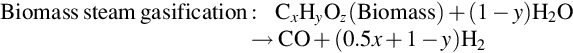Biomass gasification for synthetic liquid fuel production
H. Yang; H. Chen State Key Laboratory of Coal Combustion, Huazhong University of Science and Technology, Wuhan, PR China
Abstract
As the fourth energy resource, biomass is carbon neutral and renewable. Gasification is the most promising technology for converting biomass to high quality syngas (H2 and CO). This chapter first introduces the specialty of biomass materials, as well as the in situ status of biomass gasification with tar removal and char conversion. It then describes the novel development of biomass gasification technologies that reduce tar and produce high purity H2, such as staged gasification and CaO-based steam gasification. Simultaneously, the mathematical simulation of process thermodynamics and kinetics is summarized.
Acknowledgements
The authors wish to express their sincere thanks for the financial support provided by the National Basic Research Program (973 project: 2013CB228102) and the National Natural Science Foundation of China (51376076, 51306067, and 51306066).
11.1 Introduction
Biomass is organic material that has stored sunlight in the form of chemical energy, and this resource has the advantages of high yield, low pollution emissions, carbon neutrality, and wide availability. Biomass contains mainly carbon, hydrogen, oxygen, and traces of nitrogen and sulfur, and biomass can be converted to fuel and chemicals using the technology for converting fossil fuels. However, biomass is different from fossil fuels, such as coal, in that it has high moisture and volatile content, lower carbon content, higher oxygen content, and a lower heating value, not to mention more sodium, potassium (alkaline), and chlorine. Hence, the thermochemical conversion behavior of biomass is very different from the behavior of coal and other fossil fuels. It is therefore necessary to fully understand the properties and mechanisms of biomass conversion in detail.
Gasification is the conversion of biomass to gaseous fuel by heating the biomass in a gasification medium such as air, oxygen, steam, or their mixture. Distinguished from combustion, gasification converts the intrinsic chemical energy of the carbon in the biomass into a flammable gas. During biomass gasification, biomass feedstock is promptly heated up and devolatilized, forming tar, permanent gas, and solid char. Then the tar and solid char undergo cracking, oxidization, and reduction to form gaseous products as the final products. These products mainly consist of carbon monoxide, carbon dioxide, methane, hydrogen, water vapor, and some light hydrocarbon, known as syngas, which can be used to power gas engines and gas turbines or as a chemical feedstock to produce high rank fuels, such as liquid fuels, hydrogen, and carbon-containing chemicals.
Gasification is a complex process. It is affected by many factors, such as reactor configuration, operating conditions, gasifying agent, biomass properties, and so on. When air is used as a gasifying agent, the heating value of the gas product is very low, only 3-4 MJ/m3, while it increases to over 10 MJ/m3 with pure oxygen. Moreover, the gas product contains more H2 and hydrocarbon with a heating value of 13-20 MJ/m3 with water steam. Also, high temperature and longer residence time might be favorable for more H2.
The basic reactions occurring in the gasification process are mainly the thermal cracking of biomass, which comprises complete and partial reactions, the water gas shift reaction, and the methanation reaction. Higher temperatures are favorable for char gasification and water gas shift reaction, as more H2 and CO are formed.
In ideal gasification systems, there should be no excess tar, no nitrogen, and no methane in the gaseous product, and the gas yield should be over 80%. Although remarkable progress has been achieved in recent years in gasification technology, low gas productivity and high tar content in the gas are still two bottlenecks that have blocked the wider utilization of biomass gasification. The main problem might be attributed to low carbon conversion and high tar content. It has been noted that increasing char conversion improves efficiency, while increasing tar conversion improves gas utilization.
Removal of tar has been one of the most important technical subjects in the development of biomass gasification. Tar is formed during the biomass pyrolysis process, and it experiences cracking, condensing, and reformation. The tar is changed from mixed oxygenates to larger polycyclic aromatic hydrocarbon (PAH) dominated with temperature increasing from 400 °C to more than 900 °C. Higher temperatures are favorable for tar cracking, but the efficiency is limited. Catalytic cracking is the prevalent and efficient choice for tar cracking. Three distinct groups of catalyst materials have been the subjects of published research on biomass gasification. They are dolomite catalysts, alkali metal and alkali earth metal catalysts, and nickel catalysts. The nickel-based catalysts showed excellent catalytic effects on tar cracking, especially for H2-enriched gas.
Currently, many novel technologies have been invented to reduce tar and upgrade syngas quality, and biomass-staged gasification is one of the most promising. During staged gasification, biomass was pyrolyzed at 500 °C to organic vapor and solid char; after pyrolization, the volatile material was catalytically reformed at 800-1000 °C, while the char was combusted to provide heat for pyrolysis. As organic compounds contain more oxygen, which is easily cracked, the tar content in syngas is quite low. Because of the special properties of biomass char, which is characterized by high porosity and rich alkali content, this char was also can be used as a catalyst, and organic vapor reformed through the char bed was combined with char water shifting during steam gasification. It has been shown that char is a very efficient catalyst. Recently, a three-staged fluidized gasifier showed cold gasification efficiency of ~ 82% and carbon conversion at 97%.
As a result of the high purity of H2, the sorption-enhanced steam gasification of biomass is a novel one-step conversion technology that is being developed. CO2 sorbents (CaO, etc.) are introduced into the process of biomass steam gasification to continuously remove the CO2 in situ as soon as it is formed during the gasification process. The Ca-Ni complex attracted great interest because of the high CO2 capture and tar-cracking feature that resulted in a higher purity of H2. It has been pointed out that 80% (vol.) H2 can be derived in syngas. Also, some metal oxides were involved with chemical looping biomass steam gasification.
Mathematical and computational modeling is used to easily illustrate the expected results at low financial cost, and such modeling has support a wide range of investigations related to biomass gasification. Mathematical modeling can be categorized into three sections: equilibrium, kinetic, and neural networks, each of which might play a critical role in the development of biomass gasification.
Biomass gasification has shown unique advantages; however, with syngas, there are still some challenges, such as particulates, hydrocarbons, and alkali compounds in gas products.
11.2 Properties of biomass resources
11.2.1 Background
The world’s current energy requirements are largely met by fossil fuels, such as oil, coal, and natural gas, which are estimated to account for 80% of the world’s energy consumption (Fernando, Adhikari, Chandrapal, & Murali, 2006; Xiao, Meng, Le, & Takarada, 2011). The increase in fuel costs, limited fuel sources, and environmental problems, such as global warming and acid rain, are all problems caused by the usage of fossil fuels. These crises have prompted mankind to look for renewable energy in order to meet the increasing energy demand. Among renewable energy resources, biomass is the only one that can produce not only heat and electricity, but also fuels.
Biomass-based energy accounted for roughly 10% of world’s total primary energy supply in 2009. Most of this biomass energy is consumed in developing countries for cooking and heating via very inefficient open fires or simple cook stoves with considerable impact on health (smoke pollution) and the environment (deforestation). Modern bioenergy supply, on the other hand, is comparably small, but has been growing steadily in the last decade. A total of 280 TWh of bioenergy electricity, or 1.5% of the world’s electricity generation, was produced globally in 2010, and 8 EJ of bioenergy for heat were used in the industrial sector (Xiao et al., 2011).
11.2.2 Origins of biomass resources
Biomass is plant material derived through photosynthesis, a set of reactions in through which CO2 in the air, water, and sunlight produce the carbohydrates that form the building blocks of biomass.
The solar energy that drives photosynthesis is stored in the chemical bonds of the structural components of biomass (Peter, 2002), and biomass is the term used to describe all biologically produced matter. Biomass resources include wood and wood wastes, agricultural crops and their waste by-products, municipal solid waste (MSW), animal wastes, waste from food processing, and aquatic plants and algae. On average, the majority of biomass energy is produced from wood and wood waste (64%), followed by MSW (24%), agricultural waste (5%), and landfill gases (5%) (Demirbas, 2000).
11.2.3 Properties of biomass materials
Biomass can be used for fuels, power production, and products that would otherwise be made from fossil fuels, and it can provide an array of benefits. The use of biomass energy has the potential to greatly reduce greenhouse gas emissions. Biomass releases carbon dioxide that is largely balanced by the carbon dioxide captured in during its formation. Burning biomass produces 90% less sulfur than burning coal, and the use of biomass can reduce dependence on foreign oil because biofuels are the only renewable fuel that can be transported as a liquid (Demirbas, 2001). However, when compared to coal, biomass also has several shortcomings.
