already forcing) researchers worldwide to develop new ideas, to formulate new hypotheses, and to build new models and theoretical frameworks that will account for the major challenges in thinking. The following is a discussion of the major recent trends in this direction.
14.5.1 Hybrid coatings
Hybrid coatings are systems which are created from two (or more) components of different origin, such as an inorganic and an organic phase. The major benefit of this approach is that it is possible to combine any feature from each individual component, thus obtaining multifunctional materials with remarkably improved performance. The organic phase usually affords tenacity, flexibility, and adhesion to the polymeric substrate, whereas the inorganic phase provides toughness and thermal, chemical, and flame resistance, as well as improved gas barrier properties (Minelli et al., 2010). However, such combinations are not without their problems; they can have compatibility issues that, in many cases, hinder the possibility of blending molecules with no affinity. In these cases, the use of either compatibilizers (which modify the interface of the immiscible polymers, thus stabilizing the blend) or the appropriate chemical routes (enabling in-situ modifications, thus inducing the desired affinity) can help to avoid separation phenomena.
One established way to produce these hybrids is the sol-gel method, a chemical synthesis that allows the formation of a nanosized network with tailored properties (optical, barrier, electrical, etc.) in which the organic and the inorganic phases are highly interconnected. In addition, the flexibility of such a process makes it possible to tune the properties of the final materials between those of the polymeric component and an inorganic glass, through the proper selection of the starting molecules and accurate control of the organic/inorganic ratio. The hybrid materials obtained through this route are known as 'ceramers', 'ormocers' (organically modified ceramics), or 'ormosils' (organically modified silicates). So far, major applications of hybrid coatings in the field of food and beverage packaging coatings have been the use of poly(vinyl alcohol), poly(vinylpyrrolidone), and poly(vinyl acetate) as the organic phase, with silicon alkoxide being the preferred inorganic precursor. To date, hybrid coatings have been recognized as one of the most valuable alternatives to overcoming the main drawbacks of traditional coating solutions (aluminum-metalized films, PVdC, and EVOH) in terms of water sensitivity, transparency and environmental impact. In addition, hybrid coatings exhibit greater suitability for industrial operations compared to transparent metal oxide coatings, e.g. SiOx, which are brittle and fairly expensive. Just as important is the fact that hybrid organic–inorganic coatings may be laid onto the commonly used packaging substrates using routine coating operations, i.e. roll-to-roll or spraying, without the necessity for special equipment or costly modifications of the already existing plants. To our knowledge, only three types of hybrid coatings for food and beverage packaging applications are currently available on the market: KuraristerTM by Kuraray Co. Ltd. (Japan), BeselaTM by Kureha Co. (Japan), and Oxaqua® by Metalvuoto SpA (Italy). All of them have been specifically developed to improve the gas barrier properties of the plastic substrate, as well as for other applications.
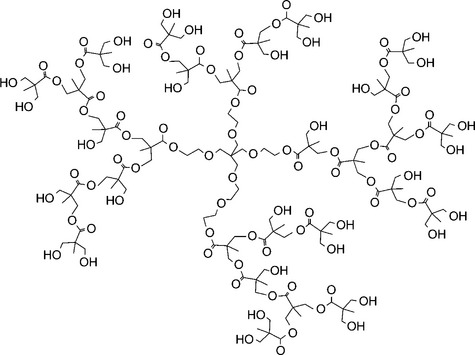
14.5.2 Hyperbranched polymers (HBP)
Together with dendrimers, hyperbranched polymers belong to a group of macromolecules called dendritic polymers. 'Perfectly' branched dendrimers are obtained through a step-by-step synthesis, as opposed to 'dirty', imperfect hyperbranched polymers, which can be fabricated by means of a one-step synthesis (Voit, 2000). Due to this difference, HBP have potential applications at the industrial level. The interest in HBP lies in the possibility of tailoring the properties of macromolecular materials by modifying the molecular architecture. Hyperbranched polymers are, nowadays, regarded as a potential solution for the development of new coatings with high barrier properties, whilst being mechanically flexible as well as resistant to water. Some of these main features make HBP exceptional when compared to conventional branched polymers. The peculiar and unique properties of HBP are linked to their highly branched backbone, with a large number of functional groups that can be favorably exploited to prompt further modifications or new reactions (see Fig. 14.5). The structure of HBP dramatically affects their functional properties, such as flow and processing properties. Hyperbranched polymers exhibit higher solubility and lower solution viscosity with respect to linear polymers of similar molecular weight (Sanger-mano et al., 2009). In practice, the use of HBP for coating fabrication can impart outstanding advantages for both the manufacturing process (tunable viscosity, high reactivity) and the final properties of the coating (improved toughness). HBP has already been used for powder coatings (Johansson et al., 2000), high solid coatings (Gopala et al., 1999), flame retardant coatings (Johansson and Hult, 1995), UV-curable coatings (Fogelstrom et al., 2006), and barrier coatings for flexible packaging (Lange et al., 2001).
