CHAPTER 6
Design at the Point of Care
Elena’s Story: Taking a Serious Turn
Elena’s personal schedule gets the best of her. She continues to take her thyroid medicine, but her commitment to personal lifestyle changes falters less than a month after reasonable adherence. The stress in her life picks up, her diet remains uneven, and she is unable to keep a consistent exercise schedule.
Two months after the first incident, Elena finds her heartbeat racing, with the feeling of a flutter rather than a beat, and she faints again. When she comes to, she is lightheaded, dizzy, and pale, with a pounding and fast heartbeat. This time her daughter is at home to catch her swoon, and unsure why this has happened again, Andrea calls her grandfather for help. He shows up as quickly as he can, assesses that his daughter has suffered a serious spell, and the three of them hurry to the local hospital emergency room.
At the hospital, the admitting nurse asks Elena for the usual information: complaints, family doctor, insurance provider. Elena volunteers her prior incident, but the hospital does not have access to her records. Because the latest occurrence was clearly an acute episode with a prior history, more complete tests and procedures are called for.
Elena is given a full electrocardiogram (ECG) in an attempt to capture and isolate the pattern of the arrhythmia. She undergoes full blood tests for sugar, cell count, and cholesterol levels. Having caught the fainting incident quickly enough to detect the originating cycle this time, the ECG shows the telltale pattern of a heart condition known as supraventricular tachycardia (SVT), a regular but very rapid heartbeat originating “above the ventricles,” resulting in dangerous heartbeat fluctuations that may occur acutely and without warning. Although SVT is not the first diagnosis for fainting, it finally explains why Elena fainted—the rapid arrhythmia, worsened by a blood sugar drop, led to a loss of consistent blood flow to the brain.
Elena’s blood sugar levels now show the approach of Type 2 diabetes (at 120 mg/dl) and her blood pressure is again abnormally high. Her thyroid pill, though, has worked well—her TSH (thyroid-stimulating hormone) levels are normal. It remains unclear to Elena how her conditions are systemically related, and if they are. Every condition is treated as a separate issue, with different medications and advice. She cannot make sense of the diseases as a whole, making it harder to see how her life choices will improve her overall health.
Elena is started on blood pressure medication and is referred to a cardiologist. She is advised to return to her family doctor to address the possibility of diabetes. Back at home, she goes online to investigate the options suggested to her. The SVT diagnosis and cardiologist referral confirm that her situation is a serious one. She naturally wants to increase her understanding of the condition and to learn about the risks and effects of the possible treatment options. Elena follows up by scheduling a consultation at a cardiac care center.![]()
Better Practice Is Better Service
From the clinical perspective, Elena’s diagnosis and even her advanced treatment are routine. Dozens of patients a week are referred to hospitals and cardiac clinics with diagnosed arrhythmias. To the health seeker, the diagnosis may come as a shock and the treatments may seem exotic or risky. As always, a huge gap in knowledge, power, and comfort exists between specialists and their patients. These gaps seriously influence how health seekers experience their care.
The information gap of medical knowledge has narrowed with the ready accessibility of Web publications. The meaningful gap is the one between information and knowledge—knowing how to judge and act on the information.
A service experience gap also exists. The clinician retains the power to diagnose, prescribe, and deliver treatment. A typical patient possesses limited knowledge of what constitutes “good service” in totality. The threshold of “bad service” may come close to malpractice, but the range of a normal care standard is quite broad and experienced differently by individuals.
Will the future patient be treated as a customer to be satisfied at every step? A compelling candidate for future patient experience is found in personalized medicine, which is not personalized care, but rather technology-enabled personalized diagnostics. Based on sensor and genetic information gathered for an individual, early detection of emerging diseases and appropriate medication can be prescribed. The trade-off is that individual adherence and self-care will be expected. Although the medical technology may be manufacturable, personal behavior is not so controllable. Adherence will become more of a designed experience.
Comfortable care experiences cost more than do-it-yourself care. The direction of future policy is oriented toward compensating providers based on outcomes. The unforeseen effect could be that services become stripped down and efficient, aligning expected outcomes to the most efficient billable treatments. As clinicians are evaluated more on outcomes than on procedures, service quality as experienced could actually be perceived as worse than today.
How should established clinical practice models be changed to improve patient experience? Will improving experience at the patient touchpoints (appointments, reception, consult, preparation, treatment, recovery, follow-up) make a significant difference to the patient? Or done wrong, could it have a negative impact on provider efficiency?
Clinical care and care teams are not service industry employees. Hospitals cannot change service practices overnight. The regimes of primary care, emergency, acute, and critical care, surgery, and long-term care are taught and developed in education and certified in board exams and licensure. Very few services are as locked in to their educational processes as healthcare.
Business, law, and even engineering teach principles but not practice. Medical education requires that trainees spend more time in practice than in school. Changing healthcare service means changing training, and vice versa. This will be an evolutionary process, and many will call for rapid change. But medical education is already changing at a surprising rate.
A Design Opportunity in the Care Crisis
Primary care providers (primarily generalist physicians, but also now nurse practitioners and physician assistants) are essential to improving and sustaining good health at a community level. The primary practice is the first stop for any person with an undiagnosed illness or nonemergency condition. In the US healthcare system, primary care may be the only point in the health-seeking journey where a true patient-centered relationship can develop. Primary care physicians have the ability to integrate knowledge and experience of individuals and their histories and lifestyles. Although much of the emerging consensus in healthcare design focuses on patient behaviors and (rightfully) on prevention, primary is mainly where the care lives. Most people who become sick or injured show up first at primary care, regardless of their technological sophistication.
The societal need for primary care increases with an aging population, as multiple chronic diseases emerge, and older people are at risk for cancers and systemic diseases. The largest population cohort in history is now reaching that age, putting pressure on the system to adapt. A crisis is foreseeable, as fewer physicians have chosen family medicine or primary care after their education. Family doctors and pediatricians have increased caseloads over the years, resulting in greater numbers of episodic patients, more stress on the system, and unsustainable practices. The situation in the United States (and Canada) has reached the point of a declared crisis.
The developing crisis of not enough primary care providers for the population affects society on a large scale. The American Academy of Family Physicians estimates that by the year 2020, the United States will face a shortfall of 40,000 primary care physicians. This 51% shortage based on anticipated need will increase costs across the system and reinforce the existing trend toward specialization.
Higher salaries drive higher service and educational costs as a systemic effect, yet the onus is often placed on physician choices. But are there deeper causes? Has the fee-for-service business model and pay-per-procedure accounting of health insurance made primary care complicated and untenable? Are residents specializing because of their affinity for a specialty, or to afford more mobility and career choice?
Family doctors mitigate costs because they assess the whole person and take into account a wide range of life and personal options, recommending exotic procedures only as a last resort. They closely monitor possible medication problems. Family physicians promote whole family and community health by advising and facilitating personal lifestyle changes; simpler, lower-cost treatments; and prevention. Each step toward prevention saves costs incurred later by more serious problems and emergency room visits.
New primary care services are being designed and prototyped within the accountable care organization model, which brings a wider range of advanced and primary clinical services to smaller, distributed community care centers. Several early-stage organizations have formed (Kaiser Permanente, Mayo Clinic), and it is notable they are referred to as prototypes. That does not, however, suggest that these new practices have been designed. A design-led (D3.0) management process would facilitate integration of care and service systems, architecture and workflow, organizational resources, IT, public and patient communications, and the new business model.
Many of the incentives for change associated with the human dimensions of a service system (education, salary, workload, lifestyle, prestige) are not designable functions within the service. Education in particular has significant long-term influence on practice, as new entrants into any practice tend to bring techniques and new thinking to the field. But it is changed within its own system, renewed by integrating changes from practice trends, emerging societal needs, and new learning. Though highly standardized, clinical education is changing rapidly for a new generation of medicine, bringing with it the potential for system-wide change in healthcare.
Where and How to Innovate
Herbert Simon was an early proponent of treating medicine and management as design practices because they “change existing situations into preferred ones.”1 How is medicine like design practice? Medicine is a knowledge process that bases care decisions on information, technology, and resource availability. Doctors quickly research a patient’s situation based on evidence, frame the problem state, and design care plans that facilitate preferred health outcomes. We might call this Clinical Design 1.0 (CD1.0).
Clinical Design 2.0 (CD2.0) redesigns care procedures and services that systematically improve outcomes. Medical researchers evaluate new surgical and intervention techniques in rigorous, controlled comparative trials to ensure that the procedure performs reliably, is repeatable, and is ultimately better for the patient. Medical device designers transfer emerging technologies—sensors, electronics, fabric and materials—into improved products for technical procedures. These may dramatically change the opportunities for future products and health management, such as the noninvasive skin sensor developed by C8 MediSensors that could replace the daily invasive ritual of blood sugar monitoring with a wearable device.