Table 11.1 shows the comparison of fixed carbon content, volatile matter, moisture content, heating value, and bulk density between coal and biomass. As can be seen, coal has a higher bulk density, higher heating value, and lower moisture content. Compared to pulverized coal fuels, biomass is generally more volatile, has more moisture, and has a lower heating value than coal. In general, biomass energy densities are approximately one-tenth of fossil fuels, such as petroleum or high-quality coal. In coal, the ratio of volatiles to fixed carbon content is low, always less than one. In biomass, however, this ratio is as high as four. This volatile-to-fixed-carbon ratio describes how easily a fuel is volatilized and affects the subsequent system and products. The high volatility is considered an advantage for biomass and allows the fuel to burn at a high power output (Demirbas, 2004).
Table 11.1
Proximate and ultimate analysis of biomass resources
| Samples | Proximate analysis/wt.% | Ultimate analysis/wt.% | |||||||
| Mad | Vad | Aad | FCad | C | H | N | S | Oa | |
| Cotton stalk | 5.10 | 72.98 | 3.09 | 16.73 | 45.22 | 6.34 | 1.15 | 0.34 | 46.94 |
| Corn stalk | 5.02 | 70.17 | 8.25 | 16.56 | 42.68 | 6.21 | 1.22 | 0.32 | 49.57 |
| Rape straw | 5.49 | 74.32 | 6.27 | 13.93 | 44.87 | 6.60 | 0.82 | 0.20 | 47.51 |
| Wheat straw | 4.38 | 68.52 | 12.91 | 14.20 | 40.36 | 5.95 | 0.55 | 0.27 | 52.87 |
| Rice straw | 5.04 | 82.12 | 7.74 | 5.10 | 37.52 | 5.92 | 0.86 | 0.14 | 42.78 |
| Tobacco stem | 3.64 | 68.52 | 21.7 | 6.14 | 36.10 | 4.85 | 2.64 | 0.77 | 55.63 |
| Pine | 15.30 | 70.40 | 0.20 | 14.19 | 51.01 | 6.00 | 0.10 | 0.02 | 42.90 |
| Poplar | 6.80 | 79.70 | 1.30 | 12.20 | 41.39 | 5.27 | 0.25 | 0.27 | 39.13 |
| Bamboo | 4.60 | 72.83 | 0.73 | 21.70 | 48.37 | 6.11 | 0.27 | 0.08 | 45.17 |
| Rice husk | 6.33 | 60.35 | 16.75 | 16.57 | 48.61 | 5.45 | 0.45 | 0.13 | 55.36 |
| Peanut shell | 9.13 | 56.62 | 1.52 | 31.86 | 60.53 | 7.12 | 1.92 | 0.35 | 30.08 |
| Coal | 2.29 | 30.65 | 28.07 | 36.84 | 56.72 | 2.76 | 1.05 | 0.53 | 2.00 |

ad, based on air dried basis; a, determined by difference.
The difference between the O/C and H/C ratios of solid fuels can be illustrated using a Van Krevelen diagram, as shown in Figure 11.1. The composition of the ash-free organic components of biomass is relatively uniform. The major components are carbon, oxygen, and hydrogen. Most biomass also contains a small proportion of nitrogen. A comparison of biomass with coal shows clearly that there are higher proportions of oxygen and hydrogen in biomass. Consequently, the higher oxygen and hydrogen content reduce the energy value of biomass as a fuel, due to the lower energy contained in carbon–oxygen and carbon–hydrogen bonds, than in carbon–carbon bonds.

Research shows that the mineral composition of coal and biomass has a strong impact on processing, application, and environmental and technological concerns related to these fuels. For biomass, variability in mineral content among plants can be considerable, as it depends on genetic and environmental factors, as well as physiological and morphological differences between crops. Table 11.2 presents the ash compositions of typical biomass and coal samples. It clearly shows that the ash compositions of biomass and coal are different. Biomass ash is mainly composed of K, Na, Mg, Al, Ca, and P, in the form of oxides, silicates, and chlorides, while coal ash consists mainly of Al and Si in the form of oxides. The ash from biomass also contains high alkali metal content, which can lead to corrosion in the gasifier instruments and downstream setup.
Table 11.2
Ash composition of typical biomass
| Samples | Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | K2O | CaO | Fe2O3 | Cl |
| Corn stalk | 0.68 | 3.55 | 1.75 | 40.97 | 5.81 | 3.74 | 25.14 | 6.48 | 0.59 | 11.15 |
| Wheat straw | 1.13 | 0.96 | 1.51 | 53.76 | 2.75 | 3.71 | 21.33 | 4.20 | 0.59 | 10.09 |
| Rice straw | 0.96 | 2.33 | 0.91 | 51.99 | 2.49 | 6.50 | 17.81 | 7.68 | 0.84 | 7.09 |
| Poplar | 0.74 | 4.14 | 6.85 | 26.83 | 7.03 | 5.52 | 8.21 | 34.8 | 3.76 | 1.49 |
| Cotton stalk | 2.43 | 6.40 | 5.82 | 18.21 | 7.14 | 9.45 | 17.07 | 26.09 | 3.8 | 2.76 |
| Rape straw | 1.06 | 0.38 | 0.21 | 4.05 | 2.69 | 21.23 | 35.40 | 25.70 | 0.71 | 8.22 |
| Tobacco stem | 0.38 | 5.88 | - | 0.12 | 4.05 | 7.96 | 21.20 | 31.36 | 0.08 | 28.16 |
| Pine | 12.84 | 5.56 | 6.50 | 16.47 | 2.42 | 7.64 | 7.76 | 24.89 | 4.57 | 8.77 |
| Bamboo | - | 4.48 | - | 19.22 | 6.36 | 8.18 | 49.22 | 6.02 | 3.13 | 1.06 |
| Rice husk | - | 0.84 | 1.06 | 87.47 | 0.81 | 1.30 | 3.02 | 1.64 | 2.38 | 0.52 |
| Peanut shell | 0.20 | 4.76 | 8.21 | 23.11 | 8.20 | 10.63 | 25.69 | 11.07 | 6.07 | 1.09 |
| Coal | - | - | 29.7 | 50.4 | 1.1 | - | 3.6 | 1.9 | 7.9 | - |

11.3 Biomass gasification
Biomass gasification is an important thermal chemical process that converts any carbonaceous biomass to gaseous products. Compared with traditional coal gasification, biomass gasification takes place at a lower temperature (~ 900 °C) due to the essential nature of biomass. The high content of volatiles and some intrinsic catalytic metals (like potassium, calcium) in biomass also tend to increase its reactivity. Additionally, biomass makes no contribution to net green house gas emissions, and its low sulfur and nitrogen contents make it a greener and cleaner option to fossil fuels.
The product of gasification, syngas, mainly contains H2, CO, CO2, CH4, and some C2 + hydrocarbons. Different uses of syngas show the flexibility of biomass gasification and thus allow it to be integrated with various industrial routes, such as gas engines for power generation, Fischer-Tropsch (FT) Synthesis for DME, methanol, carbon-containing chemicals, methane, substitute gas, H2, and gas fuels (Delgado, Aznar, & Corella, 1997).
Biomass gasification involves a complex series of chemical reactions, as shown in Figure 11.2. A good understanding of the basic biomass gasification reactions is fundamental to the planning, design, operation, troubleshooting, and process improvement of a gasification plant. In a typical gasification process, the following stages are usually take place: drying, pyrolysis, char and tar gasification. The detailed reactions that occur during gasification are summarized in Table 11.3.
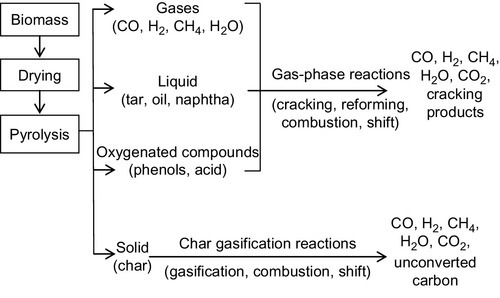
Table 11.3
Main chemical reactions of biomass gasification
| Reaction | ΔH298, kJ mol− 1 | Number |
| Pyrolysis | ||
| Biomass → char + tar + H2O + light gas(CO + H2 + CO2 + CH4 + C2 + …) | Endothermic | R1 |
| Char combustion | ||
| C + 0.5O2 → CO | − 111 | R2 |
| C + O2 → CO2 | − 394 | R3 |
| Char gasification | ||
| C + CO2 → 2CO | 172 | R4 |
| C + H2O → CO + H2 | 131 | R5 |
| C + 2H2 → CH4 | − 75 | R6 |
| Homogeneous volatile oxidation | ||
| CO + 0.5O2 → CO2 | − 254 | R7 |
| H2 + 0.5O2 → H2O | − 242 | R8 |
| CH4 + 2O2 → CO2 + 2H2O | − 283 | R9 |
| CO + H2O → CO2 + H2 | − 41 | R10 |
| CO + 3H2 → CH4 + H2O | − 88 | R11 |
| Tar reactions | ||
| CnHm + (n/2)O2 → nCO + (m/2)H2 | Endothermic | R12 |
| CnHm + nH2O → nCO + (m/2 + n)H2 | R13 | |
| CnHm → (m/4)CH4 + (n − m/4)C | R14 | |
| CnHm + (2n − m)H2 → nCH4 | R15 | |

Source: Zhang et al. (2010).
