Controlled release food and beverage packaging
Abstract:
This chapter introduces controlled release packaging, which is an emerging technology by which active compounds such as antimicrobials or antioxidants are first incorporated into the package and then released to the food in a controlled manner to inhibit microbial growth or oxidation, thereby extending shelf life of the product. Scientific evidence is provided to support the soundness of this technology. A conceptual framework is also provided as a research roadmap to facilitate the development of this technology.
2.1 Introduction
Controlled release packaging (CRP) is defined as a new generation of packaging materials that can release active compounds such as antimicrobials and antioxidants at desirable rates to extend the shelf life of a wide variety of foods. CRP may also refer to the technique of using these new generation materials to enhance food safety and quality.
An advantage of CRP is its ability to provide a sustained supply of active compounds at suitable rates for food protection. Traditionally, active compounds such as antioxidants, antimicrobials, and anti-browning agents are incorporated into food formulations; however, once these active compounds have been consumed in reactions, protection ceases and food quality degrades rapidly. CRP can overcome this limitation by continuously replenishing active compounds via controlled release from the package to provide sustained food protection. Another advantage of CRP is that a smaller quantity of active compounds can sometimes be used to provide the same or better levels of protection than are achieved when adding larger quantities of active compounds directly to food. For example, microbial problems are known to occur mostly on food surfaces, and in CRP antimicrobials are released directly to the food surface where antimicrobials are most needed. Adding antimicrobials directly to food is less effective and may result in overloading, since most of antimicrobials added using this method end up inside the food where microbial growth is of less concern. Still another advantage of CRP is its ability to protect unstable active compounds from degradation until they are released. For example, when tocopherol (a common antioxi-dant) and nisin (a common antimicrobial) are added to food formulation, unused amounts of these active compounds may undergo rapid degradation resulting in significant loss and thus much reduced levels of active compounds for food protection at later time. Our experimental data indicate that CRP can prevent this problem by storing tocopherol and nisin inside the package and thus protecting them from degradation until their release to the food.
CRP may also be described as an active packaging system that uses the package to deliver active compounds in a controlled manner. Active packaging has been defined as a group of technologies that actively modify the internal package environment through physical, chemical, or biological interactions between the package, the food, and the headspace for the purpose of enhancing food quality and safety (Rooney, 1995; Brody et al, 2001). CRP modifies the internal package environment by releasing active compounds in a controlled and desirable manner. The CRP technology is particularly useful for controlling food degradation reactions that are continuous and increase exponentially, such as microbial growth and lipid oxidation, as constant replenishment of inhibitory active compound can prevent these runaway deterioration processes.
2.2 Useful terms for controlled release packaging (CRP)
The following terms are defined to facilitate discussion of CRP in this chapter. ‘Instant addition’ means adding the entire amount of active compound to the initial food formulation. ‘Slow release’ means releasing the active compound over a period of time, either in a controlled or uncontrolled manner. ‘Controlled release’ is a special form of slow release, in which the active compound is released over time in a controlled manner. Controlled release may be achieved using a precision device such as a syringe pump to release the active compound at predetermined rates, or using controlled release packaging technology. ‘Controlled release packaging’ is a sophisticated form of controlled release, which uses the package as a delivery system to release the active compound in a controlled manner. The ability to control release is achieved by quantifying the functional relationships in the conceptual framework described later.
