Chapter 8
Microfabrication for Novel Products in Drug Delivery
An Example
Chapter Contents
8.1 Microneedle Research at University of Twente and its Spin-Off
8.1.1 Desk Research: Microneedle Arrays, Microfabrication and Transdermal Delivery of Insulin
8.1.2 Is There a Need for Microneedles?
8.1.3 Microneedles by Microfabrication Technologies
Fabrication and Design Concepts
Ceramic Nanoporous Microneedles
Summarizing the Technology Requirements from a Commercialization Point of View
8.1.4 Are Microneedles Ready for Insulin Delivery?
8.1.5 Design Aspects for Microneedle Insulin Delivery
Fabrication Attributes and Their Clinical Relevance
8.2 MNA-4-Insulin: A Brief Evaluation
8.3 Conclusions
In Chapter 1 the importance of technological innovation in the market place was discussed, and the previous chapter gave a successful example of technology transfer from university to spin-off. This chapter is dedicated to academic-driven product innovation. A new technology starts with results from various different scientific disciplines, together with an analysis of the existing state of the art in industry. One may suggest that there is already enough knowledge (business intelligence) in industry, and universities (operating on public money) are not the place that competitive products should be developed; they should stick to the provision of stimulating educational environments. Universities do, however, have a clear responsibility to take their place within the innovation chain. But what is that place exactly? Is it possible to provide the best academic training for new engineers without engineering sciences leading to products in the (competitive) industrial market place? I would immediately say that it must be both. With respect to modern learning, we believe that experience with real-world problems is almost as important to a student’s education as instruction in the basics of his/her discipline.
Universities, therefore, have to create a motivating environment, in which the students actually get in touch with real-world problems, at least to a certain level. Normally, as an individual student and as a member of society, we easily recognize this need for gaining knowledge within universities. So, what is in this for industry? Since industry is simply the profit-making part of society, universities should bring their results to the attention of the industry, and similarly industry should bring their pre-competitive research questions (encountered in the real world) to the attention of academic scientists. Today, both actors operate together in a variety of extensive networks, in part stimulated by public money. Within these networks, an individual’s effort can become linked to others and impact is created.
Well, can one expect that every individual’s research effort makes a difference? This is unrealistic, but the sum of all the incremental results clearly makes a difference to society. Here, I would like to focus on a specific example, in which the results gained from researching a new technology are pre-competitive and open access, but the utilization thereof may well not be. Public resources must be used to benefit society as a whole, without discrimination and without private economical benefit to the individuals within the research teams. However, patents can still be obtained and the technologies described therein can be validated on industrial terms.
Technology transfer occupies the interface between knowledge creation and product development. Part of this process is the selection of which products to develop. Ideas which present immediate answers to specific problems in a market place have the highest chance of being developed; however, it is not always obvious which ideas will have value after a product development path which takes five, ten or more years towards completion prior to emerging on the market. For technology to emerge into the market place, visions are important.
This chapter focuses on the development of a new technology for drug delivery through the skin. In it, we will discuss state-of-the-art microneedle arrays, and a specific microfabricated device which can transport a drug or vaccine through the skin by means of nanoscale pores.
Before building a future scenario for the use of this type of device, I have to first thank the co-workers at University of Twente’s MESA+ Institute for Nanotechnology, specifically Dr. S.N. Bystrova, M. Domanski and Dr. A.J.A. Winnubst, for their technological and scientific inputs in the development of a novel nanoporous microneedle array technology. The support of Prof. Dr. J.A. Bouwstra of the Leiden/Amsterdam Center for Drug Research (LACDR), Leiden University, is also acknowledged. She helped to define the real-world problem (enhancement of transdermal drug delivery) to us as engineering scientists and supported the valorization project through her expertise in the field of the characterization of skin-transport processes, and kindly suggested that fluorescent ovalbumin can be used as a model compound for the evaluation of the nanoporous microneedle array technology developed at University of Twente’s MESA+ Institute for Nanotechnology spin-off MyLife Technologies. Further, I would like to acknowledge the financial support of the original research project, as well as the valorization project related to MyLife Technologies, from the Dutch Science and Technology Foundation STW, Applied Science Division of NWO, and the technology program of the Ministry of Economic Affairs, The Netherlands. Finally, I would like to mention that dedicating time and expert knowledge to the compilation of this chapter would not have been possible without the funding of my personal VENI grant, also by STW.
8.1 Microneedle Research at University of Twente and its Spin-Off
Chapter 7 discussed the LICETAS project. In this section, we will discuss novel products based on microneedle array technology (MNA) from the point of the commercial exploitation of high-tech research results by academically-driven product development. This route involves obtaining a patent and consists of two main elements: (1) desk research and (2) validating research for technology–product combinations. An effective academic-driven product development and innovation path always requires the collection of insight into the current needs of society. However, in science-driven technological development this path may take quite a long time in order to fully comprehend all its complexities. From idea, to proposal writing, to receiving the grant and hiring personnel takes approximately 1.5 years. Conducting the research and interpreting the results before being able to actually attract industrial attention may take another 2–3 years.
I have focused on the practical application of a specific microfabrication technology for microneedle arrays since 2006. This technology was developed by publically funded research at the MESA+ institute between 2003 to 2007 in collaboration with Leiden University. We investigated the enhancement of transdermal drug delivery processes within a pre-competitive research setting. In 2006, the project team at MESA+, headed by Prof. Dr. Ir. van den Berg (BIOS, The Lab-on-a-Chip group), made a patent application. To enhance the chance of utilizing the collected know-how, and to offer interested industrial partners (of which some were already part of the user committee consulting on the original research project) the opportunity to evaluate this technology further, the creation of a spin-off activity was initiated, and granted with a first phase valorization grant by STW of 25,000.00 Euros.
This money was required to transfer the know-how gained from the research results in the project into the first non-tangible asset of the spin-off: a patent application. Similarly to the LICETAS project, the first step in the project was necessary to secure a second stage pre-seed technology fund, which would then allow the technology to be developed to its full potential, thus reducing the economical risk involved in pre-clinical evaluation of the MNA technology. Based on the know-how built in the valorization team and the prospective patent application different business cases could be developed. This second, STW funded, valorization project (conducted from 2008 to 2010 within MESA+ at the research group Mesoscale Chemical Systems, headed by Prof. Dr. Gardeniers) increased the chances of a start-up within the highly competitive, but highly conservative medical field. This 200,000.00 Euro project allowed the team to validate the technology to identify a specific technology–product match, to create a first business niche for the novel patented nanoporous microneedle array product. We did not just look at the drug delivery market. To increase the opportunity for initiating a successful start-up, it is important to identify the unique selling points of the technology at hand. The publicly supported valorization project allowed us to bring the potential of the original research results to the attention of different parties with specific application needs. In November 2010, indeed, The University of Twente’s MESA+ Institute for Nanotechnology spin-off MyLife Technologies received an initial, first phase (50,000.00 Euros), life-science, pre-seed grant to evaluate the use of nanoporous MNA technology for making an ultra-minimally invasive, easy-to-use vaccination patch.
Through this process of pre-seeding, the organizational actors in the network could evaluate the business potential of the MNA technology, and support the efforts of the valorization team by the suitable instruments of technology transfer to the market. These efforts reduce the financial risks for an entrepreneur, allowing the public sector to get involved in the process of patent application roll-out to the national phase. At present, the MyLife Technologies team is negotiating the full terms and conditions of commercial exploitation of the patent with the university board and its funding agent. Bringing these negotiations to a successful close will enable the birth of a new company.
In the LICETAS case a company (Medimate BV) was started quickly after finalizing the original research project and know-how was transferred to this start-up directly. Why was this not possible in the MNA case?
In brief, the idea for LICETAS started from a project proposal inspired by the needs of a practicing psychiatrist, and funding was received in 2001. The PhD project was entirely dedicated to one specific appliation: quantitative lithium measurement from a droplet of finger stick blood. In 2005, a full proof-of-principle was presented to the user committee of the STW project, and at the same time it happened that a young entrepreneur was looking for a business case to start his own company. With the initial support of the original research team, and members of the user committee, it was decided to initially make the entrepreneur a part of the research team, through (1) an extension of 25,000.00 Euros of the original project and (2) the subsequent preparation of an STW valorization phase-one grant. Similarly to MyLife Technologies, it was because of this initial seed money that Medimate BV was able to take off. The entrepreneur was not part of the academic community; he had to provide a different instrument to become one of the actors in the network, by starting his privately held company (S. Staal BV) directly in the very first valorization phase. Eventually, after settling the knowledge transfer conditions with the university board and its funding agent STW, S. Staal BV and the other involved parties became owner of Medimate BV to continue the valorization path. Since this spin-off was created by the original research from STW funds, the University of Twente was also eligible to apply for funding in the second STW valorization round with its spin-off Medimate BV as the project conducting valorization team.
It is probably the case that a BV could not be started straight away in the MyLife Technologies case because different actors, at a different time, in a different location and in different circumstances of the actors’ career paths were involved. Also, when the LICETAS research project had been finalized, no patent had been applied for, and the risk of shelving the project results was extremely high. Capillary electrophoresis on chip was not novel by itself, and detecting lithium by this technique in body fluids was not novel either, as it had been previously performed in serum. At the time, the accurate detection of lithium in a whole blood sample by capillary electrophoresis on chip might have had the potential to be patentable, but when the decision was made to transfer the know-how to the entrepreneur he already had a company in place, which increased the chances for utilization of the research results gained in the research project.
This scenario was not an option for MyLife Technologies, which brings me to another question: What can I do as an academic actor to manage the risk of shelving valuable research results versus the financially high risks involved in high-tech innovation? How can I conduct my academic research so as to generate output that is of direct value to a privately-held business that interacts with the academic network?
