Active nanocomposites for food and beverage packaging
J.-M. Lagarón and M.A. Busolo, Instituto de Agroquímica y Technología de Alimentos (IATA-CSIC), Spain
Abstract:
This chapter presents some recent developments in the area of active nanocomposites for food packaging applications. Active nanocomposites are advanced alternatives to conventional active plastic technologies or active sachets for the extension of the quality and safety of packaged food products. They are polymer blends that contain nanostructured materials which confer activity to the food packaging structures. The nanostructured materials are inserted into the packaging materials either by melt compounding or solvent casting. Examples detailing the development of highly dispersed oxygen scavenging, free radical scavenging and antimicrobial nanoclay-based nanocomposites are presented.
4.1 Introduction
Oxygen (O2) and reactive oxygen species (ROS) are either directly or indirectly responsible for most of the degradation reactions that take place in food products. Direct oxidation reactions cause the deterioration of food organoleptic and nutritional properties (e.g. degradation of oils, fats, pigments and vitamins), while an example of the indirect action of O2 on food quality is food spoilage by aerobic microorganisms.
There have been a variety of developments in packaging materials to reduce oxidation in food products over the years. Some examples include the introduction of polyvinylidene chloride (PVdC)-coated films, the incorporation of polyvinyl alcohol (PVOH) as an oxygen barrier layer, and the use of vacuum-deposited aluminum to reduce oxygen penetration into packaged products. Vacuum packaging and inert atmospheres have also been used to significantly extend the shelf life of many food products.1 Oxygen scavengers and free radical scavengers are now also incorporated into packages and packaging materials to produce so-called active packaging and active plastic packaging materials, which reduce the deleterious effects of oxygen and ROS on food products. Unlike traditional or passive packaging, which cannot remove or reduce the oxygen present, oxygen scavenging active packaging and packaging materials can reduce oxygen to levels of below 0.01%2 in the packaging headspace and maintain these conditions during product storage. Free radical scavenging active packaging can trap free radicals, thus reducing oxidative deterioration of foods.
Most oxygen scavenging systems are based on the oxidation of iron and depend on the incorporation of an oxygen-scavenging component into the package, rather than an active packaging material. Ferrous iron powder is often used as the active agent. It is typically contained in a small high oxygen-permeable sachet to separate it from the food product (see Fig. 4.1). A disadvantage of systems of this type is that the water activity of the product must be high enough for the moisture to trigger the scavenging reaction.3 The sachet also poses a real risk to consumers as large amounts of iron could be accidentally ingested and there is the danger that it could leak, causing contamination or undesirable changes in the odour and flavor of the packaged product. It is clear that active packaging solutions that do not depend on sachets would be an improvement. There have been attempts to disperse ferrous iron compounds directly into polymer matrices; however, this causes their effectiveness to diminish as the active compound is poorly dispersed or quickly deactivated. The development of new active oxygen scavenging packaging materials with higher levels of efficacy would be of benefit to the food industry. Some oxygen scavenging films have been developed by adding titanium dioxide (TiO2) nanoparticles to different polymers, but since TiO2 acts by a photocatalytic mechanism, its major drawback is the requirement of UV light for activation.4,5 Aerobic microorganisms have also been used as oxygen-scavenging ’active compounds’ in hydroxyethyl cellulose and PVOH active coatings for high humidity foods,6 but their efficiency is restricted by the hydration conditions and the matrices in which they are entrapped.
Common synthetic antioxidants have been incorporated into packaging materials for many years to reduce the oxidation of packaged foods. Their function is to interrupt free radical chain reactions by reacting with ROS by hydrogen donation, hence preventing the attack of ROS on unsaturated molecules present in foods. Flexible and thermoformable plastic packaging materials containing i-butylhydroquinone (TBHQ), butylated hydroxytolu-ene (BHT), propyl gallate (PG), butylated hydroxyanisole (BHA), among others, have been developed and commercialized. The antioxidants can be incorporated into mono- or multi-layer structures.3 These antioxidant materials are particularly interesting in those systems where a specific quantity of oxygen makes a packaged product appear fresher and more desirable to the consumer, i.e. in the case of the appearance of fresh red meat. However, the toxic and carcinogenic effects of BHA, TBHQ, PG, BHT are of concern,7,8 and their use in food packaging has been restricted. The replacement of synthetic and toxic free radical scavengers by, for instance, natural compounds such as a-tocopherol,9 is therefore considered beneficial and is also thought to be viewed positively by consumers.
