8 Modeling and Analysis of the Cellular Mechanics Involved in the Pathophysiology of Disease/Injury
Benjamin E. Reese, Scott C. Lenaghan and Mingjun Zhang
CONTENTS
8.2 Modeling, Analysis, and Control of Cellular Mechanics in Disease/Injury
8.4 Applications in Cardiovascular Disease
8.5 Advances in Experimental and Imaging Techniques (BioMEMS/NEMS)
8.6 An Example: Cardiomyocyte Mechanics
8.6.1 Experimental Setup/Design
8.1 Introduction
Many complex biological processes require continuous multifactorial monitoring, at various time and length scales, in order to elucidate the underlying physiological changes that result over the course of a disease. The technical challenge that this type of monitoring presents is significant to the scientists and clinicians attempting to monitor the pathophysiology of disease. An inability to effectively analyze the events that occur during disease progression further reduces the likelihood of effective therapy and early detection. Additionally, in order to understand complex biological responses, a large quantitative dataset is necessary for analytical analysis, which may provide insight into the underlying mechanisms associated with a behavior or response. Further, the collection of highly quantitative data for biological systems is often costly and time consuming due to the complex equipment and methods associated with their collection. At the single cell level, however, many of the complications associated with in vivo data collection can be avoided. Similarly, in most in vitro studies, cells are not synchronized, leading to variations among the population that contribute to noisy data collection which can be avoided at the single cell level. Ultimately, an understanding of the pathophysiological response at the single cell level may provide insight into disease diagnosis and treatment.
While experimental studies remain the “gold standard” for the development of preventative, diagnostic, and therapeutic strategies, the complex nature of disease relative to the simplicity of experimental studies often leads to the analysis of only a single variable at a time. As a result, it is often beneficial to use mathematical modeling, in addition to biological testing, to gain further insight into these complex processes. The flexibility of models allows them to be used to rapidly generate preliminary data that can lead to the formulation of testable hypotheses in a quick and inexpensive manner. Further, the ability to analyze and manipulate multiple parameters with larger datasets can lead to more comprehensive and robust preliminary investigations, in preparation for further in vitro or in vivo studies. For these reasons, mathematical models are being developed for multiple applications within the fields of biology and medicine to assist in the study of diseases at varying scales ranging from subcellular elements to more complex tissues and organ systems. The following sections illustrate those areas in which the modeling, analysis, and control of cellular mechanics can lead to advancements in the understanding of the pathophysiology of disease.
8.2 Modeling, Analysis, and Control of Cellular Mechanics in Disease/Injury
One of the most difficult aspects of model construction remains in the estimation of model parameters and the identification of the structural and regulatory behavior of biological networks. Generally, this is dependent on the understanding of the system being modeled and the availability of data that can be used to describe the system. Of particular interest to pathologists are changes in the mechanobiology of cells associated with disease. Mechanobiology focuses on how physical forces or changes in cell mechanics contribute to the development and physiology of cells and tissues. Structure–function relationships such as those involved in mechanobiology are known to regulate many biological processes, spanning multiple levels and length scales. As a result, numerous subcellular features, such as those involved in cytoskeletal rearrangement, influence the dynamic behavior of individual cells, which often affects surrounding cells through downstream signaling. This downstream signaling often serves as the origination signal of an injury or disease, and consequently, can be used for early detection of a pathological condition. Subtle modifications in the shape or structure of a cell could represent some of the earliest distinguishable factors indicating the onset of disease. For instance, mechanical forces applied to cells have been shown to regulate the progression of atherosclerosis and influence the transformation from a normal to malignant phenotype in certain cell types [1]. Not only can these external forces directly affect the mechanical response of cells, but they can also trigger the generation or suppression of biochemical and molecular signaling. Both the passive sensing and active modifications exhibited by different cell types due to these forces have an influence on the overall dynamics of cells and tissues. The ability to monitor and quantitatively measure these characteristic changes as a result of pathological events could facilitate earlier detection and provide further insight into healthy and diseased states.