2.3 Scientific evidence to support controlled release packaging (CRP)
Why use CRP instead of adding active compounds directly into food formulations? The soundness of CRP is supported by our research using antioxidants and antimicrobials (Zhang et al, 2004; Zhu, 2008). As an example, we compared the effectiveness of the antioxidant tocopherol under slow release conditions compared to instant addition using linoleic acid as food model. (The term ‘slow release’ was used because insufficient information was available at the time of the experiment to achieve ‘controlled release’.) For slow release, tocopherol was incorporated into low density polyethylene (LDPE) and polypropylene (PP) films and an appropriate amount of cut film was submerged in linoleic acid, allowing the tocopherol to be released slowly. For both instant addition and slow release, the same amount of tocopherol was added into the same volume of linoleic acid, the resulting solution (300 mg/kg) was then exposed to air, agitated with a shaker, and stored in the dark at room temperature (23 °C). Conjugated dienes, the oxidation products, were measured over time to determine the onset of lipid oxidation. Figure 2.1 shows that, for control samples without tocopherol, oxidation of linoleic acid started after 2 days. For instant addition, oxidation occurred after 19 days. For controlled release using LDPE and PP, onset of oxidation was significantly delayed to 23 and 31 days, respectively. The results show that slow release from film was more effective than instant addition in retarding oxidation. A possible explanation for this observation is that tocopherol is unstable in linoleic acid, and thus unused tocopherol from instant addition can degrade rapidly resulting in much lower antioxidant effectiveness, while the film can protect tocopherol from degradation until its release.
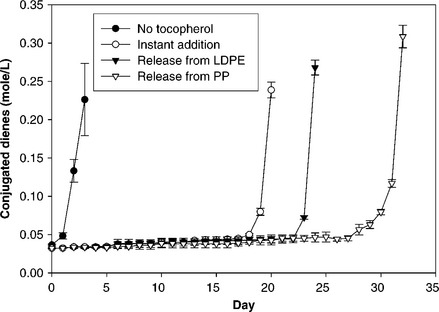
Fig. 2.1 Generation of conjugated dienes in linoleic acid at 23 °C under various conditions. Vertical bars are standard deviations.
As another example, we also compared the effectiveness of the antimicrobial nisin in inhibiting the growth of Micrococcus luteus, a model microorganism, under conditions of instant addition versus controlled release (Balasubramanian et al., 2011). Controlled release was achieved using a computerized syringe pump system to mimic the release of nisin from packaging film, which was characterized by an initially fast rate which slowed as time progressed. The release rate of nisin from the syringe pump was programmed using various diffusivities (D) and an appropriate Fickian model to mimic the release of nisin from the film, assuming the release was limited by Fickian diffusion of nisin in the film. A higher diffusivity corresponds to a faster release from the film.
Figure 2.2 compares growth kinetics of M. luteus under the conditions of absence of antimicrobial, instant addition, and controlled release. The slowest release profile (D = 1.53 × 10– 12 cm2/s) did not cause a decrease in cell number. With faster release profiles (D = 6.12 × 10– 12 cm2/s or above), complete inhibition of M. luteus was observed for at least 48 hours. The minimum D value required to effectively inhibit microbial growth was about 6.12 × 10– 12 cm2/s, which corresponds to a total amount of nisin released of 0.227 μmol or a final concentration in the media of 1.14 × 10– 3 μmol/mL after 48 hours. This total (i.e. 0.227 μmol) is equal to 15% of the amount used for the best result obtained under instant addition: 1.49 μmol. Under instant addition, growth inhibition was not sustained after 12 hours. Therefore controlled release was a more effective antimicrobial delivery method than instant addition. This observation is all the more striking when it is considered that these better results were obtained when using only 15% of the nisin used to obtain the best results under conditions of instant addition. The results show that the controlled release profiles used in this study are highly effective. The fast initial rates of these profiles are necessary to provide lethal stress to kill or injure the cells, while the subsequent slower rates with persistent release of small amounts of nisin are sufficient to suppress recovery of the injured surviving cells. Thus the combination of initial fast rate and subsequent slower rate provides good overall microbial inhibition.