The unwanted growth of microorganisms in packaged foods is also an issue of concern. Antimicrobial materials are becoming increasingly important in active packaging to control the growth of pathogenic microorganisms. These antimicrobial active packaging systems need to exhibit a combination of desirable attributes such as strong antibacterial efficacy, environmental safety, low toxicity, cost effectiveness and ease of fabrication. Silver is well known for its antimicrobial properties. Recent technical innovations and findings have increased the availability of silver products and facilitated their incorporation in a wide range of materials. Novel antimicrobial formulations are therefore available for the development of improved antimicrobial active packaging materials.10,11
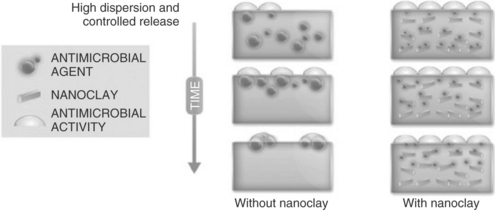
Active nanocomposites are advanced alternatives to conventional active plastic technologies or active sachets for the extension of the quality and safety of packaged food products. The term active nanocomposite generally refers to a plastic composite (i.e. a polymer blend), containing an active nanostructured material which confers an activity on the plastic matrix (see Fig.4.2). At least one of the dimensions of the active nanostructured material must be below 100 nm in size. Nanoclays can be used as carriers for the active agent. The efficacy of the active agent is enhanced because it is highly dispersed in the polymeric matrix and hence exposed more efficiently to the substance on which it is required to act (see Fig. 4.2). This chapter describes some antioxidant and antimicrobial packaging nanocomposites of interest in food packaging applications which have recently been developed in the authors’ groups.
4.2 Free radical scavenging nanocomposites
For an antioxidant to be of interest for inclusion in melt compoundable packaging films, it must provide no colour or odour and must be thermally resistant so that it can withstand conditions encountered during polymer processing. The DPPH inhibition assay,12 with some modifications, is a method typically used for the evaluation of the radical scavenging capacity of active films. Typically, film samples of 30 mg are put in cap vials with 1 ml of methanolic 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) 0.05 g/L stock solution. All samples are shaken and kept without being exposed to light at room temperature for 24 h. The film samples are subsequently removed from the solution and their absorbance measured at 517 nm. To study film ageing, films are typically exposed to artificial light at room temperature for several days before being analysed. The radical scavenging results are usually expressed as percentage inhibition of DPPH, as follows:
The DPPH inhibiting properties of EVOH nanocomposites containing O2Block™ RS-R (RS-R) (NanobioMatters S.L., Paterna, Spain) (a proprietary natural bioactive radical scavenging system supported on nanoclays), other conventional antioxidants such as butylated hydroxytoluene (BHT) (Guinama, Spain) and the commercial natural antioxidant extracts CCX45%LS and Cocoanox12% (Natraceutical Group, Spain) were tested. The antioxidant capacity of RS-R was higher than that of other non-traditional additives in similar concentrations and similar to that of BHT (see Fig. 4.3). The nanoadditive RS-R is therefore an alternative to the traditional antioxidant BHT, which is of concern from a toxicological point of view. Additionally, with ageing the efficiency of the nanoadditive system appears to increase. This behaviour could be due to the slow release of the antioxidant from the matrix to the measuring medium under test conditions.

Fig. 4.3 Comparative percentage Inh DPPH of EVOH and its composites with RS-R and other commercial antioxidants.
The excellent optical properties of multilayer films containing RS-R in both the EVOH layer and in the low density polyethylene (LDPE) food contact layer can be seen in Fig. 4.4.
4.3 Oxygen scavenging nanocomposites
Various oxygen scavenging nanocomposites of active nanoclays carrying iron and food packaging and beverage polymers were also developed by melt compounding. The oxygen scavenging capacity of films of these materials were determined by measuring the oxygen content in a vial headspace as a function of time (days). Four proprietary O2Block® grades of nanoclay additives (NanoBioMatters S.L., Paterna, Spain) namely F3, F4, F5 and F6 were used. F3 and F5 are iron-based nanoclays and F4 and F6 are iron-based organomodified nanoclays. The iron nanoparticles within the clay layers were seen to range typically from 27 nm up to ca. 150 nm in diameter. Iron metallic nanoparticles of a similar grade were also reported on the nanoclay surface through observation by transmission electron microscopy (TEM).13 Film samples were placed inside gas-tight septum-equipped vials of 40 ml capacity. Blank vials with virgin polymeric films were also prepared. The vials were subsequently sealed and placed in a room at 24 °C in atmospheric air, rather than in a modified atmosphere. The headspace percentage of oxygen (%O2) was determined using a PBI Dansensor CheckPoint (PBI Dansensor AS, Ringsted, Denmark) at different time intervals.