Though not defined as a design practice, Clinical Design 3.0 (CD3.0) transfers knowledge and practices from organizational design and sociotechnical systems toward high-performance care organizations. The initial toolkit of research methods and design processes deploys organizational innovation tools such as business and practice model design. CD3.0 generally covers the range of practices that involve all members of an organization, across services and departments. At this scale, stakeholder co-creation and innovation management guide organizational design, whereas CD1.0 and CD2.0 are driven by expert knowledge and user response data and can be considered more evidence-based.
Clinical Design 4.0 (CD4.0) co-creates practices of caring in a community or societal context. CD4.0 care is exemplified by the systematic design thinking being introduced into public health problems by innovative physicians such as Mike Evans and the Health Design Lab (see Chapter 4’s case study). Large-scale public health initiatives (such as the successful Smoke Free Ontario program) are planned as multiyear research and intervention programs at the public system level. Reviewing each level of clinical design, we have:
• CD1.0: Individual human use of products and devices that meet a specific individual need, such as personal test kits with instructions, or insulin injection kits for diabetics. These are responses to conditions, with products designed by vendors and provided by physicians.
• CD2.0: Healthcare services provided by teams in clinics, institutions, and online. Care activities are provided as services to health seekers, but are performed as work activities by a service team.
• CD3.0: Clinical and educational institutions, including clinical education, organizational management, and service program development.
• CD4.0: Social and policy design for the healthcare industry includes health system planning, community and public health strategies, and national and regional systems.
Each level calls for different methods suitable to accomplish its use cases. Adapting product design methods to the organizational practice context (CD3.0) would fail because of a mismatch of method to desired outcome. Adapting systemic design to the individual (CD1.0) is overkill.
Medical practice has a deeply established culture and will have to change systemically to change substantively. But professional cultures resist intervention by foreign practices; design processes will prove themselves in the new world of outcome-driven healthcare.
Design education and design practices are not organized to serve healthcare today. Most large health systems are largely advised by the expert-led management consulting model that has been predominant in corporate cultures for more than two decades, and the recognition of value from design firms is negligible overall. Yes, large design firms may have dedicated healthcare practices, but there are few clinically experienced designers. Furthermore, in care organizations, unlike in product or service companies, designers do not yet have leadership or ownership of the design outcomes. In most cases (not all), their work conforms to the direct needs of organizational management as essentially “work for hire.”
The integration of new disciplinary practices into a deeply set culture such as medicine can easily take a decade, but many institutions are clearly seeking the dramatic improvements in value of design to patients and healthcare business. The early adopters of design—clinical and management leaders—may initially see value in tactical improvements and may not recognize the possibilities for systemic change and inevitable barriers in staffing up to design better services and IT. A model such as the CD1.0–4.0 domains may enable better-informed strategic planning for the development of an integrated design research discipline in healthcare.
Different Design Modes of Care
A design process should match the level and variety of a context to accommodate its complexity, as shown in Figure 6.1. Each arc represents a different level in the sociotechnical system, and each requires a different design process with different stakeholders. Each corresponds to a significantly different context with differing systems, interests, and values.
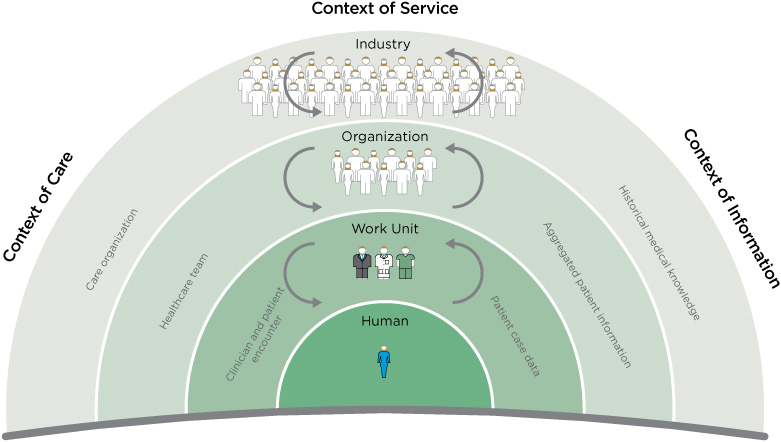
FIGURE 6.1
Contexts of service in care design.
Three contexts for innovation are shown: care, service, and information. Each context encompasses some services and excludes others. In any given project, care delivery, service provision, and IT might be interwoven. Yet one of these contexts will lead and define the purpose (e.g., IT) and another may be the internal client (e.g., pediatric care). Design and research methods selection and deliverables may differ significantly as a result.
What are the substantive differences among healthcare tasks, services, and the “system level”? The four dimensions of service in Figure 6.1 range from individual behavior to industry dynamics, aligned with the CD1.0–4.0 progression:
- Care design for the health seeker: with the aim of fostering self-care and preventive awareness in individuals
- Clinician-patient encounter: direct exam, diagnosis, and treatment of a health seeker by a care team
- Healthcare team: care planning and role configuration for the healthcare professional teams and practice areas
- Care organization: organizational strategy, business model design, and organizational development
Each level endorses a different concept of “care,” a complex of values, orientations, delivery models, and motivations that currently do not map across these levels of activity. The care encounter and care organization encompass different functions of care.
The context of care information forms a hierarchy of data sources for service management and decision making. A series of transformations follow the Data-Information-Knowledge-Wisdom (DIKW) hierarchy. The DIKW schema was developed for management applications by systems theorist Russell Ackoff2 and has been extended to knowledge management and, more recently, design research analysis.
• Data: Data acquired by sensors, self-documentation, and care practitioners, often recorded in discrete fields associated with a single record (e.g., a patient’s record from a single appointment).
• Information: Patterned formulation of data that indicates semantic meaning. Information may range from documented content to medical articles to summarized patient narratives (e.g., a trend pattern distinguished across multiple data entries).
• Knowledge: Cognitive understanding of the meaning of information to relevant problems, sufficient for competent action. Knowledge can be represented as a verb, not a thing, as in knowing how (e.g., recognizing that a trend pattern of glucose levels signals the onset of Type 2 diabetes).
• Wisdom: The ability to act on information with an understanding of short- and long-term consequences (e.g., hypothesizing the source of a disease condition and recommending systematic changes instead of medication).
As innovators, we face the challenge of enabling outcomes of healthcare through a supportive, nondirective design context. In large institutions, in particular, a given “problem” will have multiple contexts, agents, communication technologies, and organizational requirements. Our role may be subsidiary, a frustrating place for those making change. Even recognizing the plurality of meanings of “care,” designing for care is a new organizational role and there will be friction. Design research methodologies will also find resistance and need to adapt to context. It does not serve us well to declare that any preferred design methodology (e.g., experience design) is optimal for a given problem, because our methods may bias the solution toward only the outcomes the method enables.
Designing Health Education
Clinical education introduces long-term influences on the complex system of healthcare. Changes in practice and method consistently taught will propagate throughout a national healthcare system, but can also promote near-term shifts in clinical culture and practices.
Clinical practice is a Western cultural form with its roots in the initial successes of the adoption of scientific medicine in the early 20th century. Yet the way doctors (in particular) are educated for practice is by extended residency apprenticing with senior clinicians, similar to ancient guilds. Residency is a sophisticated immersion of learning in action, technical work, and scientific culture, a process not found in other professions.
Since the 1960s, articles in the medical literature have called for changes in the long-standing models of residency and medical education. Though not obvious to patients or others outside medical education, pedagogies and curricula have made huge changes in the last decade and are continuously moving toward student-centered education. Molly Cooke and a University of California, San Francisco team advocated new roles for promoting clinical teaching, a policy change they call “the Academy movement.”3 She identified the certification and development of education, the university model, and professional values as opportunities for change. Related concerns include the increasing numbers of complex and chronic patients and the need to focus on outpatient and community (and home) settings, rather than only on teaching hospitals. These are all rich opportunities for new design practice in healthcare.
Educating Designers to Educate Clinicians
Clinical education in all fields has only recently transformed after roughly 100 years of traditional science-based training and apprenticeship. Reforms in educational content, working conditions, and educational financing have shifted in just the last few years. Change may appear slow because clinical practice is highly constrained, the medical school and residency regimes are deeply ingrained, and clinicians are not faced with the institutional crises that necessitate change. Medical education has responded to demands from certifying bodies and clinical employers, and even to changes in culture and mindset from incoming students.
Consider the extraordinary range of information and services available in healthcare education. Everything from medical textbooks and e-learning systems to content delivery to research translation for practice can be considerably improved and integrated as D2.0 products. However, the educational process is not just a D3.0 institutional change; it is a socially transformational D4.0 process. Educational innovations affect all related institutions over time, so a significant design-led intervention can pay off.
Educational systems are notoriously difficult interventions—they are institutionalized, highly stable, and continuous yet complex. As with other social change problems, schools and educational services require a long period of adaptation for recommendations to show sustainable improvements.
Professionals do not generally like change when it happens to them. Designers might better understand why their contributions may be greeted with skepticism. Design interventions represent changes to careful daily routines, which require unlearning the old process and learning a new one. Even something as simple as a universal checklist can present a cognitive burden. Cognitive workload and technical work intensity are already at maximum levels for residents. So where might we find support for assisting clinical educators in rethinking the long-established order?