Due to the unknown nature and ambiguous interpretation of many biological processes, the ability to address and translate changes from the molecular level to their subsequent physiological influence is a powerful advantage that can be realized with the selection of an appropriate modeling approach. As a result, models that are capable of integrating components from radically different time and/or length scales are highly sought after. Several examples incorporating multiscale models are accomplished using agent-based modeling. This type of modeling allows for the investigation of the collective behavior of many individual units and the patterns or properties that emerge as a response to certain stimuli [2]. These are typically used when modeling at the tissue level, where changes may have compounding or distinctly different effects at the single cell and systemic level. One recently developed example of using this approach is the Delaunay Object Dynamics (DOD) framework [2]. This framework has been applied to the compartmental homeostasis of B lymphocytes and T lymphocytes in secondary lymphoid tissue. Secondary lymphoid organs are well known for their role in adaptive immunity, and their cellular organization/migration is considered to be critical for the efficient initiation of the mammalian immune response at these sites. DOD simulations involving contact-dependent forces between cells, such as those that influence topology, have been used to accurately describe these interactions based on Delaunay triangulation [2]. This method allowed for the calculation of several parameters such as the size and position of cells, which are conditional upon the local environment. Using these values further led to the determination of shape and surface properties, such as adhesion and friction forces, stemming from cellular interactions. The dynamics of this system were described based on Newtonian equations of motion, while the forces involved in these interactions included those provided by the internal cytoskeleton, as well as external forces exerted by the extracellular matrix. Using this type of framework, discrete properties, along with continuous spatial properties, including contact area and the positions of cells, were integrated, allowing the impact of cellular properties on tissue organization to be characterized and explained in the form of a multiscale model. This example illustrates the coupling that exists between cellular mechanics and the organization or function of diverse cell populations in certain tissues. As researchers continue to search for the underlying causes of many diseases and the injuries they can induce, multiscale models will see even further development to increase understanding of the effects of cellular interactions on these complex processes.
Computational modeling can be utilized for the analysis of various cellular processes from both normal and pathological tissue, such as cell migration or adhesion. Epithelial cells, as well as many other cell types, have the ability to form unique shapes and structures in two and three dimensions. Biomechanical properties and the relative forces generated or experienced by individual cells are fundamentally important to understanding how these cells are capable of accomplishing such highly cooperative tasks. Many pathological events occur in response to biomechanical factors and rely on cells’ ability to communicate with one another while orchestrating the collective behavior exhibited during these processes. Other examples in which modeling is able to mimic the behavior of individual cells within various tissues was investigated during monolayer formation and tissue morphogenesis [3]. The model used for this study included many different cellular components such as the cytoskeleton, membrane, and cytoplasm, while further accounting for growth, division, motility, apoptosis, and polarization. Simulations using this model were able to show how individual cellular biomechanical properties affected tissue growth and other morphological changes that are critically important to these processes. Experimental studies regarding these processes can sometimes be confined to a two- dimensional substrate or only allow for two dimensions of measurable data, whereas a model such as this could be used to analyze these processes in three dimensions such as that found in vivo. Many times the transition of such a model to three dimensions can be achieved by extending all of the equations to include a third axis. Although this requires a considerable increase in computational cost, it affords the opportunity to study these events in a way that might not be possible using traditional experimental techniques.
Experimentally driven models typically rely on the collection of specific parameter values in order to derive accurate mathematical representations describing a cell’s behavior or response. A review of parameter estimation techniques discusses several different approaches for model construction based on this relationship [4]. Traditionally, a forward or bottoms- up modeling approach has been used, where several components are isolated and processed individually. Multiple constituents are then merged together to form an integrative model, such as those used in metabolic pathways, which utilize the kinetic properties of individual enzymes and basic rate laws [5].
Steady-state data are also commonly used in stoichiometric or flux-based approaches in order to estimate parameter values. These types of analyses are usually done by modifying input concentrations for a specified parameter while holding the remaining parameters constant. The effects that these perturbations have on the steady- state values of the system are then studied and recorded for further regression analysis. Due to the reliance of many models on the acquisition of comparable sets of experimental data, the recent advances in biological equipment have had a great influence on the development of cell- based modeling. High- throughput techniques allow for the generation of time series data that can characterize the dynamics of genomic, proteomic, metabolic, and physiological responses for the estimation and identification of important parameters and relationships through the use of a top- down or inverse approach. This type of model construction allows for multiple parameters to be monitored throughout the course of a dynamic biological process, leading to more information on potentially transient or brief deviations occurring at various time points throughout the duration of a study.