14.5.3 Blends
Blending consists of the physical mixing of two (or more) different components, without the occurrence of any chemical synthesis. The properties of the final blend will, therefore, fall between those of the components, although synergisms may greatly improve the ultimate properties of the blend. The main advantages arising from the blend are mostly linked to the induced changes in morphology. For instance, when higher barrier properties are required, blending can allow the development of new structures by maximizing the tortuosity of the diffusion path (Lange and Wyser, 2003). The final morphologies will therefore arise from the combined effect of both processing conditions and the physico-chemical characteristics of the polymers. In this case, compatibilizers are necessary to enhance the affinity between components. One of the best examples of the potential of blending was provided a few years ago by research studies from Rutgers University and Clemson University (La Coste et al., 2005). They developed a new approach called 'smart blending', enabling the fabrication of polymer blends with deliberately induced morphologies, with the aim being to control the release of active compounds from the package, and the subsequent transfer onto the food. The final morphologies that can be attained by this approach range from multilayer systems to more fibrous patterns (see Fig. 14.6). Blending has gained increasing attention since the end of the 1990s. EVOH in PP (Faisant et al., 1998) or PA (De Petris et al., 1998), PA in PP (Citterio et al., 1999), and liquid crystalline polymer (LCP) in PE (Flodberg et al., 2000) are the most successful pairs developed by both active companies in this area (e.g., Du Pont and Eastman) and research groups from academia to the present day. However, blends developed so far have mostly been applied to polymeric systems intended for extrusion operations, rather than coating. Therefore, the challenge will be in extending the same basic principles underlying the development of new blends for film manufacturing and applying them to coating technology.
14.5.4 Combined strategies
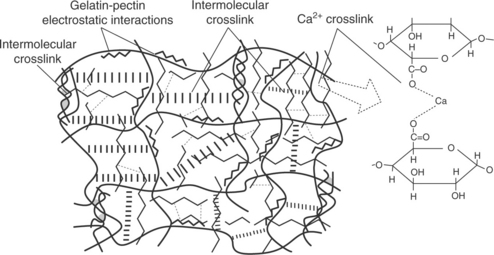
In an attempt to overcome the typical drawbacks of bio-based polymers (moisture sensitivity, poor mechanical properties, etc.), utitlizing only one 'corrective' strategy is unlikely to serve the purpose of making these materials suitable for the market. A combined strategy is more likely to represent the key to success in this case. Dealing with natural bio-polymers (proteins and polysaccharides, in particular), researchers have suggested quite a large number of tools for improving their final properties, thus increasing the possibility of using them for practical applications. Among these strategies, the use of crosslinkers, the generation of the so-called interpenetrating polymer networks (IPNs), chemical functionalizations such as grafting, and physical approaches such as layer-by-layer deposition deserve to be mentioned. In a recent paper (Farris et al., 2009b), an integrated approach supported by fundamental chemistry was proposed for the development of water-based hydrogels, especially intended for coating deposition onto the plastic substrates commonly used in the food and beverage packaging industry. Using the gelatin-pectin association as a model system, the authors discussed the great potential for producing advanced packaging materials (e.g., coatings) through the sequential combination of three different concepts (physical hydrogel, chemical hydrogel, and IPN). The resulting complex structure ('permanent interpenetrating polyion-complex hydrogel'; see Fig. 14.7) combines some of the benefits of the three component elements (physical hydrogel, chemical hydrogel, and IPN), which allows the overcoming of issues such as mechanical strength and water sorption limitations, which are found in simple biopolymer films. This strategy is just an example of how different approaches can be joined creatively to generate new families of coatings from biopolymers.
14.6 Nanotechnology and the development of coatings for food and beverage packaging materials
It is not rash to say that nanotechnology is probably the greatest ongoing revolution in polymer science and has been over the last two decades. Although skepticism has been raised about the real contribution of nanotechnology to everyday life, the impact of nanotechnology on many research activities is indisputable. This interest is justified as there is immense potential involved in the fascinating world of nanotechnology, which is only now being understood and governed. It would be prohibitive to include an exhaustive description here of nanotechnology and how it is applied to the world of polymer science; however, this section tentatively provides an overview of the main trends linked to nanotechnology from the perspective of developing new materials, with a special emphasis on coatings intended for food and beverage packaging applications.
As far as the polymer field is concerned, the term nanotechnology refers either to new materials with nanosize features (nanostructures) or to small inorganic particles that are included in the polymer matrix to create so-called nanocomposites. These distinctions clearly reflect the two main widely accepted definitions of the term nanotechnology. In the first instance, nanotechnology accounts for the development of new materials whose properties start changing as the structure of the system approaches nanosize. In other words, the 'nano' feature of the system is key for the achievement of new properties that are otherwise unattainable. The ability to design, create, and model nanosystems will, therefore, drive the fabrication of new materials (e.g., coatings) with outstanding properties. Making things smaller is commonly called top-down processing. The 'assembly' approach (self-assembly or directed-assembly), especially at the atomic, molecular, and supra-molecular level (so-called bottom-up processing) has been demonstrated as the more suitable for producing multifunctional, hierarchical structures to be integrated into mesoscale and macroscale systems (Baer et al., 2003).
Two main routes of self-assembly leading to nanostructured films and coatings are currently attracting large amounts of attention at both academic and industrial levels. The first one consists of building blocks that can be either attached to surfaces or grown into large units. In building block systems, the bonds involved in the original block units are, generally, stronger than those in the next-generation assemblies. This approach has been used for the formation of molecular crystals (Braga and Grepioni, 2002), in which non-covalent or 'secondary' bonding processes (hydrogen bonds, electrostatic forces, and van der Waals' interactions) yielded a periodic supermolecule that can be advantageously used for the generation of new coatings with unique crystal architectures (Baer et al., 2003). The second approach envisages the development of nanodimensional structures through specific chemical pathways involving, in contrast to the previous case, cova-lent bonds that produce a nanosized network not existing before (see Fig. 14.8). An example of this is provided by the self-assembled nanophase particle (SNAP) coating process. This approach lies in a sol-gel reaction consisting of two sequential steps (hydrolysis and condensation) on an organic-inorganic system to form hybrid nanoparticles. Self-assembly of such nanoparticles eventually occurs as the solvent evaporates onto the coating deposition (Vreugdenhil et al., 2001).