Service Design Issues in Clinical Education
Any educational system presents a complex challenge for service design. Learners are not customers, and education is not understood as a service by its administrators. Some educational processes—online resources, distance education, curriculum management—may be innovated as service concepts. But education is based on public service traditions. Conforming education to service methodologies could instill biases toward commercial values that might conflict with academic values. Many educators would argue that service design’s pursuit of efficiency and value delivery are not suitable for educational practice and excellence in learning. Yet academic leaders have been calling for the redesign of clinical education for decades. How might we best approach this problem?
Services have been “designed” since the first modern service was created. Consider that Thomas Jefferson designed the US patent office in 1790, based on the British patent registry, and that the original criteria and even the legal process have changed little since then. Government services, such as the US Social Security program, were carefully conceived as standards across national or industry levels. Unlike products designed for sale through retail distribution networks, services are resource networks that can satisfy transaction requests from any customer through mediated channels. Successful services have always been designed and supported as social and technological systems, requiring sufficiently flexible standards decoupled from their technologies. Yet healthcare may be one of the last industries to adopt a designed regime to produce sociotechnical services.
Innovating Big Box Clinical Education
Hospitals have leveraged their “big box” administration to heavily invest in advanced medical and information technology. Hospital services have retained their essential patterns, however, and differ little in 2012 from 1982. In fact, a description of hospital organizations in 1888 is surprisingly similar to today’s structure.
Hospitals are organized into separately staffed and managed departments that treat patients by acuteness and temporality. The introduction of associated outpatient or urgent care in the 1980s reduced the burden on emergency departments, but many service units have remained remarkably unchanged over the years. With the cost and societal pressures of various healthcare reforms, the basic model of the clinic has not yet been successfully challenged. Could the clinic be transformed by the newly educated clinical leaders entering hospital services, with support from their faculties? Should system-level change start with education? How could education drive institutional innovation? What if clinical education were reformulated as a service (D2.0), organized and led at the institutional level (D3.0), and transferred across the educational sector (D4.0)?
As both a local and transnational institution, medical education is complex and crosses levels of design process. For this reason, the “target level” of design method is the position that gives effective access to the adjacent two levels most crucial for the outcomes. Positioning education as a D3.0 project, we can inherit the service design methods of D2.0 and “reach up” to the adjacent strategic scale of D4.0. The rationale for this process is that an organizational leader can transfer practices across institutions (D4.0), but service-level projects (D2.0) may not reach across organizational boundaries.
The need for a new frame of design thinking and research is driven by the order of complexity. D2.0 is driven by customers (or users), and a D3.0 institutional context is driven by organizations, regulators, and patients.
The complexity (and risk of getting it wrong) in D3.0 increases by an order of magnitude.
The opportunities for designers to contribute to service transformation are enhanced and expanded when embracing the D3.0 perspective. Rather than merely producing visual and digital artifacts as end products enabling clinical activities, designers in the D3.0 context are involved in:
• Embracing organizational-level services design as a participatory process.
• Employing creative and analytical problem-solving tools with diverse multidisciplinary (clinical and staff) teams.
• Coordinating and facilitating innovation processes for organizational teams.
• Evaluating new service proposals and their fit to other services and systems in use.
• Facilitating visual integration of concepts and proposals to communicate design options.
Taking a D3.0 position sets priorities. Organizational outcomes take precedence over resident education, but there is no reason not to improve all touchpoints. As in any D3.0 project, the needs for change are apparent, so where do we invest? Today’s clinical practice is the outcome of a long process of education, one that generates well-qualified specialists. But the traditional focus on technical practice and medical knowledge ignores emerging social and public aims for healthcare improvement and a healthy society.
Doctors can be forgiven for replicating the same educational system—after all, it produces clinical excellence and arguably achieves its primary objectives. Success makes any system more difficult to change. Also, the most difficult competencies to teach, if not to learn, are those that may change a practice that trainees have not even become competent at managing.
Contexts and Content of Clinical Education
Medical school is only the beginning of a lifelong career of learning and practice. Postgraduate education and residency establishes the physician in a career timeline that requires constant updating of knowledge and performance, from passing board exams to periodic maintenance of certification. The level of performance required to manage a concentrated workload of 80 to 100 patients a day is learned in a 3- to 5-year residency (7 years for surgery and some subspecialties), where 80-hour workweeks are typical.
Education is more complex than clinical practice because it has multiple foci and measures of success. It usually involves research, which practitioners do not do.
Clinical work involves much more routine than residency. Education places residents and faculty within the operational environment of live clinical practice, then layers multiple paths of learning and practice onto the routines. Residents learn in a high-tempo, high-risk mix of scheduled and improvised occasions. Education has multiple outcomes, whereas clinical encounters may have a single path from admission to discharge. Residency is mediated by multiple entities, ranging from the university and hospitals to the American Council of Graduate Medical Education and the academy boards that certify practitioners. Making simple changes to education does not necessarily scale beyond the institutional boundary.
Clinical work is technical and physical work. Not only is it not online, it is only peripherally enabled by information resources. Most of what we consider user experience is tangential to technical work. In fact, paper records (whether patient records or clinical notes) have been found in numerous studies to communicate more effectively and accurately within clinical teams than electronic records systems.4 Information resources for clinical diagnostic and treatment decisions are common when learning in residency, but once in practice are used infrequently. No experienced doctors prepare to perform a common procedure by brushing up online first (although they may review the steps of a complicated technique to refresh their memory). Clinical work is not automated, nor should it be if the object is care.
The outcomes of clinical education (and all post-secondary learning) are social and public goods. Social goods include access to healthcare, public health and community care, the ethical use of appropriate technologies and procedures, and the movement toward a patient-centric vision of healthcare. These outcomes are associated with a shift from D3.0 to D4.0.
Design Strategies for Clinical Education
How do doctors do what they do every day? How do they acquire their extraordinary blend of human, physical, cognitive, and technical skills and manage to maintain superhuman schedules from medical school through practice? How is their training designed to develop these skills and mindset?
Medical practice has been based on craft skill since the founding of the clinical tradition, and long before as guilds of trained healers and surgeons. It is a sophisticated craft, but one of a tradition of independent practitioners with significant autonomy and professional prestige that lives in cultural conflict with the bureaucratic mode of the hospital.5 Residents train in an apprenticeship to learn the hands-on work and manners of their attending physician mentors. Once accepted in a residency, doctors are socialized until they attain a defined level of skill and pass final board exams. That is, much of their training happens as a result of continual exposure to the culture of medicine. This cultural socialization forms the persistent mental models that maintain the profession and its practices.
Culture reproduces itself for our own survival, and it outlasts all of us and our interventions. An understanding of culture is necessary if we are to change it or propose service design models. Designed services can bring great productivity and enhance knowledge sharing and production, but interventions in process and technology have done little to change the essential routines of clinical training. As a stable, widely distributed, high-performance social system, clinical education is not easily changed by “design thinking.” There are few entry points for designers to study, observe, define, and introduce prototypes. Changes to education take years for adoption, overcoming barriers to co-creating, evaluating, and diffusing new processes.
The progression of medical education (Table 6.1) indicates very different educational approaches (learning modes) and objectives for each stage of education or practice. These translate to differing information needs and resources used in each stage, and different service models.
TABLE 6.1 LEVELS AND MODES OF MEDICAL EDUCATION
Practice stage |
Learning modes |
Objectives |
Medical school |
Didactic, instructor-led courses in dedicated university programs; extensive reading and testing; problem-based small group learning; clinical practice training (third and fourth years) |
Completion of program with high marks in courses; high scoring on board exams; learning practice of “doctoring”; licensure |
Residency (dedicated training in elective specialty) |
Apprenticeship by rounds (observations), doing procedures under guidance of attending, and teaching; typically includes a research rotation |
Mastering procedures and clinical routines; passing specialty boards; acceptance into fellowships; entry into practice |
Fellowship (subspecialty training and research) |
Research, advanced procedures training, and clinical practice; often involves continuous teaching and practice in related specialties |
Completion of program required for specialized practice or academic role |
Practicing physician (private or group practice) |
Learning in reflective practice on the job; learning how to manage a practice; logging clinical learning hours for continuing medical education |
Growing a practice and maintaining currency in the field |
Attending physician (medical faculty and practicing clinician) |
As faculty, learning advanced clinical practice; clinical research; continuous learning in course development |
Academic progression; senior staff roles; publication |
Trends in clinical education change slowly because disrupting the education of doctors midstream may introduce unforeseeable risks that outweigh the value of innovation. Educational IT has always lagged practice and cannot be counted on to drive professional change. To be acceptable across institutional contexts, online resources must precisely represent acknowledged canons of education and the baseline of current knowledge, not the cutting edge of new procedures and devices.
Fundamental changes to education are formulated as top-down strategies. Yet innovations in technique and technology are often created as bottom-up practices that are advanced further in the training context. These are very different types of innovation.