These examples help demonstrate how various approaches can be taken in order to apply quantitative measurements to the biological processes being described, such as disease pathology. By integrating a model along with experimental studies, researchers are able to further analyze and relate the growing amount of data that can be collected and used to help characterize and describe these complex biological functions. Modeling these events can also allow for control theory to be incorporated into these studies so that predictive or therapeutic strategies can be applied to enable a more in- depth understanding of how these parameters are related and ultimately provide researchers and clinicians with a clearer picture of how to address or control those diseases.
8.3 Applications in Cancer
Cancer prognosis and treatment have benefited greatly from studies involving gene expression profiles and other clinical or pathological variables. A recent study involving the construction of a prognostic model combining these two approaches showed even further improvements in the accuracy of predictions when compared to either type alone [6]. This model was able to combine two separate methods of prognostication and utilize the statistical analysis of multiple datasets in order to derive a better predictive model that can potentially be used for determining cancer therapy responsiveness and patient outcome. Prognostic variables such as tumor size and genetic signatures were identified and associated with their respective outcomes based on data provided by patients from a combination of multiple clinical trials.
The results from this research show that a model of clinical and genomic variables used in combination with each other had a greater prognostic capability over either predictor individually. This example demonstrates how modeling can combine experimental data spanning different levels of characterization to form a powerful tool for predicting or analyzing complex biological processes such as cancer.
Another example in which modeling has been applied to cellular mechanics involved the quantification of metastatic potential and invasiveness of chondrosarcoma cells [7]. This study developed a thin- layer viscoelastic model for stress relaxation in which the mechanical properties of chondrosarcoma cells of different configurations were quantified using microscopy- based indentation tests with an atomic force microscopy (AFM). The viscoelastic properties of both rounded and spread cell morphologies from several cell lines were acquired and characterized using the derived model to interpret their results. The selection of an appropriate theoretical model for contact geometry is a crucial determinant for the accurate quantification of biophysical properties using this technique. Force- versus- distance curves were taken on each of the different cellular configurations/cell lines and further analyzed using a Hertz- based fit in order to determine Young’s modulus. The full viscoelastic response of the cells during stress relaxation tests required a thin- layer correction in order to accurately describe the experimental conditions during the relaxation phase. Darling et al.’s findings were able to demonstrate the effective characterization of different cells using the derived thin- layer viscoelastic model, and further indicated that more aggressive cells exhibited lower moduli. This study was also able to conclude that cell deformability can accurately reflect certain phenotypic characteristics that are related to metastasis, including release from the original tumor site, along with penetration and invasion of the vasculature. Modeling the mechanical properties of these cells led to the confirmation of the hypothesized inverse relationship between metastatic potential and stiffness while providing new insight into therapeutic strategies that might be used to inhibit metastasis by targeting cytoskeletal structures that regulate stiffness and motility.
8.4 Applications in Cardiovascular Disease
As previously mentioned, the role that mechanical forces play in the regulation of atherosclerosis has been recently investigated in terms of the adaptive response of cells in the vasculature under varying flow conditions [8]. Mechanical signaling and mechanotransduction, which describes the molecular mechanisms occurring in response to mechanical cues, are critical determinants of both morphogenesis and function in pathological states such as hypertension and atherosclerosis. Vascular smooth muscle cells (VSMCs) and endothelial cells (ECs) that line and help form the vasculature are capable of morphological remodeling in order to accommodate shifts in blood pressure and shear stress over relatively long time scales. Chemical cues and other circulating factors are also released upon acute changes in blood flow and the resulting shifts in mechanical forces as an attempt to maintain vascular homeostasis. From this study, it is believed that the ability to adapt to changes helps protect regions of the vasculature from the onset and progression of inflammatory diseases such as atherosclerosis. Other studies have shown that areas in which smooth, laminar flow and vascular homeostasis are maintained lead to a protective effect that decreases EC and VSMC death, promotes cell alignment, and stimulates increased levels of antiinflammatory and antioxidative mechanisms. The extracellular matrix (ECM) has also been shown to undergo changes at atherosclerosis- prone sites of fluctuating mechanical forces by shifting from a normal basement membrane (BM) consisting of collagen and laminin, to proteins characteristic of wounds and inflammation such as fibronectin (FN), fibrinogen (FG), and thrombospondin. Similar studies found that downstream signaling was also affected by these same changes, which then further induced altered gene and protein expression. All of these subsequent changes that occur in response to chronic fluctuations in mechanical cues illustrate the importance of cellular mechanics in relation to the onset and progression of atherosclerotic plaques.