The second definition of nanotechnology relies on the use of nanometric inorganic particles, also called nanofillers, with high aspect ratio, generally in the form of platelets 100–1000 × 1 nm in size. The most commonly used nanofillers for polymer nanocomposites are sheen silicate minerals (phyl-losilicates) pertaining to the main groups of clays (e.g., montmorillonite, kaolin, vermiculite, talc) and micas (e.g., illite and muscovite). These nanoparticles are included in a polymer matrix to generate nanolayered nanocomposites, with the scope for improving specific properties such as barrier properties, fire retardancy, and wear resistance. This approach, first adopted by Toyota within the automotive industry in the 1980s (Usuki et al., 1993), was later extended to the packaging field; specifically it was applied to the extrusion of polymeric resins. It has broadly been demonstrated that barrier properties (e.g., against oxygen) can be dramatically improved (~50 orders of magnitude) even at very low filler loadings (~ 2-4 wt%). This can be achieved by increasing the tortuosity path across the thickness of the film, thus placing an additional physical obstacle in the path of the permeant. To make the nanofillers effective, it is of utmost importance to make sure that the platelets are positioned with length and width perpendicular to the permeant transport direction, and that they are homogeneously dispersed in the polymer matrix (intercalation and, especially, exfoliation are the targets) (Paul and Robeson, 2008).
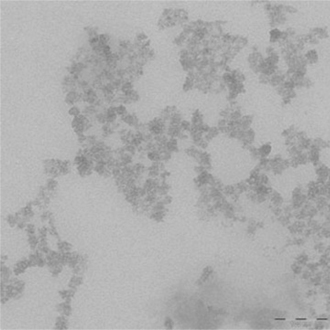
As far as food and beverage packaging applications are concerned, the use of nanofillers may be seen as a powerful tool for enhancing the barrier properties of plastic substrates by means of nanocomposite coatings. As far as we know, there are few academic works on nanocomposite coatings that can be found in the literature. There are also very few commercially available solutions (Harrison et al., 1996; Carlblom and Seiner, 1998), indicating that issues of various origins (technical, legislation, etc.) are hindering the marketability of this new generation of coatings. Feasible dispersion (see Fig. 14.9) of the inorganic fillers, at a reasonable cost, without compromising the original properties of the substrate (e.g., transparency), is the major future goal for the fabrication of successful nano-coatings.
14.7 Active coatings for food and beverage packaging materials
Within the broad field of packaging, active packaging is, undoubtedly, one of the top research subjects, having captured the attention of many scientists over the last decade. The interest in this topic lies in the potential impact that may arise from such technology. Active packaging has the capability to modify the packaging sector to a great extent, by producing new concepts at both the theoretical and practical levels. Traditional packaging is defined as 'passive' since it provides some specific functions (e.g., containment, protection, and communication) passively, i.e. in a 'static manner'. In this sense, the barrier properties of a package (against gases, UV light, etc.) fall into the passive category, as they are innate within the material. By contrast, the term 'active' refers to any package which is able to interact with the surrounding environment, namely the food system and the surrounding space. Therefore, active packaging is better defined as as an 'environment/ package/food' system.
The ultimate purpose of active packaging is the enhancement of food quality, safety, and convenience. Examples of active packaging technologies include absorbing systems and releasing systems. The first group includes, oxygen and carbon dioxide scavengers, humidity absorbers, exuded absorbers, and so on. However, the majority of research studies on active packaging for food and beverage applications focus mainly on the development of antimicrobial and antioxidant systems, which fall into the second group. Antimicrobial and antioxidant active packaging systems are intended as controlled release packaging (CRP), which are a new generation of materials that can release active compounds at different controlled rates, suitable for improving the quality and safety of foods during storage (La Coste et al., 2005). This packaging behaves effectively as a reservoir to the active compound, which is released into the food according to a predetermined pattern, in a controlled manner, throughout the entire expected storage time. To achieve this goal, the active molecules need to be encased properly in the packaging, ensuring that the release is neither too slow nor too fast. Therefore, factors such as the chemical affinity between polymers and actives, potential chemical interplays, and physical factors (e.g., entanglement, hindrance, free volume, morphology, etc.) have to be carefully taken into account during the design and development of active packaging.
So far, efforts have concentrated mainly on the incorporation of the active compounds directly into plastic films (Wessling et al., 2000; Vartiainen et al., 2003, 2005; Plackett et al., 2006; Suppakul et al., 2006; Conte et al., 2007; Silveira et al., 2007; Nerín et al., 2008; Camilloto et al., 2009; Del Nobile et al., 2009; Mascheroni et al., 2010). However, several drawbacks have been encountered during the production of plastic films loaded with active molecules. The stability of the active compounds is an important issue, especially in light of the high shear and thermal stress which the plastic film endures during processes such as extrusion, which can lead to a pronounced depletion in the activity of the added molecules. Moreover, when volatile compounds have to be added, the working temperatures (especially of the equipment) are a limiting factor, since temperatures which are too high may dramatically increase the amount of volatiles lost into the air, thus affecting the efficiency of the whole process. Owing to these considerations, coatings can pave the way for new patterns to be created for the next generation of active packaging systems. The main benefit arising from this choice lies in the fact that coatings can be used as an additional layer to embed and release the active compounds, with no additional functions (e.g., barrier, optical, mechanical properties), which are still guaranteed by the plastic substrate. Thus, for the conceived coatings to work efficiently, one option is to make them able to 'sense' external stimuli acting as triggers (e.g., pH, temperature, relative humidity, etc.) for the release of the active compounds. Once these triggers have been selected, adjusting other parameters, such as raw materials, composition, and morphology, can allow the release to be achieved in a controlled manner. In addition, as coating processes can be considered 'mild' compared, for instance, to extrusion, such a technology can address the limitations linked to both high temperatures and processing conditions, as mentioned above.