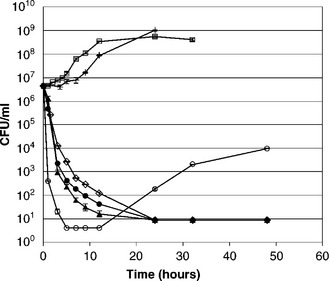
Fig. 2.2 Effect of controlled release profi le of nisin on growth of M. luteus in 200 mL tryptic soy broth (TSB) at 30°C. (![]() ) cultures in absence of nisin (control), (
) cultures in absence of nisin (control), (![]() ) growth of M. luteus with instant addition of 7.45 × 10– 3 μmol/mL of nisin; (
) growth of M. luteus with instant addition of 7.45 × 10– 3 μmol/mL of nisin; (![]() ) growth for diffusivity of 1.53 × 10− 10 cm2/s, (•) growth for diffusivity of 3.83 × 10− 11 cm2/s, (◊) growth for diffusivity of 6.13 × 10− 12 cm2/s, (+) growth for diffusivity of 1.53 × 10− 12 cm2/s. Standard errors calculated based on plate count from eight plates.
) growth for diffusivity of 1.53 × 10− 10 cm2/s, (•) growth for diffusivity of 3.83 × 10− 11 cm2/s, (◊) growth for diffusivity of 6.13 × 10− 12 cm2/s, (+) growth for diffusivity of 1.53 × 10− 12 cm2/s. Standard errors calculated based on plate count from eight plates.
2.4 Conceptual framework
CRP is as an emerging technology being developed by our and other laboratories around the world (LaCoste et al., 2005). The key word in controlled release packaging is ‘controlled’, and a major challenge is to deliberately control the release of active compounds at rates suitable for a wide range of food products and specific degradation reactions, since there is a lack of fundamental understanding of the factors governing the release of active compounds from packaging materials. To minimize empirical testing and achieve better results, we have developed a systematic approach based on the conceptual framework in Fig 2.3 to elucidate the relationships between important variables in CRP systems.
Four groups of variables are identified in this conceptual framework. The first three groups (‘process variables’, ‘structure variables’, ‘property variables’) are related to ‘packaging research’ and development. The process variables are those that can be manipulated directly by the designer to develop CRP packages. The structure variables and property variables are those package variables that cannot be manipulated directly; however, once the process-structure–property relationships are established, desirable package properties such as release behavior of active compound can be obtained by properly manipulating the process variables. The fourth group of variables (‘food variables’) is related to ‘food research’ to determine the ‘target release rate’ necessary to develop CRP systems. These four groups of variables and the target release rate will be described in later sections.
The conceptual framework provides a research roadmap for the systematic study of these variables and the relationships between composition, processing, structure, and properties. For example, proposed in this framework are three types of effects contributing to the observed properties: compositional effects, processing effects, and structural effects. As shown in Fig 2.3, the ‘compositional effects’ depend on the active compounds and polymer composition (polymer type and polymer ratio), which can affect properties either directly (via pathway ➊) or indirectly through its influence on the film structure (via pathways ➋ and ➌). The ‘processing effects’ depend on the processing method, which can affect the structure and properties (via pathways ➍ and ➌) and the stability of active compound (via pathway ➎). The ‘structural effects’ depend on compositional effects and processing effects, which can affect the release and other physical properties (via pathway ➌), although probably not the stability of active compound.
2.5 Process variables
The process variables may be divided into three sub-groups relating to active compounds, polymer composition, and processing methods, which can be manipulated directly by the designer to achieve a package with the desired properties.
2.5.1 Active compounds
The first consideration is to select effective food grade active compounds suitable for the application; for example, antimicrobials are used to inhibit microbial growth, and antioxidants are used to retard oxidation. Other possible active compounds include enzymes, flavors, nutraceuticals, etc. Sometimes two or more active compounds may be used to provide the desired results. Natural compounds (e.g., tocopherol extracted from nature sources and thymol extracted from essential oils) are preferred over synthetic compounds. Two or more active compounds may be used when necessary.
The second consideration is whether the active compound is volatile or nonvolatile, because the active compound must make contact with the food before it becomes effective. Volatile compounds such as sesamol or butyl-ated hydroxytoluene (BHT) are necessary for products that do not have direct food package contact, such as breakfast cereal in a plastic bag. They are first released from the package, then vaporized into the headspace, and finally condense onto the food surface. Non-volatile compounds such as tocopherol and nisin may be used for products that have direct food package contact, such as a pouches containing meat and gravy.