A comprehensive answer is probably not possible within the context of this book, hence I will restrict myself to the context of microfabrication for industrial applications. One future application of MNA technology may be the creation of a method for treating diabetes.
Today, insulin is given to a diabetes sufferer to control their glucose level. It is estimated that there will be 230 million diabetes cases worldwide in approximately 20 years from now, and today’s methods are insufficient for providing a solution to this problem, so novel technologies will be required.
Many different researchers world-wide have been involved in the development of microneedle array technology for the ultra-minimally invasive delivery of insulin via the skin. Due to my involvement in publicly funded research on microneedle array technology for drug delivery and the patenting of a technology based on the research result we gained from the academic project, I was able to put my entrepreneurial head on and think towards a unique selling point for this technology in the identified application of insulin delivery. Is it a new business case? A significant amount of desk research was needed to answer this question. In the following sections I will describe its results and evaluate in depth a possible match for microneedle array technology with insulin delivery.
8.1.1 Desk Research: Microneedle Arrays, Microfabrication and Transdermal Delivery of Insulin
Microneedles are a next-generation drug delivery device, that targets the skin. As yet, few original research papers consider this type of insulin delivery, so a review of microneedle array microfabrication technology and its possible applications in transdermal delivery of insulin is presented. A small sub-set of publications has been identified that demonstrate the proof-of-principle for insulin delivery by microneedles. The results of these papers are compared. However, the specific criteria that a microneedle device must fulfil to be used in this way are unclear, and a variety of hypotheses can be derived from this desk research.
A variety of microneedle designs (tip shapes, length, diameter, materials, etc.) in three distinct design categories have been produced. These are: silicon micromachined, replicated and fine-mechanically manufactured arrays. Despite the fact that microneedles can be used to target the skin as a route for delivery in a nearly painless manner, none of the designs show an appropriate level of technological readiness for clinical applications. Many designs have been presented in the scientific literature, but so far, clinical evaluation is limited. To bring any of these minimally invasive devices to market, one has to develop a suitable model which allows clinical researchers to benchmark the emerging microneedle techniques with respect to the conventional hypodermic needle injection for insulin. The case study discussed here will highlight the essential research and development stages concerned with microfabrication of microneedle arrays.
8.1.2 Is There a Need for Microneedles?
An arsenal of specific microfabrication techniques has been developed for miniaturization technologies, of which photolithography has been the most successful. Inspired by the success of the microelectronics industry, a wealth of devices, not necessarily electronic, has been developed where small size is a prerequisite. Some contain both electronic and non-electronic components. These devices are called microsystems or micro(opto)electromechanical systems (MEMS, MOEMS). Since the 1980s, these architectures also have been explored for the manipulation of minute amounts of fluids, as has been discussed in preceding chapters. This activity has presented us with an entirely new field of research named microfluidics [1], which has made its mark in the life sciences by the introduction of so called Laboratory-on-a-Chip (LoaC) devices. These devices now present us with a multitude of applications, of which some are concerned directly with clinical applications [2]. One type of device is the microneedle array, either developed as a stand-alone device or integrated with sensors and actuators [3]. They may offer advantages over hypodermic needles or chemical penetration enhancers. For certain applications, whereby the target is actually in the skin, the direct insertion of active compounds into the viable epidermis will also increase efficacy of the therapy [4–8]. This is especially beneficial in the cases where the delivery of macromolecular compounds is desired, e.g., DNA vaccine [9]. The viable epidermis hosts antigen-presenting cells and these can be addressed by microneedle array therapy directly (see work by Birchall’s group, Cardiff University, UK) [10–13]. Bouwstra’s group at the Leiden/Amsterdam Center for Drug Research (LACDR), Leiden University, NL, presented two other examples in this area describing “Immune modulation by adjuvants combined with diphtheria toxoid administered topically in BALB/c mice after microneedle array pre-treatment” and “Microneedle arrays for the transcutaneous immunization of diphtheria and influenza” by Ding et al. [14, 15]. In some studies, microneedle arrays are used for skin pretreatment [16–19]. Other, more sophisticated miniaturized devices for drug delivery also exist [20–22].
Reviews of microneedle technology published to date [23–30] show they are virtually pain free compared to the hypodermic needle. Micromachined needles are intended to be limited in their penetration depth and should be painless in use because of their very sharp tips and defined length. Of course, passive patches and topical creams also avoid pain, however, they often work only for very potent, small molecule drugs, or in specific formulations with chemical penetration enhancers. Microneedle array devices, by contrast, create a multitude of highly-defined pathways through the stratum corneum, which allow the passage of molecules without the side effects of chemical enhancers.
Can this be applied to insulin delivery? When it comes to the micro-manufacture of microneedles two distinct concepts exist: (1) the in-plane and (2) the out-of-plane approach, as depicted in Figure 8.1. During in-plane manufacture, the microneedle tips lie within the plane of the material. In the out-of-plane approach the microneedle tips evolve perpendicularly to the plane of the material’s working surface, which offers two important advantages: (1) a huge number of parallel injection sites, and (2) seamless integration of micro/nanofunctionality from the needles into the back plate. The schematic top and the cross-sectional views shown in Figure 8.1 present an overview of the key parameters which define needle geometry. While in-plane processing can deliver very long microneedles (Lshaft), the out-of-plane process is generally preferred because of its shallow, self-limiting insertion depth. However, the preferred technique depends on the individual application.

Figure 8.1 In-plane and out-of-plane micromachined microneedle arrays. (A) In-plane microneedles. (Reproduced with kind permission from reference [31]. Copyright © 2000 Springer Science+Business Media, LLC.) (B) Out-of- plane microneedles. (Reproduced with kind permission from reference [32]. Copyright © 2007 Springer Science+Business Media, LLC.) Schematic top and cross-sectional view of the in-plane (C) and the out-of-plane (D) microneedle designs, indicating the key design parameters for both concepts.
Combinations of these two approaches can be applied, for example Martanto et al. [33]. Microneedle fabrication uses the MEMS technologies, as outlined in Chapters 2 and 3, with the sequence of processing steps based on whether the needles are to be in-plane or out-of-plane, and can differ quite significantly per type of microneedle array (MNA).
The development of a clinically superior MNA delivery technology has been hampered by a limited understanding of the underlying transport mechanisms and the pharmacodynamics of the different tissue layers. The delivery of insulin, in particular, has been paid less attention than the more obvious applications of targeted delivery for vaccines and immuno-enhancing agents (adjuvants). Because diabetes is a severe disease with a rapidly growing patient population, it is important to investigate whether MNA devices possess distinctive benefits for its treatment.
In summary, this desk research provides a model case for entrepreneurial science and is organized in three sections. First a general literature screen of recent fabrication technology for microneedles is performed, including a discussion of the choice of materials and the technology requirements for commercialization. In the second section, a literature review of microneedle insulin delivery is presented. Thirdly, a sub-set of eight publications is derived, that describe successful experimental studies reaching proof-of-principle for insulin delivery by microneedles. In the latter section, specific attributes of these microneedle designs are discussed in more detail, which can eventually help to decipher the required criteria for the application of MNA to insulin delivery. Finally, the conclusion reflects on the state-of-the-art microneedle arrays for insulin delivery in clinical applications.
8.1.3 Microneedles by Microfabrication Technologies
Research Status
Pioneering research in the field of high-precision out-of-plane microneedle array fabrication and their medical applications were first studied by research groups at the Berkeley Sensor and Actuator Center, University of California (Berkeley, CA, USA), the Royal Institute of Technology (Stockholm, Sweden), MESA+ Institute for Nanotechnology, University of Twente (Enschede, The Netherlands) and the Georgia Institute of Technology (Atlanta, GA, USA) [3, 34–41]. A large number of publications have appeared over the last 15 years concerning the fabrication and testing of microneedles in a diversity of applications, including brain–computer interfaces, microneedle probing and stimulating for various brain regions, scalp electroencephalography, drug delivery, diagnostics and applications where spiked microstructures are used as nozzles and pipettes.
MNAs containing microneedles of diverse shape, length, diameter and density have been demonstrated to be capable of bypassing the skin barrier. Both in-plane and out-of-plane fabrication are well suited to the creation of microneedle arrays, but each requires a different approach if it is to be integrated into a skin patch. Only the out-of-plane method will directly produce a two-dimensional array seamlessly connected to a back plate, which is preferable for integration into higher functionality [3]. MNAs are also fine-mechanically manufactured by assembly from conventionally produced hypodermic needles, and successfully demonstrated in certain types of transdermal delivery studies [42]. Nonetheless, lithographic exposure techniques utilizing microsystems technology (MEMS), (i.e., reproducing a structural element multiple times from a mask or mold), are now established in drug delivery research.
Lithographic techniques result in arrays with a tip radius of < 100 nm, microneedle outer diameters below 300 μm, very well-defined length in the range of a few to hundred micrometers, and varying needle densities. Batch processing allows some cost reduction. Hollow MNAs require a flow-through delivery system and have been tested by gluing the back plate of the MNA to a syringe. These devices are not yet applicable to self-administered transdermal insulin delivery, but they demonstrate a clear step forward. Figure 8.2 summarizes complementary examples of out-of-plane MNAs, and demonstrates early prototypes of microneedles coupled to a syringe for intradermal drug delivery [33, 43–46]. Recent publications have discussed microfabricated microneedle technology, and evaluated their performance against other insulin delivery techniques [24, 47–49].