Within this scenario, coatings made of bio-macromolecules (proteins, carbohydrates, and lipids) may be more easily applicable than oil-derived polymers. This is because there are no safety hazards with regard to migration into food (nevertheless, compliance with migration limits as defined by law have to be fulfilled in every instance). Finally, it is expected that the development of the thriving area of active packaging can gain a significant surge from innovations involving the development and applications of new systems such as coatings.
14.8 Applications of the latest developments in coating technologies to improve product quality
The increasing demand for innovation has pushed researchers dealing with food and beverage packaging to design and develop new structures, which are able to afford better functional properties, whilst also fulfilling the current trends with respect to environmental aspects and, at the same time, being cost-effective. Several encouraging results have been achieved so far, especially at the academic level, which, nevertheless, implies that most novelties still remain at the lab scale. Among other reasons, this may be because the link between academia and industry is often too weak to make the implementation of the academic findings possible. The synergy between these two entities (academia and industry) is a crucial point for the scale-up of many meaningful ideas. Here, some representative examples of advancements in the field of new coatings for food and beverage packaging applications are discussed.
14.8.1 Oxygen and water vapor barrier coatings
The food industry always shows a vivid interest in new solutions to help control food deterioration, which is caused by oxidation phenomena and water vapor transfer across the package. Therefore, controlling oxygen permeation through the appropriate packaging is of utmost importance, and coatings with calibrated barrier performance are, from this perspective, very welcome. Recent trends are predominantly aimed at the development of very high performance barrier coatings. These coatings are intended not only to protect oxygen-sensitive foods from outside oxygen permeation but also to keep constant the internal atmosphere of modified atmosphere packaging (MAP) products over time. At the same time, these coatings are required to possess high transparency, resistance to the relative humidity - %RH (i.e., the barrier against oxygen should be the same throughout the entire relative humidity range) - and, no less important, to be low cost (i.e., they should clearly offer an economic advantage over the already existing solutions).
In a recent work (Minelli et al., 2010), a high oxygen barrier coating showing all of the aforementioned properties has been described. In particular, the authors solved the moisture sensitivity limitation of a high oxygen barrier polymer (PVOH) using a physico-chemical approach, by generating a hybrid organic-inorganic coating. This was made possible by combining the vinyl polymer with an ethyl ester of orthosilicic acid (tetrae-thoxysilane), which acted as the inorganic counterpart, using a sol-gel process. During this process, comprising a hydrolysis step first, then followed by a condensation reaction, the formation of both hydrogen and covalent bonds between the two phases was postulated, which finally led to the synthesis of a new hybrid three-dimensional network. The sol-gel solution was deposited on different polymer substrates (polyethylene tere-phthalate, oriented polypropylene, cast polypropylene, linear low-density polyethylene, and a blend of the same with co-extruded low-density polyethylene) by dip coating. Surprisingly, the permeability tests showed that coatings of 1–2 μm thick were able to reduce the oxygen transmission rate by approximately two orders of magnitude (1.7 × 10− 4 cm3(STP)/cm2 24 h atm compared to 1.8 × 10− 2 cm3(STP)/cm2 24 h atm of the uncoated substrate). More notably, it has been observed that the oxygen permeability of the hybrid coatings remained extremely good (7.7 × 10− 4 cm3(STP)/cm2 24 h atm) even after prolonged contact with water (immersion in saturated water vapor for 4 days and evacuation), which is a strong solvent for PVOH. However, it must be pointed out that the only suitable support for the present coating was PET, to which the previous results have to be referred.
In another, similar work (Iotti et al., 2009), a hybrid coating was obtained from poly-ε-caprolactone, polyethylene oxide, or polylactic acid (as organic phase) and silica from tetraethoxysilane (as inorganic phase) using a sol–gel approach. In this case, the coatings were laid onto polylactic acid films for food packaging applications in order to improve the barrier properties against oxygen and water vapor. The maximum reduction of the oxygen transmission rate due to the coating deposition was approximately 48% (60 cm3/m2 24 h compared to 115 m3/m2 24 h for the uncoated polylactic acid film). The authors concluded that this result, although not completely effective in achieving a strong barrier against the oxidative processes, can be useful for medium shelf life product applications coupled with MAP (e.g., fresh meat, fish, cheese). Therefore, this achievement can be seen as a potential tool for driving polylactic acid penetration in additional markets than that of fresh, minimally processed food with a short shelf life (from 3 to 5 days, usually under refrigeration). The same coating was, however, ineffective against water vapor permeation, which was attributed to the inherent hydrophilic nature of the coatings.
Once the existing issues have been addressed (e.g., the adhesion of the coating to the polyolefinic substrates and the barrier against water vapor), this kind of organic-inorganic thin layer can be profitably used as a valuable alternative to metalized films, at least as far as the barrier properties against oxygen and water vapor are concerned.
