2
A Comprehensive Review of the Li-Ion Batteries Fast-Charging Protocols
Talal Mouais1 and Saeed Mian Qaisar1,2*
1Electrical and Computer Engineering Department, Effat University, Jeddah, Saudi Arabia
2LINEACT CESI, Lyon, France
Abstract
One of the significant drawbacks of renewable energy sources, such as solar and wind, is their intermittent pattern of functioning. One promising method to overcome this limitation is to use a battery pack to enable renewable energy generation to be stored until required. Batteries are known for their high commercial potential, fast response time, modularity, and flexible installation. Therefore, they are a very attractive option for renewable energy storage, peak shaving during intensive grid loads, and a backup system for controlling the voltage drops in the energy grid. The lithium-ion (Li-Ion) is considered one of the most promising battery technologies. It has a high energy density, fair performance-to-cost ratio, and long life compared to its counterparts. With an evolved deployment of Li-Ion batteries, the latest trend is to investigate the opportunities of fast Li-Ion battery charging protocols. The aim is to attain around the 70-80% State of Charge (SoC) within a few minutes. However, fast charging is a challenging approach. The cathode particle monitoring and electrolyte transportation limitations are the major bottlenecks in this regard. Additionally, sophisticated process control mechanisms are necessary to avoid overcharging, which can cause a rapid diminishing in the battery capacity and life. This chapter mainly focuses on an important aspect of realizing the effective and fast-charging protocols of Li-Ion batteries. It presents a comprehensive survey on the advancement of fast-charging battery materials and protocols. Additionally, the state-of-the-art approaches of optimizing the configurations of concurrent fast-charging protocols to maximize the Li-Ion batteries life cycle are also presented.
Keywords: Lithium-ion battery, renewable energy, lifetime of a battery, control system, fast charging, protocols, battery materials, system optimization
2.1 Introduction
A battery is a very significant energy storage device. Each battery converts chemical energy to electricity, and it contains three main parts: the electrolyte, electrodes, and a separator. A lithium-ion battery is considered one of the most promising battery technologies because it has a high energy density, a low falling cost, and a long lifetime [1]. A comparison among the energy densities of various rechargeable batteries is displayed in Figure 2.1. It shows the outperformance of Li-Ion batteries over their counterparts.
The Li-Ion battery has many important applications. For instance, in the medical field, Li-Ion batteries are used in implantable cardiac pacemakers as shown in Figure 2.2. A device sends an electrical pulse to the heart at the right strength to stimulate it at the right pace.
Also important is the use of Li-Ion batteries for energy storage in the space exploration as shown in Figure 2.3. In 2010, NASA replaced all Ni-H2 batteries in the International Space Station (ISS) with Lithium-ion (Li-Ion). Li-Ion battery is also used to power the spacesuits of astronauts.

Figure 2.1 The energy densities and specific energies of several rechargeable batteries.
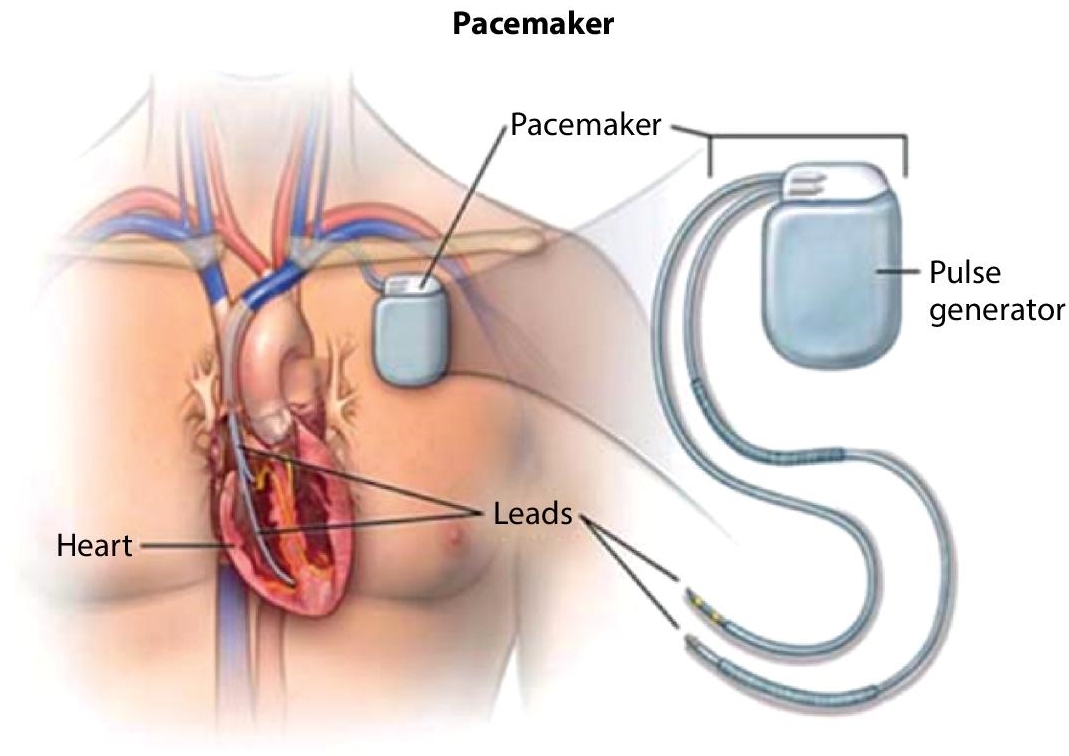
Figure 2.2 Cardiac pacemakers. Source: Adapted from [2].
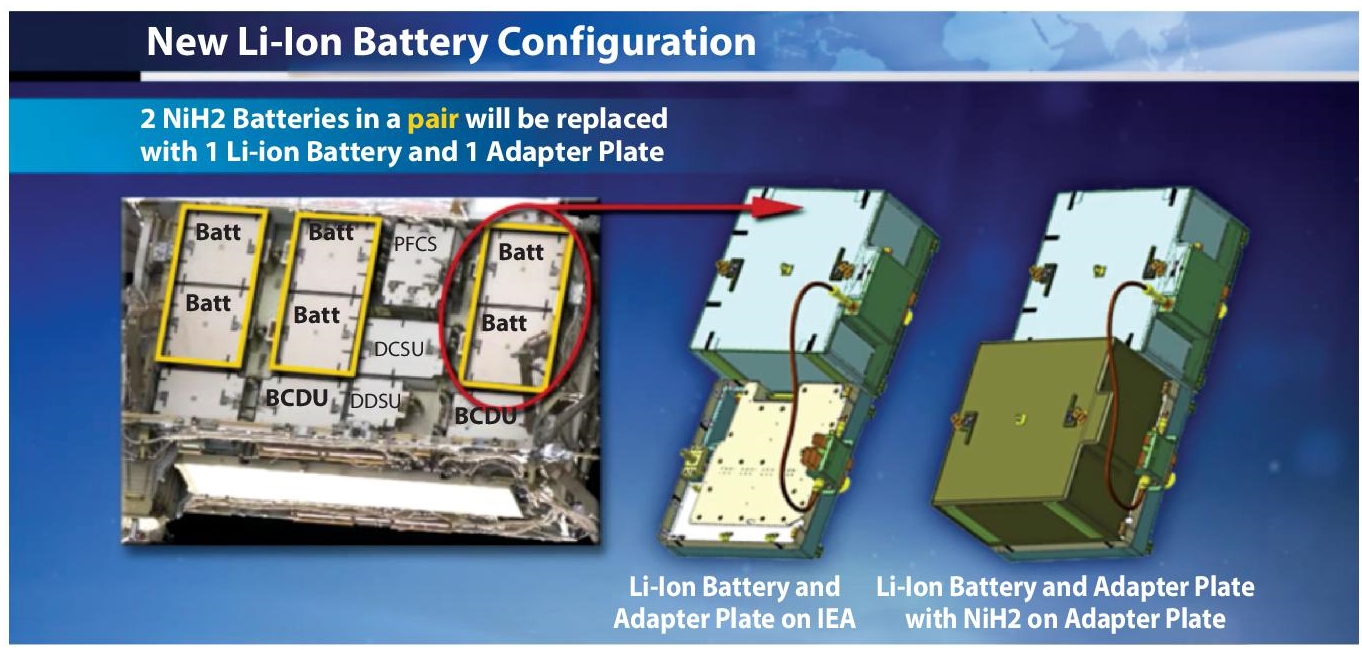
Figure 2.3 Li-Ion batteries used in the international space station. Source: Adapted from [3].
The Li-Ion battery shows promise as a technology that can store energy for renewable energy technologies, such as wind and solar. A major challenge of renewable energy is that it supplies energy intermittently; the electrical output varies depending on the sun’s intensity, wind speed, and other factors. Overcoming the intermittent energy supply problem, Li-Ion batteries can store the energy and supply it to the grid during energy supply shortages. The basic battery working principle during discharge/charge cycle is shown in Figure 2.4.
The development of electric vehicles has resulted in a greater need for batteries with large storage capacities and quick-charging capabilities. For these vehicles, lithium-ion batteries have shown to be one of the most promising energy storage systems. In comparison to other battery types, Li-Ion batteries offer a high power and energy density. The battery offers a number of advantages over other types of batteries. Li-Ion batteries, for example, may be charged from a variety of sources, including the AC grid (through a power electronic converter). Electric motors that are powered by an engine or that are in regenerative mode can also be used to charge Li-Ion batteries.
The term “battery” refers to a device that produces electrical energy from chemical reactions. Different types of batteries exist using different chemicals. Usually, a battery cell has two chemicals with different loads connected with a negative electrode and a positive electrode. The cathode supplies a current of electrons to a connected appliance while the anode accepts the electrons. Figure 2.4, adapted from [4], shows the basic battery working principle during discharge/charge.
Energy storage for renewable energy sources requires rechargeable batteries [5]. The available rechargeable battery technology includes lead-acid, Nickel-Cadmium, Li-Ion batteries, and Sodium-ion batteries, each of which has advantages and disadvantages. When selecting a rechargeable battery for a renewable energy system, the significant factors to consider include energy density, capacity, cell voltage, efficiency, and lifetime.
The amount of energy held in a battery per unit volume is referred to as energy density. It can be stated either gravimetrically or volumetrically. The quantity of energy contained in a battery in relation to its weight, given in Watt-hours per kilogram (W-hr/kg), is referred to as gravimetric energy density. Volumetric energy density, on the other hand, refers to energy measured in Watt-hours per liter (W-hr/l) [6]. The Ragone figure, shown in Figure 2.5 [7], demonstrates the volumetric energy density vs. the gravimetric energy density of several battery systems.