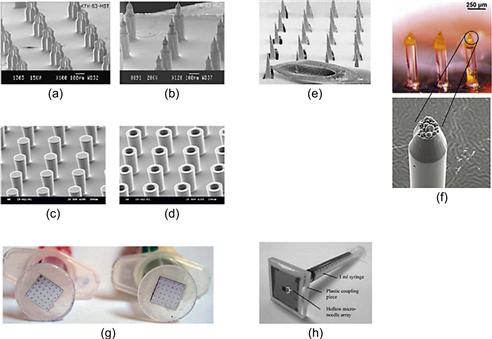
Figure 8.2 Different types of out-of-plane microneedle array technologies used for transdermal applications. (a, b) Sharp-tip silicon microneedle arrays. (Reproduced with kind permission from reference [43]. Copyright © 2008 Springer Science+Business Media, LLC.) (c, d) 80 and 100 μm diameter solid and hollow microneedle arrays without sharp tips. (Reproduced with kind permission from reference [44]. Copyright © 2005 Springer Science+Business Media, LLC.) (e) Stainless steel laser cut microneedle arrays. (Reproduced with kind permission from reference [33]. Copyright © 2004 Springer Science+Business Media, LLC.) (f) Two different types of dissolving polymer microneedle arrays by micromolding. (Reproduced with kind permission from reference [50]. Copyright © 2006 Springer Science+Business Media, LLC.) (g) Device prototypes used for clinical pain testing. (Reproduced with kind permission from reference [46]. Copyright © 1996 Springer Science+Business Media, LLC.) (h) Device prototype with 10 by 10 hollow silicon microneedles as shown in (d). (Reproduced with kind permission from reference [44]. Copyright © 2005 Springer Science+Business Media, LLC.)
Fabrication and Design Concepts
Some processes form the MNA device directly, while others first produce a master. Silicon micromachining and stereomicrolithography are two examples of direct techniques forming three-dimensional work pieces [3, 37, 51]. SU-8 photolithography on pre-patterned silicon substrates combining lithography with micromolding, titanium micromachining, and the use of two photon induced polymerization of organic–inorganic hybrid biomaterials [52–54] have also been attempted experimentally.
Some remarkably simple methods have been recorded for micromolding microneedles. For example, Lee et al. introduced drawing lithography for the three-dimensional fabrication of an ultrahigh-aspect-ratio microneedle, and Zhu et al. recently demonstrated a fabrication process that uses chemically etched silica needles as a template for a replication process [55, 56]. Unfortunately, Zhu et al. [56] omit a detailed description of the fabrication of the silica template. Although their paper does not describe the fabrication method of the template the authors clearly identify micromolding as a low-cost manufacturing scheme, which is suitable for further drug delivery research.
Many different processes have been tried to make extremely sharp microneedles. Unfortunately, there is no consensus on how one should define sharpness in this context. Many different research groups have elaborated on various fabrication processes to generate particular needle geometries, with the intention of reducing the tip radius, which will obviously lower the insertion energy. The density, the total number of needles within a defined array area, and the mechanism of insertion (manual or impact) are likely to be the major factors influencing insertion performance. Knife-like features, such as that introduced by Gardeniers et al. [38], should be assessed against other designs with respect to pain sensation, skin reaction, intrinsic skin variations from volunteer to volunteer, place of incision and relevant parameters of wound healing in relation to the size and geometry of the incision. Development of a benchmark for these parameters is not trivial because the skin is an anisotropic biomaterial, which changes its mechanical characteristics under stress. Moreover it is unclear how the tip radius of a microneedle (i.e., sharpness) can be translated into a meaningful parameter for such an incision experiment.
Some studies have explored needle radius together with other geometrical and application parameters through mechanical modeling [57, 58]. However, the ease of insertion of the microneedles or the successful introduction of drug does not just depend on the individual needle tip, or the configuration of the array, but also on the apparatus that is used to administer the MNA device to the skin and the drug formulation [59]. Furthermore, a trade-off between sharpness and microneedle stability must be sought for a clinical application. The insertion of microneedle arrays in human subjects was reported in a study by Gill and Prausnitz [60].
Materials
Microneedles are made either directly from active agents embedded in a dissolving material, or the microneedle platform acts as a carrier, which releases active compounds from a coating (solid microneedles), through a fluid conduit from a reservoir (hollow microneedles) or from the material’s intrinsic pores (porous microneedles). The range of materials used in research or commercial activities includes glass or silica, glass-like plastics and ceramic composites, silicon, silicon nitride, stainless steel, nickel-plated needle arrays and titanium, amongst various types of polymers, carbohydrates and micro-nanoparticle hybrid materials. Each of these materials may require their own fabrication techniques, for example, stainless steel or titanium microneedle arrays are cut by a laser, which limits their physical dimensions to the resolution of the laser cutter. This limited resolution will also affect the radius of the needle tips, and the overall geometrical dimensions of the needles.
Silicon, which forms a native oxide layer, is much used in microsystems technology and has been accepted for use in transdermal pre-clinical research, although no commercial application yet exists. Ji et al. have done some preliminary work in the area of porous microneedles. They used selective electrochemical etching, with a photoresist as a protective mask to form the porous silicon tip, thus forming one of the first silicon out-of-plane microneedle arrays that contain tips of two types of materials with very distinct properties [61]. Other publications also discuss the range of materials used in out-of-plane micro- and nanomanufacture of MNAs. For example, Park et al. investigated a polymer double-layer molding process, as shown in Figure 8.2f, which was generated from a mold structure. The mold was fabricated by means of a combination of SU-8 photolithography, reactive ion etching and polydimethylsiloxane soft lithography [45, 50]. They produced tapered tips, using poly(lactic-co-glycolic acid) as a material for the formation of dissolving microneedles and encapsulated calcein in the tips. The particle structure remains intact inside the molded microneedle.
Similarly, Moon et al. fabricated a mold by soft-lithography, from a master made by inclined deep x-ray exposure, then the soft-mold is used for the replication of MNAs into biodegradable poly-L-lactide acid [62]. Carbohydrates have also been used for molding MNAs, and Donnelly et al. discussed the processing difficulties and instability of the material in MNA production [63]. Kolli and Banga, and Li et al. have found maltose to be more easily adapted to molding microneedles [64, 65]. Both Kolli and Li are members of Banga’s group affiliated with the Mercer University (Atlanta, GA, USA), which holds an impressive record on microneedle array technology.
Ceramic Nanoporous Microneedles
Within microneedle research, some groups are attempting to make ever sharper and smaller point-like microfeatures, while others are looking further on in the development chain, towards low-cost production and applications. One particularly exciting concept is the possibility of making arrays of microneedles in one platform, so combining a multitude of microneedles in parallel without the need for additional assembly steps. Such arrays could be used to make hundreds of miniscule incisions into the skin in one step, without causing pain. These incisions could then be used to transport molecules across the skin barrier. Figure 8.3 shows a ceramic-based nanoporous MNA platform technology developed at University of Twente’s MESA+ Institute for Nanotechnology (patent pending WO2009/113856). Here, the MNA is replicated from a soft-mold that is generated by polydimethylsiloxane soft-lithography from a master – this technique was discussed in Chapter 3, Section 3.3.4. A specific master was designed and manufactured by a combination of SU-8 photolithography and silicon micromachining that also integrates knife-like features in the tip with similar dimensions as introduced in previous work by Gardeniers et al. [38].
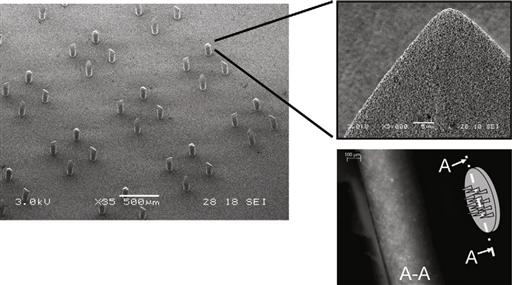
Figure 8.3 Scanning electron micrograph of a nanoporous microneedle array developed at MESA+ Institute for Nanotechnology, University of Twente. Inset (top right) shows a scanning electron micrograph revealing the porous structure of an individual needle tip. Inset (bottom right) depicts an optical micrograph showing a schematic of the array and its cross-section, demonstrating that the resulting nanopores can be filled with fluorescent ovalbumin (45 kDa), e.g., by incubating the nanoporous ceramic material in the protein solution over night.
Image courtesy: S.N. Bystrova, University of Twente, 2009.
A nanoporous microneedle has a porosity of approximately 30%, and an average pore size of 100 nm (see inset Figure 8.3, top right), which is large enough to hold model molecules such as fluorescent ovalbumin (inset Figure 8.3, bottom right). Further investigation is necessary to determine the extent to which such non-dissolving, nanoporous platforms can be of use in the delivery of macromolecules such as insulin.
In general, transdermal MNA devices are classified by the Food and Drug Administration as an invasive device, since the skin barrier is bypassed and also probably ruptured at deeper layers than the stratum granulosum. In most cases applying such devices to the skin surface will allow substances other than the drug to enter the body. Any skin rupture event will interfere with the body’s defence mechanism, generating additional adverse reactions in the skin, or even unfavorable systemic effects through infection or poisoning, hence this classification is necessary. All components of the microneedle administration device that come into direct contact with tissue, or risk contact during operation of the device must, therefore, obey stringent rules to gain medical approval.
At this stage of development, the exact material properties of a MNA are not fully understood, and have to be assessed as they stand and also when they are packaged within a drug-applying device. Despite their general potential being recognized in the drug delivery community the benefits of a specific material for insulin delivery still have to be addressed for all the different novel platform technologies described above.
Figure 8.4 gives an overview of the nanoneeedle fabrication process developed at University of Twente’s MESA+ Institute for Nanotechnology. This process starts with a hard mold, manufactured by photolithography and silicon micromachining, to combine a pre-patterned silicon substrate and a thick-film photoresist (SU-8) into one hard-mold structure for the fabrication of a soft mold for subsequent multiple production of ceramic green bodies. The individual MNAs are cut from the green body and sintered, forming the semi-finished product ready for integration into a delivery patch.