14.8.2 Sealant coatings
Seals play an important role in the shelf life of foods and beverages, as they ensure the integrity of the package throughout the distribution channel. For this reason, hermetic seals are required, which can be accomplished using either a thermoplastic polymer (e.g., polyethylene) as the inner layer of a multilayer structure (laminated or co-extruded) or by the deposition of a coating with excellent sealing attributes. Heat-sealing (at temperatures above 100 °C) involves heated pressing of the interfaces followed by cooling, whilst cold seals are obtained by only the effect of pressure or, more rarely, dissolving the interfaces with a solvent (Lee et al., 2008). Cold-sealing avoids using heat, which is necessary when either the packages suffer as a result of high temperatures or when dealing with heat-sensitive foods (e.g., chocolate-based foods).
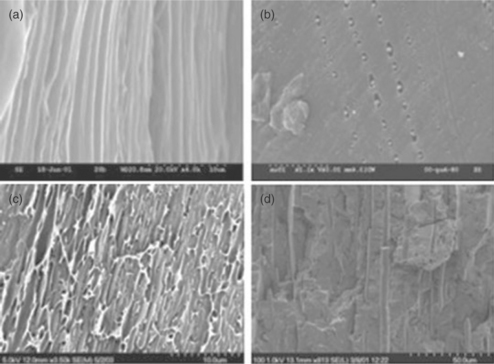
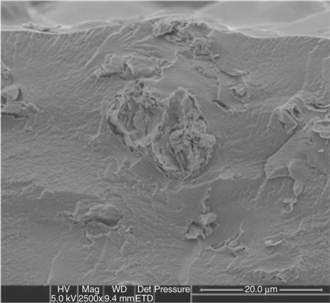
Recently (Farris et al., 2008; Farris et al., 2009c; Farris et al., 2010), a sealing coating (~ 1.5 μm thick) has been proposed that can be obtained entirely from bio-macromolecules, namely gelatin, acetic acid esters produced from monoglycerides, and glycerol. The special features of this new coating rely on the adhesive properties of gelatin, which allow sealing temperatures ranging from 50 °C to 90 °C to be achieved, depending on the specific formulations. The seal strength, which is assessed in terms of both maximum force to break the sealed joints and strain energy, was approximately 0.6 N/15 mm and 20 N mm, respectively. These are typical results for easy-opening peel seals. The authors explained the influence of the main process factors with regard to seal strength, and observed that the most important one was the 'bar pressure'. In particular, the negative effect on seal strength caused by an increase in bar pressure (from 2.5 to 4.5 bars) was explained in terms of a 'squeezing' effect, i.e. by increasing the bar pressure, the intimate contact between the two coated sides of the plastic film is exaggerated such that the bio-coating comes out from the sealing contact area. In addition, the authors provided an exhaustive explanation regarding the mode of failure underlying the physics of the seal separation. As revealed by microscopic investigation, seals failed in both peeling and tearing modes (see Fig. 14.10). These new bio-based sealant coatings appear particularly attractive for the replacement of typically thick heat sealant plastic polymers (e.g. polyethylene), thus drastically reducing the upstream amount of plastics, which, in turn, may contribute to managing the waste disposal issue. Moreover, the natural origin of these coatings would mean that any contact with packaged food would not be considered hazardous, as the whole formulation is composed of substances listed by law as either food ingredients or food additives. Consequently, the potential application of such 'green' layers could be a replacement for the so-called cold sealant, thus avoiding the expensive and time-consuming process of depositing the sealing layer only onto the edge of the package.

Fig. 14.10 SEM images of the heat-sealed interface of OPP-coated strips after rupture: (1) the substrate (OPP); (2) the coating initially lay on the upper OPP strip, de-bound from the substrate after rupture; (3) the coating initially lay on the lower OPP strip, still bound to the substrate (adapted from Farris et al., 2010).
14.8.3 Antimicrobial coatings
In recent decades, many research groups have focused their activities on the field of antimicrobial packaging. However, few examples concerning the assessment of antimicrobial coatings onto packaging materials can be retrieved in the scientific literature. The following examples aim to encourage further investigations in this direction, demonstrating that the strategy of incorporating active compounds within a coating may help to overcome many drawbacks related to direct incorporation into the plastic substrate.
One of the landmark works in this field was published in 2007 by Nerín's team (Rodriguez et al., 2007), who first coined the term 'active coating'. The authors tested the antimicrobial activity of four essential oils (clove, cinnamon, oregano, and cinnamaldehyde-enriched cinnamon essential oils) incorporated into paraffin-based coatings for paper packaging materials, intended to come into contact with foods. The microbiological tests were carried out on several common fungal and bacterial contaminants of foods. Among others, the cinnamaldehyde-enriched cinnamon essential oil exhibited the best antimicrobial performance. This essential oil was used in preliminary trials on two varieties of strawberries, which maintained their original attributes and were unchanged (microbiologically and by quality) throughout the whole testing time (7 days under refrigeration at 4 °C), thus allowing the food to meet the shelf life requirements of the market.
In the same year, another work was published dealing with the development of antimicrobial coatings on plastic films intended for food packaging applications (Marini et al., 2007). A hybrid coating was proposed that was obtained using the sol-gel approach and loaded with silver salts such as AgNO3 and AgCl, which were suggested as a more economical solution compared to the most common silver nanoparticles. The active thin coatings (0.6-1.1 μm) were then deposited on polyethylene and polyvinyl chloride films, and the antibacterial activity of the coated films was tested against Gram-negative (Escherichia coli) and Gram-positive (Staphylococcus aureus) bacteria. The results obtained were extremely interesting, suggesting that the antibacterial activity of the coated films was linked mainly to the diffusion capability of Ag+ ions within the organic phase of the coating.
