5.2.5 Steam Rankine Power Stations
5.2.5.1 Reheating-Regenerative Steam Rankine Cycle
The vast majority of power stations are based on reheating-regenerative steam Rankine cycles. These types of cycles are very complex as they comprise many types of components, including preheater, boiler, superheater, reheater, closed (CFWH) and open (OFWH) feedwater heaters, mixer, condenser, deaerator, pumps, and steam traps (ST). The cycle design depends on the type of heat source and heat sink. For most cases of large power generation the heat source is derived from coal combustion.
The heat sink is in general a lake; however, there are many cases in which power plants use air as a heat sink. In this case the plant is equipped with cooling towers—either with natural convection or with forced convection. In some situations (e.g., in colder regions) the heat sink is represented by a district heating system for cogeneration. In this section we give an illustrative example of a reheating-regenerative steam Rankine cycle as presented in Figure 5.23. This power plant comprises eighteen main components. There are also four steam extraction ports with corresponding steam extraction fractions denoted with f12, f17, f20, f21. The thermodynamic analysis of the cycle becomes complex because multiple variables and equations are involved and optimization is required for energy efficiency maximization. We will illustrate in detail how the cycle can be analyzed using balance equations and how the optimization problem for maximum efficiency can be formulated.
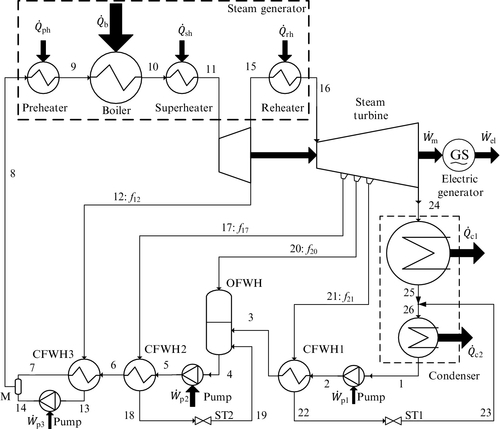
Mass, energy, entropy and exergy balance equations must be written for each component of the power plant in order to form a system of equations that can be solved to determine thermodynamic parameters defining each state. The following observation is very useful for simplifying the mass balance equations: in state 11 (turbine inlet) the mass flow rate is the maximum for the plant; also ![]() . Hence, the steam injection fractions are defined with respect to
. Hence, the steam injection fractions are defined with respect to ![]() as follows:
as follows:
![]()
In addition, in Figure 5.23 it can be observed that mass balance equations can be reduced to the following set of equations expressed as a function of flow fractions in other state points of the power plant, defined by ![]() , namely:
, namely:
![]()
![]()
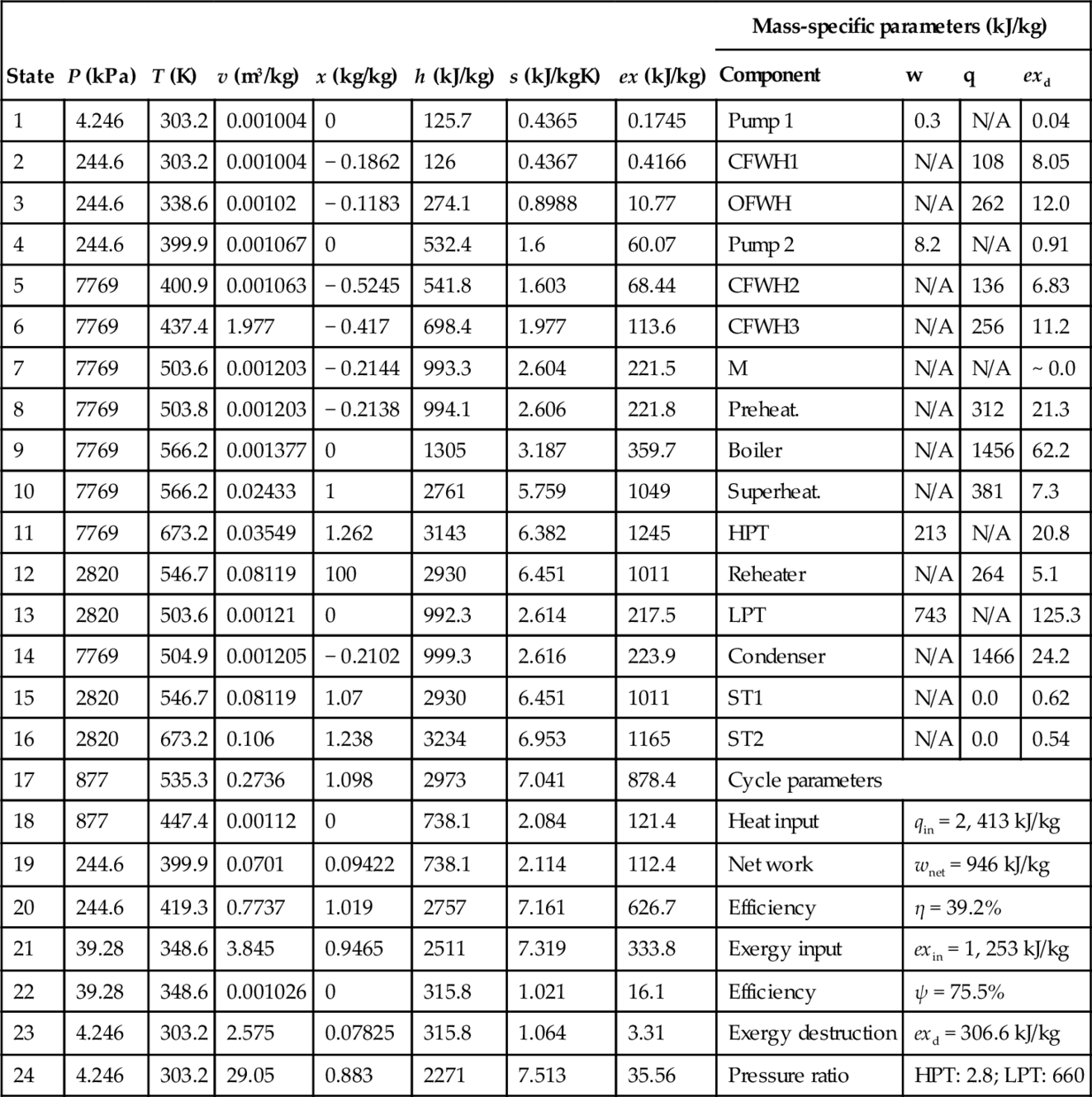
Using the flow fractions fi, the energy, entropy, and exergy balance equations are written for each component as indicated in Table 5.11. The balance equations together with the parameters that quantify exergy destructions (e.g., isentropic efficiencies of pumps and turbines, temperature differences due to heat transfer) form a closed system of equations which can be solved for temperatures, pressures, and specific enthalpy, entropy, and volume at each state point provided that all extraction fractions are specified (f12, f17, f20, f21) and the condensation, boiling, and superheating temperatures (T1, T9, T11) are given.