Figure 2.4 Basic battery working principle during discharge/charge.
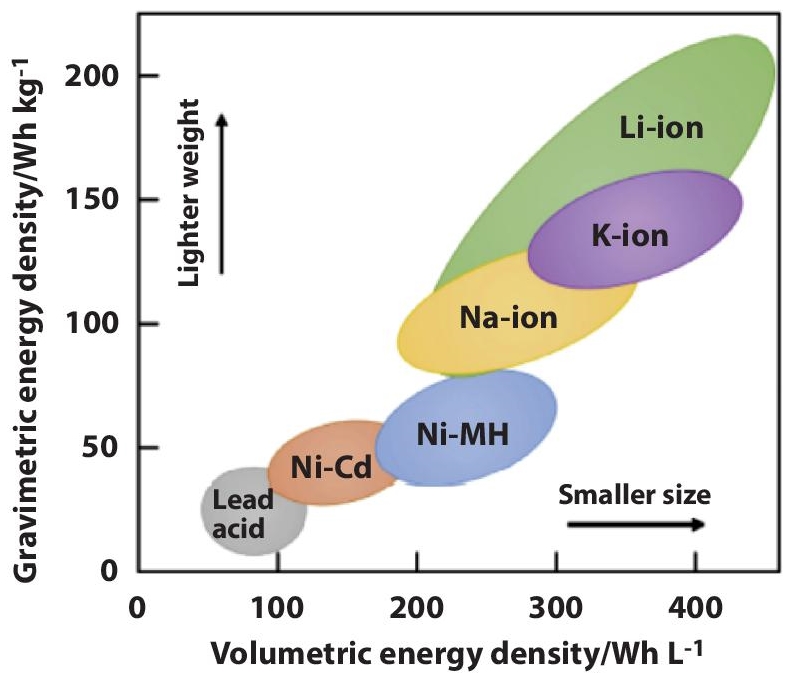
Figure 2.5 The volumetric and gravimetric energy densities for various batteries.
This study will focus on one of the most critical aspects of BMSs: the implementation of successful Li-Ion battery fast-charging methods. A complete overview of the evolution of rapid charging battery materials and processes will also be presented. We will also go through the most up-to-date methods for maximizing the life cycle of Li-Ion batteries by optimizing the configurations of concurrent fast-charging protocols.
2.2 The Literature Review
Climate change has become a menace in recent years, making governments and environmental agencies suggest strategies to solve the problem. The transportation sector is considered one of the key contributors to pollution leading to climate change. Pollution comes from combustion engine vehicles that primarily rely on carbon-based fuels that emit high levels of greenhouse gases to the atmosphere. The development of lithiumion-powered electric vehicles is among the solutions to combating global climate change. Car manufacturers have made efforts to include EVs in their range of vehicles. However, recent studies have identified significant gaps in the level of acceptance of these vehicles, especially those that are battery-powered [8]. Vehicle users have always pointed out long charging times and range anxiety as among the core reasons they are not ready to leave their combustion engine vehicles, which they can refill easily within a short time [9]. This has made fast charging capabilities the primary focus for battery developers and electric vehicle manufacturers. Tomaszeweska et al. [8] recognize that manufacturers have made efforts to create fast-charging batteries. However, charging at fast rates to reduce the overall charging time causes battery deterioration due to increased degradation.
Additionally, fast charging leads to increased heat generation, making it difficult to remove. These factors have led to a lot of safety and degradation concerns that most studies are yet to address. It is evident that existing literature has significant knowledge gaps, despite addressing the need for fast-charging protocols for batteries used in electric vehicles.
2.2.1 Overview of Lithium-Ion Battery Working Principle
The outer shell of the lithium atom has just one electron. As a result, it has the greatest chance of losing an electron (pure lithium is a very reactive metal, even it reacts with water and air). Lithium, on the other hand, is stable in its metal oxide state. The occurrence of Lithium in the periodic table is shown in Figure 2.6. The following are the basic phenomena of power production in Li-Ion batteries:
- The lithium atom is separated from the lithium metal oxide.
- Directing an electron that has been lost from a lithium atom to an external circuit.

Figure 2.6 Lithium in the periodic table.
![]() During Charging Lithium-Ion battery:
During Charging Lithium-Ion battery:
When a Li-Ion battery is connected to a power source, the positive side attracts and removes electrons from the Lithium oxide. These electrons pass through the external circuit because they cannot reach the graphite layer through the electrolyte. Meanwhile, the positively charged Li-Ion is drawn to the negative terminal and passes through the electrolyte. When Li-Ion reaches the graphite layer, it is trapped and stored between the layers of carbon graphite. Lithium will be able to act as a wedge between each layer. Intercalation is the term for this occurrence. A Li-Ion battery is seen charging in Figure 2.7 from [10].
![]() During discharging Li-Ion battery:
During discharging Li-Ion battery:
During discharge, the Li-Ion wants to return to its stable state, so the lithium ions go through the electrolyte to the metal oxide. On the other hand, the electron will go to the external circuit. Figure 2.8 from [10] shows the Li-Ion battery during discharging.
The liquid electrolyte will dry up if the cell’s internal temperature rises owing to abnormal circumstances. This might result in a fire or an explosion. An insulator layer called a separator is put between the electrodes to prevent this [11]. Because of its microporosity, the separator is permeable to lithium ions. The electron, on the other hand, will not be able to travel through it. The electrochemical process in a Li-Ion battery is illustrated with the help of Figure 2.9.
SEI refers to a significant occurrence that occurs in a Li-Ion battery when it is charged for the first time (solid electrolyte interface). Solvent molecules in the electrolyte cover the lithium ions as they flow through the electrolyte; when they reach the graphite, the lithium ions in the solvent molecules react with the graphite and produce an SEI layer. This layer is important because it avoids direct contact between the electron and the electrolyte, which protects the electrolyte from deterioration. The process of creating the SEI uses 5% of the life of a Li-Ion battery [13]. The remaining 95% of Li ensures that the battery continues to function.

Figure 2.7 Li-Ion battery during charging.
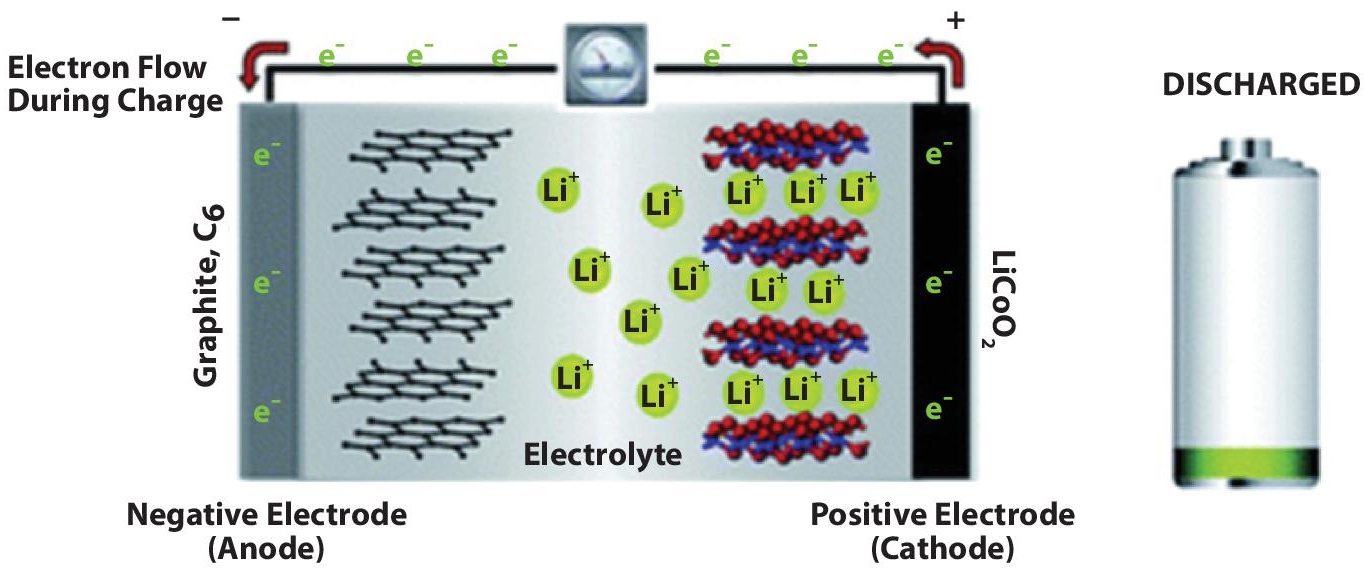
Figure 2.8 Li-Ion battery during discharging.

Figure 2.9 Illustration of the electrochemical process in a Li-Ion battery [12].
The dimensions and chemistry of the SEI layer can be optimized to improve the Li-Ion battery’s performance. We do not discuss this further here. Figure 2.10, adapted from [14], shows a scanning electron microscope (SEM) image of the SEI layer (Figure 2.10a) and its cross-sectional microstructure (Figure 2.10b).

Figure 2.10 Scanning electron microscope image shows the SEI layer’s morphology formed on graphite at 60 °C after the first cycle.
2.2.2 Principles of Battery Fast-Charging
The deployment of electric vehicles depends on the availability of batteries with efficient life and charging capacities. An ideal battery is expected to exhibit a long lifetime, with sufficient life and power densities. Such batteries would allow EV users to achieve long-range travel and fast recharge despite location and weather. However, it is not easy to achieve all these characteristics in a battery, leading to trade-offs during the manufacturing process [15]. For instance, thicker electrodes have been used to achieve high energy density. However, the electrodes are prone to extreme gradients and concentration due to fast charging [16]. Battery operation depends on effectively combining the physical properties of manufacturing materials with devices that have temperature-dependent behaviors. The operating temperature has been identified as the major barrier to fast charging as rapid temperature increases pose severe safety risks. Therefore, charge rates are always kept low to maintain the required ambient temperatures [15]. The lithium-ion batteries, the lithium plate’s risk is significantly high when temperatures decrease. This affects the overall capacity retention of the battery. The minimum temperature threshold at which the lithium plate is exposed to risk depends on various factors. Common factors that determine the level of risk that the plate is exposed to risk include cell age, parameters, and the C-rate. Various scholars have cited 25 degrees Celsius as the minimum temperature for this [17].
Fast-charging batteries are preferred for two primary reasons [18]. First, the batteries reduce the overall charging time. Second, fast-charging batteries reduce degradation levels and ensure that heat generation remains as low as possible since these are the two main challenges that are preventing electric vehicles from becoming fully adopted in the modern transport market [49].
The constant current-constant voltage (CCCV) charging procedure is used by lithium-ion battery chargers [18]. There are two steps to this charging technique. The battery is first charged at a steady current until it meets the manufacturer’s limit. The constant voltage stage follows, with the voltage remaining at the upper limit to guarantee that charging does not continue until the charging current falls below the pre-determined battery cut-off value. The CCCV protocol can be used to accomplish quick charging. However, because the constant current must be set at a high level in the initial part of the technique, it has undesirable consequences, such as strong polarization. Furthermore, strong polarization can increase the charging voltage to its top limit, causing the charging mode to flip from the first to the second stage. As a result, the CCCV protocol is used to charge the Li-Ion battery at a low rate, requiring additional charging time, because attaining quick charging with this protocol results in strong polarization, which can lead to lithium plating.
Fast-charging protocols have been suggested as the solution to high-voltage current created when the charging current is high. The proposed protocols use decayed charging current profiles to ensure that the charging voltage does not increase during fast-charging processes. The multi-stage constant current protocol is one of the recommended procedures (MSCC). The protocol has the potential to minimize total charging time and heat, hence enhancing overall charging energy efficiency [19]. Up to four layers of constant current charging are used in the multi-stage constant current technique. Because a large current may be used during the first charging stage and a low current can be used during the final charging stage, it is simple to avoid polarization during charging when these different levels are employed. The variable current decay (VCD), linear current decay (LCD), and constant voltage-constant current-constant voltage (CV-CC-CV) methods have also been proposed as fast-charging protocols [20]. These procedures follow the same guidelines as the MSCC.
2.2.3 Multi-Scale Design for Fast Charging
As noted in several studies, the degree and mode of degradation resulting from fast charging depends on battery material components [8]. The components include electrode and electrolyte properties, operational conditions, manufacturing processes, and pack design [8]. Despite these challenges, the multi-scale design provides significant opportunities for developing efficient and fast-charging batteries.
2.2.4 Electrode Materials
Electrode materials are crucial to the cell design of any form of battery. According to Tomaszeweska et al. [8], selecting suitable electrolyte and electrode combinations in the batteries’ physics to achieve high capacities is one of the most challenging aspects of the manufacturing process. Extensive research has been conducted in developing lithium fast-charging anode materials that can limit the possibility of lithium dendrite formation as temperatures change during charging and after [8]. There is a significant success in these researches as some materials, such as carbon-based alternatives like graphite and alloy composites, have proved to be effective.
Traditional graphite anodes are chosen for generating maximum cell energy density because they have a high potential for lithium/lithium-ion production. The anodes, on the other hand, are prone to lithium plating. As a result, changing the anode material is the most effective way to improve the quick-charging capabilities of Li-Ion batteries. The reversible capacity of surface-engineered graphite with a 1 wt percent Al2O3 covering demonstrates this. At a rate of 4000 mAg1, graphite reaches a reversible capacity of 337 mAh g-1 [8]. Because they do not have lithium plating issues, LTO lithium-titanate-oxide (LTO) materials are also attractive for batteries with quick-charging capabilities and extended lives. However, the materials’ working potentials are exceedingly high, resulting in poor cell voltage and energy density. LTO has undergone some changes, including the addition of carbon sources and a coating. The main goals of these changes are to improve electric conductivity and active powder content. “Vanadium disulfide flakes with Nano layered titanium disulfide coating as cathode materials in lithium-ion batteries,” according to “Vanadium disulfide flakes with Nano layered titanium disulfide coating as cathode materials in lithium-ion batteries.”
Because vanadium disulfide (VS2) is very conductive, replacing cobalt oxide in the cathode will result in quicker charging. Coating vanadium disulfide (VS2) with TiS2 also serves as a buffer layer, holding the VS2 material together and giving mechanical support.
2.2.5 Fast-Charging Strategies
Many material solutions suggested in the study show promising results. However, most of these solutions are not expected to reach the broader market level in the future, considering that more countries are adopting electric vehicle technologies. Therefore, more researchers have shifted attention to approaches that can be implemented in actual-world systems within a short time [8, 21]. More focus is now on the cell, and pack level approaches. The effectiveness of these solutions primarily depends on the charging strategies used to determine the current density.
2.2.6 Types of Charging Protocols
There are several charging protocols for the Li-Ion batteries: standard protocols, multi-stage constant current protocols, and pulse-charging protocols. A comparison of different charging protocols is presented in Table 2.1.
![]() Standard protocols
Standard protocols
Constant current-constant voltage is the most common charging protocol for these batteries. The CC-CV protocol has a constant current charging stage. In the CC phase, the battery voltage increases steadily until it reaches the cut-off value. The CV phase follows, and the constant voltage does not change. Constant voltage starts to change after the current falls to near zero. In the CV phase, there is high capacity utilization because the concentration gradients within electrode particles can easily disperse.
Moreover, there is no need for increasing the voltage as the utilization occurs within the normal range [22]. The charging time during CV increases because of the decreasing current. CC-only charging also has some weaknesses despite having lesser charging time. Therefore, CV-CC has been applied as the standard charging protocol in various applications as it has significantly low charging times and is easy to implement. However, other charging protocols have exhibited fast-charging capabilities, increased efficiencies, and better power retention than the standard CV-CC protocol. However, other charging protocols have exhibited fast-charging capabilities, increased efficiencies, and better power retention than the standard CV-CC protocol (cf. Figure 2.11).
The effects of the CC-CV charging strategy on reversible capacity and anode potential development have been studied. Zhang investigated these effects using graphite cells with reference electrodes in an experiment published in [23]. Temperatures ranging from 2 to 10 degrees Celsius were used to charge the electrodes. C-rates on the electrodes ranged from 0.16C to 1.2C. The results of this experiment revealed a link between increased charging current during the CC stage and increasing time during the CV stage. As a result, even though the current was continuously increased, the total charging time did not decrease, and this phenomenon was linked to the CC-CV association.
Table 2.1 Comparison of different charging protocols.
| Approach | Advantages | Disadvantages | Key elements |
|---|---|---|---|
| CC | Simple implementation | Low capacity | Terminal condition for charging at a constant current rate |
| CV | Simple implementation. Steady terminal voltage | It is simple to induce the battery’s lattice to collapse. | Terminal condition for charging at a constant current rate |
| CC-CV | High capacity and steady terminal voltage | It is difficult to strike a balance between goals like charging speed, energy loss, and temperature change. | In the CC phase, the current rate is constant. In the CV phase, the voltage is constant. |
| MCC | Simple implementation and fast charging | It is difficult to strike a balance between goals like charging speed, capacity use, and battery life. | The number of CC stages and their constant current rates |