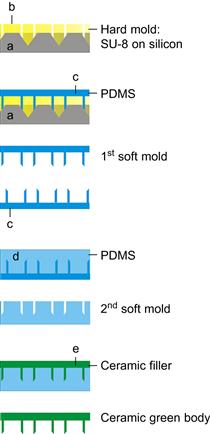
Figure 8.4 Process sequence of micromolding for ceramic microneedle arrays including the fabrication of a soft mold for production. Patent pending. (Explanation of a–e can be found elsewhere, [patent pending WO2009/113856])
Figure 8.5 depicts the integration of the ceramic MNA into a patch-type system. With a resulting materials porosity of approximatly 30%, a single microneedle within the array has a liquid loading capacity in the order of 1–2 nl, and the backing plate can contain up to 5 μl. These minute amounts may suffice for vaccination purposes. Currently, the application of University of Twente’s MNA technology is being investigated in collaboration with Prof. Dr. Scheper’s and Dr. de Gruijl’s groups at the VUmc Free University Medical Center, Amsterdam, The Netherlands, for the purpose of vaccine delivery. This type of nanoporous MNA is probably not appropriate for insulin delivery, but one needs further research to be conclusive.

Figure 8.5 Envisaged ceramic microneedle array integrated skin patch.
Summarizing the Technology Requirements from a Commercialization Point of View
Microfabricated devices compete with the delivery of insulin by standard, ultra-fine needle systems, either integrated in a patient-friendly, pen-like delivery system, or as a classical hypodermic needle mounted on a syringe. This type of needle costs about 0.05–0.20 US Dollars per disposable needle. MNA replication technology can probably compete with such a low pricing regime, but the pharmacodynamics of insulin uptake from the epidermal layer needs much more research, and is not yet clinically identified as a beneficial route for insulin delivery.
Small-molecule-releasing skin patches are already far better understood, and have been marketed at a large volume in industrial pharmacy. Fitting microneedles into one of the existing skin patch platforms may accelerate its integration into a technological solution. An example of successful, interdisciplinary, transdermal, drug delivery co-development is evident in current products in the iontophoretic patch market. These products combine microelectronics integrated current-controlled power supplies with skin patch technology into a patient-controlled iontophoretic transdermal system, providing on-demand systemic delivery of substances such as fentanyl (Ionsys™) and lidocaine (LidoSite®) [66]. Clinical trials are under way for both these systems. These electrically-controlled systems are quite complex, so it is surprising that passive microneedle arrays in a skin patch lag behind them in the market.
Another very promising example of MNA technology in a transdermal application is being commercialized by Zosano Pharma Inc. Zosano Pharma’s Macroflux® transdermal microprojection delivery system combines the convenience of a needle-free administration process with room temperature stability for various therapeutic peptides, proteins, small molecules and vaccines. The Macroflux® microneedle array component has been described in the scientific literature for more than 10 years.
Upgrading MNA technology from a device that just creates microscale aqueous conduits into the skin barrier into a fully packaged and safe drug delivery system comparable to Macroflux® will clearly require more than just engineering ingenuity. A device platform must be designed and manufactured, that will not only allow testing of a small number of demonstrators for transport studies, but must also present a better solution for diabetes management, justifying patient trials supported by the shared risk of private–public partnerships. Once such a microneedle technology platform is confirmed by evidence-based clinical translational research, it can be re-engineered into a more flexible system that will accommodate and address individual patient’s needs through the appropriate industrial channels for market introduction. The state of the art in microneedle array technology as it exists for insulin delivery in the scientific literature is discussed in the next two sections.
8.1.4 Are Microneedles Ready for Insulin Delivery?
In the last two decades a number of papers discussing the fabrication of microneedle arrays, either starting from the in-plane or the out-of-plane manufacturing concept, have been published. An evaluation of their application to insulin delivery is described in this section. One of the earliest papers addressing insulin delivery by means of MNA is the publication “Solid and hollow microneedles for transdermal protein delivery” by McAllister et al. [40]. Solid silicon microneedles were inserted across the stratum corneum and painlessly disrupted the skin’s barrier function, as demonstrated by an increase in permeability to calcein by four orders of magnitude. Additionally, an in vitro experiment was carried out that delivered insulin across the human epidermis at approximately four orders of magnitude larger levels than passive permeation.
These experiments utilized solid microneedles, and were carried out as a microneedle pretreatment intended to generate aqueous pathways into the epidermis. The publication also addressed fabrication concepts for hollow silicon and metal (nickel) microneedles, producing out-of-plane microneedles that permit fluids (water) to flow through their bores for the first time. Although this work was highly experimental, it provided a clear milestone in the field of microneedle array fabrication technology. The two pioneering researchers in this field were A. Pisano and M. Prausnitz. Although this review focuses on out-of-plane microneedles, Pisano and collaborators have also published an in-plane polysilicon micromolding process that was developed to fabricate hollow tubes by a combination of various micromachining techniques (see Figure 8.1(A) in Section 8.1.2). The researchers claimed that these types of devices could be useful for microfluidic applications, specifically including the continuous delivery of insulin to a diabetic patient [31]. In 2000, Zahn et al. (Pisano’s group) also considered the use of their microneedles for sample collection in biological analysis [31]. Their findings particularly mention microneedle strength and achievable bending moments, and optimization thereof. They further studied the flow capacity of their microneedles given a pressure at the inlet up to 138 kPa. Despite their application claim, no results on insulin delivery were described at this stage of their research. In the same year an overview of microneedle technology was published by the Prausnitz’s group which extended the list of possible microneedle applications to gene and macromolecular drug delivery, and that may include insulin [67].
Trebotich et al. then presented a thorough study of the geometries and flow characteristics of microneedles [68, 69]. Although the analysis of in-plane design differs from the required analysis for out-of-plane designs, it is worth mentioning that these mechanical parameter studies should be translated to experiments evaluating skin insertion performance. Zahn et al. have also published “Components of an integrated microfluidic devices for continuous glucose monitoring with responsive insulin delivery” in Diabetes Technology and Therapeutics [70], in which they suspect that their microneedle drug delivery technology could be applied to the intradermal injection of insulin. They hypothesized that intradermally injected insulin is quickly absorbed by the capillary bed into the bloodstream – an important postulation that still requires further clarification. They also postulate that the microfluidic administration of insulin could be performed by an integrated active device and may therefore allow complex drug delivery profiles compared to the classical bolus injection of insulin.
The methods under investigation to pass the skin’s barrier have been reviewed, as have fabrication methods and transport studies of microfabricated needles [41, 71]. Amongst other large molecular weight compounds and nanoparticles, this study included the first proof-of-concept that showed the flow of microliter quantities of insulin into skin utilizing an individual hollow glass microneedle in vivo, and reducing the blood glucose level in diabetic rats.
The fabrication review was a follow-up of the preliminary work carried out on hollow microneedles by McAllister et al. in 1999 [40]. Based on silicon micromachining, a novel out-of-plane microneedle fabrication technology was demonstrated by Gardeniers et al. in 2002 [38]. In their follow-up publication in 2003, they demonstrated in vivo insulin delivery into rats for the first time utilizing these hollow silicon microneedles coupled to a syringe [3]. Prausnitz and his various co-workers have also continued their work on insulin delivery at different levels of integration. For example, in 2004 Martanto et al. described laser-cut, stainless steel microneedles for transdermal delivery of insulin in vivo by a solid microneedle pre-treatment [33]. Prausnitz then showed that microneedles can dramatically increase transdermal delivery rates, especially for macromolecules [24]. These findings confirmed the earlier postulation of Zahn et al., although the mechanism of insulin uptake still remains unclear [70]. Together with an overview of proven transport studies, including the example of insulin facilitated by microneedle pre-treatment, Praunsnitz’ findings incorporate the concern of skin insertion force (i.e., margin of safety). Prausnitz also extended his view on advanced delivery mechanisms that could be achieved by combining microneedles with iontophoresis. To the best of my knowledge, this concept was first demonstrated for insulin delivery by Chen et al. in 2009 [17].
Inspired by the side-open microneedle design introduced earlier by Griss and Stemme [37], Zhang and Jullien described “Microneedle arrays for drug delivery and fluid extraction” including a side-open microneedle out-of-plane design with very sharp points, optimized through the use of a bi-mask technique [72]. They claimed that their needles are useful for insulin delivery into the human body, amongst other applications. Unfortunately, they did not include any results of a study on humans. In 2009, they used the same microneedle array design to demonstrate ink delivery into chicken skin [73]. Similarly to Gardeniers et al., Teo et al. coupled their microneedle arrays to a syringe [3, 44] and performed experiments with straight-walled microneedle arrays made from silicon. These results are of a controversial nature, since they used blunt microneedles despite all other systems using pointed ones. They demonstrated an increase in transport rates through dermatomed skin, while their findings in vivo restrict the potential benefits of this MNA design. Teo et al. state the following in their conclusion:
“In vitro tests using isolated skin demonstrated that the application of the microneedles resulted in a 10–20 fold increase in transdermal transport depending on the diameter of the microneedles.”
Furthermore, Teo et al. concluded:
“In vivo test in diabetic animals, however, demonstrated that arrays of these microneedles were not effective in the delivery of a systemic drug, such as insulin.”
However, in the same year an additional study by Davis et al. (Prausnitz’s group) presented further work on insulin delivery enabled by the microneedle array technology introduced by McAllister et al. [74, 41]. These contradictory findings may be explained solely by the difference in the geometry of the needles, their array density and the experimental differences between the set-ups of the in vivo trials.
In 2006, other work on MNA technology for transdermal insulin delivery was published. For example, Ma et al. introduced “A PZT insulin pump integrated with a silicon microneedle array for transdermal drug delivery” [75], but this research does not demonstrate any insulin delivery either in vitro or in vivo. Lv et al. published “Modeling of transdermal drug delivery with a microneedle array”, in which their theoretical studies were directed at insulin delivery [58]. Computer modeling of the fluid injection performance from a MNA is an important design aspect in the development of such applications. For example, transport into the skin depends on factors such as injection velocity and blood circulation. These initial modeling efforts are extremely valuable in optimizing this class of devices for clinical practice, and they must be continued as an integral part of product development.