Biomass materials are preheated and dried at 100-200 °C, before undergoing the pyrolysis stage. As the initial stage of gasification, pyrolysis partially removes carbon from the feed but does not add hydrogen. It takes place at relatively low temperatures in the range of 200-700 °C, without the use of a gasifying agent. During pyrolysis, a portion of biomass is transformed into condensable hydrocarbon tars, gases, and solid char (R1). Thereafter, a series of reactions occur in the gasifier, including a homogeneous gas-phase reaction and a heterogeneous gas-solid char gasification reaction shown as reactions (R2-R14). Char experiences partial (R2) and complete combustion (R3), as well as water gas reaction (R5) and hydrogasification (R6), which involves adding hydrogen to carbon to produce fuel with a higher hydrogen-to-carbon (H/C) ratio. Among all the reactions, R3 releases the most energy. In gas phase gasification reactions, volatiles undergo oxidation (R7-R9), steam reforming (R13), and cracking (R14). The water-gas shifting (WGS) reaction (R10) is of great importance because it plays a significant role in generating hydrogen (Matsumura et al., 2005). The methanation reaction (R11) always proceeds in the absence of any catalyst. Both R10 and R11 can proceed in either direction, depending on the specific temperature, pressure, and concentration of the reacting species. Above all, it can be seen that the product gas from gasification is a mixture mainly consists of H2, CO2, CO, CH4 and water vapor.
11.4 Biomass gasification properties
11.4.1 Influence of feedstock characteristics
11.4.1.1 Biomass type
Different biomass with different physical and chemical characteristics, such as particle size and moisture content, may affect the gasification behavior.
Van Der Drift, Van Doorn, and Vermeulen (2001) investigated gasification behaviors of ten biomass feedstocks in circulating fluidized bed (CFB) with air at 850 °C, as shown in Figure 11.3. It was found that the main combustible gases are CO (10%) and H2 (~ 8%), with trace amounts of methane and ethane (3-4 vol.%). The higher heat value (HHV) of the gas product was quite low, around 5MJ/m3. However, gasification of different biomass samples resulted in variant fuel gas properties. The HHV of gas fuel decreased significantly with the increase of water ash content in the biomass samples. For samples 7 and 8, the results might be attributed to some volatiles being removed by biocomposition, and because the ash and water content was very high.

Herguido, Corella, and Gonzalez-Saiz (1992) investigated the steam gasification behavior of four different biomass types. They found that the gas yield that resulted from sawdust and straw gasification was much higher than that from chips and thistles. The higher yield might be attributed to the higher volatile content in sawdust and straw. However, they also pointed out that the different sizes and shapes of the particles of each biomass is also a concern. As char from the sawdust particles showed much larger porosity and smaller particle diameter, the gasification reactivity of the solid sawdust char is much higher. In regard to the gas distribution, a clear difference based on the different biomass used. Sawdust showed higher H2, while straw produced higher CO content, but the lowest H2 yield. However, the variation decreased with the increase in gasification temperature, as shown in Figure 11.4.

Gani and Naruse (2007) analyzed the effect of cellulose and lignin content on biomass pyrolysis and combustion. For the biomass with higher cellulose content, the pyrolysis rate became faster, while the biomass feedstock with higher lignin content gave a slower pyrolysis rate. Thus, the cellulose and lignin content in the biomass were two important parameters used to evaluate the pyrolysis characteristics. Lv et al. (2010) found that gasification activity was considerably influenced by the content of cellulose and lignin in biomass.
Fushimi and Tsutsumi (2012) studied the gasification of cellulose and lignin and found that cellulose is easy to convert with higher reactivity, while lignin is quite difficult to convert at lower temperature. Wu, Wang, Huang, and Williams (2013) found that cellulose produces the highest amount of hydrogen, 5.8 mmol H2 g−1 sample during gasification in the absence of steam and catalyst, while lignin produced only a 1.8 mmol H2 g− 1 sample with more CH4 being formed. Also, cellulose pyrolysis/gasification produced the highest CO concentration (44.4 vol.%), but the highest CO2 concentration (27.3 vol.%) was observed for hemicelluloses (Wu, Wang, Huang, & Williams, 2013). More tar was also found to result from hemicellulose gasification, indicating that hemicelluloses might not produce high quality syngas (Fushimi & Tsutsumi, 2012).
11.4.1.2 Particle size
As mentioned above, particle size shows great effects on the gasification operation and the product gas composition. Lv et al. (2004) selected four size ranges of biomass particles to be used in a fluidized bed gasifier (Table 11.4). They found that fine particles are favorable for gas production with higher heat values and carbon conversion efficiency of biomass. As gas yield and composition are related to the heating rate of the biomass particles, high heating rates produce more light gases and less char and condensate. Smaller particles have larger surface area, and as a result, they also have a faster heating rate.
Table 11.4
Influence of particle size on the properties of biomass gasification (Lv et al., 2004)
| Biomass particle size (mm) | 0.6-0.9 | 0.45-0.6 | 0.3-0.45 | 0.2-0.3 |
| Average size (mm) | 0.75 | 0.53 | 0.38 | 0.25 |
| Gas yield (Nm3/kg biomass) | 1.53 | 1.93 | 2.37 | 2.57 |
| Gas LHV (kJ/Nm3) | 6976 | 7937 | 8708 | 8737 |
| Carbon conversion efficiency (%) | 77.62 | 84.4 | 90.60 | 95.10 |
| Steam decomposition (SD) (%) | 32.34 | 42.55 | 52.67 | 56.45 |

Source: Lv et al. (2004).
However, size control is expensive and energy intensive, so obtaining the optimal biomass particle requires a trade off. Fine and irregular-shaped feed particles may impede gas flow through the bed and result in increasing carbon conversion and a large pressure drop leading to irregular axial temperature profiles and “rat holes” or channeling of the pyrolysis and combustion zones (Cummer & Brown, 2002). On the contrary, fuel reactivity, such as slow gasifier startups and poor gas quality, may be a problem with excessively large particles. Due to the high degree of turbulence and good heat-transfer characteristics, fluidized bed gasifiers tend to be more forgiving to smaller-sized fuel particles.
11.4.1.3 Moisture content
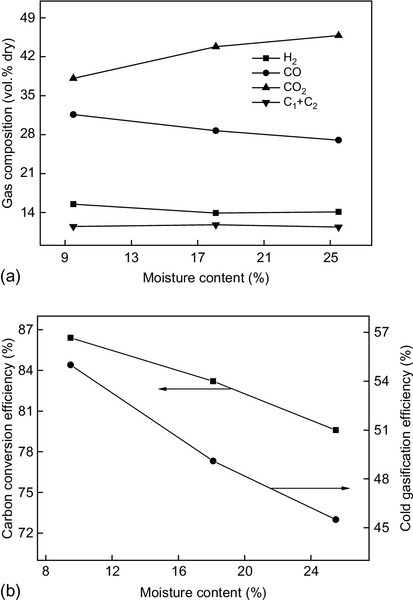
The high moisture content of feedstock has shown a negative influence on the thermal process efficiency and is usually the most energy-intensive part of the gasification process. High-moisture fuels will result in more tar formation and low gasification temperatures. As seen in Figure 11.5, a decrease in moisture content from 25.5% to 9.5% can result in increases of 8.5% for CCE and 20.8% for CGE. In high moisture conditions, the gas also tended to have lower H2 and CO contents and a higher CO2 content (Kaewluan & Pipatmanomai, 2011). The influences of the moisture content of biomass on tar species are available in detail in other literature (Ahrenfeldt, Egsgaard, Stelte, Thomsen, & Henriksen, 2012). Processing wet fuels can also bring about erratic gasifier operation, longer startup times, and higher energy consumption. Hence, air-drying or some other form of pretreatment, such as torrefaction, should be an efficient way to remove excess moisture.
11.4.2 Gasification parameters
There is a series of parameters that are crucial for the efficiency of gasification, and thus the optimum values of these factors should be maintained to ensure a constant quality with high process performance.