Figure 2.11 Constant current-constant voltage protocol.
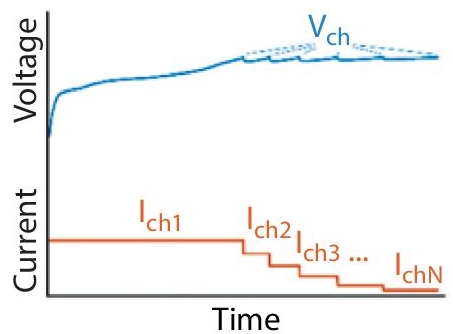
Figure 2.12 Multi-stage constant current (MSCC) charging.
Additionally, increasing the charging current and decreasing ambient temperature reduced the anode potential. The researchers associated the reduced anode potential as the primary cause of lithium plating, particularly when it gets to a negative value. However, other methods were not experimented to confirm the reduced temperatures and plating occurrence.
![]() Multi-stage constant current protocols
Multi-stage constant current protocols
Adjusting current levels during charging can help minimize cell degradation and achieve fast charging. This consequently reduces the charging time [20, 21]. Approaches that involve current level adjustment depend on reducing overall heat generation during the charging process, eliminating or minimizing conditions that enable lithium plating, and reducing mechanical stress that occurs when Lithium-ion diffusion does not happen smoothly. The MCC protocols are among the first types that were designed when the need to achieve fast charging emerged (cf. Figure 2.12). Unlike the CC-CV protocol that consists one of each, MCC protocols have more than one CC stage, followed by a CV phase. When charging batteries that use these protocols, higher current levels are used during the initial stages because the chances of the anode potential becoming negative are low. However, some researchers have done the reverse by increasing the current level during later stages [23]. The latter MCC-CV approach exhibited fast capacity loss than the CC-CV protocols [23]. However, more experimentation is needed because the protocols are yet to be experimented on other cells as their application is limited to pouch cells.

Figure 2.13 Pulse charging (PC).
![]() Pulse-charging protocols
Pulse-charging protocols
These types of charging protocols are also common in literature. The pulse charging protocols involve periodically interrupting the charging current (cf. Figure 2.13). The interruption is achieved using either short rest periods or discharge pulses. The primary purpose for interrupting charging current is to reduce concentration polarization and minimize the risk of the anode potential becoming negative, leading to plating. The strategy also reduces overall mechanical stresses that emerge from uneven insertion and when lithium is extracted from solid particles. Some studies have also revealed that pulse charging can also inhibit dendrite propagation [24]. However, the implementation of this protocol has been associated with a lot of complexity. Moreover, there is increased level of uneven heat generation when using this protocol caused by rebalancing effects as pulses keep changing. There are no studies addressing the implications of these effects on the charging process and cell life.
![]() Literature review on the Fast Charging of Li-Ion Batteries
Literature review on the Fast Charging of Li-Ion Batteries
The ability to achieve fast charge and slow discharge are crucial in the most current utilities of battery systems. For this reason, Battery Management System (BMS) has a significant role in enhancing the performance of batteries to allow for the fastest charging and slowest discharge possible. Today, advancement in BMS systems has allowed many manufacturers to produce electric vehicles [26]. However, the length required to charge their batteries and controlling their energy use is still a major concern to their developers. While in operation, EVs require a high current to facilitate acceleration, which has the drawback of reducing the efficiency of the battery. Achieving fast charging is a significant matter that requires atomic as well as system-level understanding.
Numerous experts have focused on studies on achieving fast charging of lithium-ion batteries [25, 26]. They identify several factors that affect the speed of charging of a lithium-ion battery. The factors include the temperature under which the battery is subjected, the level of current used in charging, the use and maintenance of the battery, and operational factors such as the frequency of use of the battery. Charging at a low temperature significantly boosts the speed of charging [25]. For this reason, numerous researchers have focused on methodologies that utilize low-temperature charging to help in the application of batteries in EVs. Focus has also been placed on controlling the degradation of the lithium plating, which also reduced the battery’s charging times and energy-holding capacity. Low-temperature charging is the most widely utilized approach because it provides the potential of developing onboard low-temperature charging methods [26]. Diverse methods are used to facilitate low-temperature charging methods, with other strategies focusing on combining it with managing the lithium plates through onboard systems. Some thermal management methods entail cooling the batteries during charging during normal weather and preheating them in excessively cold weather. The ability to achieve a balance between high-speed charging and operational temperature for the battery system is significant for the optimal performance of EVs.
Monitoring the degradation of the lithium plating is a difficult task that has not been achieved through onboard methods today. As a result, EVs have an extra burden of requiring examination of the battery system from the service station [26]. Consequently, achieving fast charging of batteries for EVs has been a major concern for experts. Therefore, approaches for detecting the degradation of the lithium plating in batteries or the breaking of the plating have been based on examining the voltage plateau [26]. However, there are significant challenges in distinguishing between the stripping of lithium from other factors that also induce a voltage plateau.
Similarly, detecting the degradation of the plating where a plateau has not occurred is another challenge, owing to the fact that the plateau does not always follow a deterioration of the lithium plating [26]. Nevertheless, many experts suggest key approaches of causing fast charging of lithium batteries. They include searching for a possible material to replace the lithium plating, exploring new modeling methods that could offer better charging speeds, and expanding studies on low-temperature charging, which could offer more benefits to the system.
Experts continue to search for other electrode materials for batteries that could address the plating degradation phenomenon of lithium batteries [27]. While they suggest quite a number, there is a need to examine them to ensure that they can match the stability, cost, and ease of manufacture of the lithium plating. Graphite is the primary material currently used for lithium plating. It is likely to continue to be the most preferred material despite its observed drawbacks in slowing down the charging process. Significantly, it is easy to manufacture and does not involve high costs, which makes it a viable option so far.
Exploring additional models of enhancing the charging speed of batteries is another possibility among researchers. Specifically, the subject of study regarding lithium batteries is broad and unexhausted, giving opportunities for further studies. Existing models have substantial limitations. Additional methods could be established of examining the internal conditions of batteries and providing the necessary changes to facilitate optimal operations. Only a few physics-informed methods have the capacity to perform such examinations, but they still have a limited range of use and cannot be utilized in physically abusive conditions that EVs are bound to encounter. Therefore, conducting studies on fast-charge models could provide new models that could offer viable options.
A study by Xing et al. also affirms the limitations of numerous models of implementing current fast-charge capabilities in battery management systems [28]. The author recognizes the need for conducting studies and evaluations of the performances of the system in all possible temperature and weather conditions. Significantly, the batteries are bound to undergo numerous environmental conditions with varying degrees of several factors. Notably, the range of possible temperature experiences are wide as EVs may be required to operate in high-temperature situations with low humidity to excessively low ones with high humidity [28]. Furthermore, the possibilities of having various combinations of temperature, humidity, and other astute environmental conditions are numerous, making the study significantly complicated. Fortunately, the presence of numerous possible environmental conditions provides numerous possibilities for developing advanced systems that offer the best solutions to the limitations currently experienced on the systems.
Xing et al. focus their study on measuring the various “states” of lithium batteries, including the state of charge and state of health. The authors review numerous studies on evaluating the state of the batteries and reveal significant challenges in findings that differed in respect to specific environmental conditions [28]. Similar to other researchers, they recognize that batteries for use in EVs and HEVs have to offer fast charge by having a high operational current and voltage. As a result, batteries for EVs and HEVs are significantly more complicated compared to those used in other diverse types of devices and equipment. According to the researchers, a layered decentralized topology is the most appropriate for fast charge and long-term use batteries [28].
Additionally, they recognize the need to implement systems that could offer real-time evaluations of the parameters experienced by the battery. These include their thermal conditions in each cell, the discharge strategy, the cell equalization, and charge times. Evaluating the state of the battery provides essential information about the temperature condition that the battery is subjected. Additionally, it provides an array of other useful factors, such as the operations of the charging and discharging mechanisms that facilitate cell balancing. One of the primary challenges is offering onboard systems to conduct the evaluations because the systems will also be subjected to the diverse environmental conditions that the battery is exposed [28]. Consequently, their calibration may be altered, leading to erroneous assessments. Considering that the continual assessment is essential to control the entire system, the failure of the onboard system could cause significant damage to the battery or explosion of the system that could have disastrous effects on the EV or its occupants.
The authors also point out the complications of developing a model for the battery system. They argue that modeling for state of charge encompasses applying various charging networks that have diverse material characteristics and numerous possible results based on the diverse requirements for accuracy [28]. For this reason, current models provide insufficient conditions to offer substantive rate of charge evaluations in diverse environmental conditions. Consequently, the reliability of battery management systems in diverse environmental conditions is difficult, which creates significant challenges for BMS systems for EVs and HEVs.
To achieve cell balancing in EVs and HEVs, a common approach is using parallel wiring systems that lowers the effective load of the system. As a result, the parallel systems have lower current but relatively higher voltages [29]. To balance out the need for a high voltage, some systems in the electronic model are wired in series, which enhances their voltage without necessarily affecting the current. However, the two setups create complications because weaknesses or defects in one of the cells in a series connection style affect the rest of the cells [28]. For this reason, overcharging in one of the cells could affect the state of charge of other cells, which deteriorates the state of the entire system. The cell balancing functionality works by keeping the state of charge of all cells contained within the entire battery system as close as possible to minimize the effect of any defect in one of them.
2.2.7 Li-Ion Battery Degradation
Li-Ion battery degradation is caused by physical and chemical mechanisms that impact the battery’s components, including the electrolyte, the electrodes, the separator, and the current collectors [30]. The capacity of a deteriorated Li-Ion battery is lost, as is the ability to retain a charge. Resistance increase is defined as a decrease in the rate at which electrical energy may be received or released by the battery as a result of such deterioration.