Roxhed et al. have presented a higher level integration concept for drug delivery by introducing “Compact, seamless integration of active dosing and actuation with microneedles for transdermal drug delivery” [76], giving the example of insulin, which has been eventually proven in a publication by Nordquist et al. entitled “Novel microneedle patches for active insulin delivery are efficient in maintaining glycaemic control: an initial comparison with subcutaneous administration” [32]. This paper provides the first proof-of-principle of insulin delivery by an integrated microsystem that uses a hollow silicon microneedle array in the form of a patch.
At the same time, Ito et al. introduced their microneedle concept for the percutaneous absorption of insulin, in the European Journal of Pharmaceutical Sciences [77]. In this study, a total of five microneedles delivered insulin into the skin. The microneedles were fabricated forming a thread with polypropylene tips. This technique is not comparable to the high-precision microfabricated array described previously, but their work eventually led to the development of micromolded, self-dissolving microneedle arrays for insulin delivery, introduced in 2009 [78].
Just one year before, Roxhed et al. presented controlled administration of insulin in their publication on “Painless drug delivery through microneedle-based transdermal patches featuring active infusion” in rats [79], which complemented their earlier studies on “Membrane-sealed hollow microneedles and related administration schemes for transdermal drug delivery” [43], which covers a specific design element of a dissolving or bursting membrane at the tip, gaining a higher integration level and thus potentially more control over the various parameters involved in the delivery mechanism. A relatively new review by Prausnitz and Langer published in 2008 two additional research papers, and discuss how insulin delivery depends on MNA geometry. They were followed in 2009 by “Optimizing microneedle arrays for transdermal drug delivery: extension to non-square distribution of microneedles” [57, 80, 81].
These three papers are entirely dedicated to modeling and their findings must be evaluated further in clinical translational experiments. Finally, a relatively recent study by Gupta et al. shows data that compare human in vivo studies by means of microneedles with bolus injections [49]. Although this very recent study by the Praunsnitz’s group uses a single pulled glass needle for the insulin delivery experiments, as opposed to their earlier out-of-plane hollow microneedle arrays, it is still important in evaluating the key parameters of microneedle insulin delivery. In summary, only a few significant out-of-plane microneedle technologies have been presented throughout the last decade. Figure 8.6 depicts the three design categories. Category (I) illustrates direct silicon micromachining from top to bottom: blunt needles by Teo et al., sophisticated knife-like needles by Gardeniers et al., very pointy needles with side open release channels introduced by Griss et al., optimized by Roxhed et al., and finally utilized patches in insulin delivery experiments by Nordquist et al. [44, 3, 37, 76, 32].
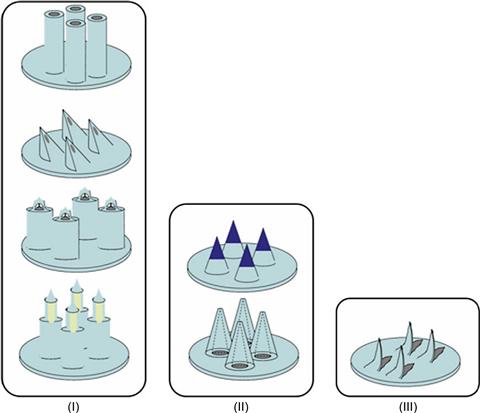
Figure 8.6 Design categories: (I) direct, (II) replication, (III) fine-mechanical approach. The drawings reflect on the alternative designs. The generated shapes and dimensions are strongly linked to a selected fabrication process. All design varieties have proven to rupture the skin. Every specific shape, i.e., fabrication process, has dimensional restrictions.
Category (II) introduces replication processes containing two extreme examples of this category: a dissolving polymer microneedle introduced by Ito et al. [78] and the metal microneedles described by Davis et al. [74]. Category (III) illustrates one of the examples of a fine-mechanical approach. This needle is manufactured by laser cutting and bending of a metal sheet. This type of microneedle has been used as a pre-treatment device, by means of which insulin delivery in a diabetic rat model has been confirmed [33]. All these designs are inherently different, however, they all give a proof-of-principle for microneedle insulin delivery. These design differences can only be further evaluated within an application-oriented design process.
As an example given on insulin, innovation in medical interventions is focused on the development of more effective therapies, having less side effects and lower costs. Injection by a needle-and-syringe system for the administration of drugs or vaccines is an example of such a medical intervention. Although this method is well established and very effective in medical practice (inserting a molecular substance in to the body that normally would not pass the skin passively) it does have drawbacks. The most obvious is that a needle generally causes pain, when it contacts the sensitive nerves inside of the skin sending signals of pain to our brains. Therefore, a variety of other mechanisms of transdermal delivery and micromachined microneedle systems have been invented, including microneedle patches as described above for the delivery of insulin. Figure 8.7 gives another example of such a transdermal application, whereas a commerically available microneedle system acts as a skin portal, which is developed by Zosano Pharma Inc. (previously ALZA Corporation).
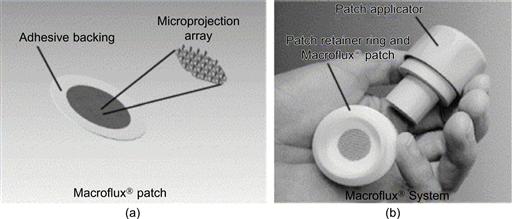
Figure 8.7 Macroflux® delivery system. The patch comprising the coated microneedle array affixed to an adhesive backing is illustrated in figure (a). The patch loaded on the disposable retainer ring and the reusable applicator are illustrated in figure (b) with its applicator device.
Reproduced from reference [82].
8.1.5 Design Aspects for Microneedle Insulin Delivery
At present, different types of insulin formulations are available with different pharmacological specifications. They are needed because patients require both a basal level of insulin and rapid-acting insulin to deal with food intake and exercise. Clearly, to be useful to patients, microneedle delivery must be able to supply both long- and short-acting insulin supply. Eventually, clinical scenarios could include the use of different microneedle configurations and the use of different insulin formulations.
However, as yet, only eight publications were found that confirm the proof-of-principle of using MNAs in insulin delivery in the diabetic rat model, while one additional publication [33] discusses microneedle insulin delivery in human subjects of Diabetes Type 1 using a single glass microneedle [3, 17, 32, 43, 44, 74, 78, 79].
Fabrication Attributes and Their Clinical Relevance
Shaping of the MNAs directly, by silicon micromachining techniques (category I, Figure 8.6), is considered to be an expensive technical solution due to the relatively high cost of high-precision silicon wafers as a base material. These techniques allow a high level of integration, and are advantageous when a certain amount of electronic integration is needed for the device. These processes will also be capable of producing very sharp tips, with radius less than 100 nm. This reduces the insertion force needed for such devices, thus enabling ease of use for manual administration. A first comprehensive trial, benchmarking important delivery parameters in the diabetic rat model, was carried out by Nordquist et al. [32]. Figure 8.8 shows two of their findings: (1) the decreasing plasma glucose level, showing the effect of the route of administration by microneedles (Figure 8.8a), and (2) the effect of the rate of intradermal administration on blood glucose (Figure 8.8b).
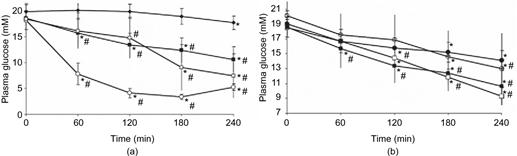
Figure 8.8 Plasma glucose measured in the diabetic rat model. Asterisk(*) denotes a difference compared to the first measurement in that same group and number sign(#) denotes differences when comparing to vehicle-treated animals within the same time period. For all measurements, P < 0.05 was considered statistically significant. (a) The effect of route of administration of insulin lispro on blood glucose. Filled square denotes microneedle-aided intradermal infusion (intradermal low-rate delivery rate, 2 μl/h, 100 IU/ml, n = 9), open square denotes subcutaneous infusion (2 μl/h, 100 IU/ml, n = 9), filled diamond denotes time control, and open circle denotes intravenous infusion (2 μl/h, 70 IU/ml, n = 7). (b) The effect of rate of intradermal administration of insulin lispro on blood glucose. Filled square denotes microneedle-aided low-rate intradermal infusion (2 μl/h, 100 IU/ml, n = 9), open square denotes microneedle-aided high-rate intradermal infusion (4 μl/h, 100 IU/ml, n = 9), filled circle denotes microneedle-aided intradermal passive infusion (0 μl/h, 100 IU/ml, n = 9), and open circle denotes rats whose skin was penetrated with microneedles, and then passively infused (2 μl/h, 100 IU/ml, n = 9).
Figures and caption adapted from Nordquist et al. by permission of the author and the publisher. Reproduced with permission from reference [32].
The second category (II), Figure 8.6, covers replication processes from a master. In general, these are cheap production schemes at a relatively low level of integration. Polymer micromolding processes incorporating insulin into a matrix material were introduced by Ito et al. This group used a relatively expensive, lithographically-defined master to yield very well-defined features in a biodegradable matrix [78]. As an alternative, batch-replication by electrodeposition from a relatively cheap sacrificial master, made by laser machining is less accurate but generates a very specific design with a through hole [74]. These two process types may each have benefits and opportunities for cost reduction. From the affordability point of view, high-volume replication from a sustainable master structure is the most feasible route.
Clinically, however, these two replication concepts are very different. The dissolving polymer microneedle can only allow a fixed amount of insulin release per time interval. On the other hand, the hollow metal microneedles can be coupled to an insulin reservoir [74, 78], which can in turn be linked to a closed-loop feedback sensor, which triggers release of insulin on demand. This is similar to continuously controlled release from an implanted or percutaneous drug delivery pump, and thus should bring high patient compliance. Such concepts anticipate high efficacy and reduced side effects during therapy. Other hollow micromachined microneedles, e.g., as given in category (I), also offer this kind of advantage.