8.5 Advances in Experimental and Imaging Techniques (BioMEMS/NEMS)
Experimental biologists often utilize analytical models by providing measured values for a set of parameters extracted over a time course from a representative study. These are subsequently input into a model to perform simulations and make analytical calculations or predictions based on the data that have been collected. Once validated, models such as these are useful tools because of their ability to identify the most significant factors influencing those changes that are characteristic of a specific disease or injury. From these simulations, parameters having the largest impact can then be targeted and further studied in future experiments to develop more rapid diagnostics and better treatment options.
As made evident by the previous examples, advanced detection and sensing devices can provide a higher level of accuracy to models by acquiring more precise values for measurable parameters. One such field that has emerged due to the increased need to extract similar measurements with improved spatiotemporal resolution from single cell studies is the micro-/nano- fabrication of BioMEMS/NEMS devices. This area has allowed for a higher degree of control and characterization to be applied to, or extracted from, a cell’s micro- environment. Cells are most commonly cultured on dishes that are not only stiffer and flatter than most cells’ native tissue, but they also lack the appropriate chemical and mechanical signals that cells experience in vivo due to these interactions with micro- environmental cues [9]. A majority of the signaling taking place at the cellular or molecular level during the early stages of disease is initiated before the larger- scale, downstream effects can be detected. In order to develop more efficient drugs, and fully comprehend many of the unknown mechanisms presented by different diseases, it is necessary to address this issue and perform studies using this level of characterization. These devices provide a better understanding of how specific interactions affect cells and cause the changes that take place in response to different stimuli by more accurately representing the local environment at the cellular level.
Some of the primary tools and techniques currently being used for acquiring this type of experimental data are in common use at various levels of characterization [4]. Examples at the genomic level include microarrays, real-time PCR, and RNA- based gene silencing. These allow for the generation of time series data, including expression profiles throughout the course of a study or the system’s response to various gene knock- outs. Gel electrophoresis and mass spectrometry (MS) are available for characterization at chosen time points on the proteomic level, while dynamic metabolic studies can also be obtained using nuclear magnetic resonance (NMR), MS, and high performance liquid chromatography (HPLC). These modern techniques provide a clear advantage in terms of the amount of data available for model construction and the ability to extract multiple parameters simultaneously from a single study.
A review of BioMEMS/NEMS illustrates how these devices are improving the depth of understanding for many different biological processes at the cellular level by better representing a cell’s native environment [9]. Some of the areas in which improvements have been made due to advances in this field include cell adhesion and traction- force measurements, microfluidics, and micromanipulation. For example, smooth muscle cells lying on an array of microneedles can provide the increased sensitivity and lower detection limit required to measure forces within an appropriate range of nanonewtons [10]. The use of an array also provides higher spatial resolution for the forces generated during the attachment or contraction of cells, lending further knowledge to the force distribution at various locations throughout the cell. In addition to the acquisition of such small forces, AFM cantilevers can be used to either apply or monitor an even broader range of nano-newton forces depending on the selection of an appropriate cantilever and the desired application. Another example utilizes microfluidics in order to simulate the mechanotransduction response of endothelial cells to varying fluid shear stresses and pressures associated with atherosclerosis, allowing for the effects of each parameter to be more closely studied at the cellular level. The development of these and other microfluidic devices have been used to mimic microcirculatory processes, study particle- cell interactions in the targeted delivery of therapeutics or nano drug carriers, and create realistic in vitro models of the microvasculature and its surrounding tissue [11]. Chemical gradients and molecular signaling can also be more precisely controlled and directed through the use of these techniques and devices. The ability to isolate and study many of these events at the single cell level has further advanced modeling capabilities for many of these mechanisms. The use of mathematical models and computational fluid dynamics (CFD) based approaches have also been developed to better interpret many of the complex flow patterns in the microvasculature [11]. BioMEMS/NEMS enable the level of control necessary to re-create and simplify many of the complex networks and reactions experienced during biological processes by isolating and reducing the uncontrollable variables involved in many tissue-level responses.