Figure 2.14 The main stages in the capacity fade vs. time.
The aging characteristics of most batteries are nonlinear [31]. Figure 2.14 depicts the three major stages of capacity fading as a function of time. The battery’s capacity rapidly decreases in the first stage owing to SEI development. Because of many side reactions that occur inside the battery, battery performance gradually deteriorates in the second stage. The rate of capacity loss accelerates in the third stage, and cell breakdown happens swiftly. The following factors contributed to the failure:
2.2.8 Factors that Cause Battery Degradation
![]() Calendar life (Time)
Calendar life (Time)
Calendar life is shelf life. It is the time before a battery, whether in inactive use or inactive, becomes unusable. Balagopal et al. [36] state that calendar aging has an important impact on a battery’s performance. Over time, the capacity of the battery declines, and it causes degradation of the battery. Degradation occurs because during manufacturing a thin SEI is formed to prevent active lithium ions and the electrode material from being exposed to the electrolyte. With battery use, degradation occurs because as the SEI grows, it reacts with electrode material and Li-Ion. Figure 2.15, adapted from [36], shows the impact of calendar life on battery capacity.

Figure 2.15 Battery capacity vs. the time curve.
![]() Temperature effects
Temperature effects
Temperature plays an important role in Li-Ion battery degradation because it affects chemical reactions that occur inside the battery. The Arrhenius equation, shown below, describes the relationship between the rate of chemical reactions and the reaction temperature. The equation clearly shows that the reaction rate inside the battery increases exponentially as the temperature rises.
Where k is the rate constant, A is the frequency factor, R is the gas constant, EA is the activation energy, and T represents the temperature.
Table 2.2, adapted from information obtained in [30] and [37], shows the impact of high and low temperatures on Li-Ion batteries.
![]() High SoC & Low SoC
High SoC & Low SoC
The ratio of the available capacity in the battery to the maximum potential charge that may be held in a battery is known as the state of charge (SoC) [38]. The cell is considered to be completely charged if its state of charge is 100%. The cell is entirely drained when the SOC reaches 0%.
Table 2.2 The effect of extreme temperatures on Li-Ion batteries.
| Low temperature | High temperature | |
|---|---|---|
| Impact on battery | Li+ plating | The development of the solid electrolyte interphase is accelerated (SEI) |
| Decomposition of electrolytes | ||
| Decomposition of the solid electrolyte interphase (SEI) | ||
| Decomposition of binders | ||
| Dissolution of transition metals and development of dendrites |
The capacity fading processes, according to [39], are extremely complicated and little understood.
Changes that occur in a low state of charge and changes that occur in a high state of charge can both be categorized as mechanisms. A low charge induces structural changes in the electrolyte, such as a breakdown process and Mn2+ dissolution. Thermodynamic instability and electrolyte oxidation are caused by a high state of charge.
![]() Charge-discharge cycles
Charge-discharge cycles
The capacity of the battery is reduced as it is charged and discharged. Excess Li-Ions are deposited on the electrode’s surface in a Li metal layer termed lithium plating when current is driven into the battery during the charging process. Lithium plating begins to form in a tiny area of the anode near the separator after a number of cycles, according to Yang et al. [32]. The chemical conversion process of the active material cannot meet the output of the battery current after too many discharges. Figure 2.16 [16] displays the number of cycles vs. capacity at various charge and discharge rates. As the number of cycles rises, battery capacity declines. Figure 2.17 [13] indicates that as the number of cycles grows, so does lithium plating and the SEI.

Figure 2.16 Number of cycles vs. capacity at different rates.

Figure 2.17 SEI build-up with the increasing numbers of cycles.
2.2.9 Degradation Mechanism of the Li-Ion Battery
The degradation mechanism of the Li-Ion battery is a chain of events that causes degradation effects. The following are the common degradation mechanisms of the Li-Ion battery [30]:
![]() Loss of Li inventory
Loss of Li inventory
Lithium ions are eaten by parasitic processes, which combine electrochemical and chemical reactions [30, 40], resulting in a loss of Li inventory. SEI growth, decomposition reactions, and lithium plating are examples of parasitic processes. Li ions are no longer accessible for cycling between the positive and negative electrodes when the Li inventory is depleted.
![]() Loss of active anode material
Loss of active anode material
When the active mass of the anode is not available for the insertion of lithium, the active material of the anode is lost [30]. This can happen in any of the following scenarios:
- Particle shattering
- A break in electrical contact
- The use of resistive surface layers to restrict active areas.
![]() Loss of active cathode material
Loss of active cathode material
When the cathode is no longer available for the insertion of lithium, there is a loss of active cathode material [30]. This might happen in any of the following scenarios:
- Disorganization of the structure
- Cracking of particles
- A break in the electrical connection.
![]() Increased electrical resistance
Increased electrical resistance
The internal resistance of a Li-Ion cell can be increased by delamination of the current collector from the electrode owing to gas development in the electrolyte [41, 42]. Decomposed electrolyte molecules combine with lithium to produce insoluble compounds, which become part of the SEI and raise the cell’s inner resistance [43]. Other degradation events, including as cathode deterioration and SEI film formation on the anode, are accelerated by the higher temperatures, resulting in capacity fading and a rise in internal resistance [44].
Figure 2.18, adapted from [30], provides an overview of the main degradation mechanisms in Li-Ion battery. The degradation mechanisms are very complex because the side reaction inside the cell is related to the materials [35], and the degradation mechanism is caused by different degradation mechanisms and at different rates. Due to the complexity of the degradation mechanism, physics-based models focus only on the dominant mechanisms of degradation, such as the solid electrolyte interphase (SEI) [45, 46]. Tables 2.3, 2.4, and 2.5 provide an overview of the factors, causes, and effects, respectively, of degradation mechanisms and the associated degradation.

Figure 2.18 An overview of the main degradation mechanisms in the Li-Ion battery.
Table 2.3 The root causes and consequences of Li-Ion degradation.
| Root cause | Consequences | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | SEI growth | ||||||||||
| High Temperature | SEI growth | Electrolyte decomposition | SEI decomposition | Binder decomposition | Transition metal dissolution/ Dendrite Formation | ||||||
| High SoC | Electrolyte decomposition | SEI decomposition | Binder decomposition | Transition metal dissolution/ Dendrite Formation | Graphite Exfoliation | Li+ plating | |||||
| Low SoC | Current collector Dissolution | Loss of electrical contact | |||||||||
| High Current | SEI growth | SEI decomposition | Graphite Exfoliation | Loss of electrical contact | Structural disordering | Particle Cracking & Island formation | |||||
| Low Temperature | Li+ plating | ||||||||||
Table 2.4 The causes of the degradation mechanism in the Li-Ion battery.
| Degradation mechanism | Caused by | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Loss of cycable Li | SEI growth | Electrolyte decomposition | SEI decomposition | Li+ plating | |||||||
| Loss of Active Anode Material | Graphite Exfoliation | Current collector Dissolution | Loss of electrical contact | Particle Cracking & Island formation | |||||||
| Loss of Active cathode Material | Binder Decomposition | Transition metal dissolution/ Dendrite Formation | Current collector Dissolution | Loss of electrical contact | Structural disordering | Particle Cracking & Island formation | |||||
| Increased Electrical Resistance | Binder Decomposition | Loss of electrical contact | Structural disordering | Particle Cracking & Island formation | |||||||
Table 2.5 The degradation mechanism and its effect on the Li-Ion battery.
| Effect on battery | Degradation mechanism | ||||
|---|---|---|---|---|---|
| Capacity Loss | Loss of Cyleable Li | Loss of active anode material | Loss of Active cathode material | ||
| Resistance Rise | Loss of active anode material | Loss of Active cathode material | Reduced Kinetics | Increased Electrical Resistance | |
2.2.10 Electrode Degradation in Lithium-Ion Batteries
Electrodes play a significant role in the degradation of Lithium-Ion batteries. Understanding electrodes degradation and the behavior is very significant to allow us to design the best fast-charging protocols. Therefore, in the next section, we will review electrode degradation in Lithium-Ion batteries.
According to Mei [47], the physical properties of electrodes have a significant impact on the rate of heat generation and energy density of batteries. Thicker electrodes, according to Zhao [48], can increase the fraction of active materials and hence enhance energy. However, increasing electrode thickness can have a detrimental impact on Li-Ion battery thermal and electrochemical performance. He also shows that when batteries with a thicker electrode discharge at the same rate, they have more intense and uneven temperature reactions across the electrochemical cell. This can result in active components being depleted and the battery’s capacity diminishing faster. Furthermore, a Li-Ion battery with a thicker electrode is more susceptible to a higher discharge rate, he finds. This is due to the formation of ohmic heat, which causes the Li-Ion battery’s health to deteriorate.
Figure 2.19 depicts the weight % breakdown of a commercial Li-Ion battery per component. The electrodes are definitely the most important material components of the Li-Ion battery, as seen in the diagram. Because electrode deterioration has such a substantial influence on Li-Ion battery performance, and because electrodes are the biggest and heaviest material components of the Li-Ion battery, electrode degradation has a considerable impact on Lithium-Ion battery lives.
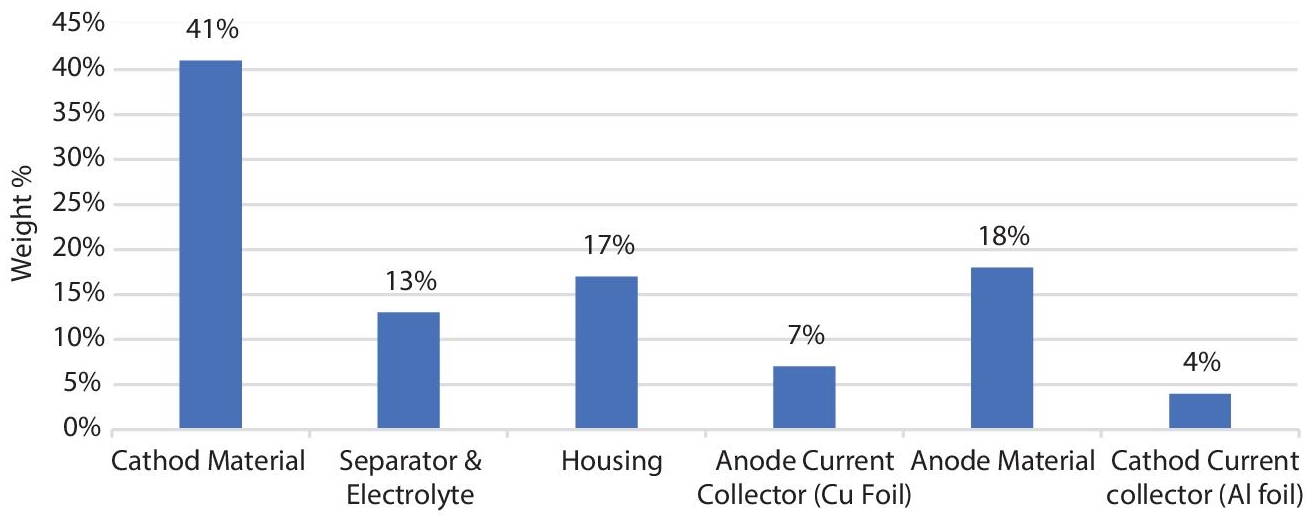
Figure 2.19 The breakdown of the weight percentage by the components of a commercial Li-Ion battery.
![]() Degradation mechanisms on the anode materials
Degradation mechanisms on the anode materials
The lithium-ion will be associated with the electrolyte breakdown while charging at a fast rate. The SEI is a passivation protective layer formed on the surface of the anode electrode as a result of this breakdown (solid electrolyte interface). After charging and discharging, the volume of the anode material varies by 10% [35]. This is due to lithium ion intercalation and deintercalation (cf. Figure 2.20). This shift in anode volume can cause the SEI to break, resulting in a lithiated graphite and electrolyte reaction that consumes the Lithium-ion and electrolyte. As a result, the SEI coating on the graphite anode’s surface thickens.
![]() Degradation mechanisms on the cathode materials
Degradation mechanisms on the cathode materials
The deterioration of the cathode in LiFePO4 cathode material is caused by the breakdown of the binder. Another source of cathode deterioration, structural disordering, is current collector corrosion driven by parasitic processes [50, 51]. Insoluble species that migrate through the separator and dissolute in the electrolyte may cause side reactions at the cathode in some situations [39, 63]. The oxidation of electrolyte components and the development of surface films are two further degradation mechanisms [39] (cf. Figure 2.21). Surface electrolyte reactions [39] and the consequent creation of fissures in particles cause gas evolution.