The devices in category (III), Figure 8.6, are made by fine-mechanical machining and are perfectly suitable for a relatively small number of devices that bring a technical solution to a drug delivery niche market, but they remain rather expensive for high-volume manufacturing, so their widespread application is limited. A variety of studies are described that use fine-mechanically manufactured out-of-plane microneedles. Martanto et al., for example, experimented with different insertion configurations [33]. The microneedles were inserted by a specific impact device and then removed after 10 s, 10 min, and 4 h, respectively. Leaving needles in the skin for longer led to smaller reductions in blood glucose levels, and multiple insertions of the device created more pathways into the skin. In these studies the area of skin that was treated with microneedles was covered with an insulin-containing reservoir. Although some variations in transport were observed during these different application procedures, the general conclusion was that the microneedles created aqueous pathways. Utilizing this fairly simple pre-treatment mechanism, an overall pharmacodynamic response to insulin delivery was demonstrated, in which blood glucose levels decreased in a similar manner to subcutaneous hypodermic injection.
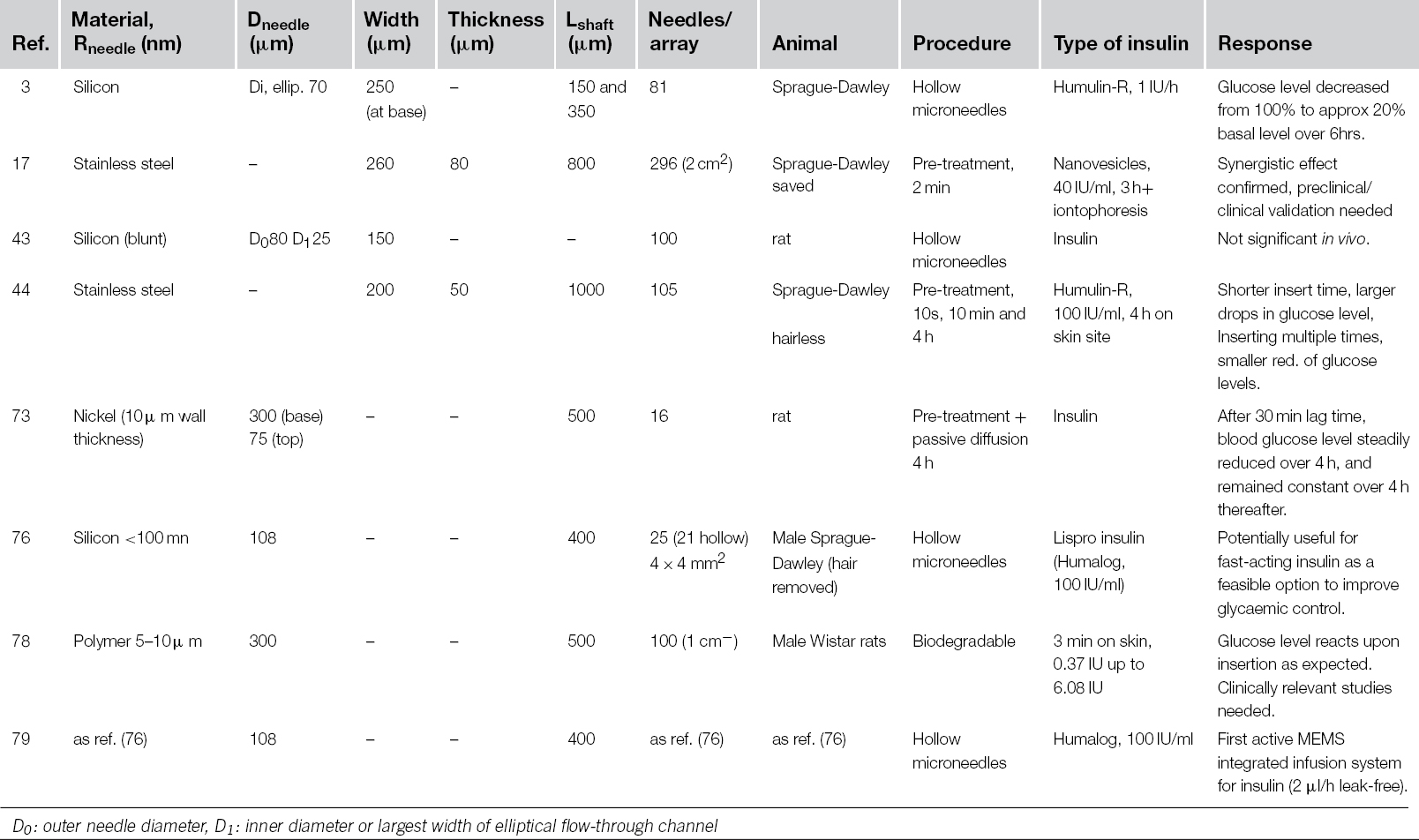
Table 8.1 compares the different microneedle configurations that were used in the rat model, with reference to the parameters illustrated in Figure 8.1. So far, there is no clinical evidence that the more precise shapes lead to a higher delivery efficacy. The device design depicted in category (III) of Figure 8.6 allows very simple and straightforward integration with a drug-filled adhesive patch by lamination [83]. The patch releases its cargo through openings in the back plate. The release profile will depend on the interplay of the type of incisions made by the spikes with the opening in the back plate, and varies quite significantly amongst different skin types or age groups of patients. It is difficult to say how robust the different design categories actually are for insulin delivery in the real world, and if they could produce the best type of incision. Further clinical research is required.
Table 8.1 Microneedle Arrays for Insulin Delivery in the Diabetic Rat Model
The various tips depicted in Figure 8.6 all present design varieties that create a “mechanical cut” and may be defined by their main function: “cutting”. However, their actual function is to release a controlled dose of insulin, and clinicians need to know how the exact size, type and geometric shape of the microconduits into the skin influence its delivery mechanism. When studying the pharmacodynamics of insulin release, one should not forget that the skin is an immunosensitive organ. When incisions are made in it, allowing foreign bodies to enter, an immune response may be triggered, which may alter the efficacy of insulin uptake, and also provoke unfavorable side-effects. This may also be an explanation for the smaller reduction in blood glucose levels observed by Martanto et al. when microneedles were inserted for a longer time or more than once [33]. Quality control of sterility, biocompatibility and efficacy of drug delivery are key for the final product.
Quantification of insertion and delivery processes, as well as the visualization of the incisions made by the individual types of microneedles in combination with the use of specific applicator devices, are therefore very important factors in these devices. A comparison on different types of insertion procedures and microneedle designs in transdermal drug delivery has been made by the Bouwstra group, but as yet not with respect to its effect on controlled insulin delivery [84]. The cost of statistically relevant clinical studies are high, and require a sustained supply of the devices which need approval by an ethical-medical committee. All the design varieties introduced and discussed above show a certain level of technological readiness that supports this request.
Clinical Perspective
From a clinical point of view, the microneedle array devices summarized schematically in Figure 8.6 are all of preliminary but potential interest, since the only studies that have used them have been small-scale animal trials (n < 10). Figure 8.9 shows two extreme design alternatives that have achieved a certain full proof-of-principle for insulin delivery, using out-of-plane microneedles as depicted in Figure 8.1(B) and (D). Figure 8.9(A) illustrates one such device integrated with a special actuator for the active release of insulin that is stored in a reservoir in liquid form. Figure 8.9(B) shows a simpler device, which releases insulin by a phase change, dissolving the polymeric material into the skin without any further active device components [78]. These self-dissolving microneedles encapsulate the insulin in a chondroitin sulfate matrix during micromolding of arrays, which is done by using flexible silicone (PDMS) molds. Both design alternatives demonstrate a viable solution for ultra-minimally invasive insulin release.

Figure 8.9 Illustration of design alternatives for insulin-releasing mechanisms. (A): integrated microneedle-micropump patch design. (Reproduced with permission from reference [43]. Copyright © 2008 Springer Science+Business Media, LLC.). (B): partially self-dissolving micropiles fabricated by micromolding in arrays from 10 × 10 microneedles. (C): skin prior to insertion (a), 5 min (b), 1 h (c) and 2 h (d) after release of insulin into rat skin. The dark color due to Evans blue staining disappeared and there was no damage on the rat skin. (Reproduced with permission from reference [78]. Copyright © 2009 Springer Science+Business Media, LLC.)
Computer-assisted design studies, in combination with the appropriate testing in a clinical laboratory, are needed to prove the microneedle concept. For diabetes care management, the devices introduced by Stemme’s group offer stop-and-go delivery, and keep the device attached to the skin [32, 43, 76, 79], which may be of particular importance during food intake or exercise. It is not yet clear which type of device offers the best number and type of pathways through the skin. One technical solution for the investigation of the pharmacokinetics of insulin is to use a hollow microneedle array together with sensor augmented active pumping. The device introduced by Roxhed et al. currently shows the highest level of integration for such a class of drug delivery device [43]. Measuring glucose by a user-independent integrated sensor is also in development, as briefly mentioned in the previous section [70]. The augmentation of the released insulin dosage will require such feedback parameters, which can significantly vary with the patient’s specific health status.
Any decision taken in the conceptual design phase of a new device will impact the type of experiments being carried out in drug delivery research. The choice of technology will therefore influence the data that can be collected for a clinical model. Insulin release by microneedle array pre-treatment has been taken to a fairly high level of testing, yet there are little or no data available that compare this novel transdermal mechanism of insulin release with the gold standard (bolus injection) within a clinically relevant number of human diabetic subjects. Although the working principle of insulin microneedle delivery has been confirmed by Gupta et al., utilizing a single, pulled glass needle at various insertion depths [49], there is still insufficient evidence to show that a delivery system equipped with an out-of-plane microneedle array can deliver insulin in a clinical application.