Elsabee et al. (2008) developed an active coating on polypropylene substrates with potential application for post-harvest crop protection. The novelty of their work lies in the fact that they produced an antimicrobial coating without incorporating active molecules such as essential oils or enzymes into the coating. Indeed, the antimicrobial feature was an inherent property of the coating itself. To this purpose, they prepared a coating based on chitosan, which is well known for its antimicrobial activity and has previously been exploited, especially in the biomedical field. The final coating was a multilayer, composed of alternating layers of pectin and chitosan. The antimicrobial activity was evaluated on different strains of fungi and bacteria. Tests conducted on tomatoes packaged with polypropylene films coated with different pectin/chitosan layers showed that vegetables remained intact with no apparent rotting for 13 days. In addition, it was found that increasing the amount of chitosan on the surface of corona-treated PP films by multilayer formation increased the antimicrobial effect.
The last example concerns a work published very recently by a team including researchers from Clemson University and Sealed Air Corporation (Matthews et al., 2010). In this work, the authors evaluated the anti-listerial activity of two nisin blends, one in combination with lysozyme and the other in combination with rosemary extract, in solution and on fresh beef, when incorporated into a cellulose coating applied to a barrier film. The innovation involved in this work is a new strategy to face contamination caused by food-borne pathogens of public health, such as Listeria monocytogenes. Instead of using only one active molecule (e.g., nisin, which has already been demonstrated to be a powerful bacteriocin in a number of food systems), a combined approach has been suggested, i.e. the use of a 'cocktail' of antimicrobial molecules in the coating, in order to exploit the synergies that may arise from such a combination. The shelf life of food products can be further extended in consideration to the different release rates of the active compounds from the coating to the food matrix. This means that a 'cascade' mechanism can be achieved: the effect of the second antimicrobial agent starts virtually as soon as the first ceases, thus prolonging the overall antimicrobial effect.
14.9 Future trends
Although many steps forward have been taken during the last few years, further innovations are expected within the field of food and beverage packaging. Coatings have been recognized as a potential research topic that can contribute a great deal to new high-performance materials. As we have shown in this chapter, several achievements have characterized recent research in this field. However, for the most part the findings attained so far are still under development, and the applications in the marketplace are limited. Therefore, further developments are necessary, but what is required needs to be considered within the context of some of the main trends.
Firstly, the environmental aspect is probably the most important driving force for the next few years. Many companies are looking with increasing interest to new high-performance materials that are perceived as environmentally friendly and safe by consumers. In addition, legislation also forces new trends. Due to new volatile organic compound (VOC) level restrictions implemented in North America as well as in Europe, the change from solvent-borne coatings to waterborne systems will increase considerably. Since water-based coatings are expected to play a major role in the future, a drastic change in direction is therefore urged. Presumably, this will impose some technical changes. Indeed, due to both the use of water as a solvent and the necessity for high-speed production lines, faster drying systems have to be developed, assuring high throughput and keeping the coating costs low.
With regard to the topic of packaging safety, another expected development is the use of coatings as functional barriers (FB) to prevent the migration of unwanted contaminants into food. The concept of a functional barrier relies on the use of one or more layers in order to:
• ensure that the migration of authorized substances from the package is below the specific migration limit (SML);
• reduce the migration of non-authorized or non-intentionally added substances (NIAS) to a 'non-detectable' level (10 ppb has been proposed).
Since the 1990s (Gilbert et al., 1994) this concept has been stressed as one of the major research requirements to support legislation in the area of food packaging, in particular in light of the potential use of recycled materials as food packaging materials.
Another trend concerns avoidance of aluminum and chlorine-based coatings, together with the replacement of metal and glass, with plastic packaging. From this perspective, more attention should be paid to natural, readily available bio-macromolecules, such as protein, polysaccharides, and lipids, which can be used profitably to generate high-performance coatings whilst reducing the upstream consumption of plastics.
Finally, the widely used term 'sustainability' should also apply to the development of the next generation of coatings. The word 'sustainable' refers not only to the environmental aspects, but also to social and economic aspects. Indeed, any new product should provide real benefits to the people coming into contact with it (social component), and any new material should arise from an overall process that is able to guarantee financial profitability (economic component). In other words, new coatings intended for food and beverage packaging applications have to be high-performance and cost-effective whilst also being socially and environmentally sensitive.
14.10 Sources of further information and advice
Brown, W.E. Plastics in Food Packaging: Properties, Design, and Fabrication. New York: Marcel Dekker; 1992.
Robertson, G.L. Food Packaging: Principles and Practice. New York: Marcel Dekker; 1993.
Ryntz, R., Yaneff, P. Coatings of Polymers and Plastics. New York: Marcel Dekker; 2003.
Selke, S.E.M., Culter, J.D., Hernandez, R.J. Plastics Packaging: Properties, Processing, Applications, and Regulations. Munich: Carl Hanser; 2004.
Tracton, A.A. Coatings Technology Handbook. Boca Raton, FL: CRC Press; 2006.
Yam, K. The Wiley Encyclopedia of Packaging Technology. New York: John Wiley & Sons; 2009.
http://www.european-coatings.com/
http://ec.europa.eu/environment/air/pollutants/stationary/solvents.htm
14.11 References
Baer, D.R., Burrows, P.E., El-Azab, A.A. Enhancing coating functionality using nanoscience and nanotechnology. Prog Org Coat. 2003; 47:342–356.
Baxter, B.R. 'The polyurea revolution: protective coatings for the 21st century'. In: Tracton A.A., ed. Coatings Technology Handbook. Boca Raton, FL: CRC Press, 2006. [52-1-52-4].