Figure 2.20 Degradation mechanisms on the anode material.
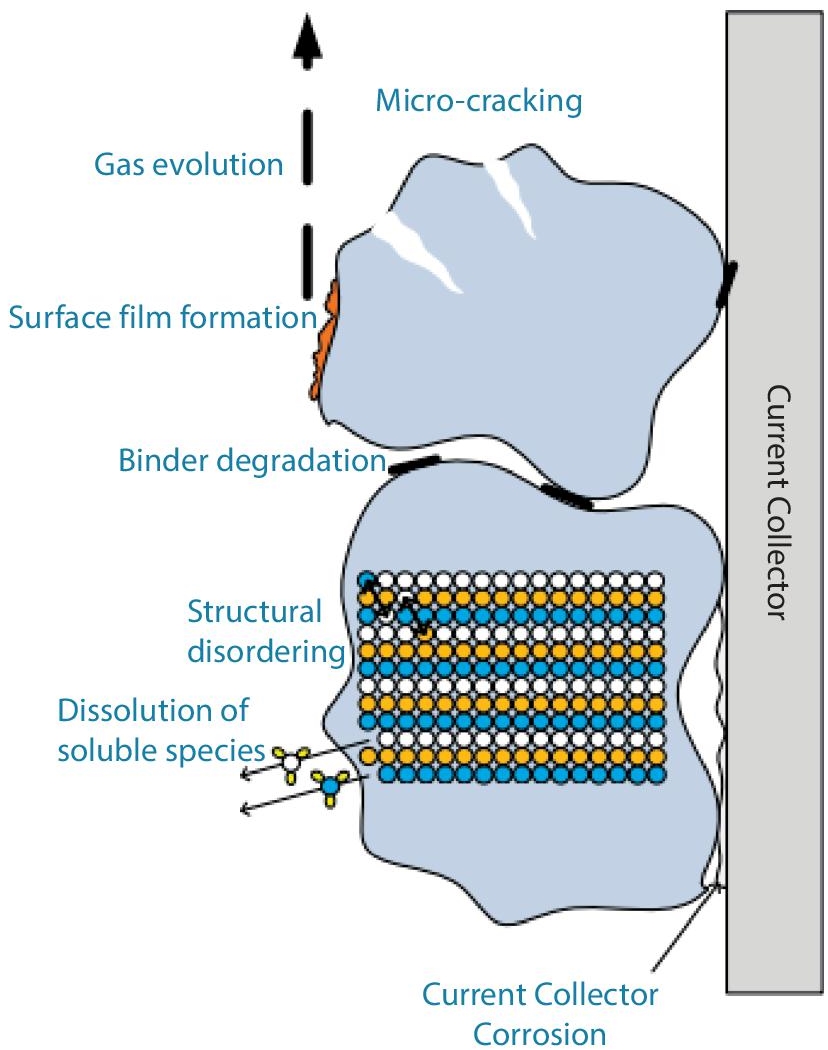
Figure 2.21 Degradation mechanisms that affect cathode materials.
2.2.11 The Battery Management System
A battery management system is a crucial strategy for extending the battery’s life and managing quick charging. The Battery Management System (BMS) has several functions, including protecting the operator’s safety, detecting unsafe operating conditions, protecting battery cells from damage in failure, and informing the application controller how to make the best use of the pack right now (e.g., power limits), and controlling the charger.