The third consideration is the release kinetics of active compound, which depends on the interactions between the active compound, the package, and the food. The effectiveness of active compounds can be greatly influenced by the quantity released to the food and the rate at which this takes place.
2.5.2 Polymer composition
Although CRP may be in various forms, we consider here CRP films that are made of common polymeric packaging materials such as polyethylene and polypropylene. ‘Polymer composition’ refers to the film composition and its structure. A simple film may have a single layer consisting of a single polymer. A sophisticated film may have more than one layer, with each layer consisting of two or more polymers in which the ratio of polymers may be varied. The combinations of different layers and polymers allows the production of films with different release rates suitable for the wide range of foods in the market.
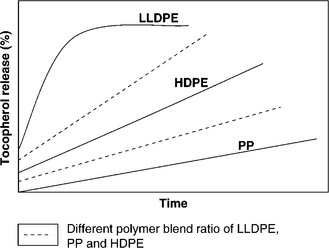
In Fig 2.4, three different single polymer (linear low density polyethylene, LLDPE; high density polyethylene, HDPE; and polypropylene, PP) films were used to investigate the effects of polymer type on tocopherol release (pathways ➊ and ➋ in Fig. 2.3). Tocopherol release over time from each of the single polymer films was measured at 25 °C using 95% ethanol as a food simulant. It was found that the tocopherol was released most rapidly from LLDPE, followed by HDPE and PP, likely due to their physical properties (density, crystallinity, and glass transition temperature). The same trend was observed at an accelerated temperature of 40 °C.
The evaluation of estimated diffusion coefficients using single polymer types assisted the realization that a range of release rates could be achieved by combining two or more individual polymers at different ratios in a polymer blend. The dotted lines in Fig 2.4 show the intermediate release rates obtained by blending LLDPE/HDPE/PP at different ratios. A wide selection of release rates can be obtained by varying the polymer type and polymer ratio.
2.5.3 Processing methods
There are various methods of producing CRP films, which can greatly affect film structure and properties. The most common commercial processes of producing packaging films are the cast film and blown film processes, which involve melting a polymer resin, extruding the polymer melt through a die, and stretching and cooling the polymer melt into a film. Depending on processing conditions such as the feed rate, screw speed, barrier temperature, and extruder configuration, CRP films with significantly different properties may be obtained.
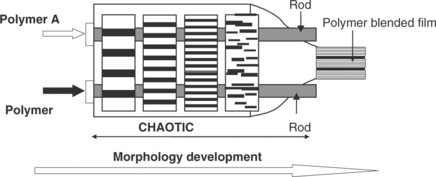
When two or more polymers are used to form a polymer blend film, an innovative processing method known as smart blending technology based on the principle of chaotic advection may be used to produce polymer blend films with different film morphologies (Zumbrunnen and Chhibber, 2002). The heart of this method is a chaotic mixer consisting of two rotating rods (Fig. 2.5). Two immiscible polymers are first melted using an extruder and then the melts are forced through the chaotic mixer. By varying the speeds and number of turns of the rotating rods, different film morphologies in the forms of droplets, multilayers, interconnected layers, platelets, and fibers can be produced. Fig 2.6 shows examples of polymer blend morphologies and their influence on release of tocopherol. These different film morphologies can greatly influence the release of active compounds and thus a wide range of release rates may be obtained for different food applications.
Lamination, coextrusion, solution casting, and coating are also processing methods that can significantly influence release kinetics and other film properties.
2.6 Structure variables
As shown in the conceptual framework, structure variables can be influenced by the composition effects (via pathway ➋) and processing effects (via pathway ➍).
2.6.1 Polymer blend morphology
When two or more polymers are used to produce a blend film, the film morphology becomes an important variable. The term polymer blend morphology is used here to describe polymer film structures at microscopic level, observable by scanning electronic microscope, and displaying distinguishable phases that are formed by two or more immiscible polymers. Examples of polymer blend morphologies are shown in Fig. 2.6.