Because of the added manipulability of BioMEMS/NEMS devices, experiments are able to more closely mimic biological conditions and detect more subtle changes. Microfluidics and micro-/nanoscale fabrication has enabled researchers to better design and re-create environments like those naturally occurring within the body, with the added advantage of being able to direct and manipulate many of the variables that are much more difficult to regulate in vivo. As this field continues to develop, the level of accuracy afforded by cell- based models will also improve and provide scientists with more data and knowledge regarding many complex biological processes.
8.6 An Example: Cardiomyocyte Mechanics
To further illustrate the potential and future directions of this field, an example focused on cardiomyocyte mechanics applied to the study of ischemia and reperfusion injury is proposed in which advanced experimental/imaging techniques and mathematical modeling of single cells can be utilized, followed by a discussion on some of the advantages to this approach.
8.6.1 Experimental Setup/Design
The direct mechanical effects of interactions involving subcellular, cell– cell, and cell– substrate, and those occurring between cells and their local environment have shown their importance in mediating a variety of biological processes. One advantage that has been demonstrated thus far is the fact that these interactions can be described using representative mathematical expressions built on physical principles in order to determine the influence that each parameter has at either the single cell and/or multiple cell levels.
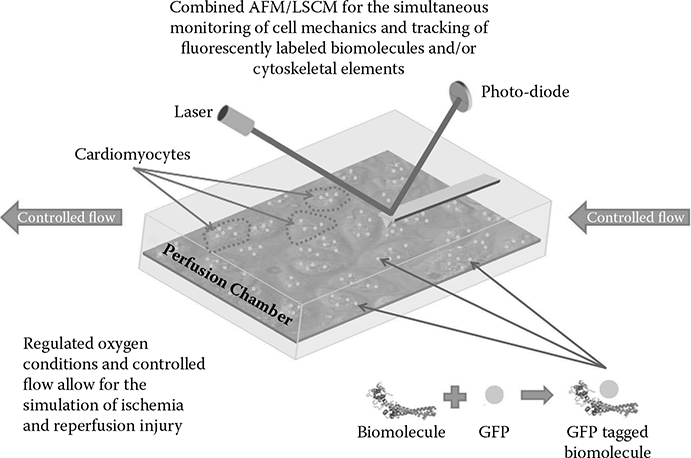
FIGURE 8.1
Experimental setup for the study of ischemia and reperfusion injury utilizing fluorescent monitoring with an LSCM and physical characterization using an AFM for nanoindentation.
In addition to those examples already discussed, the study of cardiomyocyte mechanics is of particular interest due to the unique set of dynamic properties related to their autonomous beating such as stiffness, cytoskeletal arrangement, and a variety of factors including protein or ionic concentration that can be directly related to function and physiology. Advanced imaging used in combination with BioMEMS/NEMS devices or specialized techniques such as nanoindentation can be utilized to acquire enhanced spatiotemporal resolution enabling the characterization and monitoring of events at the single cell or micro-/nanoscale level. An exemplary setup, as shown in Figure 8.1, includes a combined laser scanning confocal microscope (LSCM) for the inter/intracellular fluorescent monitoring of targeted biomolecules or the arrangement of cytoskeletal elements, along with an AFM for nanoindentation that is capable of characterizing the mechanical properties of single cells, such as elasticity or beat- related parameters including force, frequency, and amplitude in real time. The simultaneous acquisition of localized fluorescent intensity and physical properties would then facilitate time-based studies for the examination of pathophysiological events associated with disease or related injuries. Equations derived from indentation can be used to relate these physical parameters to a cell’s mechanical properties. Modeling the coordinated efforts of molecular signaling in relation to beating dynamics could provide the information necessary to uncover those mechanisms involved in the transition from normal beating, seen under representative physiological conditions, to arrhythmias or tissue damage seen during ischemic conditions and reperfusion injury. The molecular changes occurring as a result of these states, which can be quantified using techniques such as genomic, proteomic, or metabolomic profiling discussed previously, can also be integrated into a cell- based model through the application of BioMEMS/NEMS devices, further advancing the amount of data and information that can be used to characterize these events. By doing so, not only could a model such as this account for those changes directly related to the physical forces being experienced by the cell, but it could also incorporate the molecular signaling or composition of cells as they constantly undergo changes in response to these fluctuations in a feedback and feed- forward manner. This type of model could then be further expanded to larger, multiscale networks as discussed earlier by involving the interactions of multiple cells with one another and their surroundings. Using this model, one could then look at how modifying single cell or micro-/nanoscale parameter values would affect cell beating and/or its genotype/phenotype from a mechanical and/or biological perspective at a more physiologically relevant tissue level in relation to disease or injury. It is this relationship between the physical and biological features that could ultimately lead to a more in- depth understanding of how a cell functions under various conditions.