Information Practices in Clinical Education
Medical, nursing, and other clinical education programs require intensive didactic learning, journal reading, anatomical dissection, patient interaction, hands-on procedure training, and technology-based simulations. Few educational experiences match the intensity and variety of teaching methods found in clinical education. It involves learning medical skills, technical and machine skills, communications practices, coordinated team activities, institutional rules, and industry regulations in a demanding institutional setting. Learning takes place across multiple knowledge domains—physical, biomedical, biological, technical, organizational, and informational. It demands full attention to the clinical tasks at hand, and optimal access to facts, formulas, lists, and techniques from memory. The trainee’s information tasks cannot be separated from care practice or the learning process, and because medical research, knowledge sharing, and technology never go idle, these drivers continue throughout the entire life cycle of professional practice.
Clinical education is not yet directly delivered online to the extent we find in administration (trainee evaluations and logs), clinical data (health records), or clinical decision making (online research resources). But it is rapidly changing. Education is a complex multichannel activity, and not every task can be—or should be—automated or socialized online. Most medical schools video record all basic science lectures, and students download and watch them on their own time and often at rapid speed. Students now spend more time in small problem-solving teams than in lecture halls. These are contextual changes, not technologies. Allocating mere content and clinical media to the Web as if it were facilitating education would be a significant mistake.
Health Information Technology as a Learning Ecology
Residents are both doctors and advanced students, and are constantly learning from experience with patients and colleagues in clinical situations. Although they have access to a wide range of resources to answer questions and concerns, many often start with Google, using specific terms to filter out nonclinical references. Residents report that they generally select only high-credibility site references (not Wikipedia).6 They may start an information search on familiar sites, but then follow completely different information paths than nonprofessionals.
Residents show a different path through the information ecology than other clinicians. They navigate between resources located in care networks (electronic and social system networks supporting direct care services) and learning networks (networks and resources enabling tools for education and research) during their training. They may start and stay with learning networks in their internship and second year, then use care networks more often in their final years in residency as they learn the core curriculum and move fully into practice. When preparing for board examinations, residents cite a 3- to 6-month return to resources in learning networks.
Within a given day or week, residents may navigate among all the health IT (HIT) domains. Figure 6.2 shows the pathways among information resources followed by a typical resident during the course of a clinical day. In this scenario, a junior (first or second year) internal medicine resident may interact with seven different HIT resources during her first half-hour in the office:
- She begins by reviewing the case schedule for the day to consider the treatments and procedures that will be needed and to prepare for questions that may come up during the rounds. She scans the patient list by unit in the Epic EMR. The EMR is efficient because the records of a given patient can be selected to view their current (or overnight) status and any updates to a condition or schedule.
- An older inpatient being treated for liver complications from diabetes was to be discharged, and was observed with emergent vertigo. The case was moved to the top of the list, so the resident takes a few minutes to search the issue on UpToDate to help determine a care plan.
- UpToDate does not help diagnose the causes of vertigo, so she scans for a clinical review article from recent research. Suspecting a drug interaction as the cause, she continues to research quickly for another 5 minutes, evaluating the scheduled medication.
- A lung cancer patient requires a chest tube insertion to drain fluid accumulation. Although she has seen many of them performed, she is scheduled to perform the next one herself, so she scans the steps on Procedures Consult and watches the insertion steps on video. She prints the Quick Review as a checklist, just in case.
- Reminders from the McKesson practice management system identify two patients to see before their release from the hospital.
- Reminders from the hospital laboratory service indicate that new images and blood tests are available. She scans a blood workup and then consults UpToDate on the values.
- She refers to Epocrates to determine medications and dosages.
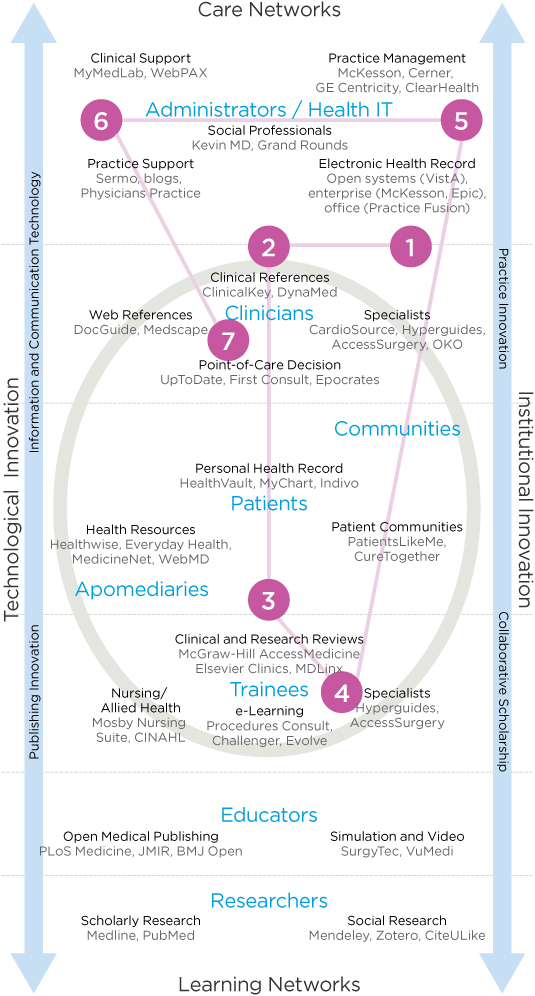
FIGURE 6.2
Pathways through the health information technology ecology.
There is no “one-stop shop” that integrates these resources, and as obvious as a single-source portal may seem, there have been no successful innovations of such a resource. Each is optimized for a specific information task, and that purpose may be incommensurate with even closely adjacent tasks. Billing and diagnostics are different use contexts, and superficial integration can be counterproductive. For example, attempts to integrate point-of-care information (e.g., UpToDate) with the EMR’s diagnoses and lab results have not caught on. The assumption that patient condition data in the EMR might trigger a search is backward—the search for a condition at the point of care will assist in diagnosis, which would then be entered in the EMR.
Even if learned in coursework, clinical informatics are first used while on the job. Except for the EMR, only minimal training at best is expected or provided to busy doctors. The constant time pressure in a typical clinical day reveals the need for extremely concise information objects and minimal cognitive overhead. UX and information design enhancements can make a significant difference in “time yield,” and saving a clinician a few seconds in a page view or navigation will be noticed and immensely appreciated.
Practice Research for Institutional Innovation
Practice-based clinical research is conducted to improve health outcomes by evaluating new routines based on empirical evidence from practice. Simple yet high-impact studies have led to better infection prevention, procedural error reduction through checklists, or better patient care flow to reduce wait times. Practice research can make a more significant and immediate contribution to patient health than traditional biomedical research, although disease research and drug trials make news and get funding. Practice studies are often overlooked as boring and administrative, yet recommendations can be applied generally and immediately.
Practice research can have a direct impact on individual patients’ lives. The current mandate toward adopting preprocedure and patient checklists in hospital care is the result of practice research on the clinical effectiveness of checklists in preventing iatrogenic infections. The simple “undesigned” checklist reminds residents and surgeons of basic steps in both complex and simple tasks. Physician and author Atul Gawande attests to the impact of this simple solution in his popular book The Checklist Manifesto.7
Gawande reports on the adoption problems associated with the very introduction of checklists into the work ecology. Checklists are generally associated in clinical culture with nurses and management, and doctors resist the idea of using checklists on a clipboard to remind them of the most basic steps in a process. Yet, studies performed by Peter Provonost at Johns Hopkins demonstrated that a basic infection-control checklist reduced deadly complications with central venous catheters, a common infection vector.8 In the initial experiment, the 10-day infection rate dropped from 11% to zero. In a 15-month trial, only two infections were reported. The study indicated that the checklists prevented up to 43 infections and eight deaths and saved at least $2 million in associated costs.
The institutional checklist is typically a printed form with a single page of brief items to check and perhaps sign as an accountability routine for clinical care teams. Checklists are provided for both standard patient protocols and procedures. Checklists are starting to appear in online form as a guideline or reminder. A standardized protocol for basic procedure safety and accountability is shown (in part) in Figure 6.3.

FIGURE 6.3
Universal protocol for procedure safety. (From the Joint Commission, http://jointcommission.org/standards_information/up.aspx)
Why do such obviously beneficial instruments find resistance in the regulated, risk-averse hospital environment? Two cultural factors work against the acceptance of checklists: clinical culture and research reputational culture. Senior residents often admit that they would not personally use (visibly) a universal protocol checklist, online or printed, of the type deployed in these studies. Universal protocol checklists cover patient identification, informed consent, marking the right site, sterile hygiene, and a “time out” to ensure general clinician situation awareness. Where an institutional mandate exists to use checklists, nurses or first-year interns typically follow the resident’s procedure against the checklist as a delegated duty.