2.6.2 Package structure
Package structures are related to package design. For example, a design may involve a packaging material of a three-layer structure, the outer layer consisting of a gas barrier polymer, the middle layer containing the first active compounds, and the inner layer containing the second active compounds. By manipulating factors such as loading of active compounds and thickness of layer, different release rates can be obtained.
2.6.3 Localization of active compounds
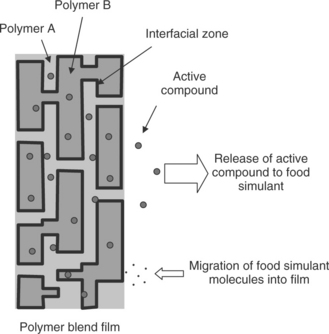
When there are two or more immiscible phases in a polymer blend film, localization refers to distribution of active compound in these phases, which can greatly influence the release of active compounds. The release of a compound from a polymer film to a food simulant involves three steps (Fig. 2.7): molecular diffusion within the polymer film toward to the film/food interface, mass transfer across the interface, and dispersion into the bulk food. In most cases, diffusion is the rate controlling step due to the high diffusion resistance in the polymer matrix.
Diffusion of active compounds through two immiscible phases in CRP depends on many factors: size, shape, and distribution of active compounds in the polymer matrix; polymer morphologies including density, crystallinity, tortuosity, degree of crosslinking and branching, and glass transition temperature; molecular interactions of the compounds and host polymers; ther-modynamic properties such as polarities and solubilities; and the nature of the food or food simulant in the package. With high loadings, the active compound may also reside in the interfacial zone. In addition, sorption of solvent molecules by the film may result in an increase in free volume and swelling of the polymer film, leading to an increase in diffusion coefficient of the active compound in the film. This swelling may be both an advantage and a disadvantage. A better understanding of the factors affecting diffusion will provide many ways to tailor the release of active compounds for a wide range of food packaging applications.
2.7 Property variables
These variables describe the properties most important to controlled release packaging films. The desired properties can be directly obtained by varying the polymer composition (pathway ➊), by varying the structure through composition and processing (pathway ➌), or by varying the processing method and conditions (pathway ➎).
2.7.1 Release properties
The ability to release of active compounds is the most important property of CRP packages. The release rate of antimicrobials or antioxidant should properly match the microbial or oxidation kinetics of the food and the shelf life requirement. Since different foods have different requirements, it is necessary to have the ability to produce CRP materials of different release rates for a wide range of food products.
The release of active compounds such as tocopherol and nisin may be studied using the single-sided diffusion cell developed in our earlier studies (Chung et al., 2001). As shown in Fig. 2.8, a 30.68 cm2 CRP film is secured on the bottom of the diffusion cell, and a 100 mL food simulant solvent (such as water, 95% ethanol, and oil) is added and agitated with a magnetic impeller to enhance interfacial mass transfer and thorough mixing. Effects of temperature may be studied at 10, 25, and 40 °C. Release of active compound may be measured as a function of time and temperature. Partition coeficients of active compound between food simulants and polymer ilms may be determined, and ‘overall diffusion coefficients’ of active compound in the film may be calculated using the Fickian or non-Fickian models as appropriate.
2.7.2 Other film properties
These are important properties for CRP film including heat sealability, ability to be laminated, tensile strength, and gas permeability. The active compounds may act as plasticizers that decrease mechanical properties and gas permeability, usually slightly since concentrations of active compound are low. These decreases are not a concern in situations when CRP is used as a functional layer in a multilayer structure, with other layers (such as an aluminum foil) that provide strength support and gas barrier.
2.7.3 Stability of active compound
This is of great concern as CRP involves intentional release of active compounds into food, and the compounds may lose activity, generate toxic products, or remain unchanged during severe processing conditions involving high heat and shear. To gain FDA approval, it is necessary to demonstrate that the compounds are not significantly degraded or converted to potentially toxic products. Analytical methods such as gas chromatography (GC) and liquid chromatography/mass spectometry (LC/MS) can be used to identify and quantify potential harmful volatile and non-volatile compounds.