8.6.2 Model Development
The development of a cell- based model for the study of ischemia and reperfusion injury should be able to utilize the increased sensitivity and time-dependent data afforded by the proposed experimental setup. A previous model of ischemia and reperfusion was able to describe the relationship between these two conditions based on ionic concentrations of various intra- and extracellular components such as sodium, potassium, and calcium, as well as the inclusion of pH dependence and ATP levels as shown in Figure 8.2 [12]. The total allosteric regulation of the sodium– proton exchange (NHE) was calculated using the following equation,
where and represent intracellular and extracellular proton concentration, nNHE,hi and nNHE,he represent the intra- and extracellular Hill coefficients for binding protons, and the dissociation constant was shown as KNHE.
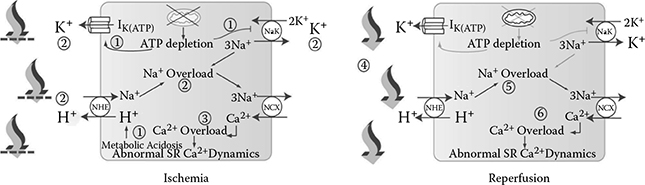
FIGURE 8.2
Models of ischemia and reperfusion as developed by B.N. Roberts and D.J. Christini [12] illustrating the effects of each condition on various ionic exchange pumps in relation to sodium, potassium, and calcium concentrations, pH, and ATP levels. During ischemia, ATP depletion leads to sodium and calcium overload along with increased anaerobic metabolism through various ionic exchange pumps, producing metabolic acidosis, lowered pH levels, and proarrhythmic behavior (1–3). Upon reperfusion, the washout of acidotic fluid reduces extracellular potassium and protons with an increased flux through ionic exchange pumps resulting in a proton gradient exacerbating intracellular sodium and calcium overloads, along with additional proarrhythmic behavior (4–6).
The function for calculating the effects of varying ionic concentrations tied to the amount of water flux in and out of the cell was as follows:
where Lp is the hydraulic conductivity of the membrane, R is the gas constant, temp is temperature, [Na +], [K +], [Ca2+], [Cl –], and [X zi] represent internal/external concentration levels of sodium, potassium, calcium, chlorine, and impermeable osmolytes, respectively, and Volmyo/ Volexternal stands for the volume of the myoplasm and external compartment. To simulate the pH changes during ischemia the following equation was used:
describing the changes seen as a function of time, where t is the ischemic time in minutes. Separate equations were also derived to describe the extracellular pH and intracellular anionic changes associated with each time step to include the effects of acidosis as pH decreases. Another marker associated with ischemia and reperfusion includes calcium channel availability with fluctuating ATP levels which was described by
representing ATP concentration with [ATP] as kATP is the k1/2 for the binding of ATP to ICa(L) channels. Finally, the sarco/endoplasmic reticulum Ca2+- ATPase (SERCA) pump cycling rate was translated to calcium flux using the following equation:
where cycSERCA is the raw cycling rate of the SERCA pump and VSERCA is the output resulting in appropriate Ca2+ transients. Through simulations, this model was able to discern one potential cause for the lack of clinical efficacy in NHE inhibition by demonstrating the lack of desired reductions in sodium and calcium overloads during the ischemia– reperfusion event, resulting in the suppression of pH recovery and the inability of the sodium– potassium exchanger to remove sodium from the cell [12]. All of the values and concentrations needed for this model could be accurately characterized and monitored with the use of fluorescence or downstream detection as shown in the specialized platform described previously. Additionally, other signaling molecules such as troponin I, which is known to be released upon myocardial damage, could be integrated into this base model for ischemia and reperfusion in order to monitor the effects that these ionic concentrations, pH changes, ATP levels, and exchange pump rates have in relation to cell damage and the time dependency for the loss of viable tissue in response to the simulated conditions of ischemia and reperfusion.