Practice research is also overlooked due to reputation factors. Although major published studies have demonstrated the efficacy and significant cost savings for guiding technical work with simple checklists, this practice research is often treated as second class by the scholarly world. As professor of dermatology Jonathan Rees pointed out, the funding and status bias of medical research relegates practice to scholarly irrelevance:
Most medical researchers rarely, if ever, see patients. . . . However, many of the major discoveries that have had a direct impact on clinical practice arose from clinical disciplines rather than from generic biomedical approaches—consider hip replacements, cataract surgery, the importance of Helicobacter pylori, phototherapy, in vitro fertilisation, and minimally invasive surgery. In the biomedical model, these successes are brushed aside as being of historical interest only. From finding genes to gene therapy and stem cell therapy, both the public and research community itself are fed a biased view of medical advances. Cancer cured (in mice) again.9
Innovations Emerge from Practice Needs
High-impact innovations are more likely to originate from observing common situations in everyday practice. Many of the popular clinical information resources originated as simple content services created by a physician who saw a need in everyday practice or learning (including UpToDate, the Wheeless and OrthoBullets services for orthopedics, and the VuMedi surgical video–sharing service).
Sermo, a specialized physician networking service, was started as a way for doctors to exchange answers and ideas, and to replace the collegiality that doctors miss from their residency days. Sermo also originated from observations of a practice need, as founder Daniel Palestrant recognized that small-practice physicians have few ways to ask questions and exchange information with other doctors in an environment of trust and privacy.
These products all followed the essential Christensen model of disruptive innovation in their respective markets.10 Harvard professor Clayton Christensen famously established the pattern of disruptive innovation, where the entry of a noncompetitive new product, serving an emerging market niche, can rapidly grow by increasing technology performance to overtake existing markets. The theory distinguished disruptive from sustaining innovations, which can be significant developments in their own right (e.g., new EMR systems that replace outdated products), but do not form new markets.
Each of these services started as a niche product in the physician marketplace, originally envisioned by a fellow doctor who observed a need from his or her own practice. Most of these introduced alternatives to long-standing practices, where a different approach was not yet obvious. An emerging innovation will be ignored by competitors because it focuses on an unprofitable and small market and poses no threat to their share. But some of these local solutions could grow quickly into popular platforms and replace current paradigms entirely. UpToDate has grown with the widespread use of the Web and has replaced the reference books and literature searches prevalent before its success. As an early innovator, it was able to enter and capture the emerging market.
Given the sheer size of the healthcare market, it is telling that the disruptive innovation model has not been widely successful. Few sustainable successful digital innovations or online resources have been developed from the findings of clinical research. There are no successful health records platforms developed as disruptive innovations. Several well-funded, high-profile innovations (such as Google Health and Revolution Health) did not capture markets as expected. Cost savings and user experience are also not (yet) the significant drivers they are in other markets, so designing a better user experience is not necessarily a sustainable advantage. The healthcare sector is different.
Improving Clinical Decision Making
Clinical point-of-care and clinical decision reference systems are ubiquitous in residency. Interns now carry smartphones loaded with Epocrates and eMedicine. Today’s residents are digital natives, having grown up with the Web, and are now the first cohorts of e-doctors. Since the early 2000s, hospitals have placed networked desktop computers in every ward, meeting room, and common area. Many clinics have switched their staff from pagers to smartphones. Physicians have access to high-quality online clinical references and research at any location or time, from the desktop or mobile devices.
As institutions implemented enterprise EMRs, one of the attempts at integration was to directly augment clinical interaction associated with patient records.11 Available for over a decade in Web and handheld formats, clinical references are being integrated with patient records as EMR assistants. This HIT integration leverages the idea of actuating information in context, enabling the clinician instant access to drug or diagnostic test information from a preferred clinical reference database when a question arises or when prompted by values in the patient record. It is presumed this functionality will increase practitioner satisfaction with and use of the EMR as well.
There are two problems with this idea: the assumption that the EMR is actually at the doctor’s point of care, and that the EMR displays terms that may require clarification by a reference search. These problems can be resolved by reframing how decisions are actually made.
The literature is inconsistent regarding the true effectiveness and appreciation of clinical decision support systems (CDSS) at the point of care.12 In most cases, these studies should be considered a snapshot of the state of the art of the time. Though the trend 5 years ago was to integrate information at the point of the EMR patient record, the main users of the clinical reference at the EMR would be junior staff, as they would have both the need (based on less experience) and inclination. Experienced physicians are not inclined to use these resources when at the EMR. This attempt at integration may only be adding cognitive complexity to an already overloaded interface and task.
Experts and Clinical Information Practices
How do clinicians mobilize information at the point of care to form diagnoses, determine treatments, or make patient care decisions? My research on clinical information practices explained the differences in information drivers and online practices between residents and senior clinicians, all with access to the same HIT resources.13 Differences are found in the intended clinical uses of information, but these applications also reveal differences in decision-making style associated with experience. Junior residents deal with relatively common, everyday situations that they might be able to resolve from clinical references such as UpToDate. Less experienced doctors need to understand the current state of the practice, any controversies regarding treatments, and pitfalls in therapies they may consider. These are issues well covered by online references.
Expert clinicians refer to different sources and content types than residents, and read them differently. Senior clinicians (5 or more years postresidency) may be both teaching faculty and practitioners. The more experience clinicians have, the less need they have to refer to a common condition. They are also less likely to see routine cases that residents can handle, so they do not require access to a comprehensive database of medical conditions outside their specialization. Because senior clinicians see the tough cases, they refer to primary journal sources, using searches in PubMed or Medline. There they are confronted by the problem of searching a vast biomedical literature that is not indexed by diagnosis or clinical condition but by research topic.
All doctors are time limited. Senior physicians may have much less time available than a resident for problem solving, and they deal with more complex cases. Senior practitioners (in an inpatient setting) have less need to look up general points of clarification for symptoms or diagnoses because they have a broad personal repertoire that surpasses the simplified outlines in the references. They may also specialize (if in a group practice) and can refer to another specialist for a unique problem they may encounter, again more reliably and probably more quickly than with online research. Practitioners usually develop expertise in an area and are known for and relied upon for complex or exceptional situations.
These findings are consistent with cognitive scientist Gary Klein’s view of expert reasoning, known as recognition-primed decision making.14 Physicians’ decisions for complex and ambiguous clinical situations appear to be intuitive and personal, not rote or formulaic. Intuitive mastery comes from the ability to adapt deep patterns developed in the clinician’s experience. This reasoning has been discovered across domains of expertise.
Clinical Sensemaking
We can observe two cognitive modes of information behavior—clinical decision making (a task supported by online resources) and clinical sensemaking for complex cases (a problem-solving characteristic of experts).
Decision making selects an optimal choice from options presented in response to a case. Because trainees do not yet have a deep repertoire (1,000 or more cases) from which to make self-informed judgments, they are obligated by training and liability to formulate decisions supported by evidence, rules, and best practices as documented in current informatics resources.
Open-ended navigation of a difficult problem based on limited information and an unknown content base is a classic sensemaking situation. If clinicians analyzed complex cases (such as rare diseases or odd symptom patterns) using a process of elimination or exhaustive search strategies, their solution-finding process could take longer than a patient might live! Sensemaking involves the assimilation of multiple streams of information and observations, testing hypotheses drawn from experience, using intuition to resolve gaps in knowledge, and accommodating (not necessarily resolving) ambiguity.
The New York Times Magazine’s “Diagnosis” column presents true stories of people dealing with complicated health conditions. These diagnostic puzzles are not easily solvable by logical analysis and are often resolved by an individual with unique prior experience with the exact condition. The column reveals the power of recognition as opposed to analysis. Some nonphysicians are able to correctly identify a rare disease from a brief narrative because they have personally encountered the disease and relied on pattern recognition of the symptoms and unique characteristics.
A clinician faced with a complex disease presentation, such as an unforeseeable complication, has the goal of resolving the patient’s source condition. A truly complex situation involves not just information and a course of action and observation, but might entail patient culture and family conditions, personal history, unique biochemistry and medications, prior diseases, and unknown allergic responses.
Gary Klein takes expert sensemaking further toward decision making. Sense-making is not a set of discrete steps, but rather a continuous process of testing the data of reality against different frames or models that the clinician poses to the data. Klein’s data-frame sensemaking presents the mental construct of the frame guiding the interpretation of data (symptoms, signs, signals, information) to achieve an outcome or resolution to a problem.15 Think of a frame as a mental model about a problem area populated with known facts, opinions, and memories from personal experience. A frame selects the perception of cues in the data and environment, enabling the efficient selection of possible meanings. Experts “make sense” by preselecting information that counts, rapidly sifted by their frames.
Unlike trainees, experts exhibit confidence in their frames and are willing to commit to them sufficiently to explore hypotheses available within the frame. They serve as filters or assessments to test how well a situation matches the frame. Sensemaking pulls together plausible narratives from the data that enable the expert to present a coherent “answer” rapidly.
No checklist is thorough enough to document and resolve the unique challenges of the human body in its environments. Many diseases behave as wicked problems, with multiple interacting sources and the inability to clearly determine an effective course. And each decision counts, as the opportunity cost of inappropriate treatment can be the loss of health or life.