2.8 Food variables
‘Food variables’ including food composition, food/package contact area, storage condition, shelf life requirement, and other factors are important for the development of CRP packages. For example, food composition determines whether antimicrobials or antioxidants or both are required for food stability; whether the food is in solid or liquid form determines whether a volatile or a non-volatile active compound is suitable; storage conditions and shelf life requirements determine how much and how fast active compounds need to be released.
The study of food variables involves what the conceptual framework describes as ‘food research’. Scientific research is needed to study microbial and oxidation kinetics of food as a function of slow or controlled release of active compounds. The research will require great effort because most data of microbial and oxidation kinetics in the literature are conducted under the condition of instant addition. For more sophisticated CRP systems, studies of kinetics for more than one active compound may be needed. With the encouraging data obtained in our laboratory during the past several years, we are convinced that the food research for CRP will likely be a good investment.
2.9 Target release rate
‘Target release rate’ is a new concept proposed by our laboratory to serve as a bridge between the packaging research and food research in the conceptual framework (pathways ➏ and ➐ in Fig. 2.3). The successful development of CPR food packages requires the collaboration of a packaging engineer and a food scientist. The packaging engineer is responsible for producing the package, and the food scientist is responsible for making sure that the package serves the purpose of extending the shelf life of the food. To design the package, the packaging engineer needs to know the target release rate of active compounds. The food scientist needs to provide this target release rate based on the knowledge gained from the food research in the conceptual framework. After the target release rate is provided, the packaging engineer can then design and produce the package based on the knowledge gained from the packaging research in the conceptual framework.
The target rate release describes how fast and how much active compound should be released for a particular food application. The rate of release is not constant since the release of an active compound from a packaging film is usually controlled by diffusion of the active compound in the film, characterized by fast release initially followed by progressively slower release as time passes. Determining the target release rate is rather challenging since it involves many factors and considerations. This new concept is being developed in our laboratory.
2.10 Potential food applications
Controlled release packaging has a wide range of applications for improving food quality and safety. CRP using antimicrobials may be used for short or intermediate term microbial inhibition for highly perishable foods such as fresh meats, seafood, fruit and vegetables. CRP using antioxidants may be used for long-term retardation of lipid oxidation for shelf stable foods such as ready-to-eat meals containing fatty components susceptible to oxidation.
2.11 References
Balasubramanian, A., Lee, D.S., Chikindas, M.L., Yam, K.L. Effect of nisin’s controlled release on microbial growth as modeled for Micrococcus luteus. Probiotics and Antimicrobial Proteins. 2011; 3:113–118.
Brody, A.L., Strupinsky, E.R., Kline, L.R. Active packaging for food applications. Lancaster, PA: Technomic Publishing; 2001.
Chung, D.H., Papadakis, S.E., Yam, K.L. Release of propyl paraben from a polymer coating into water and food simulating solvents for antimicrobial packaging applications. Journal of Food Processing and Preservation. 2001; 25:71–87.
Lacoste, A., Schaich, K.M., Zumbrunnen, D., Yam, K.L. Advancing controlled release packaging through smart blending. Packaging Technology and Science. 2005; 18:77–87.
Rooney, M.L. Active food packaging. New York: Blackie Academic & Professional; 1995.
Zhang, Y.C., Yam, K.L., Chikindas, M.L. Effective control of Listeria monocytogenes by combination of nisin formulated and slowly released into a broth system. International Journal of Food Microbiology. 2004; 90(1):15–22.
Zhu, X. Development of target release rate concept for controlled release packaging. New Brunswick, New Jersey: Department of Food Science, Rutgers University; 2008. [Ph. D. Thesis].
Zumbrunnen, D.A., Chhibber, C. Morphology development in polymer blends produced by chaotic mixing at various compositions. Polymer. 2002; 43(11):3267–3277.