Along with the molecular and ionic fluxes being represented in association with the dynamics of cardiomyocytes, additional physical monitoring and the acquisition of mechanical properties and dynamics such as cell stiffness, beat force, beat frequency, or beat amplitude, increase the level of characterization available with an advanced platform. The dynamic properties can be acquired by collecting the deflection signal from the AFM. These properties can all be derived from this signal through the following relationship taken from Hooke’s law, F = kx, where F is the force between the tip and the sample, k is the spring constant of the cantilever, and x is the deflection of the cantilever. The real- time deflection signal using this technique is shown in Figure 8.3, allowing for frequency, force, and amplitude to be derived and constantly monitored throughout the course of a study.
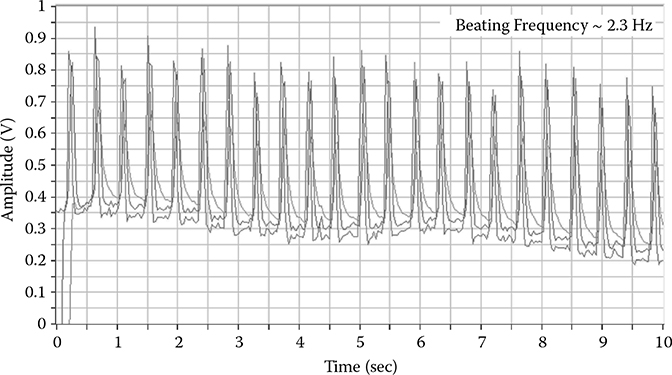
FIGURE 8.3
Deflection signal of a cantilever over a beating cell as shown in Figure 8.1.
In order to measure elasticity, the use of the Hertz theory for calculating the contact area and forces resulting from the tip geometry and cell surface interactions can be applied as previously described [13]. This theory assumes completely elastic behavior and a homogeneous sample. The following example will assume spherical tip geometry and a flat, planar sample surface but can be adapted to various geometries based upon the desired application. Upon indentation and the resulting force-versus-indentation curves, the modulus value can be derived based upon the relationships presented in the following equations:
where ks and kc are the stiffness values for the sample and cantilever, Zc is the deflection of the cantilever and δ is the indentation depth, which is the elastic deformation of the sample calculated by the addition of the deflection signal of the cantilever and the height position of the piezo, Zp, as shown below.
where D is the tip-sample separation, assuming an equilibrium once in contact where D = 0. Making substitutions, the following equation is obtained:
and keff ≈ ks when ks «kc. The stiffness of the sample is then related to Young’s modulus by
where vt, Et, vs and Es are the Poisson’s ratio and Young’s moduli of the tip and sample, respectively, Etot is the reduced modulus, and a is the tip- sample contact radius. If the tip is much stiffer than the sample and the deformation of the tip is neglected, then this equation can be approximated by
to describe the elastic deformation of the sample using the Hertz theory. The Hertz model neglects the adhesion between the tip and sample and can be used as an approximation when the adhesion force is much smaller than the maximum load. The contact portion of force-versus-indentation curves are then fit with the Hertz model to obtain the elasticity of the sample. Incorporating this information into the cell-based model, mechanical properties, and other beating parameters can be used to determine the resulting structure– function relationships along with the molecular– biochemical profiles obtained during ischemia and reperfusion during a time study. The ability of the proposed experimental platform to produce this level of characterization and monitoring could enable scientists to uncover the mechanisms responsible for these injuries or other simulated conditions. Quantification from modeling further improves upon the analysis and predictive nature of the system and allows for the study of advanced therapeutic agents or treatment strategies based upon the relationships described within the developed model.
8.6.3 Discussion
The advantages provided by using an advanced experimental platform in conjunction with mathematical modeling for single-cell studies improve upon the current abilities of each approach separately, and further surpass traditional methods in the level of characterization and quantification that can be attained. Single-cell approaches are becoming more relevant as the level of control over experimental conditions improves with the use of BioMEMS/NEMS and other advanced imaging techniques. The ability to monitor changes at the micro- and nanoscale will eventually provide clinicians and researchers with the information needed to uncover the mechanisms involved in complex biological processes. As control over these conditions improves, the computational power associated with the modeling, analysis, and control of these studies will allow scientists to more accurately describe biological functions, further demonstrating the advantages of this combinatorial approach. The current level of characterization described by this proposed setup could help determine the underlying causes of diseases and their associated injuries by combining physical, optical, and chemical techniques with mathematical modeling. While limitations from both modeling and biological perspectives still exist, combining these techniques advances the capabilities currently provided by other methods and opens up the possibility for extending single-cell characterization and monitoring to study complex biological systems and the pathophysiology of disease.