Delivery technology research can be carried out for the creation of a multitude of parallel injection sites with the preliminary devices presented here, but some level of concern remains regarding the degree of control that is exerted over the device insertion mechanisms. For example, applying high pressures during insertion will affect the skin properties, and thus the results of the transport studies. A delivery platform that includes sensor-augmented release is clearly desired, but at this stage of development many kinds of out-of-plane MNA devices can be already translated from the pure engineering sciences to research in drug delivery technology. Patient trials, especially those related to comfort, user-friendliness and pain sensation during the use of such devices, have by now demonstrated an appropriate level of technology readiness for clinical translational research. The initial studies by Gupta et al. [49], photographed the insertion sites to qualitatively evaluate the difference between injection depths. Much more indepth studies of the efficacy of MNA injections will be needed to prove their clinical effectiveness. For micromachined microneedles, new benchmark methods must be adopted to evaluate the clinical relevance of the different designs and materials. Both of the two concepts presented in Figure 8.9 provide a starting point for a further analysis of the specific design criteria for the application of MNAs in insulin delivery.
From the viewpoints of microengineering, drug delivery technology and clinical translation, the current microdevices show clearly that microsystems engineering presents certain benefits compared to those designs that use simple fine mechanical solutions or even mechanical assembly. A formal design approach can offer an exquisite tool for finding appropriate technological solutions for the development of transdermal insulin delivery, based on the aforementioned out-of-plane microneedle designs [85].
8.2 MNA-4-Insulin: A Brief Evaluation
In addition to developing MNA fabrication technology, it is also important to create a clinical focus for the use of microneedles in insulin delivery. This review gives an overview of the different types of microneedle technologies, which already refer to the proof-of-principle of microneedle insulin delivery. With respect to drug delivery research, devices from the three well-known categories of technology (silicon micromachining, replication and precision engineering) show enhanced transdermal delivery rates for insulin. Specific efficacy/bioavailability data has not yet been gathered, however. The devices show few side effects (including safety), and little discomfort during application, but other clear advantages over the hypodermic needle/syringe concept cannot yet be confirmed, and there is a lack of data on pharmacodynamics for systemic human insulin uptake. Nevertheless, there is no doubt that passive microneedle integrated skin patches could be an affordable solution for the painless intradermal delivery of insulin. In conclusion, a sufficiently high technology readiness of a variety of MNAs has been demonstrated. However, none of the systems discussed in the literature are ready for the market, mainly due to the lack of clinically confirmed reference data. Verification of the device quality and pharmacokinetics must be benchmarked first.
8.3 Conclusions
Is it too early or is it already too late to start a profitable business based on MNA technology? Based on this discussion of the application needs for insulin, it appears that there is not yet any significant business based on using microneedle arrays. Does this mean we just sit and wait for someone to take an initiative, and find a solution for the year 2030? Obviously, this is a rhetorical question. At the current stage of development it is not possible to judge which type of MNA will best deliver insulin via the skin. There are, of course, also other means of delivery, and other treatments for diabetes, but the delivery of insulin by transdermal means should be studied seriously. A great deal of clinical and business study is needed before further clinical decisions can be taken. Further, it is important to realize that high-tech business development benefits from the collaboration between different research disciplines at the cross-over point: the application. For drug delivery applications it is important that researchers from the pharmaceutical, drug delivery and the engineering sciences meet as early as possible to define a common aim to work on.
Having conducted this desk research, I must ask myself what research questions remain for a microfabrication expert in this field, and yes, I can think of many. Obviously, it is important to ask this question from the viewpoint of each discipline taking part in the work, and then combine them into one joint-call proposal for a focused technology development in this specific sector of the life sciences to provide a solution for the 2030 problem. This should be anticipated by the researchers in this field together with appropriate industry sectors as early as possible. Only this approach will make sure that the required results from the different disciplines can come together at the required pace of development. A world-wide science and technology funding structure has been created that provides research subsidies for public–private partnership.
Lessons learned from this case study should inspire students in the engineering sciences to start talking to their colleagues in the pharmaceutical and biomedical sciences and get those partnerships running. Of course, this discussion may also inspire managers of key technologies to adopt microfabrication principles in totally different areas to those introduced here. This chapter may serve as a starting point for a new business case. An ultra early technology adapter, similar to Unversity of Twente’s spin-off MyLife Technologies, may allow many more real-world applications of micro- and nanofabrication technology.
REFERENCES
1. Hogan J. Lab on a chip A little goes a long way. Nature. 2006;442:351–352.
2. Vrouwe EX, Luttge R, Vermes I, Van Den Berg A. Microchip capillary electrophoresis for point-of-care analysis of lithium. Clin Chem. 2007;53(1):117–123.
3. Gardeniers HJGE, Luttge R, Berenschot EJW, et al. Silicon micromachined hollow microneedles for transdermal liquid transport. IEEE J Microelectromech Syst. 2003;12(6):855–862.
4. Mills PC, Cross SE. Transdermal drug delivery: Basic principles for the veterinarian. Vet J. 2006;172(2):218–233.
5. Godefroy S, Peyre M, Garcia N, Muller S, Sesardic D, Partidos CD. Effect of skin barrier disruption on immune responses to topically applied cross-reacting material, crm197, of diphtheria toxin. Infect Immun. 2005;73(8):4803–4809.
6. Partidos CD, Muller S. Decision-making at the surface of the intact or barrier disrupted skin: Potential applications for vaccination or therapy. Cell Mol Life Sci. 2005;62(13):1418–1424.
7. O’Hagan DT, Rappuoli R. Novel approaches to vaccine delivery. Pharm Res. 2004;21(9):1519–1530.
8. Barry BW. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci. 2001;14(2):101–114.
9. Kendall M. Engineering of needle-free physical methods to target epidermal cells for DNA vaccination. Vaccine. 2006;24(21):4651–4656.
10. Chabri F, Bouris K, Jones T, et al. Microfabricated silicon microneedles for nonviral cutaneous gene delivery. Br J Dermatol. 2004;150(5):869–877.
11. Birchall J, Coulman S, Pearton M, et al. Cutaneous DNA delivery and gene expression in ex vivo human skin explants via wet-etch microfabricated microneedles. J Drug Targeting. 2005;13(7):415–421.
12. Coulman S, Allender C, Birchall J. Microneedles and other physical methods for overcoming the stratum corneum barrier for cutaneous gene therapy. Crit Rev Ther Drug Carr Syst. 2006;23(3):205–258.
13. Pearton M, Allender C, Brain K, et al. Gene delivery to the epidermal cells of human skin explants using microfabricated microneedles and hydrogel formulations. Pharm Res. 2008;25(2):407–416.
14. Ding Z, Van Riet E, Romeijn S, Kersten GFA, Jiskoot W, Bouwstra JA. Immune modulation by adjuvants combined with diphtheria toxoid administered topically in BALB/c mice after microneedle array pretreatment. Pharm Res. 2009;26(7):1635–1643.
15. Ding Z, Verbaan FJ, Bivas-Benita M, et al. Microneedle arrays for the transcutaneous immunization of diphtheria and influenza in BALB/c mice. J Controlled Release. 2009;136(1):71–78.
16. Coulman SA, Anstey A, Gateley C, et al. Microneedle mediated delivery of nanoparticles into human skin. Int J Pharm. 2009;366(1–2):190–200.
17. Chen H, Zhu H, Zheng J, et al. Iontophoresis-driven penetration of nanovesicles through microneedle-induced skin microchannels for enhancing transdermal delivery of insulin. J Controlled Release. 2009;139(1):63–72.
18. Verbaan FJ, Bal SM, van den Berg DJ, et al. Improved piercing of microneedle arrays in dermatomed human skin by an impact insertion method. J Controlled Release. 2008;128(1):80–88.
19. Coulman SA, Barrow D, Anstey A, et al. Minimally invasive cutaneous delivery of macromolecules and plasmid DNA via microneedles. Curr Drug Deliv. 2006;3(1):66–75.
20. La Van DA, Lynn DM, Langer R. Moving smaller in drug discovery and delivery. Nat Rev Drug Discovery. 2002;1(1):77–84.
21. Grayson ACR, Shawgo RS, Li Y, Cima MJ. Electronic MEMS for triggered delivery. Adv Drug Delivery Rev. 2004;56(2):173–184.
22. Razzacki SZ, Thwar PK, Yang M, Ugaz VM, Burns MA. Integrated microsystems for controlled drug delivery. Adv Drug Delivery Rev. 2004;56(2):185–198.
23. Partidos CD. Delivering vaccines into the skin without needles and syringes. Expert Rev Vaccines. 2003;2(6):753–761.
24. Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Delivery Rev. 2004;56(5):581–587.
25. Schuetz YB, Naik A, Guy RH, Kalia YN. Emerging strategies for the transdermal delivery of peptide and protein drugs. Expert Opin Drug Deliv. 2005;2(3):533–548.
26. Sharma S, Nijdam AJ, Sinha PM, et al. Controlled-release microchips. Expert Opin Drug Deliv. 2006;3(3):379–394.
27. Sivamani RK, Liepmann D, Maibach HI. Microneedles and transdermal applications. Expert Opin Drug Deliv. 2007;4(1):19–25.
28. Vandervoort J, Ludwig A. Microneedles for transdermal drug delivery: A minireview. Front Biosci. 2008;13(5):1711–1715.
29. Arora A, Prausnitz MR, Mitragotri S. Micro-scale devices for transdermal drug delivery. Int J Pharmaceutics. 2008;364(2):227–236.
30. Kalluri H, Banga AK. Microneedles and transdermal drug delivery. J Drug Deliv Sci Technol. 2009;19(5):303–310.
31. Zahn JD, Talbot NH, Liepmann D, Pisano AP. Microfabricated polysilicon microneedles for minimally invasive biomedical devices. Biomed Microdevices. 2000;2(4):295–303.