Bourlier, K. 'Phenolic resins'. In: Tracton A.A., ed. Coatings Technology Handbook. Boca Raton, FL: CRC Press, 2006. [Ch. 53-1-53-5].
Braga, D., Grepioni, F. Intermolecular interactions in nonorganic crystal engineering. Acc Chem Res. 2002; 33:601–608.
Camilloto, G.P., De Fatima Ferreira Soares, N., Dos Santos Pires, A.C., De Paula, F.S. Preservation of sliced ham through triclosan active film. Packag Technol Sci. 2009; 22:471–477.
Carlblom, L.H., Seiner, J.A. PCT International patent application WO 98/24839, 1998. [(PPG INDUSTRIES INC)].
Citterio, C., Selli, E., Testa, G., Bonfatti, A.M., Seves, A. Physico-chemical characterization of compatibilized poly(propylene)/aromatic polyamide blends. Angew Makromol Chemie. 1999; 270:22–27.
Conte, A., Buonocore, G.G., Sinigaglia, M., Del Nobile, M.A. Development of immobilized lysozyme based active film. J Food Eng. 2007; 78:741–745.
Del Nobile, M.A., Conte, A., Buonocore, G.G., Incoronato, A.L., Massaro, A., Panza, O. Active packaging by extrusion processing of recyclable and biodegradable polymers. J Food Eng. 2009; 93:1–6.
De Petris, S., Laurienzo, P., Malinconico, M., Pracella, M., Zendron, M. Study of blends of Nylon 6 with EVOH and carboxyl-modified EVOH and a preliminary approach to films for packaging applications. J Appl Polym Sci. 1998; 68:637–648.
Elsabee, M.Z., Abdou, E.S., Nagy, K.S.A., Eweis, M. Surface modification of polypropylene films by chitosan and chitosan/pectin multilayer. Carbohyd Polym. 2008; 7:187–195.
Faisant, J.B., Ait-Kadi, A., Bousmina, M., Deschenes, L. 'Morphology, thermo-mechanical and barrier properties of polypropylene-ethylene vinyl alcohol blends. Polymer. 1998; 39:533–545.
Farris, S., Piergiovanni, L., Ronchi, G., Rocca, R., Edible matrices and relevant applications and preparation method (METALVUOTO S.P.A.). International patent application WO 2008/075396 A1. 2008.
Farris, S., Introzzi, L., Piergiovanni, L. Evaluation of a bio-coating as a solution to improve barrier, friction and optical properties of plastic films. Packag Technol Sci. 2009; 22:69–83.
Farris, S., Schaich, K.M., Liu, L., Piergiovanni, L., Yam, K.L. Development of polyion-complex hydrogels as an alternative approach for the production of bio-based polymers for food packaging applications: a review'. Trends Food Sci Tech. 2009; 20:316–332.
Farris, S., Cozzolino, C.A., Introzzi, L., Piergiovanni, L. Effects of different sealing conditions on the seal strength of polypropylene films coated with a bio-based thin layer. Packag Technol Sci. 2009; 22:359–369.
Farris, S., Cozzolino, C.A., Introzzi, L., Piergiovanni, L. Development and characterization of a gelatin-based coating with unique sealing properties. J Appl Polym Sci. 2010; 118(5):2969–2975.
Flodberg, G., Hellman, A., Hedenqvist, M.S., Sadiku, E.R., Gedde, U.W. Barrier properties of blends based on liquid crystalline polymers and polyethylene. Polym Sci Eng. 2000; 40:1969–1978.
Fogelstrom, L., Antoni, P., Malmstrom, E., Hult, A. UV-curable hyper-branched nanocomposite coatings. Prog Org Coat. 2006; 55:284–290.
Fogelstrom, L., Malmstrom, E., Johansson, M., Hult, A. Hard and flexible nanocomposite coatings using nanoclay-filled hyperbranched polymers. ACS APPL. Mater. Interfaces. 2010; 2(6):1679–1684.
Gallstedt, M., Brottman, A., Hedenqvist, M.S. Packaging-related properties of protein- and chitosan-coated paper. Packag Technol Sci. 2005; 18:161–170.
Gilbert, J., Sharman, M., Rossi, L. A review of the research requirements for Europe to support legislation in the area of food contact materials and articles. Food Addit Contam. 1994; 11:497–518.
Gopala, A., Wu, H., Xu, J., Heiden, P. Investigation of readily processable thermoplastic-toughened thermosets: IV. BMIs toughened with hyperbranched polyester. J Appl Polym Sci. 1999; 71:1809–1817.
Harrison, A.G., Meredith, W.N.E., Higgins, D.E. US Patent 5,571,614, 1996. [(to Imperial Chemical Industries)].
Hong, S.I., Krochta, J.M. Oxygen barrier properties of whey protein isolate coatings on polypropylene films. J Food Sci. 2003; 68:224–228.
Hong, S.I., Krochta, J.M. Whey protein isolate coating on LDPE film as a novel oxygen barrier in the composite structure. Packag Technol Sci. 2004; 17:13–21.
Hong, S.I., Krochta, J.M. Oxygen barrier performance of whey-protein-coated plastic films as affected by temperature, relative humidity, base film and protein type. J Food Eng. 2006; 77:739–745.
Iotti, M., Fabbri, P., Messori, M., Pilati, F., Fava, P. Organic-inorganic hybrid coatings for the modification of barrier properties of poly(lactic acid) films for food packaging applications. J Polym Environ. 2009; 17:10–19.