Table 5.11
Balance Equations for Ideal Rankine Cycle of Power Plant in Figure 5.23
| Component | Equations | Component | Equations |
| Pump 1 (states 1–2) | MBE: EBE: f1h1 + wp1 = f1h2 EnBE: s1 + sg = s2 ExBE: f1ex1 + wp1 = f1ex2 + exd | CFWH1 (states 2–3) | MBE: EBE: f1h2 + f21h21 = f1h3 + f21h22 EnBE: f1s2 + f21s21 + sg = f1s3 + f21s22 ExBE: f1ex2 + f21ex21 = f1ex3 + f21ex22 + exd |
| Pump 2 (states 4–5) | MBE: EBE: f4h4 + wp2 = f4h5 EnBE: s4 + sg = s5 ExBE: f4ex4 + wp2 = f4ex5 + exd | OFWH (states 3–4–19–20) | MBE: EBE:f1h3 + f17h18 + f20h20 = f4h4 EnBE: f1s3 + f17s18 + f20s20 + sg = f4s4 ExBe: f1ex3 + f17ex18 + f20ex20 = f4ex4 + exd |
| Pump 3 (states 13–14) | MBE: EBE: f12h13 + wp3 = f12h5 EnBE: s13 + sg = s14 ExBE: f12ex13 + wp3 = f12ex13 + exd | CFWH2 (states 5–6–17–18) | MBE: EBE: f4h5 + f17h17 = f4h6 + f17h18 EnBE: f4s5 + f17s17 + sg = f4s6 + f17s18 ExBE: f4ex5 + f17ex17 = f4ex6 + f17ex18 + exd |
| Mixer (states 7–8–14) | MBE: EBE: f4h7 + f12h14 = h8 EnBE: f4s7 + f12s14 + sg = s8 ExBE: f4ex7 + f12ex14 = ex8 + exd | CFWH3 (states 6–7–12–13) | MBE: EBE: f4h6 + f12h12 = f4h7 + f12h13 EnBE: f4s6 + f12s12 + sg = f4s7 + f12s13 ExBE: f4ex6 + f12ex12 = f4ex7 + f12ex13 + exd |
| Preheater (states 8–9) | MBE: EBE: h8 + qph = h9 EnBE: s8 + qph/Tph + sg = s9 ExBE: ex8 + qph(1 − T0/Tph) = ex9 + exd | Boiler (states 9–10) | MBE: EBE: h9 + qb = h10 EnBE: s9 + qb/Tb + sg = s10 ExBE: ex9 + qb(1 − T0/Tb) = ex10 + exd |
| Superheater (states 10–11) | MBE: EBE: h10 + qsh = h11 EnBE: s10 + qsh/Tsh + sg = s11 ExBE: ex10 + qsh(1 − T0/Tsh) = ex11 + exd | Reheater (states 15–16) | MBE: EBE: f15h15 + qrh = f15h16 EnBE: f15s15 + qrh/Tsh + sg = f15s16 ExBE: f15ex15 + qrh(1 − T0/Trh) = f15ex16 + exd |
| Turbine 1 (states 11–12–15) | MBE: EBE: h11 = f12h12 + f15h15 + wt1 EnBE:s11 + sg = f12s12 + f15s15 ExBE: ex11 = f12ex12 + f15ex15 + wt1 + exd | Turbine 2 (states 16–17–20–21–24) | MBE: f15 = f17 + f20 + f21 + f24; EBE: f15h16 = f17h17 + f20h20 + f21h21 + f24h24 + wt2; EnBE: f15s15 + sg = f17s17 + f20s20 + f21s21 + f24s24; ExBE: |
| ST 1 (states 22–23) | MBE: EBE: h22 = h23 EnBE: f21s22 + sg = f21s23 ExBE: f21ex22 = f21ex23 + exd | ST 2 (states 18–19) | MBE: EBE: h18 = h19 EnBE: f17s18 + sg = f17s19 ExBE: f17ex18 = f17ex19 + exd |

As seen in Table 5.11 the balance equations can be written using mass-specific quantities. These quantities are defined with respect to the mass flow rate at the HPT inlet; thus: ![]() and
and ![]() . Furthermore, the energy efficiency can be maximized according to the following objective function:
. Furthermore, the energy efficiency can be maximized according to the following objective function:
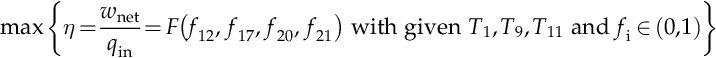
where F(f12, f17, f20, f21) represents the functional relation between steam extraction fractions and the energy efficiency of the cycle, a relation which is established by the nonlinear system of equations mentioned above.