The LDPE–iron-based nanocomposites were seen to scavenge 1.2 ml of oxygen per gram of composite in the headspace after 10 days. The so-called F3 clays were more effective than the F4 clays once incorporated into LDPE (see Fig. 4.5). This behaviour is likely to be related to the more efficient interaction with moisture in the non-organomodified system.
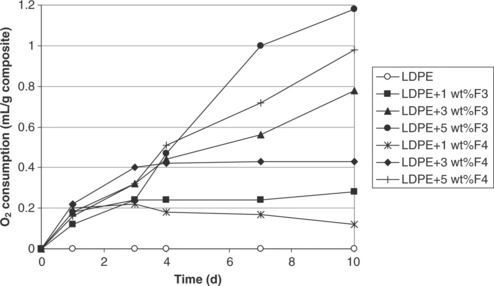
Fig. 4.5 Oxygen scavenging behaviour with time (day) of LDPE and its 1–5 wt% iron-based clay nanocomposites.
In the case of polyethylene terephthalate (PET) nanocomposites, the F3 active nanoclay once again exhibited greater efficiency than the F4 active nanoclay (see Fig. 4.6). The blend of F3-F4 iron-based nanoclays in PET showed less scavenging capacity than composites containing the pure nano-clay grades. In the best case, the PET active composites scavenged 0.6 ml of oxygen per gram of composite from the headspace. In the case of high-density polyethylene (HDPE) composites (see Fig. 4.7) maximum scavenging capacity was achieved with the F5 grade. As expected a higher scavenging capacity was also achieved with higher levels of filler.
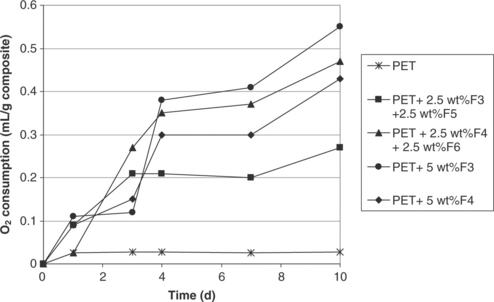
Fig. 4.6 Oxygen scavenging behaviour with time (day) of PET and its 5 wt% of iron-based clay nanocomposites.
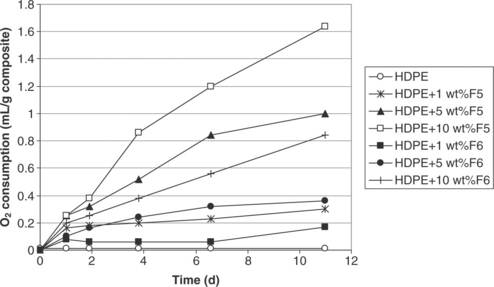
Fig. 4.7 Oxygen scavenging behaviour with time (day) of HDPE and its 1–10 wt% iron-based clay nanocomposites.
There were differences in the active performance between the various nanocomposites depending on the clay type and the iron-based chemistry used. The layered nanoclays are also inherently able to reinforce the physical properties of the polymeric matrices with little impact on optics and flexibility. Therefore it is thought that this technology can be relatively easily employed to produce tailored packaging materials with active performance and also the desired mechanical, barrier and optical properties that are very well suited to specific food preserving needs.
4.4 Antimicrobial nanocomposites
Silver systems are thermally stable and add no colour or odour to the packaging materials in which they are incorporated if they are properly formulated.10 Nanocomposites of polylactide (PLA) containing two nanoclay grades carrying silver were prepared by melt-blending routes (see Fig. 4.8 as an example). The active filler is, as in the other active systems described above, relatively well nanodispersed (particle thickness is generally below 100 nm) and intercalation and exfoliation of the layered nanoclay in the matrix has taken place. The active nanoclays used for the experiments below were Bactiblock®101 R1.51 (NanoBioMatters S.L., Paterna, Spain) which contains nanoparticles of Ag and Bactiblock®101 T1.51 which contains ionic silver. Both systems contain active organomodified nanoclays. To assess the biocide properties, the PLA nanocomposites (see Table 4.1) loaded with antibacterial nanoclays were cut into small pieces (5 × 5 cm). Subsequently, their antimicrobial properties were evaluated according to the standard JIS Z 2801 (ISO 22196), revised version from 2006. The tested microorganism was S. aureus (CECT 86). Each piece was inoculated with ca. 1 × 105 CFU (colony forming units) and incubated at 37 °C, 100% RH for 24 hours. Viable cells were determined by the agar plate count method. Four specimens for each type of sample were evaluated.