Johansson, M., Hult, A. Synthesis, characterization, and UV curing of acrylate functional hyperbranched polyester resins. J Coat Technol. 1995; 67:35–39.
Johansson, M., Malmstrom, E., Jansson, A., Hult, A.J. Novel concept for low temperature curing powder coatings based on hyperbranched polyesters. J Coat Technol. 2000; 72:49–54.
Kim, S.J., Ustunol, Z. Thermal properties, heat sealability and seal attributes of whey protein isolate/lipid emulsion edible films. J Food Sci. 2001; 66:985–990.
La Coste, A., Schaich, K.M., Zumbrunnen, D., Yam, K.L. Advancing controlled release packaging through smart blending. Packag Technol Sci. 2005; 18:77–87.
Lange, J., Wyser, Y. Recent innovations in barrier technologies for plastic packaging - a review. Packag Technol Sci. 2003; 16:149–158.
Lange, J., Stenroos, E., Johansson, M., Malmström, E. Barrier coatings for flexible packaging based on hyperbranched resins. Polymer. 2001; 42:7403–7410.
Lee, D.S., Yam, K.L., Piergiovanni, L. Food Packaging Science and Technology. Boca Raton, FL: CRC Press; 2008.
Leonard, M.W. Barrier and overprint coatings. In: Yam K., ed. The Wiley Encyclopedia of Packaging Technology. New York: John Wiley & Sons; 2009:98–103.
Lombardi, R.A., Gasper, J.D. 'Acrylic polymers'. In: Tracton A.A., ed. Coatings Technology Handbook. Boca Raton, FL: CRC Press, 2006. [46-1–16-10].
Marini, M., De Niederhausern, S., Iseppi, R., Bondi, M., Sabia, C., Toselli, M., Pilati, F. Antibacterial activity of plastics coated with silver-doped organic–inorganic hybrid coatings prepared by sol–gel processes. Biomacromolecules. 2007; 8:1246–1254.
Mascheroni, E., Guillard, V., Nalin, F., Mora, L., Piergiovanni, L. Diffusivity of propolis compounds in polylactic acid polymer for the development of antimicrobial packaging films. J Food Eng. 2010; 98:294–301.
Matthews, B., Mangalasary, S., Darby, D., Cooksey, K. Effectiveness of barrier film with a cellulose coating that carries nisin blends for the inhibition of Listeria monocytogenes. Packag Technol Sci. 2010; 23(5):267–273.
Minelli, M., De Angelis, M.G., Doghieri, F., Rocchetti, M., Montenero, A. Barrier properties of organic - inorganic hybrid coatings based on polyvinyl alcohol with improved water resistance. Polym Eng Sci. 2010; 50:144–153.
Müller, H.W.J. 'Vinyl ether polymers'. In: Tracton A.A., ed. Coatings Technology Handbook. Boca Raton, FL: CRC Press, 2006. [47-1–47-3].
Nerín, C., Tovar, L., Salafranca, J. Behaviour of a new antioxidant active film versus oxidizable model compounds. J Food Eng. 2008; 84:313–320.
Paul, D.R., Robeson, L.M. Polymer nanotechnology: nanocomposites. Polymer. 2008; 49:3187–3204.
Plackett, D.V., Holm, V.H., Johansen, P., Ndoni, S., Veggemose Nielsen, P., Sipilainen-Malm, T., Södergärd, A., Verstichel, S. Characterization of L-polylactide and L-polylactide-polycaprolactone co-polymer films for use in cheese-packaging applications. Packag Technol Sci. 2006; 19:1–24.
Rodríguez, A., Batlle, R., Nerín, C. The use of natural essential oils as antimicrobial solutions in paper packaging. Part II. Prog Org Coat. 2007; 60:33–38.
Sangermano, M., Messori, M., Galleco, M.M., Rizza, G., Voit, B. Scratch resistant tough nanocomposite epoxy coatings based on hyperbranched polyesters. Polymer. 2009; 50:5647–5652.
Silveira, M.F.A., Soares, N.F.F., Geraldine, R.M., Andrade, N.J., Goncalves, M.P.J. Antimicrobial efficiency and sorbic acid migration from active films into pastry dough'. Packag Technol Sci. 2007; 20:287–292.
Suppakul, P., Miltz, J., Sonneveld, K., Bigger, S.W. Characterization of antimicrobial films containing basil extracts. Packag Technol Sci. 2006; 19:259–268.
Usuki, A., Kojima, Y., Kawasumi, M., Okada, A., Fukushima, Y., Kurauchi, T., Kamigaito, O. Synthesis of nylon 6-clay hybrid. J Mater Res. 1993; 8:1179–1184.
Vartiainen, J., Skytta, E., Enqvist, J., Ahvenainen, R. Properties of antimicrobial plastics containing traditional food preservatives. Packag Technol Sci. 2003; 16:223–229.
Vartiainen, J., Rättö, M., Paulussen, S. Antimicrobial activity of glucose oxidase-immobilized plasma-activated polypropylene films. Packag Technol Sci. 2005; 18:243–251.
Voit, B. New developments in hyperbranched polymers. J Polym Sci Part A: Polym Chem. 2000; 38:2505–2525.
Vreugdenhil, A.J., Balbyshev, V.N., Donley, M.S. Nanostructured silicon sol–gel surface treatments for Al 2024-T3 protection. J Coat Technol. 2001; 73:35–43.
Wessling, C., Nielsen, T., Leufvén, A. The influence of a-tocopherol concentration on the stability of linoleic acid and the properties of low-density polyethylene. Packag Technol Sci. 2000; 13:19–28.