32. Nordquist L, Roxhed N, Griss P, Stemme G. Novel microneedle patches for active insulin delivery are efficient in maintaining glycaemic control: An initial comparison with subcutaneous administration. Pharm Res. 2007;24(7):1381–1388.
33. Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004;21(6):947–952.
34. Stoeber B, Liepmann D. Arrays of hollow out-of-plane microneedles for drug delivery. J Microelectromech Syst. 2005;14(3):472–479.
35. Griss P, Tolvanen-Laakso HK, Merilinen P, Stemme G. Characterization of micromachined spiked biopotential electrodes. IEEE Trans Biomed Eng. 2002;49(6):597–604.
36. Griss P, Stemme G. Novel, side opened out-of-plane microneedles for microfluidic transdermal interfacing. In: 2002;467–470. Proceedings of the IEEE Micro Electro Mechanical Systems (MEMS).
37. Griss P, Stemme G. Side-opened out-of-plane microneedles for microfluidic transdermal liquid transfer. J Microelectromech Syst. 2003;12(3):296–301.
38. Gardeniers JGE, Berenschot JW, De Boer MJ, et al. Silicon micromachined hollow microneedles for transdermal liquid transfer. In: 2002;141–144. Proceedings of the IEEE Micro Electro Mechanical Systems (MEMS).
39. Henry S, McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles: A novel approach to transdermal drug delivery. J Pharm Sci. 1998;87(8):922–925.
40. McAllister DV, Kaushik S, Patel PN, Mayberry JL, Allen MG, Prausnitz MR. Solid and hollow microneedles for transdermal protein delivery. Proc Controlled Release Soc. 1999;26:192–193.
41. McAllister DV, Wang PM, Davis SP, et al. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc Natl Acad Sci USA. 2003;100(Suppl. 2):13755–13760.
42. Verbaan FJ, Bal SM, van den Berg DJ, et al. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin. J Controlled Release. 2007;117(2):238–245.
43. Roxhed N, Griss P, Stemme G. Membrane-sealed hollow microneedles and related administration schemes for transdermal drug delivery. Biomed Microdevices. 2008;10(2):271–279.
44. Ling Teo MA, Shearwood C, Ng KC, Lu J, Moochhala S. In vitro and in vivo characterization of MEMS microneedles. Biomed Microdevices. 2005;7(1):47–52.
45. Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Controlled Release. 2005;104(1):51–66.
46. Haq MI, Smith E, John DN, et al. Clinical administration of microneedles: Skin puncture, pain and sensation. Biomed Microdevices. 2009;11(1):35–47.
47. Teo AL, Shearwood C, Ng KC, Lu J, Moochhala S. Transdermal microneedles for drug delivery applications. Mater Sci Eng. 2006;B132(1–2):151–154.
48. Aggarwal G, Garg A, Dhawan S. Transdermal drug delivery: Evolving technologies and expanding opportunities. Indian J Pharm Educ Res. 2009;43(3):251–259.
49. Gupta J, Felner EI, Prausnitz MR. Minimally invasive insulin delivery in subjects with type 1 diabetes using hollow microneedles. Diabetes Technol Ther. 2009;11(6):329–337.
50. Park J-H, Allen MG, Prausnitz MR. Polymer microneedles for controlled-release drug delivery. Pharm Res. 2006;23(5):1008–1019.
51. Matsuda T, Mizutani M. Liquid acrylate-endcapped biodegradable poly(-caprolactone-co-trimethylene carbonate) ii Computer-aided stereolithographic microarchitectural surface photoconstructs. J Biomed Mater Res. 2002;62(3):395–403.
52. Luttge R, Berenschot EJW, de Boer MJ, et al. Integrated lithographic molding for microneedle-based devices. J Microelectromech Syst. 2007;16(4):872–884.
53. Zhao G, Li W, Tang G, Chen J. Fabrication of bulk titanium out-of-plane microneedles. In: 2009;428–431. 4th IEEE International Conference on Nano/Micro Engineered and Molecular Systems, NEMS 2009.
54. Doraiswamy A, Jin C, Narayan RJ, et al. Two photon induced polymerization of organic-inorganic hybrid biomaterials for microstructured medical devices. Acta Biomater. 2006;2(3):267–275.
55. Lee K, Lee HC, Lee D-S, Jung H. Drawing lithography: Three-dimensional fabrication of an ultrahigh-aspect-ratio microneedle. Adv Mater. 2010;22(4):483–486.
56. Zhu Q, Zarnitsyn VG, Ye L, et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci USA. 2009;106(19):7968–7973.
57. Al-Qallaf B, Das DB. Optimization of square microneedle arrays for increasing drug permeability in skin. Chem Eng Sci. 2008;63(9):2523–2535.
58. Lv Y-G, Liu J, Gao Y-H, Xu B. Modeling of transdermal drug delivery with a microneedle array. J Micromech Microeng. 2006;16(11):2492–2501.
59. Banga AK. Microporation applications for enhancing drug delivery. Expert Opin Drug Deliv. 2009;6(4):343–354.
60. Gill HS, Prausnitz MR. Pocketed microneedles for drug delivery to the skin. J Phys Chem Solids. 2008;69(5–6):1537–1541.
61. Ji J, Tay FEH, Miao J. Microfabricated hollow microneedle array using ICP etcher. J Phys Conf Ser. 2006;34(1):1132–1136.
62. Moon SJ, Jin CY, Lee SS. A novel method of microneedle array fabrication using inclined deep x-ray exposure. J Phys Conf Ser. 2006;34(1):180–186.
63. Donnelly RF, Morrow DIJ, McCarron PA, et al. Microneedle arrays permit enhanced intradermal delivery of a preformed photosensitizer. Photochem Photobiol. 2009;85(1):195–204.
64. Kolli CS, Banga AK. Characterization of solid maltose microneedles and their use for transdermal delivery. Pharm Res. 2008;25(1):104–113.
65. Li G, Badkar A, Nema S, Kolli CS, Banga AK. In vitro transdermal delivery of therapeutic antibodies using maltose microneedles. Int J Pharm. 2009;368(1–2):109–115.
66. Meidan VM, Michniak BB. Emerging technologies in transdermal therapeutics. Am J Ther. 2004;11(4):312–316.
67. McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles for gene and drug delivery. Annu Rev Biomed Eng. 2000;2:289–313.
68. Trebotich D, Zahn JD, Liepmann D. Complex fluid dynamics in bioMEMS devices: Modeling of microfabricated microneedles. In: 2002;20–23. 2002 International Conference on Computational Nanoscience and Nanotechnology - ICCN 2002.
69. Trebotich D, Zahn JD, Prabhakarpandian B, Liepmann D. Modeling of microfabricated microneedles for minimally invasive drug delivery, sampling and analysis. Biomed Microdevices. 2003;5(3):245–251.
70. Zahn JD, Hsieh Y-C, Yang M. Components of an integrated microfluidic device for continuous glucose monitoring with responsive insulin delivery. Diabetes Technol Ther. 2005;7:536–545.
71. Prausnitz MR. Overcoming skin’s barrier: The search for effective and user-friendly drug delivery. Diabetes Technol Ther. 2001;3(2):233–236.
72. P. Zhang, G.A. Jullien, Microneedle array for drug delivery and fluid extraction, in Proceedings, (2005) International Conference on MEMS, NANO and Smart Systems, ICMENS Digital Object Identifier: 10.1109/ICMENS.2005.71.
73. Zhang R, Zhang P, Dalton C, Jullien GA. Modeling of drug delivery into tissues with a microneedle array using mixture theory. Biomech Model Mechanobiol. 2009;1–10.
74. Davis SP, Martanto W, Allen MG, Prausnitz MR. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng. 2005;52(5):909–915.
75. Ma B, Liu S, Gan Z, et al. A PZT insulin pump integrated with a silicon microneedle array for transdermal drug delivery. Microfluid Nanofluid. 2006;2(5):417–423.
76. Roxhed N, Samel B, Nordquist L, Griss P, Stemme G. Compact, seamless integration of active dosing and actuation with microneedles for transdermal drug delivery. In: 2006;414–417. Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS). vol. 2006.
77. Ito Y, Hagiwara E, Saeki A, Sugioka N, Takada K. Feasibility of microneedles for percutaneous absorption of insulin. Eur J Pharm Sci. 2006;29:82–88.
78. Ito Y, Yamazaki T, Sugioka N, Takada K. Self-dissolving micropile array tips for percutaneous administration of insulin. J Mater Sci Mater Med. 2009;21(2):835–841.
79. Roxhed N, Samel B, Nordquist L, Griss P, Stemme G. Painless drug delivery through microneedle-based transdermal patches featuring active infusion. IEEE Trans Biomed Eng. 2008;55(3):1063–1071.
80. Davidson A, Al-Qallaf B, Das DB. Transdermal drug delivery by coated microneedles: Geometry effects on effective skin thickness and drug permeability. Chem Eng Res Des. 2008;86(11):1196–1206.
81. Al-Qallaf B, Das DB. Optimizing microneedle arrays for transdermal drug delivery: Extension to non-square distribution of microneedles. J Drug Targeting. 2009;17(2):108–122.
82. Cormier M, Johnson B, Ameri M, et al. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Controlled Release. 2004;97(3):503–511.
83. Wermeling DP, Banks SL, Hudson DA, et al. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci USA. 2008;105(6):2058–2063.
84. Bal SM, Caussin J, Pavel S, Bouwstra JA. In vivo assessment of safety of microneedle arrays in human skin. Eur J Pharm Sci. 2008;35(3):193–202.
85. Otto KN, Antonsson EK. Trade-off strategies in engineering design. Res Eng Des. 1991;3(2):87–103.