11.4.2.1 Gasification temperature
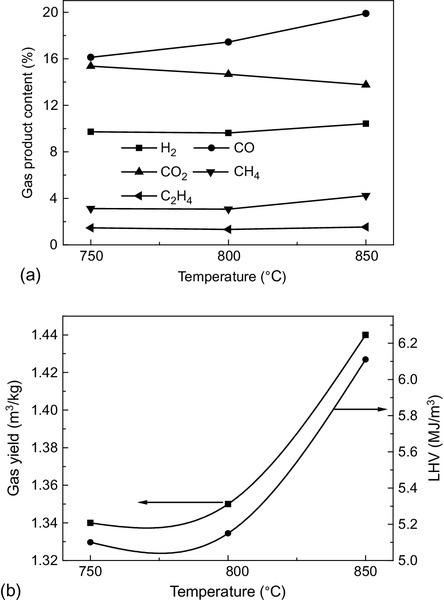
As it controls the cracking and conversion of biomass, temperature plays a vital role in gasification. Figure 11.6 provides the gasification property under different temperatures during sawdust gasification (Chen, Li, Yang, Yang, & Zhang, 2008). A higher operating temperature (> 800 °C) is always favorable for higher hydrogen and lower tar content yield (decrease from 13.2 to 6.5 g/m3) in the product gas. Meanwhile, temperature not only influences the amount of tar, but also the tar composition by changing the chemical reactions during gasification (Devi, Ptasinski, & Janssen, 2003; Meng, De Jong, Fu, & Verkooijen, 2011; Mayerhofer et al., 2012). The effects of temperature on tar are shown in detail in the section on tar. With the rise of temperature, the carbon conversion rate increases, as does the thermal efficiency, but the rising temperature may cause more severe fouling and slagging problems when the biomass has higher contents of K, Cl, and other inorganic materials. Therefore, the optimal temperature is based on the conversion and application condition.
11.4.2.2 Gasifying agent
The gasifying agent, which is normally a gas such as oxygen, air, subcritical water, carbon dioxide, or their mixtures, or supercritical water, is an indispensable medium for biomass gasification. The selectivity of the gasification reactions varies with different gasifying agents, thus affecting the composition and LHV of produced gas (Devi et al., 2003). Table 11.5 shows the gas product properties when the gasification process involves different agents (Gil, Corella, Aznar, & Caballero, 1999). A steam medium is preferred if high hydrogen content and higher heating value are required for syngas utilization, especially in a small-scale operation unit. But steam has its drawbacks, as it results in high tar content (60-95 g/kg compared with 3.7-61.9 g/kg produced by air), so downstream purification is needed. When using the medium of air or oxygen, most of the heat used to drive the reaction is generated by partial oxidation and exothermic combustion reactions inside the gasifier. Air has gained popularity as the most practical gasifying agent in biomass power plants due to its low cost and availability. However, the LHV of the product gas is quite low with air gasification due to the dilution of nitrogen. Other research has shown how the properties of the product gas can vary with the specific content of the gasifying agent, the ER (Narvaez, Orio, Aznar, & Corella, 1996), the S/B ratio (Franco, Pinto, Gulyurtlu, & Cabrita, 2003; Pinto et al., 2003), and the gasifying agent ratio(GR) (Aznar et al., 1997; Pinto et al., 2003).
Table 11.5
Gas product properties with variant gasifying agents (Gil et al., 1999)
| Air | (Pure) Steam | Steam-O2 mixtures | |
| Operating conditions | |||
| ER | 0.18-0.45 | 0 | 0.24-0.51 |
| S/B (kg/kg daf) | 0.08-0.66 | 0.53-1.10 | 0.48-1.11 |
| T (°C) | 780-830 | 750-780 | 785-830 |
| Gas composition | |||
| H2 (vol.%, dry basis) | 5.0-16.3 | 38-56 | 13.8-31.7 |
| CO (vol.%, dry basis) | 9.9-22.4 | 17-32 | 42.5-52.0 |
| CO2 (vol.%, dry basis) | 9.0-19.4 | 13-17 | 14.4-36.3 |
| CH4 (vol.%, dry basis) | 2.2-6.2 | 7-12 | 6.0-7.5 |
| C2Hn (vol.%, dry basis) | 0.2-3.3 | 2.1-2.3 | 2.5-3.6 |
| N2 (vol.%, dry basis) | 41.6-61.6 | 0 | 0 |
| Steam (vol.%, wet basis) | 11-34 | 52-60 | 38-61 |
| Yields | |||
| Tars (g/kg daf) | 3.7-61.9 | 60-95 | 2.2-46 |
| Char (g/kg daf) | naa | 95-110 | 5-20 |
| Gas (Nm3/kg daf) | 1.25-2.45 | 1.3-1.6 | 0.86-1.14 |
| LHV (MJ/Nm3) | 3.7-8.4 | 12.2-13.8 | 10.3-13.5 |
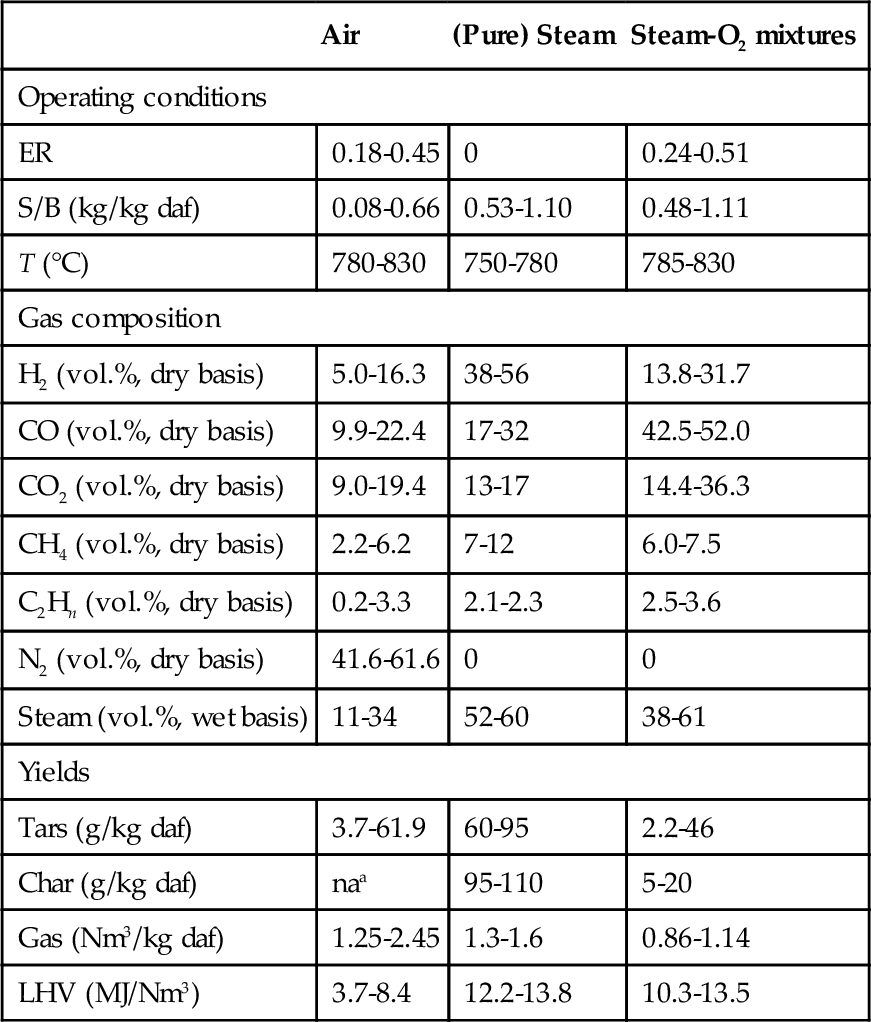
Source: Gil et al. (1999).
na, not available.
11.4.2.3 Gasification pressure
Pressure also influences gasification behavior. As shown in Figure 11.7, Mayerhofer investigated the effects of pressure on gas composition and tar content (Mayerhofer et al., 2012). The enhancement of WGS reactions under pressurized conditions makes the gas composition shift to higher CH4 and CO2 content, while CO decreases. Figure 11.7b indicates that an increase in total tar content was observed when pressure increased from 0.1 to 0.25 MPa. But an opposite trend in tar content has also been reported (Wolfesberger, Aigner, & Hofbauer, 2009). Furthermore, the syngas produced at high pressures is favorable for downstream high-pressure units, such as turbines and FT synthesis. However, high-pressure gasification seems uneconomical when extra equipment is needed to ensure the stability of the over-pressurized gasification conditions. Other operational conditions, such as the bed material in fluidized beds (Meng et al., 2011) and the biomass feeding rate (Lv et al., 2007), influence gas distribution and tar formation as well.