8.7 Conclusions
In conclusion, there are numerous experimental and model- based approaches to investigate the role of cellular mechanics in dictating cellular responses to various conditions associated with disease or other pathologies [14]. Although many of the physiological responses to alterations in these mechanics are known, the difficulty in identifying the underlying mechanisms stems from the inability of most techniques to capture or encompass the changes occurring at both the local (molecular/cellular) and systemic (tissue/organ) levels due to the range of the spatiotemporal domain needed to fully characterize these pathways. Advanced approaches utilizing mathematical modeling and newly developed experimental techniques offer ways to help connect the local and systemic changes associated with these pathologies while affording alternative methods for uncovering the fundamental mechanisms behind these complex biological processes.
Acknowledgment
This research was sponsored by the Office of Naval Research under award number ONR- N00014-11-1-0622. The authors are grateful for the support.
References
1. Hoffman, B.D. and J.C. Crocker, Cell mechanics: Dissecting the physical responses of cells to force, in Annual Review of Biomedical Engineering. 2009, Annual Reviews: Palo Alto. pp. 259–288.
2. Beyer, T. and M. Meyer- Hermann, Multiscale modeling of cell mechanics and tissue organization.Engineering in Medicine and Biology Magazine, IEEE, 2009. 28(2): pp. 38–45.
3. Jamali, Y., M. Azimi, and M.R.K. Mofrad, A sub- cellular viscoelastic model for cell population mechanics. PLoS ONE, 2010. 5(8): p. e12097.
4. Chou, I.C. and E.O. Voit, Recent developments in parameter estimation and structure identification of biochemical and genomic systems. Mathematical Biosciences, 2009. 219(2): pp. 57–83.
5. Schallau, K. and B.H. Junker, Simulating plant metabolic pathways with enzyme-kinetic models. Plant Physiology, 2010. 152(4): pp. 1763–1771.
6. Fan, C. et al., Building prognostic models for breast cancer patients using clinical variables and hundreds of gene expression signatures. BMC Medical Genomics, 2011. 4(1): p. 3.
7. Darling, E.M. et al., A thin- layer model for viscoelastic, stress- relaxation testing of cells using atomic force microscopy: Do cell properties reflect metastatic potential? Biophysical Journal, 2007. 92(5): pp. 1784–1791.
8. Hahn, C. and M.A. Schwartz, The role of cellular adaptation to mechanical forces in atherosclerosis. arteriosclerosis. Thrombosis, and Vascular Biology, 2008. 28(12): pp. 2101–2107.
9. Ting, L.H. and N.J. Sniadecki, 3.315—Biological microelectromechanical systems (BioMEMS) devices, in Comprehensive Biomaterials, Ducheyne, P., Editor-in-Chief, 2011, Elsevier: Oxford, pp. 257–276.
10. Tan, J.L. et al., Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proceedings of the National Academy of Sciences USA, 2003. 100(4): pp. 1484–1489.
11. Prabhakarpandian, B. et al., Microfluidic devices for modeling cell– cell and particle– cell interactions in the microvasculature. Microvascular Research, 2011. 82(3): pp. 210–220.
12. Roberts, B.N. and D.J. Christini, NHE inhibition does not improve Na<sup>+</sup> or Ca<sup>2+</sup> overload during reperfusion: Using modeling to illuminate the mechanisms underlying a therapeutic failure. PLOS Computational Biology, 2011. 7(10): p. e1002241.
13. Butt, H.-J., B. Cappella, and M. Kappl, Force measurements with the atomic force microscope: Technique, interpretation and applications. Surface Science Reports, 2005. 59(1–6): pp. 1–152.
14. Loh, O., A. Vaziri, and H.D. Espinosa, The potential of MEMS for advancing experiments and modeling in cell mechanics. Experimental Mechanics, 2009. 49(1): pp. 105–124.