Note that the certain additional assumptions must be made in order for the optimization problem to be completely specified. These assumptions are as follows:
• at pump suction the liquid is saturated, hence x1 = x4 = x13 = 0
• at the steam traps inlet the liquid is saturated, hence x18 = x22 = 0
• at the OFW outlet there is a saturated liquid, hence x4 = 0
• there is a minimum temperature difference at the closed feedwater heater outlet denoted with ΔThx; it is assumed that T22 − T3 = T18 − T6 = T13 − T7 = ΔThx.
5.2.5.2 Coal-Fired Power Stations
Although their flue gas stacks highly pollute the atmosphere with carbon dioxide, sulfur dioxide, nitrous oxide emissions, and other pollutants, coal-fired power stations are used in nearly every country of the world. These types of power generating stations are responsible for the majority of global electricity production. They operate based on steam Rankine cycles (generally on reheating-regenerative schemes) and comprise a coal-fired furnace as steam generator.
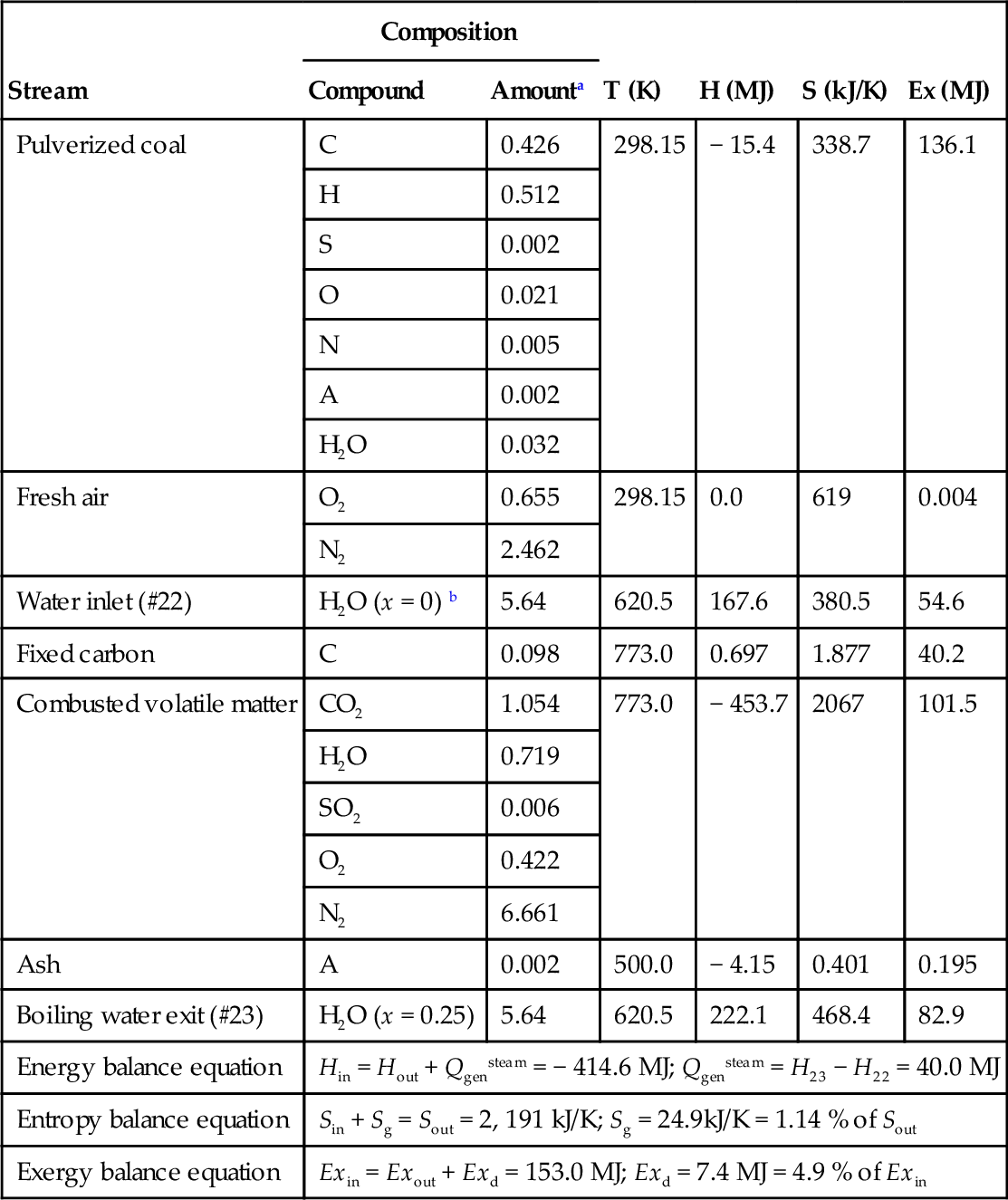
Two main combustion technologies are used currently in coal-fired power stations, namely pulverized coal boilers (the most predominant) and fluidized bed boilers (used when the coal rank is lower). Although the two technologies are different with regard to transport phenomena, the transient heat and mass transfer from a combusting coal particle to the surroundings are similar in both cases: combustion occurs at the coal particle surface, which gradually consumes while generates incandescent, hot gases. Coal is supplied at the lower part of the furnace where it devolatilizes and ignites in an oxidative atmosphere. The oxygen comes from two sources: fresh air injection and recirculated flue gases. In the first part of the combustion process, the volatiles are oxidized while the temperature of coal particle increases. Thereafter, fixed carbon oxidizes at very fast rate.
In Figure 5.25 the typical configuration of a pulverized coal-fired furnace at a conventional power station is illustrated. It has the roles of preheating, boiling, superheating, and reheating high-pressure steam. The furnace normally is in the form of a tall brick structure of a 10m to 50m height, depending on the type of coal and the residence time of coal particles required for complete combustion. Pulverized coal-fired furnaces are crucial to the utility industry. Typically, pulverized coal-fired furnaces comprise a coal mill which grinds coal to form small powder-like particles conveyed with primary air to the pulverization point, where ignition occurs.