How do we best design information tools to assist experts in sensemaking—in recognizing problems—when few have the experience to identify these unique conditions?
Fitting Informatics to Workflow
Practitioners often report that their use of online information tools declined throughout and after residency. As clinicians progress in a career, they have less need for information support at the point of care. They learn from their own case experience, establish a repertoire, and keep current using more advanced resources such as journals and review articles. This shift in information practices is consistent with recent experiments showing that senior clinicians use a “conscious reasoning” style for complex diagnostic problems, a process of supplementing or activating the scaffolds of long-term memory patterns.16 Junior doctors have not constructed deep enough experience (or frames) to pursue conscious reasoning, so tend to follow the published guidelines or procedures for an identified condition.
Other studies have found that clinical references can improve performance but are rarely used by clinicians at the point of care or with the EMR. This variance is explained by suggesting that use of references is not yet established within the clinical workflow, or that interfaces are insufficiently easy to use. It is more likely that the EMR itself is not a conducive context for information for clinical problem solving, even if helpful patient data is provided at the point of care. Enhancements in usability and content may not result in increased adoption, as we would expect in other fields. In the institutional context, clinical need, habit, and ease of access are bigger factors than interaction usability.
Well-designed information resources and EMR interfaces present information aids following a temporal clinical workflow—the series of tasks performed in their standard sequence in the clinical setting. A standard workflow starts with patient presentation and delivers information aiding for some configuration of the following standard tasks:
• Presentation: the patient’s signs and symptoms as observed
• Diagnosis: identifying and testing possible conditions that match the symptomology
• Indications and contraindications: the guidelines for treatment or avoiding certain treatments
• Treatment: therapies appropriate for the various diagnoses associated with the condition
• Procedures/techniques: steps to perform procedures to deliver treatment
• Prognosis: expectations for patient recovery or clinical outcomes
• Aftercare: guidelines for postprocedure recovery or home care
• Patient education: guidelines presented to the patient about the condition or treatment
Explicit workflow provides temporal bookmarking, giving a clinical user confidence in the regularity of content organization, which aids findability. But residents and attendings often see patients for just a single step in the workflow. They are picking up where other doctors left off, and are literally “thrown” into acting without having the ability to step back, reflect, and research. Assumptions of a primary disease and prior history precede the encounter. Other disease conditions may muddle the diagnostics, and medications may be potentially conflicting.
The conventional EMR, with its static patient record and incomplete history, cannot advise the clinician on real, implicit patient needs. If it attempted to do so, there would be little trust in the advice. The accuracy and context would be thought questionable, even if the advice was correct. One poor-fitting recommendation would doom such a feature forever. Instead, the closest approximation employed in practice is the clinical alert, the reminders and suggestions from the EMR rules database to prompt care teams to review possible conflicts or harmful outcomes from prescriptions or orders placed through the system. EMRs have been designed with a bias to push alerts to responsible caregivers, resulting in too many alerts and alert fatigue.
Several reasons for alert fatigue have been empirically discovered, but the primary reason appears to be that the vast majority of alerts are for possible drug interactions. In 2009 Harvard’s Beth Israel Deaconess Medical Center analyzed a collection of more than 233,000 alerts and discovered that 98% warned of drug interactions, and that 90% of these were overridden. At this level of irrelevance, physicians likely override or tune out other alerts as well.
One solution to this informatics problem has been to encourage smarter asynchronous alerts—the updates sent to the caregiver’s activity stream at a later point to indicate possible outcomes as a patient’s physical status changes. Not only were these overridden less often, but they were found to be more relevant because of their responsiveness to changing contexts that the clinician could not have observed.
Cognitive Aiding of Team Care
Are new types of clinical information aids needed? Do experts only need clues and tips for unusual situations to act as a nudge in the right direction? How can a caregiver’s always partial knowledge best be supplemented?
For decision aids to be effective, the information presented must be associated as specifically as possible with the context of a care situation. Yet these situations differ by specialty, clinic type, stage of care or treatment, and so on. The point of care is really a different point for every type of clinician.
The points where care occurs are spread out across clinicians and over time. Complex care is often performed by a clinical team, not by individuals working alone. Yet medical training emphasizes (and certifies) the performance and knowledge of individual physicians. Some residency education programs have reoriented their training toward team care, but these approaches are necessarily incomplete. Every residency trains doctors for a particular specialty. Coordinating team training across clinical specialties confronts a complex organizational environment that few (if any) institutions have resolved sufficiently.
Research and best practices are showing the value of developing explicit team practices in healthcare. Teamwork as a guiding process helps reduce individual and technology-induced errors, improves the coordination of care, and builds a culture of collaborative work in the clinical environment. Team-based care practices have not propagated widely in US healthcare, though they have been shown to improve patient outcomes. Healthcare management has yet to develop team approaches to process and workflow improvement, and team models have only recently been introduced into medical training. Several approaches to team development in 21st-century healthcare are recommended:
• Training team approaches in real-world settings
• Debriefing and solicitation of feedback from team members
• Including all team members in patient care planning and service decisions
• Creating processes of collaborative care as continuous improvement17
Field studies of clinical team performance in acute care,18 emergency room, and critical care operations19 reveal how complex operations and procedures are performed as integrated team practices. Technical clinical work is distributed across physicians, nurses, pharmacists, and anesthesiologists, all working on different requirements for an inpatient situation. For coordinated procedures such as surgeries and intensive care management (e.g., cardiac or respiratory incidents), standard teams of specialized clinicians are assembled as transient crews, performing roles according to expectations for well-trained situations. Teams are created and dispersed as needed, demanding a skill set of flexible collaboration by role, clear communication across roles, and giving and receiving feedback to improve collaboration.
In large hospitals, individual roles are trained by constant performance, and clinical teams conduct complex operations together with minimal planning and guidance. A visiting surgeon may work with surgical nurses and an anesthesiologist who have never met one another before the case. A well-known point for the universal checklist protocol includes a step to ensure each member of the surgical team knows everyone’s names, to promote trust and coordination. The surgeon may provide directions and exceptions to standard protocols, but for standard procedures, strict role coordination has passed as teamwork for many years.
Each member of a clinical team performs technical work assigned to their role, communicates as needed with specific team members, and uses information and cognitive artifacts pertinent to their role. Yet the true collaborative teams envisioned by progressive institutions have not emerged. The efficiency, accuracy, and completeness demanded in acute care and clinical procedures sets boundaries on the ability of clinical team members to exchange roles or collaborate. There are no agreed processes for collaboration beyond the strict assigned roles in care planning. Today’s clinical teams may believe they are working together in teams as effectively as possible. Yet a barrier to change or innovation is the belief that a current process has been optimized.
Design Sensemaking
A comprehensive picture of clinical IT domains for point-of-care, administrative, and specialist services was shown in Figure 4.4. Many of these tools help guide and educate the clinician to make better care decisions, and so should be evaluated by their effect on patient outcomes. This turns out to be a difficult result to measure.
Studies of CDSS over the last decade revealed high user satisfaction with these tools over time, but this finding may also reflect several known biases in sampling. Study participants are mostly residents, who as trainees appreciate having any information resource that helps their learning and performance. Early technology adopters (e.g., younger doctors) also are able to cognitively adapt their decision-making style to new tools and tend to be less critical of the tools’ shortcomings because they have probably not used the many alternatives. In other words, CDSS resources could be much better, and the sample has insufficient experience to make the demand. They also do not usually pay for apps and information resources, so are not behaving as vigilant customers.
In contrast, clinicians in practice are much more deliberate in adopting information technologies, whether an EMR or clinical reference. As found in studies of other professional practices, doctors become highly skilled in resources learned in graduate education, and they maintain facility with those tools that were learned in their generational period. Learning new HIT requires commitment—not just the time to learn but the cognitive burden of learning to use a resource that may be used infrequently or that may require changing a stable set of known routines.
How well do clinical references like UpToDate or Epocrates fit the clinician’s work ecology or decision making? Even though these tools may be commonly used and accessible through a university library subscription or hospital intranets, their use is always discretionary. Many different resources could be substituted for the same information need, and few institutions narrow their options to just a single provider.
Discretionary resources are third-party products that present content with no relationship to the decision context in which they will be used. For example, WebMD may provide useful (if nonexclusive) content, but the interface is overloaded with links, categories, and advertising, all of which bias the user experience as a commercial product (see Figure 2.1). The assumption is made that WebMD is being searched and read just like any other website, but the health seeker might be in a critical or compromised situation and not have the luxury of navigating the complicated interface. Health information needs, unlike most other information tasks, may indeed be urgent.
The risks of designing clinical informatics as end-user products is that the vendor can make design and content decisions without regard to the actual clinical task needs. Design decisions are often and effectively made based on inferences from a small number of observations, but the more distant these observations are from the front lines of practice, the higher the risk of irrelevance or commercial bias. The deciding factor for clinical resources should be whether the content design and user experience enhances or augments care practice and patient outcomes.