11.5 The biomass gasifier
A gasifier is the device in which biomass gasification takes place. Hundreds of different gasifier models can be categorized into three types, as shown in Figure 11.8: updraft, downdraft, and fluidized beds. All of these types have the same four reaction zones: drying, pyrolysis, combustion, and reduction. However, the zones are distributed differently in each type.

In a typical updraft gasifier (Figure 11.8a), the preheated gasifying agent enters the reactor from the bottom and flows upward, and the producer gas leaves from the top of the reactor where incoming biomass is added. This type of gasifier is more forgiving with respect to fuel moisture, as the heat transfer is enhanced with the counter flow arrangement. The disadvantage of the updraft gasifier is the high tar yield because the tar formed during pyrolysis is partly taken away by producer gas.
In a downdraft gasifier (Figure 11.8b), the reaction zones differ from those of updraft gasifiers. Compared with the updraft gasifier, some large molecular tars can be decomposed by thermal cracking in the downdraft type, leaving a clean gaseous product with less concentrations of tar and thus benefitting the downstream equipment. For this reason, the downdraft gasifier has the widest applications, especially for small-scale engines and heating supply.
In a fluidized bed gasifier, oxygen or steam enters at the bottom of the reactor, carrying biomass, which has been reduced to a fine particle size, upward through a bed of heated silica particles. The biomass is decomposed in the hot bed, forming char and gaseous product. Fluidized bed gasifiers can be further classified into bubbling fluidized bed and circulating fluidized bed (Figure 11.8c). Fluidized beds typically operate in the temperature range of 800-1000 °C, which avoids the ash agglomeration and sintering, allowing the safe operation of fuel with high ash content. Additionally, the large thermal inertia and vigorous mixing benefit the flexibility of various biomass feed rates and compositions.
The advantages and disadvantages of different types of gasifiers are summarized in Table 11.6. The gasifier plays a vital role in a gasification plant, and it is responsible for keeping syngas production as steady as possible. The selection of the gasifier type will depend on feedstock properties, the reaction conditions, the desired end use, and the quantity of the producer gas required.
Table 11.6
Properties of biomass gasification reactor types
| Advantages | Disadvantages |
| Fixed/moving bed, updraft | |
| Simple and reliable design | Large tar production |
| High carbon conversion efficiency | Potential channeling, bridging, and clinkering |
| Low dust levels in gas | Small feed size |
| High thermal efficiency | Low-output |
| Fixed/moving bed, downdraft | |
| Simple, inexpensive process | Minimum feed size |
| Low tar content in product gas | Limited ash content allowable in feed |
| Limits to scale up capacity | |
| Potential for bridging and clinkering | |
| Fluidized bed | |
| Short residence time | Low char conversion rate |
| High ash fuels acceptable | The efficiency is not high |
| Excellent heat and mass exchange | High product gas temperature |
| Flexible feed rate and composition | High tar and fines content in gas |
| Uniform temperature distribution in gasifier | Possibility of high C content in fly ash |
| High CH4 in product gas | Complicated operation |
| High volumetric capacity | |
| Able to pressurize | |

Source: Arena (2012), Knoef and Ahrenfeldt (2005), Pan et al., (1999), Sridhar et al. (2001), Wang et al. (2008), and Zhang et al. (2013).
11.6 The formation and cracking of tar
One of the main barriers for the application of biomass gasification is the presence of tar in the gas product, which may cause severe problems for downstream equipment. Tar has been widely defined in the gasification literature. Li and Suzuki (2009) considered “tars” to be condensable fractions of the organic gasification products that largely consisted of aromatic hydrocarbons, including benzene. Devi et al. (2003) describe tar as a complex mixture of condensable hydrocarbons, such as single to multiple ring aromatic compounds, other oxygen-containing hydrocarbons, and complex PAHs. The formation and cracking of tar is critical in biomass gasification in order to produce high quality gas fuel.
11.6.1 Formation mechanism of tar
The characteristics of tar mainly depend on the composition of the tar, particularly the tar’s heavy compound content. The components of tar are very complex, and more than 200 kinds can be detected in a single sample. This diversity of components is due to the fact that tar is formed from volatiles during biomass pyrolysis, and the composition of volatiles is dependent on temperature. The main composition of liquid tar obtained from variable temperatures is shown in Figure 11.9. When the temperature is below 550 °C, the volatiles are formed from the direct degradation of cellulose and hemicellulose. Therefore, most of the resulting tar is formed by low-molecular-weight and oxygen-containing compounds, such as acids, esters, ketones, furans, cyclopentene, guaiacols, and phenols. This type of tar easily undergoes further reforming. With the temperature increasing up to 650 °C, complex phenols replace these low-molecular-weight and oxygen-containing compounds (Hernández, Ballesteros, & Aranda, 2013; Zheng, Zhu, Guo, & Zhu, 2006). However, during the secondary reaction of the volatiles, the elimination reactions of the oxygen-containing functional groups produce some aromatic compounds containing benzenes, naphthalenes, biphenyls, benzofurans, and benzaldehyde. Above 650 °C, the molecular weight and the number of aromatic rings within the tar components increase significantly because of the generation of a large number of PAHs. Although complex phenols are observed in the tar obtained at 750 °C, the weight content of complex phenols is rather lower, and the branched structures become uncomplicated. As the temperature rises to 950 °C, the dehydrogenation condensation reaction generates some PAHs with more than three aromatic rings, such as acenaphthylenes, benzopyrenes, fluoranthen, and pyrene, which are the precursors of the particulate matter called “soot” (Chen, Yang, Wang, Zhang, & Chen, 2012; Qin, Feng, & Li, 2010), The thermal stability of such tar is very high, so it is difficult to crack and remove.

11.6.2 Tar cracking
Tar removal is seen as one of the greatest technical challenges to overcome for the successful development of advanced, commercially viable gasification technologies (Minlne & Evans, 1998; Li & Suzuki, 2009). Tar cracking technology can be divided into two basic methods: thermal cracking and catalytic cracking. However, the former method is not considered to be a feasible option, as it requires temperatures higher than 1100 °C to achieve high cleaning efficiency, and it also produces soot (Aznar, Corella, Delgado, & Lahoz, 1993).
In comparison with high temperature thermal cracking, catalytic cracking is highly efficient. Catalysts used in biomass conversion can be divided into three distinct groups. They are dolomite catalysts, alkali metal and alkali earth metal catalysts, and nickel-based catalysts.
11.6.2.1 Dolomite
Dolomite is a magnesium ore with the general formula MgCO3·CaCO3. The use of dolomite as a catalyst in biomass gasification has attracted much attention (Xu, Donald, Byambajav, & Ohtsuka, 2010). The chemical composition of dolomite varies from source to source, but it generally contains 30 wt.% CaO, 21 wt.% MgO, and 45 wt.% CO2. Dolomite also contains the trace minerals SiO2, Fe2O3, and Al2O3. Orío, Corella, and Narváez (1997) investigated four different dolomites from different places for oxygen-steam gasification of wood in a downstream catalytic reactor. They found that the catalytic activity was different.
Delgado et al. (1997) investigated the use of Norte dolomite and compared it with calcite (CaO) and magnesite (MgO) for the steam reforming of biomass tars. They investigated the effects of temperature, contact time, and the particle diameter of the catalysts and reported that tar conversion increased with the temperature of the catalyst bed, and complete elimination was observed at 840 °C. Vassilatos, Taralas, Sjöström, and Björnbom (1992) also studied the effect of temperature, catalyst contact time, and steam-carbon ratio. They found that higher temperatures resulted in an increase in the gas yield. An increase in the gas-catalyst contact time led to an increase in the destruction of tar present in the gas, with a maximum being reached at 0.3 kg h/Nm3. Increasing contact time produced more H2 and CO due to tar conversion reactions and the water-gas shift reaction.
Chen et al. (2008) compared the catalytic properties of dolomite, olivine, and magnesite in fluidized bed gasifier. They found that catalyst addition showed great catalytic effect on biomass gasification, and the release of light gas products (H2, CH4, and CO) was enhanced greatly. However, in the Chen study, the biomass samples showed variant adoptability. Tar removal efficiency varied from 48.1% to 70.5%, while sawdust gasification showed the highest tar removing efficiency with the addition of dolomite.
Dolomite is a cheap, disposable catalyst that can significantly reduce the tar content of the product gas from a gasifier. It may be used as a primary catalyst, dry-mixed with biomass, or, more commonly, placed in a downstream reactor, in which case it is often referred to as a guard bed.