The average temperature of gases at the lower part of the pulverized coal furnace (below point A in Figure 5.25) is typically around 650–700 K. A calandria boiler system with vertical tubes is installed at the lower half of the furnace. Between the hot gases inside the furnace duct and the calandria tubes placed at the periphery an intense radiative heat transfer occurs. Under exposure to intense thermal radiation, water boils.
The calandria system comprises a steam drum (see Figure 5.25) which accumulates liquid water at the lower part. Water flows gravitationally downward and feeds into a system formed of multiple headers arranged such that water is distributed uniformly into calandria tubes. The boiling process in the calandira system is incomplete and is facilitated by the fact that the water is close to its saturation state. Given that the boiling water is lighter than the feed water, in the calandria system is formed a natural circulation process due to gravity. In some cases circulation pumps are also used to enhance the water circulation rate.
In later stages of the combustion process, carbon oxidizes and the flue gas temperature increases further. This process occurs approximately between locations A and B in Figure 5.25. At point B, coal particles are completely consumed and the flue gas temperature reaches its maximum value (around 1200 K). In between points B and C the heat of the flue gases is transferred (mainly radiatively) to the superheater and reheaters of the steam Rankine plant. At points C and D there is a heat transfer process by forced convection and radiation between flue gases and the boiling water prior its return to the steam drum. Saturated vapor exists above the liquid in the steam drum. The next heat transfer process in the furnace occurs between D and E where the economizer is installed, which preheats water prior to feeding it into the steam drum. The heat transfer in the economizer is mostly caused by a forced convection process. After point E, a part of the oxygen-containing hot flue gases is returned to the combustion zone in the furnace. Fly ash is removed and electrostatic devices are typically used to remove particulate matter. Further, the flue gases are directed to the stack using a mechanical draft system.
An example of Rankine cycle configuration for a coal-fired steam Rankine power station is shown in Figure 5.26. The system is a double reheating-regenerative Rankine cycle. Liquid water in 1 is pumped by a low-pressure pump (LPP), preheated in a low-pressure closed water-feeding heater (LPFW), further heated in the OFW, and then is pressurized by the high-pressure pump (HPP).