• Good CD2.0 opportunities include interaction design of clinical information resources, procedure simulation devices, and interactive learning environments. These contribute to the overall redesign of healthcare education and practice.
• Educational service design needs a flexible strategy, a scalable service, and an organizational mindset. A CD3.0 strategy may integrate existing educational services (CD2.0) to adapt new resources to organizational strategy (CD3.0) and scale across the healthcare education sector (CD4.0).
• Advanced service design can provide multilayered support systems: training to support end-to-end guidance for complex procedures, scaffolding for quickly learning manual skills, and ambient and portable displays to enable distributed cognition and collaboration.
• A vast range of clinical and reference knowledge is employed in medical curricula. E-learning platforms have recently shown promise, if designed well, to improve curriculum management.
• Institutional drivers such as a certification or standards push, or even an organization-wide campaign such as an infection control strategy, may have key touchpoints with your service concept and will pull it through the organization. By aligning service enhancements to anticipated or new institutional requirements, the service changes will be accommodated as part of the expected change.
• Designers should not aim to reinvent clinical practice or workflow (not yet). Clinical work is taught and deeply ingrained, and innovations that attempt to change labels or paradigms may just be rejected as poorly adapted to clinical reality.
• An iterative and perhaps lengthy process of service prototyping is necessary for any clinical education or practice workflow redesign. Iterations should be used as opportunities for increasing the circle of stakeholders involved in evaluating the process redesign.
Case Study: IDEO Continuity of Care
The California HealthCare Foundation (CHCF) sponsored a unique design challenge, led by the global design firm IDEO, to innovate the process for managing a patient’s continuity of care. Collaborating as a team with CHCF, IDEO brought representatives together from the largest US organizations managing health information, including the US Department of Veterans Affairs and EMR system vendors Epic, Cerner, McKesson, and others, to uncover new possibilities for improving patient opportunities in the process.
The design study focused on the expressed need to improve the continuity-of-care document (CCD), which communicates and updates a patient’s medical status and complex health concerns between clinicians over the duration of their care. Physicians and staff record and transfer patient information from diagnoses and tests, from primary care to specialist referrals, and between electronic health records.
Two major design opportunities were discovered in the continuity-of-care process: the inclusion of patients in communicating their own continuity, and the enhancement of EMR systems to enable patient collaboration with their care. IDEO’s Abbe Don led the joint team’s co-creation and design process to resolve health information and sensemaking concerns from patients, caregivers, and physicians. Four care challenges were discovered:
• Patients feel they are left on their own to figure out next steps.
• Patients with serious health issues work around the system to get the best care.
• Episodic and disjointed care hides valuable connections.
• Both patients and physicians doubt the reliability of (reported) health data.
These were translated into four physician needs and seven patient drivers for continuity of care. These were considered novel because the patient is not a user of the document but is rather a real life carrier of the data. In more patient-centered terms, these issues were expressed as:
• Represent what I truly care about.
• Present information in a way I can relate to.
• Help me cross-check my facts.
• Help me close communication loops among my care team.
• Set me up to have clarifying and guiding conversations.
• Clearly lay out the next steps.
• Show my trajectory over time.
The participatory workshop drew out compelling insights for future alternatives:
• The CCD process is not patient-centered because it is a document shared between physicians. It was never designed to be a patient instrument, so these emerging patient requirements are novel.
• Patients are starting to see the need for a secure and persistent location for their health data. A simple data storage solution (“Personal Health Record 2.0”) was considered a design concept to pursue.
The research and workshop led to two design concepts and several future scenarios. Figure 6.4 shows a single page of the concept wireframe reflecting the design of patient-centered CCD data for a shared context. Patient and physician information needs (as shown above) are checked off for their relevance to the concept. Patient questions and concerns guiding the need for this view are shown in the page.
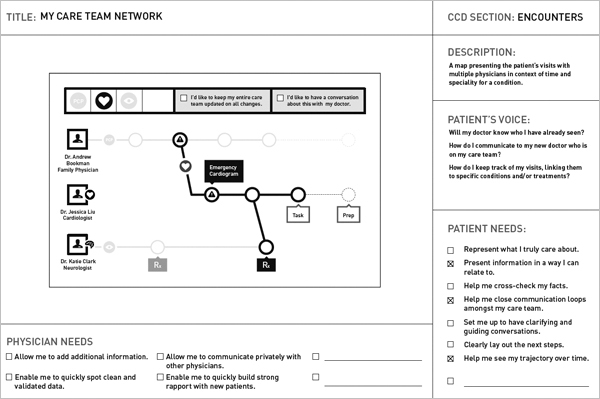
FIGURE 6.4
IDEO Continuity of Care: Project Synapse concept. (Courtesy of IDEO)
Two prototypes were prepared to reflect two modes of patient-centric CCD data presentation. The Timeline Meets Accordion concept (Figure 6.5) connected critical data elements that are today separated by multiple encounters and reports. The “accordion” view displays a section for each dimension of continuity, here shown in a website or tablet view, allowing the patient or physician to update and follow the history and current status of the healthcare journey.
A second “collage” concept presents a visual dashboard in a series of labeled visual boxes highlighting the current status or prominent data by selection. This concept suggests a future representation of patient care data in a mobile-friendly iconic display, integrated with calendar scheduling, communications, and patient health records.
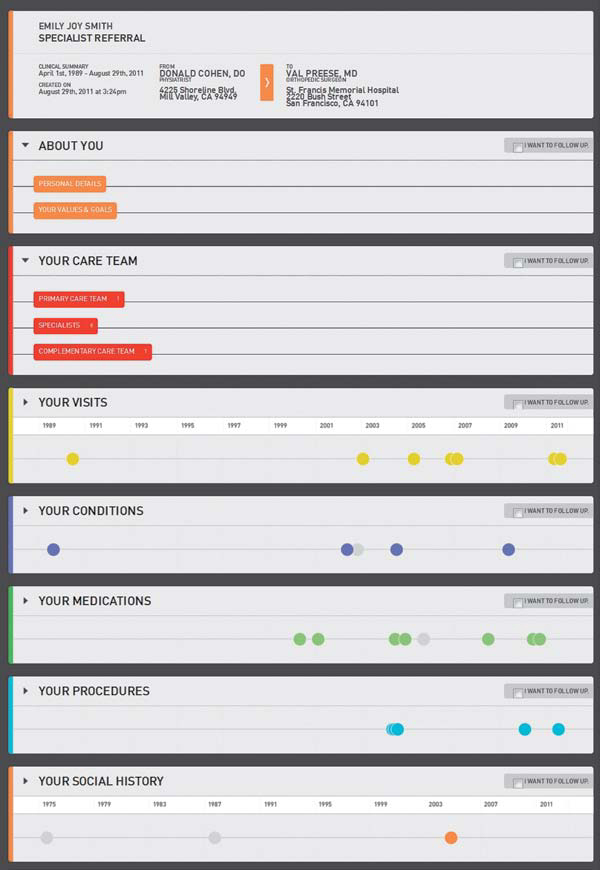
FIGURE 6.5
IDEO Continuity of Care: Timeline Meets Accordion. (Courtesy of IDEO)
These concepts support at least two scenarios or directions. One notion was a dynamic care plan process where the CCD served as an action plan for patients and a way to mutually align patient-centered goals with clinical team care and treatment schedules. Another scenario envisions an advanced EMR interface that summarizes patient data (“at a glance” summary) to create a mutual care plan. In both scenarios, the CCD process has been transformed from a passive data presentation to a patient-centered, active planning tool.
Project Synapse is presented online at CHCF (www.chcf.org/projects/2012/project-synapse-ccd-design).
LESSONS LEARNED
• Complex healthcare information problems can be simplified by articulating a new process that provides value to both providers and patients, rather than compromising on a format that serves only one or the other.
• The CCD case presents a fairly universal problem area, where many patient types might be expected to have similar information and navigation needs. A small-sample, rapid-research process was sufficient to develop the initial concepts due to the universal context of continuity of care.
• Nonprofit or agency sponsorship (such as CHCF) can support system-level design by engaging multiple vendors in the early design process, enabling all to respond to and benefit from the finding of a common research program.
• The CCD project also led to continuing universal design in the form of the patient record, with an open design contest sponsored by stakeholders from the Synapse project to solicit concepts for electronic health record design. See Blue Button: Challenge at http://blue-button.github.com/challenge/.
Methods: Stakeholder Co-creation
The Basadur Simplexity process is employed in a service design context as a powerful methodology for engaging stakeholders directly in co-creating their service concept. The second stakeholder design method relevant in the larger service context is Positive Deviance, one of a family of methods for organizational engagement. Both of these methodologies have widespread application and can be employed in other D3.0 and D4.0 contexts, such as management, customer-facing services, and information systems.
Simplexity Thinking
Min Basadur’s Simplexity process has been in continual use and development since its inception in the 1980s as a creative problem-solving technique.20 Simplexity thinking harnesses a group’s ideation to generate a series of responses to successive levels of problem inquiry, resulting in a challenge map that serves as a road map to strategic design.