Figure 2.22 Typical battery management system.
BMS manages the output of a battery and provides notifications on the condition of the battery. The BMS manages the battery’s charging and discharging processes and provides vital precautions to protect rechargeable batteries from damage [58]. Different types of BMS are used in different devices using rechargeable batteries. Battery management systems are commonly found in cars, data centers, smartphones, and mp3 players.
The BMS monitors and regulates the main power voltage, charging and discharging levels, temperatures of the batteries, and how healthy the battery is [53]. Also, the systems check and control the battery voltage and the coolant temperature [54]. Figure 2.22 shows a sample block diagram of a typical BMS system.
![]() How BMS Can Help Increase the Life and Reliability of Battery
How BMS Can Help Increase the Life and Reliability of Battery
The lifetime of a battery depends on several aspects ranging from its internal battery parameters to external parameters. The internal parameters are influenced by the manufacturers, while the external parameters are influenced by the users [55]. The external aspects can have a major impact on the lifetime of a battery. Through the use of the BMS, it is possible to control the external parameters to increase its life. Considerations of the demands and the battery life objectives help establish the correct architecture to create a BMS and charging strategy that optimizes the life of the battery [56].
BMS is needed in providing safety and proper battery performance. Improper battery conditions such as over-voltage and over-charging cause rapid aging and can lead to an explosion. BMS includes sensors that estimate vital parameters such as temperature, voltage, and current. After determining the thermal and electric behaviors of batteries, it is important to optimize the charging and discharge algorithms to provide efficiency and enhance the battery lifespan [57]. The BMS alarm and safety control module helps eliminate any abnormalities that might make the battery less reliable or shorten its lifespan [57]. The parameters of a BMS depend on the battery operation conditions. Therefore, it is significant to collect data on the battery’s behavior during the charging or discharging processes. The BMS helps to increase the life and reliability of a battery by stopping charging and discharging current if it establishes that it is necessary.
Batteries are used in electric vehicles, and BMS plays a significant role in enhancing their reliability. The battery systems of electric vehicles are made of several battery cells packed together. The systems have a high voltage rating and current, and any slight mismanagement action could lead to a disaster. In this regard, BMS plays a significant role in the safe operation of high voltage batteries used in electric vehicles. BMS monitors the state of the batteries and prevents overcharging and discharging that might reduce the capacity and lifespan of the batteries [58]. A BMS checks the voltage and shuts down or sends an alarm when the required voltage is attained. Also, battery management can relay information about the condition of the battery to the power management systems. This helps in correcting any fault before it becomes serious. Also, BMS controls the temperatures of the battery cells and their health, ensuring that the battery is safe and reliable.
![]() Thermal Management
Thermal Management
Preheating in cold conditions. Maintaining low temperatures for quick-charging Li-Ion cells, as previously stated, has proven to be a considerable difficulty. The literature seeking to address this topic has considerable gaps. Some research, on the other hand, has sought to devise charging procedures that include diverse preheating techniques. This section discusses techniques that can result in fast heating across the cell. The emphasis is on this since high speed is an important feature of any preheating technique that may be used in conjunction with rapid charging. Internal preheating is favored because it ensures that less heat is wasted to the environment, making it more efficient. Li-Ion cells that are particularly built to permit rapid preheating have been used to address fast charging without the risk of excessive temperature effects [59]. As seen in Figure 2.23 [60], this is accomplished by sandwiching electrically insulated nickel foils between two single-sided anodes. Using conventional direct current, the technique assures that the cell can raise the temperature on its own when needed [59]. In most cases, an activation switch is utilized to control the amount of current that passes through the foils [59]. Turning on the activation switch, for example, sends direct current to the foils, resulting in fast heat buildup [59].
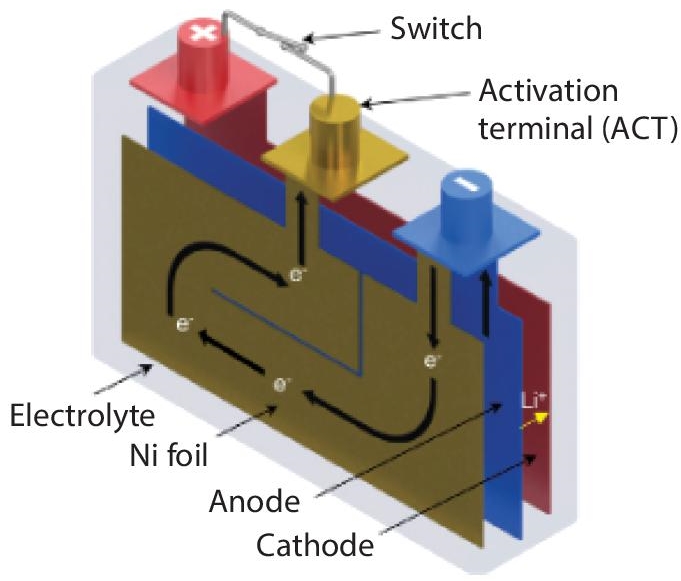
Figure 2.23 Schematic of Li-Ion cell structure with embedded Ni foil.
Turning it off, on the other hand, permits current to flow between the electrodes while the nickel foil stays separated. Internal heating techniques, according to most studies, boost efficiency and allow for uniform temperature distribution across the cell. However, little study has been done on the effects of internal preheating when utilized in conjunction with rapid charging. Experimenting with internal temperature is difficult. As a result, models that can analyze preheating when alternating current is employed are required. Preheating using nickel foil appears promising; nevertheless, it can only be utilized in non-standard cells, which adds weight and reliability concerns. Furthermore, the concept was very recently proposed, and only a limited amount of study has been conducted on its use. As a result, substantial and additional study into its performance, as well as an evaluation to establish whether the technology is cost-effective, are required before it can be completely commercialized.
2.2.12 Battery Technology Gap Assessment for Fast-Charging
As discussed in the previous sections, electrodes will impact fast charging and cell performance. In this section, we will discuss the other factors and components of Li-Ion batteries which will impact fast-charging.
![]() Electrolyte degradation
Electrolyte degradation
Electrolytes are among the cell performance determinants. The electrolyte can affect cell performance and electrode behavior. For example, the electrolyte can potentially change the structural aspects of graphite electrodes [61]. Reports have shown large and irreversible capacity loss of graphite electrodes in batteries with cells that contain propylene carbonate electrolytes. These reports hypothesized that reduction products in these cells lack the capacity to coat graphite as required. Therefore, propylene gas formed after reduction remains trapped within the electrode.
As a result, there is a build-up of high pressure, which causes exfoliation.
There is currently inadequate data on this influence on electrolyte deterioration. Lithium plating and excessive heat production are thought to decrease electrolyte conductivity, according to “Electrolyte design for fast-charging Li-Ion batteries”. The addition of low-viscosity solvents to standard carbonate electrolytes to boost ionic conductivity is one of the most promising approaches for improving electrolytes for quick charging. Low-MW esters like Methyl acetate and Ethyl acetate, as well as nitriles, are the best options.
![]() Charging protocol
Charging protocol
Several reports have been published about the effects of the mode of charging or protocol used has on Li-Ion cells performance and life. [61] identify rapid degradation when various protocols are used. For instance, one observation showed the various methods discussed earlier cause different lithium deposition levels. Like most researchers, [61] emphasize that field implementation of the charging protocols will require the development of complex algorithms, which are yet to be studied.
![]() Usage
Usage
Customer usage is a core factor in battery development. Manufacturers must consider consumer usage patterns during design and optimization processes. The customer usage pattern is considered because it affects battery life, performance, and safety; this makes the conventional CC-CV charging protocol unsuitable for extreme fast charging (XFC) because it is prone to forming a lithium plating that will compromise performance and safety. The suggested fast-charging protocols and other newer protocols should be explored. Electric vehicle users are likely to arrive at recharging stations with empty to almost full battery packs. This depends on extreme fast charging (XFC) availability, convenience, and pricing [61]. Research shows that currently, auto manufacturers assume that EV owners charge their cars at home, especially after work. Data collected from drivers who adopted EVs early has proved this assumption right as most EV users charge at home and work during their break hours. However, it is difficult to determine what the duty will look like when a larger part of the population switches to electric vehicles as more countries are encouraging the use of renewable energy resources in the transport sector. From the battery perspective, manufacturers should focus on developing batteries that correspond to aggressive use by reducing the overall charging hours and improving performance and safety.
![]() Fast-charging effects on thermal runaway characteristics
Fast-charging effects on thermal runaway characteristics
Besides degradation, fast charging has raised some safety concerns. Lithium plating and temperature rises that occur during fast charging pose potential risks. For instance, research has shown significant changes in batteries’ thermal runaway behavior after fast charging [59]. Accelerating Rate Calorimetry (ARC) tests on fast-charged high-energy batteries revealed that the self-heating temperature reduced significantly when cells were subjected to fast charging than it dropped in fresh cells. However, these effects are not permanent since, with adequate rest time, they are reversible. Besides this, overcharge-induced thermal runaway is another safety concern for fast-charging batteries. There is a possibility of having some cells in a battery pack overcharging after fast-charging. This phenomenon is often caused by inconsistencies among the battery cells.
Two design models have been proposed to increase safety in fast-charged batteries by protecting them from overcharging. One is by using an electrolyte with a higher oxidation potential. For instance, raising the oxidation potential from 4.4V to 4.7V will increase electrolyte stability. This can be achieved by including additional functional additives. Functional additives are chemical species that can be oxidized at a higher potential than the conventional safe cathode potential. Considering the unique Li-Ion cell components, there is a need for more research to determine the suitable redox shuttle species that can be used in these batteries without possible risks and effects like cell-life reduction. Secondly, increasing the temperature at which the battery thermal runaway occurs can help reduce risk levels in fast-charged batteries. The temperature can be increased by increasing the battery pressure relief design to the optimum.
2.2.13 Developmental Needs
Based on Li-Ion battery gaps assessment and challenges around this technology, some developmental needs have been proposed to enable fast charging. Changes in modeling are among the needs that [61] proposed. Extreme fast charging (XFC) requires advanced models; therefore, the current ones need to be updated. Advanced models will be incorporated with fast-charging protocols [62, 63]. Model development should also include fast-charging constraints and other design requirements to facilitate cost estimation for the entire process [64, 65].
Additionally, the updated models should include the identified temperature, and current effects on the life and performance of fast-charging-enabled battery packs. Aging and failure due to degradation are the most pertinent fast-charging mechanisms. Therefore, manufacturers need to identify and include them in the advanced models.
2.3 Materials and Methods
The research utilized secondary resources. Existing literature about fast-charging protocols for Li-Ion batteries was the primary resource for the study. Various articles assessed the electric vehicle battery technology and other factors around the fast-charging protocols. The study also relied on experimental data. Researchers assessing these technologies have used experiments to evaluate the effectiveness of different fast-charging protocols [20, 21]. The experiments use cells with different rating capacities and under varying current profiles. The experimental conditions in the reviewed literature are changed to assess the thermal behaviors of batteries when different protocols are changed. The literature also provides different models for various charging protocols. For example, in their work, [21] presented a model to calculate the charging current profile as a function of the charging stage. When designing a fast-charging protocol for Li-Ion batteries, the model revealed the significance of taking cost functions into account. The researchers recommended a multi-stage pricing methodology based on their findings [21]. When evaluated in the model, the technique was shown to be successful in lowering the capacity fade ratio.
All of the cells used in the experiment were cycled using different charging techniques, according to one of the studies published in the literature. They were, however, fired in the same manner.
The ten-minute charging methods were used to charge the cells. [66] was charged in the range of 0% to 80% SOC. After that, they were granted a five-second break. Following that, all cells were charged with an 80% to 100% SOC to the manufacturer’s specified optimal voltage and a C/20 cutoff. After another five seconds of rest, the cells were discharged with a CC-CV discharge of 4C to 2.0V and a C/20 cutoff [66]. After the discharge, the cells were allowed to rest for another five seconds before starting the charging process [66]. The lowest and upper cutoff voltages were 2.0V and 3.6V, respectively. Cycle life refers to the total number of cycles until the discharge capacity falls below 80% of the nominal capacity in all experiments conducted by previous researchers.
Using the charging and discharging procedure described above, the cell capacity of each cell was measured after every 10 cycles. Q [66], a dimensionless capacity, was introduced by the researchers. Q was the ratio of the old cell’s capacity to the new cell’s capacity. The plotting of the dimensionless values revealed a considerable increase in the aging rate. A rise in the cut-off voltage is related with rapid acceleration. Some phenomena were observed during the studies, such as a high concentration of surface ion, which limited the charging current. Concentration is advantageous because it avoids capacity deterioration.
A two-stage constant current rapid-charging methodology is evaluated in a second experiment published in the literature. The batteries are charged at 80% of the C-rate for half an hour in this experiment [20]. The goal of the study was to examine the thermal behavior of various charging current patterns [20]. The electrochemical-thermal-coupled model is used to analyze the thermal behavior and charging efficiency of this approach. For a cylindrical Li-Ion phosphate battery, the model was constructed using charge and energy conservation concepts [20]. The charging current profiles for the two-stage constant current protocol achieve higher charging efficiency and lower temperature than the quickest charging protocols published in the literature, indicating that the experiment yielded encouraging results.
Another research, [67] called “Reliability and Failure Analysis of Lithium-Ion Batteries for Electronic Systems.” The researchers in [67] used two commercial batteries designed for application in portable electronic systems were used for testing the impact of increasing charging rate for batteries with the same material but with different physical parameters. Table 2.6 below shows the physical parameters for each battery.
Table 2.6 The physical parameters for each battery.
| B1 | B2 | |
|---|---|---|
| Nominal Capacity (Ah) | 1.1 Ah | 1.35 Ah |
| Length (mm) | 50 | 50 |
| Width * Thickness (mm) | 33.8*5.4 | 33.8*6.7 |
| Weight (g) | 22 | 28 |
In this study [67], it was found that a battery with a thicker electrode will be very sensitive to the increase of the charging rate; this is due to the ohmic heat generated from the battery, which will lead to the deterioration of the battery.
2.4 Discussion
Battery technology is critical in electric car commercialization which makes a collective effort to address the climate change crisis resulting from pollution in the transport sector. Literature shows that electric vehicle manufacturers have widely adopted lithium-ion batteries. The Li-Ion battery technology has been widely reviewed, with more studies focusing on fast charging and the key aspects that limit it in these batteries. Various fast-charging protocols for Li-Ion batteries have been proposed, with some like multi-stage constant current MSC proving to be effective in enhancing fast charging. However, the literature identifies various limitations to fast charging, lithium plating being the primary challenge to the technology.
A positive electrode constructed of metal oxide is found in a conventional Li-Ion cell. Organic carbonates with lithium-bearing salt and graphite rod are also included in the cell. The negative electrode is a graphite rod, while the electrolyte is an organic carbonate mixture [61]. There is an ion movement during charging, with Li-Ions moving from the metal oxide to the negative electrode via the electrolyte as the medium. During discharge, the ions flow in the opposite direction. Electrode particles are covered with other operation products as a result of the reaction. The sold interface layer (SEI) is formed by the coating [61]. The SEI components differ depending on the electrode, with the negative electrode having a higher value.
Li-Ion batteries’ ability to maintain high power densities makes them ideal for usage in electric cars. Recharging Li-Ion batteries takes longer than fuelling an internal combustion engine car, notwithstanding its efficacy. Consumer adoption of electric vehicles has been hampered by the passage of time. Furthermore, due of the range anxiety issue connected with electric vehicles, drivers are hesitant to adopt them. The capacity of manufacturers to guarantee that cars have a shorter recharge time that is equivalent to that of internal combustion engine (ICE) vehicles is critical to achieving efficient customer adoption. Fast-charging techniques for Li-Ion batteries are used to reduce refueling time. Additionally, unlike with carbon-based fuels, battery recharge should not be confined to empty to fully charged batteries. Consumers should be able to charge their automobile batteries to any capacity they choose. The electrolyte-negative electrode contact is reached as Li-Ions are transferred from the positive electrode to the negative electrode during charging. Lithium metal is likely to plate on the negative electrode when the transportation process is quicker than the intercalation rate, according to studies [61]. Lithium plating has been shown to increase at low temperatures and with high current density [61]. Even at temperatures as low as -20 degrees Celsius, lithium plating can occur at a C-rate of 6.
System flaws have been documented to produce plating, in addition to higher current density and low temperatures. For example, plating is likely to occur if pore closure occurs in the separator or if there are large currents present throughout the operation. If the overpotential is larger than the equilibrium potential, plating is likely to occur [68]. The lithium quantity deposited on the negative electrode depends on the electrode’s capacity loading. [61] show that in some cases, the plating is removed during the discharge cycle. However, discharge is unreliable for eliminating plating because even slow discharges have been seen not to remove significant amounts of plating from the negative electrode [16]. The findings suggest that the connection between lithium deposits and the graphite rode is not electronic. Under severe circumstances, the deposits affect cell performance and cell life. Figure 2.24 below is adapted from [61], and it shows how lithium deposition increase with increasing the charge rate.
In the extant literature, there are several ways for detecting lithium plating. For example, [64] and [69] provide non-destructive ways for detecting lithium deposits on graphite rods. Volumetric measurements to identify any anomalous cell volume variations, voltage monitoring to detect high-voltage plateaus, and high-precision coulometry to detect cell efficiency changes are examples of these approaches. Furthermore, continuous current charging guarantees that the rate of lithium delivery to the negative electrode remains constant. Placing on the electrode surface is minimized by keeping the delivery rate equal to or less than the intercalation rate. Despite maintaining such conditions, however, lithium plating avoidance is not always assured since other causes, such as a rise in the local chemical potential, might produce plating.