After state 5, pressurized water is the preheated in closed feedwater heater (HPPFW) and in the economizer, and then it is fed as saturated liquid in 7 to the steam drum. Saturated vapor is extracted from the steam drum in 8 and superheated and expanded in the HPT. The expanded steam in 10 is then reheated (RH1) and expanded in the intermediate-pressure turbine (IPT). The expanded steam in 12 is again reheated (RH2) and expanded in the LPT. Steam is extracted in 15, 17, 18, and 19 to be used in the water preheating process. In the calandria system, saturated liquid water from the steam drum bottom is extracted in 22 and heated radiatively in the “Calandria HXs” and then further heated by combined convection–radiation heat transfer in the “BOILER” section. The figure illustrates the heat transfer between hot flue gases and steam as shown with bold line. The process B–C represents an approximation of the heat transfer process between the flue gases and the steam at the superheater and the two reheaters. The process C–D represents the heat transfer in the “BOILER” heat exchanger (see Figure 5.26), whereas process D–E represents the heat transfer in the preheater.
The coal type and its specific energy and exergy contents play a role mostly in the design of the furnace and the efficiency of the power plant as well as its air pollution characteristics. The gross calorific value of coal varies from 13 to ~34 MJ/kg; chemical exergy (which is sensitive to moisture content) varies from about 5 to ~25 MJ/kg; and the specific GHG emissions of coal combustion are in the range of 70–140 kg CO2 equivalent per GJ on a net calorific value basis (see Chapter 3).
For thermodynamic analysis the mass, energy, entropy, and exergy balance must be written for each component of the system. A logical sequence of computation steps requires solving the equations related to coal combustion in the furnace first.
In the actual coal furnace system oxidant in excess is used. Furthermore, additional oxygen is supplied to the combustion process from recirculated flue gases. Therefore, the combustion process can be represented by the following equation
where ![]() stands for reactant (referring to coal only) and
stands for reactant (referring to coal only) and ![]() for products (except the ash), respectively,
for products (except the ash), respectively, ![]() represents the stoichiometric molar fraction of fresh oxygen, λ > 1 is the excess air factor,
represents the stoichiometric molar fraction of fresh oxygen, λ > 1 is the excess air factor, ![]() is the flue gas recycling factor, and
is the flue gas recycling factor, and ![]() stands for the ash. It is useful to describe coal using atomic fractions expressed per mole of coal on an as-received basis. Hence, the reactants
stands for the ash. It is useful to describe coal using atomic fractions expressed per mole of coal on an as-received basis. Hence, the reactants ![]() can be represented by the following equation
can be represented by the following equation
where ![]() are determined from weight fractions as-received by dividing with molecular masses.
are determined from weight fractions as-received by dividing with molecular masses.
The reaction products, assuming that complete consumption of coal generates only the products CO2, H2O, SO2, O2, N2, and ash, is described by the following equation:
The stoichiometric molar fraction of fresh air, ![]() , results from atomic species balance:
, results from atomic species balance:
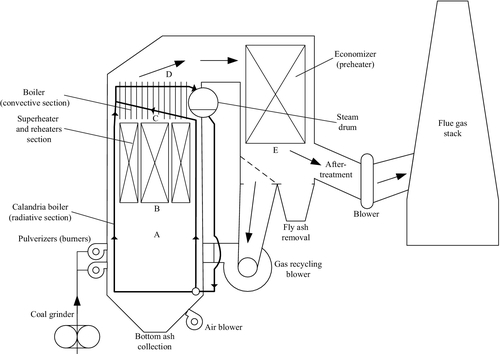
As mentioned above, the first phase of the combustion process is represented by the process of pyrolysis coal and volatile combustion and occurs in a spatial region that extends approximately between the furnace bottom and the coal’s pulverization location. Figure 5.27 gives a sketch representing the matter and heat fluxes in the ignition and volatile combustion zone of the furnace. During the pyrolysis, a series of combustible materials such as long- and short-chain hydrocarbons (e.g., CnH2n + 2) and sulfur and some amount of carbon dioxide are released. Hence, a part of fixed carbon is released during pyrolysis as hydrocarbons with a carbon-to-hydrogen ratio of ~0.25–0.47. If one denotes with ![]() the fraction of fixed carbon released during pyrolysis, then the volatile combustion phase can be described by the following chemical equation
the fraction of fixed carbon released during pyrolysis, then the volatile combustion phase can be described by the following chemical equation
where ![]() represents the combusted volatile gases defined according to
represents the combusted volatile gases defined according to
with
![]()
![]()
standing for the number of moles of components per mole of combusted coal.