11.6.2.2 Alkali metal and alkaline-earth metals catalysis
Much research has also considered the use of alkali metal catalysts for the elimination of tar and the upgrading of the product gas. These catalysts are often added directly to the biomass by dry-mixing or wet-impregnation. When added in this way, the catalyst is difficult to recover, and, as a result, this form of catalyzing tar cracking is not always cost effective for the gasification process. It also leads to an increase in the ash content remaining after char gasification, and the disposal of this ash is predicted to become a problem for the technology over the coming years.
In a study of the catalytic properties of potassium (K) on char gasification with potassium-loaded woody biomass, Sueyasu et al. (2012) found that the catalysis of K reduced the heavy tar content to 20 mg/m3 N, and the concentration of hydrogen in the product gas exceeded 50 vol.% dry. Mudge, Baker, Mitchell, and Brown (1985) studied the catalytic steam gasification of wood using alkali carbonates and naturally occurring minerals, which were either impregnated or mixed with the biomass. They considered the effectiveness of four different primary catalysts and of different catalyst concentrations at 550, 650, and 750 °C. The order of activity was reported as being potassium carbonate > sodium > carbonate > Trona (Na3H(CO3)2)2H2O > Borax (Na2B4O710H2O) Impregnated catalysts had little or no carbon deposition, as compared to the mixed catalysts, and carbon deposition resulted in deactivation. In the paper, the Mudge team also reported that the impregnation decreased particle agglomeration.
11.6.2.3 Nickel-based catalysts
The most significant body of literature published on hot gas cleaning for biomass gasification concerns nickel-based catalysts. Several groups have investigated a system of raw gas cleaning that involves a dolomite or alkali catalyst for the removal of up to 95% of the tar, followed by the adjustment of the gas composition reforming of the methane and the remaining tar using a nickel-steam-reforming catalyst. Steam and dry reforming reactions are catalyzed by metals of group VIIIA. Among these catalysts, nickel is the most widely used in the industry. Nickel catalysts are designed for steam reforming of hydrocarbons and methane. Using these catalysts at temperatures > 740 °C generally produces an increase in the hydrogen and carbon monoxide content of the exiting gas, as well as the elimination or reduction of the hydrocarbon and methane content.
Modification of nickel catalysts through the addition of promoters has also been investigated (Arauzo, Radlein, Piskorz, & Scott, 1997; Bangala, Abatzoglou, & Chornet, 1998). Arauzo et al. (1997) reported on the addition of magnesium and potassium to a nickel alumina catalyst. The magnesium substitution was made at two different levels, resulting in two catalysts, namely Ni2MgAl8O16 and NiMgAl4O8. Magnesium was added to increase the physical strength of the catalyst and its resistance to attrition. Partial replacement of nickel by magnesium improved the strength of the resultant catalyst, but the replacement also produced a 14% lower gas yield and an increase in the char yield. The CO and H2 yields decreased slightly with nickel content. The magnesium modified the catalyst structure and pore size distribution, with the unmodified catalyst containing a higher fraction of wider macropores. Arauzo and colleagues further proposed that the magnesium should inhibit the reduction of the nickel. Carbon deposition was reported as the cause of deactivation. However, the kinetics of catalyst deactivation depend on many factors, such as catalyst type, bed temperature, gas residence time, steam/biomass ratio, catalyst particle size, and, above all, the tar content of the raw gas (Herguido et al., 1992). Researchers have energetically sought a variety of reasonable catalysts for tar reforming and removal. Some catalysts performed with high tar reforming efficiency and excellent catalysis property, such as the nano-architectured Ni5TiO7/TiO2/Ti compound, palygorskite-supported Fe, and Ni catalyst. Further research work on tar elimination during biomass gasification is needed to identify reasonable catalysts that efficiently eliminate tar in an environmentally friendly manner (no secondary pollution), with low cost and easy regeneration based on tar types and content (Xu et al., 2010).
11.7 Char gasification
Another critical issue blocking the widespread use of biomass gasification is the lower carbon conversion efficiency of the process. Biomass gasification consists of two stages: the pyrolysis or release of volatiles and the gasification of the residual char. The release of volatiles is very fast, while char conversion is a gas-solid oxidation reaction, and as a result, it is the rate-limiting step in the overall conversion process (Bridgwater, 1995).
Chars from biomass tend to have higher gasification reactivity than those from coal (Miura, Hashimoto, & Silveston, 1989). As biomass contains much higher volatile content, char particles show a more porous surface structure and carbonaceous matter (Keown, Li, Hayashi, & Li, 2008). Wu, Yip, Tian, Xie, and Li (2009) found that biochar has highly heterogeneous and disordered structures, that easily react with the gasify agent. At the same time, the alkali and alkaline earth metallic (AAEM) species in biomass have a catalytic influence on char gasification. It was found that K and Ca increased the gasification rate significantly (Mitsuoka et al., 2011). Especially with the addition of K2CO3, char gasification can be shifted to a much lower temperature (600-700 °C). Furthermore, tar content in the gas product is decreased sharply as a result of this process (Sueyasu et al., 2012).
Aside from biomass composition, the pyrolysis condition also plays a critical role in char structure and gasification reactivity. Cetin, Moghtaderi, Gupta, and Wall (2004) found that char reactivity increased with increases in the pyrolysis heating rate and decreases in pyrolysis pressure. Under high heating rates, the char particles underwent plastic deformation (melted), developing a structure different than that of the virgin biomass. Pressure was also found to influence the physical and chemical structures of char particles. Klose and Wolki (2005) found that the reaction rate of biomass char is generally proportional to the reactive surface area. The surface-related reaction rates for the studied biomass chars are comparable to surface-related reaction rates for coal chars at similar reaction temperatures
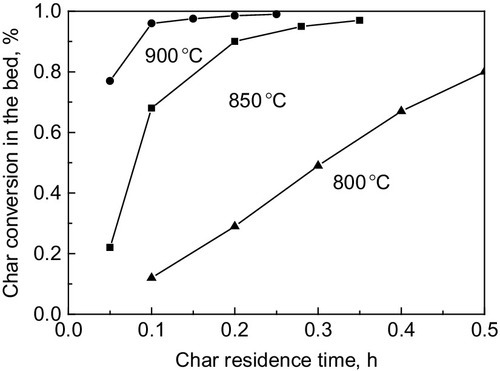
During char gasification, the structure and gasification reactivity also differ greatly. The highly heterogeneous and disordered structures in the char are selectively consumed during steam gasification, leading to the enrichment of larger aromatic ring systems, which are much more ordered and difficult to further convert (Wu et al., 2009). Consequently, a high char conversion for biomass is difficult to attain in gasification units. For example, one study found that carbon conversion reaches 80% in fluidized bed gasifier only after half an hour at 800 oC. Higher temperatures might accelerate the conversion, but 20 min is still necessary for full carbon conversion, as is shown in Figure 11.10, based on calculation (Gómez-Barea, Ollero, et al., 2013). For pilot, experimental, and industrial running, carbon conversion is much lower. Hence, measures should be taken to improve char gasification, thus ensuring a higher carbon conversion ratio and gasification efficiency.
11.8 Novel technology for biomass gasification
11.8.1 Staged gasification
Low carbon (char) conversion and high tar content in the gas are the two main problems that have blocked the development of biomass gasification (Gómez-Barea et al., 2013). Therefore, tar cracking and char gasification are two critical issues for syngas quality upgrading. Higher temperature is favorable for tar removal and char gasification (Hasler & Nussbaumer, 1999; Sutton, Kelleher, & Ross, 2001). But a new challenge is arising, as biomass facilities may be at risk of agglomeration at high temperatures, due to the release of a high proportion of alkali metals from certain feedstocks such as agricultural straws (Nilsson, Gomez-Barea, Fuentes Cano, & Ollero, et al., 2012). Staged gasification is a suitable way to reach high char conversion, while yielding a gas with a low concentration of heavy tar (Nilsson et al., 2012; Gómez-Barea, Ollero, et al., 2013). Gas produced by stage gasification is ideal for direct thermal applications, including gas engines, boilers, and fuel cells.
Staged gasification creates at least two different temperature zones and various thermal levels in the gasification bed by staging the oxidant. The biomass gets devolatilized at relatively low temperatures and then gasified at elevated temperatures with the remaining oxidant. As is shown in Figure 11.11, the process is divided into two parts: the pyrolysis stage at temperatures of 350 ~ 600 °C and the gasification stage at temperatures of 800-1000 °C. This process is convenient for the optimization of simultaneous char conversion and tar cracking.