Figure 2.24 Lithium deposition increase with increasing the charge rate from left to right.
The study literature also includes studies on the effect of capacity loading and charge rate on lithium plating [70]. Lithium plating as a function of C-rate and capacity loading is shown in [16]. Plates were predicted to appear in cells with the largest capacity fade. This was proven by the study, which found that these cells had the most lithium deposits [16]. Despite the fact that the cells were discharged at a moderate rate, lithium deposits persisted at the electrode, contrary to predictions. This shows that when lithium deposits are plated, they become electrically insulated from the negative electrode. Furthermore, the findings imply that theorized reconditioning of the battery pack by gradual discharging cannot return accumulated lithium on the graphite electrode surface to the positive electrode.
With fast charging, the lithium deposition rate increases, increasing degradation, consequently reducing the cell life. The rate at which the cell degrades and its overall performance depends on temperature. Besides the temperature at which a cell operates, other factors, such as the nature of active material and graphite rode design, also determine degradation level. Graphite is the common material used in lithium-ion battery negative electrodes. Plating occurs easily on graphite electrodes because of the local chemical and electrochemical around the material. However, other materials are explored for their effectiveness in use for the negative electrode in lithium-ion cells. These materials have a higher potential than graphite. Higher potentials are beneficial as they limit the conditions needed for lithium plating. Lithium metal is also suggested as a graphite alternative for the negative electrode. However, as evident from the studies, lithium metal is associated with dendrite formation, reducing the life cycle and increasing safety concerns [8]. As a result, before employing lithium metal as a negative electrode, these problems must be resolved. Lithium metal, according to some studies, cannot be utilized in applications that need a lot of power [61]. Lithium was discovered to be very reactive when combined with various electrode components. In addition, the metal generates a more complicated SEI layer than other materials. Dendrites still occur when lithium metal is utilized, despite the presence of the SEI layer. The surface morphology of electrodes made of lithium metal was also shown to be sensitive to current density [61]. The surface of the lithium metal altered from smooth to dendritic in most studies, making the surface rough.
When lithium metal is exposed to rapid-charging conditions, dendrite growth is accelerated, rendering the material unsuitable for the application.
(Li4Ti5O12) LTO is considered to be the appropriate graphite electrode alternative. LTO (Li4Ti5O12) can support fast charging as it has the required electrode kinetics. Several literature reports show that (Li4Ti5O12) LTO nano-particles can continuously be charged at C-rates as high as 10C without any additives [61]. Additionally, doping the material increased its electrochemical performance. [20, 21] and [59] further reported that sodium-bearing phases have a higher rater performance than (Li4Ti5O12) LTO. Like (Li4Ti5O12) LTO, doping the phases increase their superiority by improving electronic conductivity and ionic diffusivity. Silicon is another material that could be used as a negative electrode; however, its response to fast-charging conditions has not been assessed in the resources used for this study. Therefore, the viability of silicon electrodes and their stability in fast-charging applications is not currently known.
The open literature has yet to assess the impact of fast-charging conditions on the positive electrode. However, some reports show that metal oxides, which are the primary positive electrode materials, have low stability for cycling [61]. Charging involves the diffusion of ions in and out of the host material. This leads to changes in volume and concentration gradient around the positive electrode. Diffusion-induced stress can potentially cause failure, especially when the C-rate is high. Stress levels are likely to increase during fast charging, causing degradation when temperatures are non-uniform. However, there is a need for more research assessing the effects on the positive electrode as there are significant knowledge gaps on the phenomenon.
Electrode design is another factor identified to influence plating. [16] shows that electrolyte transportation and polarization can potentially cause lithium plating on the negative electrode during extreme fast charging. It was proposed that avoiding high charge current densities can limit plating. Charge currents above 4 mA should only be applied after taking necessary precautions. Similarly, as noted in several studies, Li-Ion battery power depends on temperature [18, 20, 21, 59, 61]. The total cell impedance changes significantly over a wide range of temperature changes [52]. During fast charging, resistance is high, causing heating that consequently increases temperatures within the battery cells. High temperatures reduce battery resistance as kinetics become faster. On the other hand, electronic resistance in the electrode current collectors increases as temperatures rise. This allows some power resulting from faster kinetics to be released. It is essential to avoid reaching the upper-temperature limit set by the battery manufacturer during fast charging. This is recommended because if at any point the temperature of the lithium-ion cell when it is fully charged exceeds the manufacturer’s pre-determined point, it could lead to a thermal runaway, which is a severe safety concern. Despite some systems having set points that are considered low, they should not be exceeded. Additionally, high temperatures in the electrolyte can cause slat decomposition, shortening the cell life.
Electrolyte degradation is another key aspect of fast-charging protocols discussed in many reviews. The electrolyte has significant impacts on cell and electrode behavior. One of the evident effects is its potential to change the structure of the graphite electrode. For instance, a significant capacity loss was experienced on negative graphite electrodes used in cells containing propylene-based electrolytes. It was hypothesized that reduction products did not coat graphite well. This led to the formation of propylene gas that was consequently trapped in electrode surface crevices, leading to excessive pressure buildup. High pressure caused exfoliation. Currently, there is insufficient information about fast charging on degradation. There are speculations that high heat generation during fast charging and lithium plating affects electrolyte properties. Consequently, further research is needed to determine the exact impact of fast charging on electrode performance.
Because of the necessity to increase rapid charging efficiency in Li-Ion batteries used in electric vehicles, academics have focused on fast-charging procedures. Based on the findings, it is clear that a battery’s charging technique impacts its performance decline. In [59], the authors recognize that the CCCV charging protocol has been widely adopted in many batteries and electric vehicle industries. The high adoption is primarily because this charging protocol is simple and less costly than others like the multi-step charging protocol. The CCCV, on the other hand, is linked to severe deterioration and poor performance. The procedure also offers a number of dangers by allowing lithium plating on the negative electrode. In light of these consequences, it is suggested that alternate charging techniques be utilized during high-rate charging to reduce deterioration and extend cell life.
Experiment results show that charging protocol optimization is another key area of fast-charging protocols. Charging protocol optimization is achieved through charging and discharging. [21] shows in his experiment that the charging process affects the SEI layer thickness and the cell capacity fade, whereby the ratio of the two rises as the cells are charged. On the contrary, the discharge has no impact on SEI and capacity fade [21]. When SEI increases and lithium plating occurs, capacity fade is experienced in the negative electrode of the cell. The effects of lithium plating cyclable lithium are considered to be minimal than SEI impact. Despite this, lithium plating remains a safety concern because it can cause short-circuiting in the cell. The charging process is only considered safe when the electrode potential is higher than the electrolyte potential. This scenario implies that the SEI potential is positive. Maintaining the SEI potential positive is a challenge in fast charging because fast rate charging increases the SOC, consequently decreasing SEI potential creating conditions for plating.
The study is significant in developing fast-charging protocols for Li-Ion batteries. Li-Ion batteries are promising in making electric vehicle technology a global reality by addressing the current charging limitations affecting its adoption. The study identifies various fast-charging protocols for these batteries and the level to which their effectiveness has been tested. Reviewing the protocols is vital as it provides insights to manufacturers on what to invest in and the constraints that need to be addressed. The study’s significance is also because it addresses the effects of different charging protocols on degradation and how it can be minimized to lengthen the cell life. Literature used in the research further provides recommendations for minimizing and eliminating problems that cause cell degradation, such as heating and lithium plating. Additionally, recommendations for future studies are provided, which are critical for improving the charging protocol mechanisms to meet the commercialization needs for Li-Ion batteries used in electric vehicles. Despite these benefits, there are significant knowledge gaps identified in the constraints section that might limit the effectiveness of the review in future applications.
2.5 Conclusion
The Li-Ion battery is considered the most efficient among the battery technologies. They are promising in meeting the electric vehicles commercialization needs. The Li-Ion batteries have a high energy density, low and falling cost and longer life than the counterparts. The functionality of Li-Ion batteries depends on various aspects like its internal configuration and charging protocols. The charging protocols that apply initially do not meet the commercialization needs of these batteries and their use in the electric vehicles. Therefore, a lot of investment and investigation is going on in developing the effective fast-charging protocols. Although fast charging is highly recommended, the existing fast-charging protocols have their pros and cons. They could affect the cell functionality by causing thermal runaway. The key disadvantage of the majority of existing protocols is a fast battery degradation and diminished life. Additionally, current protocols are associated with extreme heating that leads to high temperatures during charging. These factors raise safety and economic concerns that need to be addressed before the fast-charging protocols are adopted for commercial usage. Moreover, the fast-charging protocols will be used on high-voltage battery packs, which requires additional safety measures. Certain safety measures, suggested to be used in the fast-charging arrangements, include reliable time-monitoring strategies and thicker insulations. Manufacturers can also improve safety by considering recommendations like evaluating the critical issues associated with fast charging, before the commercialization. This will help in identifying and eliminating the safety concerns.
The literature reviewed in this study identifies the factors that contribute to lithium plating as this is one of the key challenges in developing the fast-changing schemes. It is recommended that the used material must have a fast kinetics. The graphite remains the most appropriate material for developing the negative electrode. The lithium-titanate-oxide is also recommended for its high kinetics potential. However, further investigation is required to assess how the lithium-titanate-oxide’s potential energy can be reduced. Li-Ion battery developers also need to invest in electrode designs that can accommodate fast diffusion. Current designs lack the capacity to facilitate the level of diffusion, which is required. Considering that the fast-charging protocols are new and still evolving, more studies are required to assess the impact of these protocols on various Li-Ion battery materials. It is evident that limited studies have addressed extreme fast-charging effects on materials. Most of these studies have focused on the graphite. Further studies should be conducted on the extreme fast-charging effects while considering the materials like silicon and lithium metal. The effects should also be assessed in electrolytes and separators to help in enhancing the cell efficiency by using materials that are least affected. Studies and improvements on methods that can be used to measure the cell parameters in real time are also needed. Also in the context of fast charging, the physical parameters of electrode play a significant role in the life and sensitivity of the battery. Therefore, it is recommended to investigate thoroughly the electrode design, which can accommodate the need for fast charging. Another key recommendation is to develop effective control and management algorithms. Cell arrangement will be different in fast-charging packs than it is in current Li-Ion batteries. Manufacturers have recommended that these batteries will have more cells in series than in parallel. This form of arrangement is beneficial as it allows better control, management and easy fault detection during charging. Additionally, future studies should be conducted to assess the cost-management strategies that can be utilized as fast-charging packs get commercialized.