In the second phase of combustion (see Figure 5.28) the remaining un-combusted fixed carbon from the first phase oxidizes and the temperature of flue gases increases. Recirculated gases are added to the process as a means of adjusting the temperature at the end of combustion phase. The combustion process (see A–B in Figure 5.25) is described by the following equation
Notice that the summation of Equation (5.19) and Equation (5.21) gives Equation (5.15), which describes the overall process of coal combustion.
The boiling process continues in the heat exchanger placed between points C and D as indicated in Figure 5.25. Once the thermodynamic states in the furnace (0, A, B, C, D, E, F, 6, 7, 8, 9, 10, 11, 22, 23, 24, 25) are completely determined the analysis proceeds further with balance equations of all other components of the Rankine steam power plant. According to energy balance, the mass flow rate in states 4, 5, 6, 7, 8, 9, and 10 must be the same. According to the overall energy balance on the boiler one has
where ![]() is the enthalpy rate of the flow.
is the enthalpy rate of the flow.
In the above equation one accounts for the fact that a saturated liquid enters in state 7 whereas a saturated vapor exits out of the drum in 8. The equation is then solved for ![]() . The enthalpy in 25 results from the enthalpy in state 24 and the enthalpies of states C and D on the flue gas side, according to the energy balance
. The enthalpy in 25 results from the enthalpy in state 24 and the enthalpies of states C and D on the flue gas side, according to the energy balance
![]()
In the example presented above the NOx emissions are neglected, which is a reasonable assumption for first hand calculations. More detailed models must include chemical equilibrium analysis of the combustion process and chemical kinetics in order to determine the NOx emissions. As a rule of thumb, NOx emissions represent half of SO2 emissions per unit of kW net power. On average, for a coal-fired power plant the specific emissions are as follows: 900 g/kWh CO2, 7 g/kWh SO2, and 4 g/kWh NOx.
In the subsequent part of this section the summary of two case studies of energy and exergy analyses of two actual power plants is given. A larger-scale power plant is selected, as Nanticoke Power Generation Station (NPGS) in Ontario, with 500-MW installed capacity per power generating unit. The exergy analysis of the NPGS power plant is summarized here based on the work of Dincer and Rosen (2013). For comparison purposes, the second case study refers to a lower-capacity power plant with a 32-MWgenerator output, operated by Tecro Power Systems Ltd. (TPSL) in India which was previously analyzed in Regualagadda et al. (2010).
The NPGS has steam-reheating power generating units with three stages of turbines (high, intermediate, and low pressure) and a water-cooled condenser. The steam generator operates on the coal pulverization principle. The feedwater system comprises LPP and HPP, two closed feedwater heaters with steam traps, one OFW, and five steam extraction points from the turbines.
The TPSL power plant has a circulating fluidized bed furnace as a steam generator with a nominal capacity of 140 t/h superheated steam. The ultimate coal analysis for TPSL is as follows: moisture 25%, ash 0.88%, hydrogen 4.06%, nitrogen 1.1%, sulfur 0.075%, oxygen 7.935%, carbon 60.95%. The feedwater system comprises a LPP, a HPP, three closed feedwater heaters, and one OFW. The steam cycle operates with no reheating and the turbine has three steam extraction points. The condenser is aircooled.