Early in the 1994, Bui, Loof, and Bhattacharya (1994) found that tar yield in multi-stage reactors for thermal gasification was a factor of 40 times lower than the tar yield produced by one-stage gasification. Henriksen, et al. (Brandt, Larsen, & Henriksen, 2000; Henriksen et al., 2006) designed a 75-kw two-stage fixed-bed gasifier that has been operated for more than 2000 h, and this gasifier has produced product with a tar content < 15 mg/m3. Šulc et al. (2012) found that two-stage gasification systems can significantly decrease aromatic compounds with two or more benzene rings. Stage gasification of biomass not only sharply decreases the tar yield, but it also can increase the LHV of the resulting syngas. Hamel, Hasselbach, Weil, and Krumm (2007) produced high calorific value gas in a bubbling fluidized bed-fixed bed stage gasification system, and when using this process, the LHV of gas from MSW steam gasification increased to 14 MJ/Nm3. As for the release of AAEM and the reaction rate of gasification at about 800 °C lower than traditional conditions, Sharma, Saito, and Takanohashi (2008) studied the effects of K2CO3 catalytic gasification characteristics on ash-washed coal, and they pointed out that the low temperature of coal gasification (< 650 °C) is feasible. Sueyasu et al. (2012) proposed a two-stage conversion of biomass into gas, during which pyrolysis occurs at 500-600 °C and steam reforming/gasification occurs at 600-700 °C, with the K2CO3 catalyst being recycled. The addition of K can obviously increase the gasification characteristics of pine sawdust at low temperature. As a reslt of adding potassium, the concentration of hydrogen in the product gas exceeded 50 vol.%, and the tar yield was as low as 20 mg/Nm3.
Furthermore, the produced char can be gasified in the second stage and as the heat-carrier or catalyst for the further conversion of tar. Gómez-Barea (Gómez-Barea, Leckner, et al., 2013; Gómez-Barea, Ollero, et al., 2013) has proposed the three-stage concept shown in Figure 11.12, which includes fluidized bed devolatilization (first stage), the non-catalytic air/steam reforming of the gas from the devolatilizer (second stage), and the chemical filtering of gas in a moving bed supplied with the char generated in the devolatilizer (third stage). Air and steam can be injected at various points, such as the devolatilizer, steam reformer, and seal, with different proportions of the two reactants. The fuel is fed near the bed’s surface and has to circulate down to the bottom before leaving the bed. The devolatilizer, where a high yield of fresh tar is generated, is operated at relatively low temperatures (700-750 °C). The fresh tar compounds are drastically reduced in the reformer, where a temperature of up to 1200 °C is created. The injection of steam into the reformer avoids coking and polymerization of the tar. The gas is then filtered through a moving bed made of char which comes from the loop seal. The loop seal can be operated as an oxidizer fed with enriched air or as a light reformer fed with H2O, depending on the fuel’s reactivity and ash properties. The char filter also cools down the gas through an endothermic char gasification reaction with steam, while the char also acts as a catalytic filter promoting tar decomposition reactions with steam.
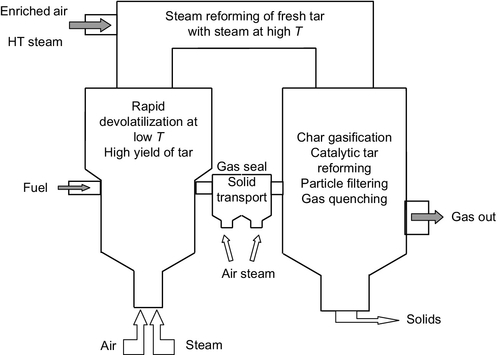
In the new three-stage system, the char conversion is up to 98%, leading to higher process efficiency. By optimizing the operating condition of air and steam in the three-stage system, the cold gas efficiency and gas HHV can increase to 0.81 and 6.9 MJ/Nm3 dry gas respectively. When using 40 vol.% oxygen-enriched air instead of typical air, the cold gas efficiency increases to almost 0.85, and the resulting gas can have an HHV of 10.8 MJ/Nm3dry gas. Given that the heavy tar content is lower than 0.01 g/Nm3, being virtually converted in the system, the obtained low dew point gas can be burned in a gas engine, and as a result, this gas is ideal for power production.
Nowadays, staged gasification tends to involve large-scale applications and continuous operation. The University of Canterbury has built a 100 kW fast internal circulating fluidized bed gasification system that incorporates two closely coupled fluidized bed stages, a bubbling bed for gasification and a fast circulating bed for combustion (Brown, Dobbs, Devenish, & Gilmour, 2006). This duality provides a medium calorific value producer gas suitable for use as a fuel in a gas engine or gas turbine. Steam gasification is used in the bubbling fluid bed, at ~ 800 °C, to form a product gas that is rich in hydrogen. Residual char is transferred with bed material to the circulating fluid bed, where it is combusted, along with LPG, to heat the bed material. The hot bed material is then circulated back to the gasification stage, providing heat for the endothermic gasification reactions. On the other hand, char, char-supported catalysts, and ilmenite are investigated for the steam reforming of biomass tar, as opposed to the use of a precious metal catalyst. Min et al. (Min, Asadullah, et al., 2011; Min, Yimsiri, Asadullah, Zhang, & Li, 2011) clearly indicated that the chars from the pyrolysis and gasification of biomass could support a new class of cheap industrial catalysts with superior performance. Char would not only disperse the catalysts, but it would also interact with the catalysts to enhance their involvement in the steam reforming of tar. The physical and chemical property of support could play important roles for the activities of the catalysts and the reaction pathways of the catalysts. The char-supported iron/nickel catalysts exhibited much higher activity for the reforming of tar than char itself. So the utilization of pyrolysis char as catalyst support is another research point.
11.8.2 Sorption-enhanced steam gasification of biomass for H2 production
Together with electricity, hydrogen is considered to be one of the two main terminal energies produced by gasification in the twenty-first century. Hydrogen can also be utilized for ammonia production and, with suitable CO concentrations, for methanol and FT synthesis. Through the use of a calcium oxide (CaO) sorbent for carbon dioxide (CO2) capture, the steam gasification of biomass offers a potential means for the renewable and sustainable hydrogen (H2) production.
Sorption-enhanced steam gasification of biomass is a novel one-step conversion technology developed for high-concentration H2 production. In this process, CO2 sorbents are introduced into the process of biomass steam gasification for the continuously in situ removal of CO2 as soon as it was formed in the gasification process. As a result, the chemical equilibrium of the gasification reactions changes, and the process produces more H2 (Hanaoka et al., 2005; Harrison, 2009). The gasification unit, the WGS unit, and the CO2 separation unit are integrated into a single-stage reactor in this process, in order to achieve in situ CO2 removal, energy integration of the endothermic gasification and exothermic WGS and CO2 sorption processes, facility and process simplification, and steam usage reduction. As a result, the overall efficiency and economic feasibility of biomass gasification are improved.
Considering the spent CO2 sorbent regeneration, the whole H2 production process is a looping system, and the circulation between sorption and desorption ceaselessly transforms each cycle. With CaO as an example, the principle of this process could be described as shown in Figure 11.13 (Koppatz et al., 2009), and the main reactions that take place in the process could be summarized as follows:
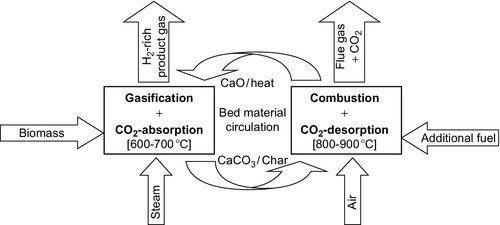
Li et al. (2011) have reported that a H2 concentration of 94.92% could be obtained at the gasification temperature of 600-700 °C, with the atmospheric pressure being appropriate for chemical equilibrium. Marquard-Möllenstedt et al. (2004) also reported that the H2 concentration could increase from 40% to about 75%, with the addition of CaO, while the CO2 concentration decreased significantly.
CaO-enhanced steam gasification of biomass still has several advantages: (1) the temperature of the CaO carbonation reaction coincides with the temperatures required for biomass gasification; (2) CaO also has a catalytic effect on the process of biomass gasification and tar cracking and reforming. Considering the appropriate conditions of the CaO carbonation reaction, the optimized carbonation temperature is between 600 and 700 °C at atmospheric pressure. In this temperature range, biomass steam gasification enhanced by CaO sorption could produce a high H2 concentration.