In future studies the potential applications and protocols specific to the power system operation will also be investigated. Another future work is to conduct a critical analysis of the fast-charging protocols by following by international standards such as the Institute of Electrical and Electronics Engineers (IEEE), American National Standards Institute (ANSI) and British Standards (BS). Moreover, another prospect is the analysis of fast-charging circuits and applications in distribution systems.
Acknowledgements
The Effat University funds this work under grant number Decision No. UC#9/2June2021/7.2-21(3)5.
References
- 1. D. Deng, “Li‐ion batteries: basics, progress, and challenges,” Energy Sci. Eng., vol. 3, no. 5, pp. 385-418, 2015.
- 2. N. T. Al-Sharify, D. Raheem Rzaij, Z. Majid Nahi, and Z. T. Al-Sharify, “An experimental investigation to redesign A pacemaker training board for educational purposes,” IOP Conf. Ser. Mater. Sci. Eng., vol. 870, p. 012020, 2020.
- 3. C. Gebhardt, “Multi-spacewalk series to replace Station batteries completed,” Nasaspaceflight.com, 20-Jan-2020. [Online]. Available: https://www.nasaspaceflight.com/2020/01/us-segment-five-spacewalk-series-iss-batteries/. [Accessed: 2 Dec. 2020].
- 4. P. Zhang, J. Liang, and F. Zhang, “An overview of different approaches for battery lifetime prediction,” IOP Conf. Ser. Mater. Sci. Eng., vol. 199, p. 012134, 2017.
- 5. “Battery Lifetime and Maintenance,” Pveducation.org. [Online]. Available: https://www.pveducation.org/pvcdrom/battery-characteristics/battery-life-time-and-maintenance. [Accessed: 2 Dec. 2020].
- 6. N. P. Ankit Gupta, “Lithium Ion Battery Market by Chemistry,” Global Market Insights, May-2020. [Online]. Available: www.gminsights.com. [Accessed: 2 Dec. 2020].
- 7. Y. Mekonnen, H. Aburbu, and A. Sarwat, “Life cycle prediction of Sealed Lead Acid batteries based on a Weibull model,” J. Energy Storage, vol. 18, pp. 467-475, 2018.
- 8. Tomaszewska, A. et al. (2019). Lithium-ion battery fast charging: A review. eTransportation. DOI: 10.1016/j.etran.2019.100011
- 9. Khan, H., Nizami, I. F., Qaisar, S. M., Waqar, A., & Krichen, M., “Analyzing Optimal Battery Sizing in Microgrids Based on the Feature Selection and Machine Learning Approaches”, Preprint, 2022.
- 10. T. Chen et al., “Applications of lithium-ion batteries in grid-scale energy storage systems,” Trans. Tianjin Univ., vol. 26, no. 3, pp. 208-217, 2020
- 11. M. Ghiji et al., “A review of lithium-ion battery fire suppression,” Energies, vol. 13, no. 19, p. 5117, 2020.
- 12. Sheng, J., Tong, S., He, Z., & Yang, R. (2017). Recent developments of cellulose materials for lithium-ion battery separators. Cellulose, 24, 4103-4122.
- 13. Mian Qaisar, S., AbdelGawad, A. E. E., & Srinivasan, K., “Event-Driven Acquisition and Machine-Learning-Based Efficient Prediction of the Li-Ion Battery Capacity”, SN Computer Science, 3(1), 1-6, 2022.
- 14. S. Bhattacharya, A. R. Riahi, and A. T. Alpas, “Thermal cycling induced capacity enhancement of graphite anodes in lithium-ion cells,” Carbon N. Y., vol. 67, pp. 592-606, 2014.
- 15. Yang X-G, Zhang G, Ge S, & Wang C-Y. (2018). Fast charging of lithium-ion batteries at all temperatures. Proc. Natl. Acad. Sci. U.S.A, 115(28):7266e71. https://doi.org/10.1073/pnas.1807115115.
- 16. Gallagher KG, Trask SE, Bauer C, Woehrle T, Lux SF, Tschech M, L. P, Polzin BJ, Ha S, Long B, Wu Q, Lu W, Dees DW, & Jansen AN. (2016). Optimizing areal capacities through understanding the limitations of lithium-ion electrodes. J Electrochem 163(2):138e49. https://doi.org/10.1149/2.0321602jes
- 17. Waldmann T, Kasper M, & Wohlfahrt-Mehrens M. (2015). Optimization of charging strategy by prevention of lithium deposition on anodes in high-energy lithium-ion batteries electrochemical experiments. Electrochim Acta, 178:525e32. https://doi.org/10.1016/J.ELECTACTA.2015.08.056.
- 18. Yin, Y., Hu, Y., Choe, S., Cho, H., & Joe, T.W. (2019). New fast charging method of lithium-ion batteries based on a reduced order electrochemical model considering side reaction. Journal of Power Sources, 423, 367-379.
- 19. Qaisar, S. M., & AbdelGawad, A. E. E., “Prediction of the Li-Ion Battery Capacity by Using Event-Driven Acquisition and Machine Learning”. In 7th International Conference on Event-Based Control, Communication, and Signal Processing (EBCCSP) (pp. 1-6). IEEE, 2021.
- 20. Xu, M. et al. (2018). Modeling the effect of two-stage fast charging protocol on thermal behavior and charging energy efficiency of lithium-ion batteries. Journal of Energy Storage, 20, 298-309.
- 21. Xu et al. (2019). Fast charging optimization for lithium-ion batteries based on dynamic programming algorithm and electrochemical-thermal-capacity fade coupled model. Journal of Power Sources, 438.
- 22. Keil, P., & Jossen, A. (2016). Charging protocols for lithium-ion batteries and their impact on cycle life—An experimental study with different 18650 high-power cells. Journal of Energy Storage, 6, 125-141.
- 23. Zhang S, Xu K, & Jow T. (2006). Study of the charging process of a LiCoO2-based Li-Ion battery. J Power Sources, 160(2):1349e54. https://doi.org/10.1016/J.JPOWSOUR.2006.02.087.
- 24. Qaisar, S. M., & Alguthami, M., “An Effective Li‐Ion Battery State of Health Estimation Based on Event‐Driven Processing”. Green Energy: Solar Energy, Photovoltaics, and Smart Cities, 167-190, 2020.
- 25. Liu, K., Li, K., Peng, Q., & Zhang, C. (2019). A brief review on key technologies in the battery management system of electric vehicles. Frontiers of Mechanical Engineering, 14(1), 47-64.
- 26. Mian Qaisar, S., “Event-driven coulomb counting for effective online approximation of Li-ion battery state of charge”. Energies, 13(21), 5600, 2020.
- 27. Fijita, Yoshikazu et al. “Development Of Battery Management System”. Fujitsu Ten Technical Journal, vol. 42, no. 2016, 2016, pp. 68-80, https://www.denso-ten.com/business/technicaljournal/pdf/42-10.pdf. Accessed 20 Feb 2021.
- 28. Xing, Yinjiao et al. “Battery Management Systems In Electric And Hybrid Vehicles”. Energies, vol 4, no. 11, 2011, pp. 1840-1857. MDPI AG, doi:10.3390/ en4111840. Accessed 20 Feb. 2021.
- 29. Qaisar, S. M., “Li-Ion Battery SoH Estimation Based on the Event-Driven Sampling of Cell Voltage”. In 2nd International Conference on Computer and Information Sciences (ICCIS) (pp. 1-4). IEEE, 2020.
- 30. Birkl, C. R., Roberts, M. R., McTurk, E., Bruce, P. G., & Howey, D. A. (2017). Degradation diagnostics for lithium ion cells. Journal of Power Sources, 341, 373-386.
- 31. Bach, T. C., Schuster, S. F., Fleder, E., Müller, J., Brand, M. J., Lorrmann, H., ... & Sextl, G. (2016). Nonlinear aging of cylindrical lithium-ion cells linked to heterogeneous compression. Journal of Energy Storage, 5, 212-223.
- 32. Yang, X. G., Leng, Y., Zhang, G., Ge, S., & Wang, C. Y. (2017). Modeling of lithium plating induced aging of lithium-ion batteries: Transition from linear to nonlinear aging. Journal of Power Sources, 360, 28-40.
- 33. Park, J., Appiah, W. A., Byun, S., Jin, D., Ryou, M. H., & Lee, Y. M. (2017). Semi-empirical long-term cycle life model coupled with an electrolyte depletion function for large-format graphite/LiFePO4 lithium-ion batteries. Journal of Power Sources, 365, 257-265.
- 34. Mian Qaisar, S., “A proficient Li-ion battery state of charge estimation based on event-driven processing”. Journal of Electrical Engineering & Technology, 15(4), 1871-1877, 2020.
- 35. Han, X., Lu, L., Zheng, Y., Feng, X., Li, Z., Li, J., & Ouyang, M. (2019). A review on the key issues of the lithium ion battery degradation among the whole life cycle. ETransportation, 1, 100005.
- 36. B. Balagopal, C. S. Huang, and M.-Y. Chow, “Effect of calendar aging on li ion battery degradation and SOH,” in IECON 2017 - 43rd Annual Conference of the IEEE Industrial Electronics Society, 2017.
- 37. S. Ma et al., “Temperature effect and thermal impact in lithium-ion batteries: A review,” Prog. Nat. Sci., vol. 28, no. 6, pp. 653-666, 2018.
- 38. Abdi, H., Mohammadi-ivatloo, B., Javadi, S., Khodaei, A. R., & Dehnavi, E. (2017). Energy storage systems. Distributed Generation Systems, 333-368.
- 39. Vetter, J., Novák, P., Wagner, M. R., Veit, C., Möller, K. C., Besenhard, J. O., ... & Hammouche, A. (2005). Ageing mechanisms in lithium-ion batteries. Journal of Power Sources, 147(1-2), 269-281.
- 40. Zeng, X., Xu, G. L., Li, Y., Luo, X., Maglia, F., Bauer, C., ... & Chen, Z. (2016). Kinetic study of parasitic reactions in lithium-ion batteries: a case study on LiNi0. 6Mn0. 2Co0. 2O2. ACS Applied Materials & Interfaces, 8(5), 3446-3451.
- 41. Qaisar, S. M., “Event-Driven Approach for an Efficient Coulomb Counting Based Li-Ion Battery State of Charge Estimation”. Procedia Computer Science, 168, 202-209, 2020.
- 42. Lu, B., Ning, C., Shi, D., Zhao, Y., & Zhang, J. (2020). Review on electrode-level fracture in lithium-ion batteries. Chinese Physics B, 29(2), 026201.
- 43. Tippmann, S., Walper, D., Balboa, L., Spier, B., & Bessler, W. G. (2014). Low-temperature charging of lithium-ion cells part I: Electrochemical modeling and experimental investigation of degradation behavior. Journal of Power Sources, 252, 305-316.
- 44. Waldmann, T., Wilka, M., Kasper, M., Fleischhammer, M., & Wohlfahrt-Mehrens, M. (2014). Temperature dependent ageing mechanisms in Lithium-ion batteries–A Post-Mortem study. Journal of Power Sources, 262, 129-135.
- 45. Pinson, M. B., & Bazant, M. Z. (2012). Theory of SEI formation in rechargeable batteries: capacity fade, accelerated aging and lifetime prediction. Journal of the Electrochemical Society, 160(2), A243.
- 46. Prada, E., Di Domenico, D., Creff, Y., Bernard, J., Sauvant-Moynot, V., & Huet, F. (2013). A simplified electrochemical and thermal aging model of LiFePO4-graphite Li-Ion batteries: power and capacity fade simulations. Journal of the Electrochemical Society, 160(4), A616.
- 47. Mei, W., Chen, H., Sun, J., & Wang, Q. (2019). The effect of electrode design parameters on battery performance and optimization of electrode thickness based on the electrochemical–thermal coupling model. Sustainable Energy & Fuels, 3(1), 148–165. https://doi.org/10.1039/C8SE00503F
- 48. Zhao, R., Liu, J., & Gu, J. (2015). The effects of electrode thickness on the electrochemical and thermal characteristics of lithium ion battery. Applied Energy, 139, 220-229. https://doi.org/10.1016/j.apenergy.2014.11.051
- 49. Pender, J. P., Jha, G., Youn, D. H., Ziegler, J. M., Andoni, I., Choi, E. J., ... & Mullins, C. B. (2020). Electrode degradation in lithium-ion batteries. ACS Nano, 14(2), 1243-1295.
- 50. Liu, P., Wang, J., Hicks-Garner, J., Sherman, E., Soukiazian, S., Verbrugge, M., ... & Finamore, P. (2010). Aging mechanisms of LiFePO4 batteries deduced by electrochemical and structural analyses. Journal of the Electrochemical Society, 157(4), A499.
- 51. Sarre, G., Blanchard, P., & Broussely, M. (2004). Aging of lithium-ion batteries. Journal of Power Sources, 127(1-2), 65-71.
- 52. Amine, K. (2002). Factors responsible for impedance rise in high power lithium ion batteries. In Fuel and Energy Abstracts (Vol. 4, No. 43, pp. 261-262).
- 53. Groot, J. (2012). State-of-health estimation of Li-Ion batteries: Cycle life test methods.
- 54. Plett, G. (2015). Battery Management Systems, Volume 1. Artech House.
- 55. Li, J., Zhou, S., & Han, Y. (2016). Advances in Battery Manufacturing, Service, and Management Systems. John Wiley & Sons.
- 56. AL-Refai, A., Rawashdeh, O., & Abousleiman, R. (2016). An Experimental Survey of Li-Ion Battery Charging Methods. International Journal of Alternative Powertrains, 5(1), 23-29. Retrieved 31 October 2020, from https://www.jstor.org/stable/26169102.
- 57. Weicker, P. (2014). A Systems Approach to Lithium-ion Battery Management. Artech House.
- 58. Xiong, R. (2019). Battery Management Algorithm for Electric Vehicles. Springer Nature.
- 59. Tomaszewska, A. et al. (2019). Lithium-ion battery fast charging: A review. eTransportation. DOI: 10.1016/j.etran.2019.100011
- 60. Zhang, G., Ge, S., Xu, T., Yang, X. G., Tian, H., & Wang, C. Y. (2016). Rapid self-heating and internal temperature sensing of lithium-ion batteries at low temperatures. Electrochimica Acta, 218, 149-155.
- 61. Ahmed, S. et al. (2017). Enabling fast charging - A battery technology gap assessment. Journal of Power Sources, 367, 250-262.
- 62. Computer Aided Engineering for Batteries. (2002). Community Battery Simulation Framework. http://batterysim.org.
- 63. U.S. Department of Energy. (2017). Computer Aided Engineering for Batteries, https://energy.gov/eere/vehicles/downloads/computer-aided-engineering-electric-drive-vehicle-batteries-caebat
- 64. Zhang S, Xu K, & Jow T. (2006). Study of the charging process of a LiCoO2-based Li-Ion battery. J Power Sources, 160(2):1349e54. https://doi.org/10.1016/J.JPOWSOUR.2006.02.087.
- 65. Dahn H. M., Smith A. J., Burns J. C., Stevens D. A. & Dahn J. R. (2012). J. Electrochem. Soc. 159, A1405
- 66. Attia, P. M., Grover, A., Jin, N., Severson, K. A., Markov, T. M., Liao, Y. H., ... & Chueh, W. C. (2020). Closed-loop optimization of fast-charging protocols for batteries with machine learning. Nature, 578(7795), 397-402.
- 67. Williard, N., He, W., Osterman, M., & Pecht, M. (2012, August). Reliability and failure analysis of Lithium Ion batteries for electronic systems. In 2012 13th International Conference on Electronic Packaging Technology & High Density Packaging (pp. 1051-1055). IEEE.
- 68. Cannarella, J. & Arnold, C.B. (2015). Electrochem. Soc. 162. A1365eA1373.
- 69. Burns, J.C., Stevens, D.A., & Dahn, J.R. (2015). J. Electrochem. Soc. 162, A959-A964.
- 70. Computer Aided Engineering for Batteries. (2002). Community Battery Simulation Framework. http://batterysim.org.
Note
- *Corresponding author: [email protected]