• Applications: The Simplexity process is used for strategic planning, ideation workshops, collaborative innovation, and participatory design workshops with clients and stakeholders. Because of the time necessary to conduct a full session, it is not generally employed with user participants as a user-centered design method. It is effective for identifying true priorities and mapping design proposals. It can be used as a macro-design structure for other methods and for project planning.
• Requirements: Simplexity workshops can be conducted with almost any group size, from 5 to 50 participants. Multiple facilitators are required for larger groups, but even with a small group, assigning a lead facilitator and a recorder/assistant is helpful. Breakout groups are typically most effective with five to seven people.
• Workshop structure: A full workshop requires an all-day commitment, or up to 2 days for larger groups or more complex problems. Planners should count on taking 30 minutes to introduce the session and orient participants, and up to an hour or more for each stage in the eight-stage process.
• Preparation: Simplexity is a sophisticated design process that requires training or experience to perform well. Trained Simplexity facilitators may require 4 to 6 hours of planning to design the sessions and organize the materials and logistics.
Simplexity guides practitioners through a series of progressive transformations of group idea generation and consensus selection. The entire process moves from generative problem formulation (A) through solution formulation (B) and implementation (C) in three phases and eight steps (Figure 6.6). This process can be followed for narrowly focused problems, such as identifying the best new features for a successful software product, or it can scale sufficiently to empower executives and stakeholders to formulate an entire business strategy and action plans.
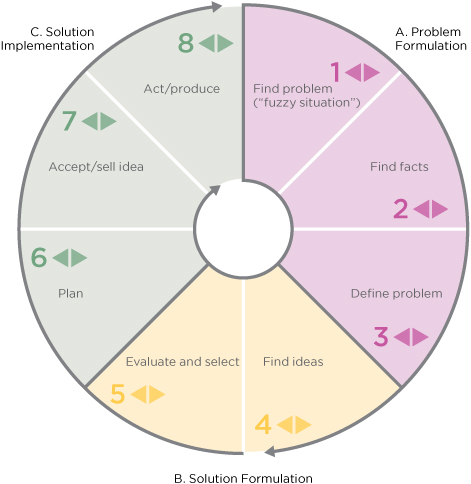
FIGURE 6.6
Simplexity method phases and steps.
Creativity is maximized by constraining the process and allowing participants to focus on content. The eight steps follow a necessary sequence to ensure that prerequisites to each step are co-created. Fact finding is enhanced by achieving a powerfully framed problem. Clarification of the elements of the problem enable problem definition, which helps people find ideas that suggest solutions. Solutions are formulated in a two-phase dialogic process (Figure 6.7), moving from divergence (the goal being quantity and a wide range of ideas) to convergence (the goal being quality and selecting consensus ideas) as final solutions are selected in a rapid evaluative process.
FIGURE 6.7
Dialogic process language.
The best ideas are meaningless in innovation unless they are packaged for the audience, owners, or customers of the proposed solution. Simplexity takes a group of participants through a collaborative thinking process documented by sticky notes on boards organized to represent individual contributions for each of the eight steps. Each step is characterized by a common process of divergence and convergence, represented by the “<” and “>” symbols, respectively.
The process is not analytical and reductive in any stage, but instead reaches “convergence” of selected ideas by group progression. It organizes group participation around a central challenge, formulated as a focus question. Used as a facilitated and consultative method, the Simplexity process enables groups to generate highly readable problem maps and consensus solution scenarios based on a high variety of scenario alternatives.
Overview of Simplexity
Simplexity begins with a fuzzy situation, a deliberately undefined problem. Viewing the fuzzy situation from a neutral position, Simplexity guides a group of stakeholders from problem formulation to solution implementation. In each step, a process leader facilitates divergence, and participants generate ideas without judging or analyzing them. After a broad range of possibilities have been generated through divergence, participants converge to select the options that will be brought forward to the next step.
The three-stage implementation process ensures that the design team’s momentum carries to the planning and promotion of the selected solution as the way forward.
Simplexity in Practice
As simple as the process appears, the power of the innovation method is in organizing ideas so that collective wisdom is generated in the process. Participants recognize the emergence of transformative patterns of ideas as they work through each phase. The continuous “testing” of group ideation through multiple cycles ensures that ideas found to be sufficiently effective and wise are raised and refined through iterations and dialogue.
The Simplexity process model draws on inductive reasoning, synthesis, and abductive reasoning. Inductive reasoning is involved in the divergent steps in each phase, wherein participants generate ideas that answer the question. In the convergence step, participants label and select appropriate example ideas.
Synthesis is involved in each phase as participants align a set of concepts with the problem carried forward into each step and fuse related ideas together in new forms.
Abduction is the style of iterative reasoning used in effective problem solving, even if we do not consciously recognize it. Abduction occurs when we reason between inductive particulars and deductive explanations and find new formulations. By associating and comparing concepts in a structured network (such as the challenge map), people can explore meaning in the relationships between items and derive net new concepts.
A challenge map represents the set of challenges identified by participants and is a dynamic mapping of the sequence of ideas generated from problem solving to solution finding. It is generated collaboratively by positioning ideas from stakeholders in a network, displaying them from specific tactical needs (at the bottom) to strategic and desired outcomes (at the top).
Simplexity is structurally similar to other systems-thinking methods. The challenge map process is analogous to the abstraction hierarchy (Chapter 7) and problematique (Chapter 8). It aligns with other design and system-thinking models that leverage multiple modes of reasoning and cyclic structures to model human systems.
Positive Deviance
Positive Deviance (PD) is a stakeholder innovation practice that guides organizations to discover native solutions to difficult problems in processes or operations. Homegrown innovations can be located by scanning the organization for effective (or positive) deviances from the standard operating procedures. Healthcare and hospitals are often managed very closely to their established standards to ensure a consistent level of performance and to mitigate risk. However, innovative problem solving that is initiated at the front lines is often overlooked because, although not harmful, the “deviant” processes are considered contrary to accepted practices.
PD is based on the recognition that in every organization a small number of individuals demonstrate uncommon behaviors and strategies that represent possibly better solutions to problems than the mainstream practices. PD originated in the health field in 1976 and became widely followed after Tufts professor Marian Zeitlin compiled surveys that revealed how “positive deviant” children in poor communities were found to be better nourished than others due to their own independent resources.21 The core goal of PD was to address childhood malnutrition at the grassroots level by identifying positive behaviors that worked well, and to reinforce the effective minority behaviors as opposed to focusing on critique and fixes. PD was further institutionalized as a process for social change, expanding to difficult problems in diverse areas such as public health, education, and healthcare.
A recent significant and successful PD application was conducted at six Canadian hospitals over an 18-month period in 2009–10. Physician Michael Gardam, director of Infection Prevention and Control, took on the seemingly intractable problem of the growth of “superbugs” that challenge hospital infection management.22 By coordinating a continuing series of open dialogues and informational workshops, Gardam was able to build awareness of the ability to significantly mitigate the hospital-based sources of antibiotic-resistant bacterial infections. Many of these infections arise from irregular and insufficient hygiene practices, such as hand washing. Other vectors, such as patient transportation and ambient surfaces and fabrics in wards, are contributing factors.
Hospitals are covered with reminders about hand hygiene, but the notorious fact is that busy healthcare workers often get caught up in their routines and fail to wash between patients or even procedures. Gardam found that peer pressure and regular routines could be initiated in places where compliance was low by following practices discovered using PD. The PD practice is not a set of techniques but rather more of a systematic management process whereby opportunities are investigated to discover and learn from local solutions to difficult problems of this kind.
PD is a bottom-up strategy. Rather than defining the problem and drawing solutions from experts, the PD approach recognizes that the best solutions are discovered by those working closest to the problem. The process starts by actively requesting the assistance and participation of people from across all levels of an organization, so that a more open culture can be cultivated to better support the emergence of effective solutions. Multiple open dialogues and facilitated sessions are scheduled, using facilitated practices such as discovery and action dialogues and improvisational theater. Eventually (in a large organization), people with insight into effective local practices are found and encouraged to contribute their own experiences and observations. High-potential actions are identified from these conversations and new practices are presented to the organization that encourage others to try the activity found successful in the “deviant” outlier.
• Stakeholder engagement methods are necessary for system-level service design. Service changes require organizational agreement and consensus for adoption, so stakeholders should be drawn from all levels in the organization.
• Multistakeholder design methods are powerful techniques for mobilizing proposals in large organizations, but their effectiveness is highly dependent on the skill and practiced ability of the facilitator. Hire an experienced practitioner or get well trained.
• Stakeholder design is a CD3.0 management care practice. Any management practices can be configured by multistakeholder design, but not all are feasible design-led projects. A good sponsor is essential.
• Simplexity and Positive Deviance have uniquely successful applications in healthcare service design and problem solving. Other facilitated methods may be compatible with these practices. Art of Hosting (Integrating Open Space and World Café) and Appreciative Inquiry also provide effective stakeholder design processes for organizational innovation.