However, due to the lower temperature (600-700 °C) of this process compared to the conventional gasification process (800-900 °C), and the reaction activity of H2O is relatively low compared to air, and although the H2 concentration is very high, the conversion rate of biomass and total carbon is still low. This low conversation rate is due to the fact that a portion of the carbon remains in the solid char, and other parts go into the liquid tars, thus resulting in the lower H2 yield, as shown in Figure 11.14. To improve the biomass/carbon conversion rate and the H2 yield, two methods could be used:

(1) Increase the gasification temperature to enhance the reaction rate and accelerate the gasification process, thereby improving the biomass conversion rate and the H2 yield. Considering that the temperature necessary for CO2 absorption would enter the high range under pressurized conditions, a feasible solution might be high-temperature pressurized gasification, such as the HyPr-RING process (Lin, Harada, Suzuki, & Hatano 2002; Lin, Suzuki, Hatano, & Harada, 2001) or pressurized fluidized bed gasification (Han et al., 2011).
(2) Introduce the catalysts into the gasification process to enhance the gasification reaction rate, as well as the selectivity and conversion rate of the reforming reaction of tars and carbonaceous gases. Introducing catalysts would realize the high conversion rate of biomass and the high H2 selectivity in the product gas at relatively low temperatures. Such catalysts include Fe/CaO (calcined dolomite) and Ni/CaO (calcined dolomite) catalysts (Di Felice et al., 2009; Di Felice, Courson, Foscolo, & Kiennemann, 2011), Ni-Mg-Al-CaO catalysts (Nahil et al., 2013), and Pd-Co-Ni + dolomite catalysts (Fermoso, Rubiera, & Chen, 2012), etc. For example, Nahil et al. (2013) found that over 80% H2 was produced with a Ni-Mg-Al-CaO bed during biomass gasification.
11.9 Mathematical simulation of biomass gasification
In order to understand the biomass gasification process and to optimize the design and operation of the biomass gasifier, implementers must conduct an extensive investigation of biomass gasification behavior and the involved operating parameters. However, performing experiments is impossible and dangerous in some cases. Instead, mathematical modeling can give us the expected result. So far, many investigations involved mathematical and computational approaches. Overall, mathematical modeling can be categorized into the use of equilibrium, kinetic, and neural networks (Ahmed, Ahmad, Yusup, Inayat, & Khan, 2012).
11.9.1 Thermodynamic equilibrium models
The “equilibrium model” refers to thermodynamic equilibrium models, such as models of chemical reaction equilibrium, which are based on the minimization of the Gibbs free energy of the system. Gibbs free energy is minimized when the species of a reaction system reach equilibrium and will no longer experience any change over time.
Mansaray, Ghaly, Al-Tawell, Ugursal, and Hamdullahpur (2000) simulate rice husk gasification based on material balance, energy balance, and chemical equilibrium relations. Their model attempted to predict the core, annulus, and exit temperatures; the mole fractions of the combustible components of the gas; the higher heating value of the gas; and the overall carbon conversion under various operating conditions, including bed height, fluidization velocity, equivalence ratio, and the moisture content of rice husk.
However, the definition of chemical equilibrium implies that the residence time is long enough to allow the chemical reactions to reach stasis, which is rarely reached in real gasifier. Hence, in some research, the models employed the restricted equilibrium method (Doherty, Reynolds, & Kennedy, 2009), and the temperature approach for the gasification reactions was specified. Li et al. (2004) introduced a phenomenological model that incorporated experimental results regarding unconverted carbon and methane to account for non-equilibrium factors, and this model predicted product gas compositions, heating value, and cold gas efficiency in good agreement with the experimental data.
Thermodynamic equilibrium models, which only take into account thermodynamic limitations, inherently disregard specific reaction mechanisms that are independent of the gasifier design (Jand, Brandani, & Foscolo, 2006). Also, the results may not be achieved at low temperatures, so the calculations may not be representative of the real situation, when kinetic constraints become the major factor (Ju et al., 2009). Hence, thermodynamic equilibrium models cannot produce accurate results, but these models are efficient for the process of optimizing and prediction (Ju et al., 2009; Mahishi & Goswami, 2007).
11.9.2 Kinetics models
Kinetic models differ from thermodynamic equilibrium models, as they describe the char reduction process using kinetic rate expressions obtained from experiments, thereby permitting better simulation of the experimental data when the residence time of gas and biomass is relatively short.
Kaushal, Abedi, and Mahinpey (2010) developed a one-dimensional steady state model with two phases (bubble and emulsion) and two zones (bottom dense bed and upper freeboard). This model was based on global reaction, kinetic, mass, and energy balances of biomass gasification in bubbling fluidized gasifiers, and it is capable of predicting temperature, solid hold up, and gas concentration along the reactor’s major axis.
Wu, Zhang, Yang, and Blasiak (2013) built a two-dimensional computational fluid dynamics (CFD) model to study the gasification process in a downdraft configuration, considering drying, pyrolysis, combustion, and gasification reactions. The gas and solid phases were resolved using an Euler-Euler multiphase approach, with exchange terms for the momentum, mass, and energy.
Miao et al. (2013) developed a new mathematical model that combined hydrodynamics with chemical reaction kinetics to predict the overall performance of a biomass gasification process in fluidized beds. The fluidized bed gasifier was divided into two distinct sections: a dense region at the bottom and a dilute region at the top. Each section was divided into a number of small cells, over which mass and energy balances were applied. The model is capable of predicting the bed temperature distribution along the gasifier, the concentration and distribution of each species in the vertical direction of the bed, the composition and heating value of produced gas, the gasification efficiency, the overall carbon conversion, and the produced gas production rate well.
Kinetic models provide essential information on kinetic mechanisms to describe the conversion during biomass gasification, which is crucial in designing, evaluating, and improving gasifiers. These kinetic models are accurate and detailed, but they are also computationally intensive. In addition, the basic experiment data is necessary when constructing and running the models. Sometimes, thermodynamic equilibrium models have been combined with kinetic models. Lee, Yang, Yan, and Liang (2007) extracted substitutable gas phase compositions from thermodynamic calculations, before entering the gas phase compositions into the Sandia PSR code to consider the potential kinetic constraints involved in the pyrolysis. Lee and colleagues also hoped to obtain the distributions of gas products. The result, in this case, is much closer to the realistic situation (Lee et al., 2007).
11.9.3 Neural networks model
Artificial neural networks are normally based on mathematical regression to correlate input and output streams to and from process units. Such models principally rely on a large number of experimental data. Guo, Li, Cheng, Lu, and Shen (2001) created a hybrid neural network model for the purpose of predicting biomass gasification profiles at atmospheric pressure with steam. Artificial neural networks differ from traditional regression because they have more potential for finding the unseen structure (Guo et al., 2001).
Each type of model has its own strengths and limitations, as mentioned above. In order to achieve efficient calculation and design, Brown, Fuchino, and Mar Chal (2006) combined the three models together, given that fuels and chars are defined as pseudospecies with properties derived from their ultimate analyses, and tars are defined as a subset of known molecular species with their distribution determined by equilibrium calculations. While the reforming of gas, tar, and char formation was explained by applying reaction temperature differences to a complete set of stoichiometric equations, the changes in temperature related to fuel composition and operational variables were determined with nonlinear regression via an artificial neural network. This method improves the accuracy of equilibrium calculations and reduces the amount of required data by preventing the neural network from learning atomic and heat balances.
11.10 Conclusion and future trends
Biomass is an accepted form of renewable energy, and it plays a pivotal role in helping the world reduce the environmental impacts of burning fossil, such as global warming and acid rain. Gasification is a versatile thermochemical conversion process that produces a gas mixture of CH4, CO, and H2. The relative yields of these substances are determined by operating conditions, such as the gasification agent and reactor configurations.
Catalyzed tar reforming is essential to biomass gasification, and this chapter summarizes the effects and activities of three types catalyst (dolomite, alkali metals, and nickel). These catalysts effectively increase gas productivity and reduce carbon deposition. Dolomite and alkali metals are often mixed with biomass feedstocks, and studies showed that Ni metal was most productive as a secondary catalyst located in a downstream reactor.
Staged biomass gasification is an optimum choice for biomass gasification with high char conversion and low tar content, and the three-stage biomass gasifier has approached 98% carbon conversion efficiency. Sorption-enhanced steam gasification, on the other hand, is a novel one-step conversion technology developed for the production of high concentration H2 from biomass. The H2 concentration could increase from 40% to about 75% or higher as CaO or a CaO-based catalyst is added.
Biomass gasification has shown unique advantages. With syngas, however, there are still some challenges, such as particulates, hydrocarbons, and alkali compounds in the gas product. A lot of research is needed regarding the gasification process, and academia and industry must continue to develop and demonstrate new technologies, with the intention of creating large-scale real-world applications.