Table 4.1
Antimicrobial activity of the tested samples against the growth of S. aureus after 24 h at 37°C


Fig. 4.8 Typical TEM micrograph of a PLA-active nanoclay nanocomposite film. The scale marker is 1000 nm.
The antimicrobial activity of the samples tested by the Standard JIS Z 2801 was determined using the following formula:
where B is the average number of viable cells of bacteria on the untreated test piece after 24 h, and C is the average number of viable cells of bacteria on the antimicrobial test piece after 24 h. When R > 2.0, the sample is considered to present biocide properties.
Table 4.1 shows that growth on packaging sheets without additive was optimal (the standard specifies that the minimal final count for untreated samples should be 1 × 104 CFU/mL). When silver-containing clays were incorporated, the R values were higher than 2, which indicate that PLA-silver based-nanocomposites exhibited the expected antimicrobial performance. However, the T1.51 nanoclay was more active than the R1.51 nanoclay, possibly due to the more readily available biocidal ionic species of silver in the former which are, in turn, more easily released into the surface.
The incorporation of nanolayered antimicrobial additives based on silver such as the ones described above in polymer formulations generates antimicrobial materials, which additionally show enhanced gas and vapour barrier due to increased tortuosity imposed on the flow of gases by the layered nanofiller.10
4.5 Future trends
Antioxidant additives based on natural bioactive free radical scavengers incorporated into food contact compliant nanoclays have shown high efficiency in the scavenging of free radicals once incorporated into packaging plastics. The natural bioactive free radical scavengers also show no toxicity, in contrast to traditional antioxidants. Oxygen scavengers can also be very efficiently incorporated into LDPE, HDPE and PET, and their impacts on oxygen scavenging, colour and transparency can be tailored. Both systems described are alternatives to current passive and active packaging materials to reduce oxidation of food products. The incorporation of silver-containing nanoclays into a PLA matrix generated very efficient antimicrobial composites, the antimicrobial activity being higher when silver was present in its ionic form. There were differences in mechanical, barrier and optical performance as well as oxygen-scavenging performance between the various nanocomposites described in Section 4.3 depending on the clay type and the iron-based chemistry used. Materials compositing to improve multiple functionalities in plastics (i.e. bringing both physical reinforcement and active performance) is one of the most innovative prospects in packaging applications for foods and beverages. The technologies discussed in sections 4.2, 4.3 and 4.4 have either recently received approval by bodies such as the Environmental Protection Agency (EPA), the Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) for use in food packaging applications or approval is being sought.
4.6 References
1. Podhajny, R.M. Paper, Film & Foil Converter, April 1 2002.
2. Ahvenainen, R.Novel Food Packaging Techniques. CRC Press, 2003.
3. Brody, A., Strupinsky, E.P., Kline, L.R.Active Packaging for Food Applications. CRC Press, 2001.
4. Mills, A., Doyle, G., Peiro, A.M., Durrant, J. J. Photochemistry and Photobiology A: Chemistry. 2006; 177:328–331.
5. Xiao, L., Green, A.N.M., Mills, A., Durrant, J.R. J. Photochemistry and Photobiology A: Chemistry. 2004; 162:253–259.
6. Altieri, C., Sinigaglia, M., Corbo, M.R., Buonocuore, G.G., Falcone, P., Del Nobile, M.A. Lebensm.-Wiss. U-Technol. 2004; 37:9–15.
7. kahl, R., kappus, H., Lebensm, Z. Unters. Forsch. 1993; 196(4):329–338.
8. Miyauchi, M., Nakamura, H., Furukawa, H., Son, H.Y., Nishikawa, A., Hirose, M. Cancer Lett. 2002; 78:19–24.
9. Byun, Y., Darby, D., Cooksey, K., Dawson, P., Whiteside, S. Food Chemistry. 2011; 124:615–619.
10. Busolo, M.A., Fernandez, P., Ocio, M.J., Lagaron, J.M. Food Additives and Contaminants – Part A Chemistry, Analysis, Control, Exposure and Risk Assessment. 2010; 27(11):1617–1626.
11. Quintana, P., Magaña, S.M., Aguilar, D.H., Toleda, J.A., Angeles, C., Cortes, M.A., Freile, Y., Torres, R.M. J. Mol Catal A: Chemical. 2008; 281:192.
12. Yen, G.C., Duh, P.D. J. Agrie Food Chem. 1994; 42:629–632.
13. Busolo, M.A., Aouad, A., Lagaron, J.M. Annual Technical Conference – ANTEC, Conference Proceedings. 2010; 1:22–25.