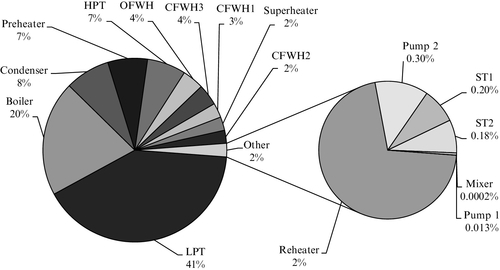
The general technical parameters of both power stations are compared in Table 5.14. The layout of a Rankine plant is described by the simplified diagram in Figure 5.29, which is applicable to both the NPGS and TPSL plants and consists of four main subsystems: feedwater system, steam generator, power production system, and condenser. The state points indicated in the diagram are described in Table 5.15, and the energy and exergy are given for each stream. In addition, the exergy destructions are given in the Table 5.16. As remarked in Table 5.14, the coal quality is much better at NPGS, hence the efficiencies are higher.
Table 5.14
General Technical Parameters of NPGS and TPSL Power Stations
| Parameter | NPGS | TPSL |
| Cycle type | Single reheating | No reheating |
| Nameplate power | 523 MW | 32 MW |
| Coal GCV | 32.8 MJ/kg | 22.0 MJ/kg |
| Coal exergy | 34.2 MJ/kg | 26.0 MJ/kg |
| Steam generator | Pulverized coal | Circulating fluidized bed |
| Condenser type | Water cooled | Air cooled |
| Sink temperature | 287 K | 314 K |
| Energy efficiency | 37% | 25% |
| Exergy efficiency | 36% | 30% |
| Specific emissions | 1.262 kg GHG/kWh | 1.218 kg GHG/kWh |

Table 5.15
Streams Parameters for NPGS (523 MW) and TPSL (32 MW) Generating Units
| Stream | Description | NPGS | TPSL | ||||
| T (K) | T (K) | ||||||
| 1 | Coal fed | 300 | 1368 | 1427 | 300 | 120 | 124 |
| 2 | Air | 300 | 0 | 0 | 300 | 0 | 0 |
| 3 | Flue gas | 393 | 74 | 62 | 500 | 26 | 9 |
| 4 | High-pressure steam | 710 | 1780 | 838 | 793 | 125 | 46 |
| 5 | Heat loses from high-temperature steam | 298 | 10 | 0 | 298 | 4 | 0 |
| 6 | Net power | n/a | 524 | 524 | n/a | 32 | 32 |
| 7 | Saturated vapor | 308 | 775 | 54 | 331 | 67 | 1.6 |
| 8 | Extracted steam | 550 | 449 | 142 | 497 | 7 | 5.6 |
| 9 | Condensed vapors | 308 | 26 | 0 | 331 | 7 | 0 |
| 10 | Heat rejected by the condenser | 308 | 746 | 0 | 331 | 60 | 0 |
| 11 | Pump power input | n/a | 13 | 13 | n/a | 1 | 1 |
| 12 | Preheated water | 500 | 487 | 132 | 473 | 31 | 4 |

Table 5.16
Exergy Destruction of Power Plant Subsystems for NPGS and TPSL
| Component | NPGS | TPSL |
| Feedwater system | 23 MW (3%) | 2.6 MW (3%) |
| Steam generator | 600 MW (75%) | 73 MW (87%) |
| Power production | 118 MW (15%) | 6.9 MW (8%) |
| Condenser | 54 MW (7%) | 1.6 MW (2%) |
| Total | 795 MW (100%) | 84.1 MW (100%) |
Due to a lower quality of coal the exergy destructions of the TPSL steam generator are relatively more important than at NPGS (87% vs. 75% with respect to overall exergy destructions). Comparatively, the feedwater system destroys 3% of exergy for both the NPGS and TPSL stations. The destruction of exergy in the power production subsystem is more important at NPGS than at TPSL (15% vs. 8%).
Conventional power generators based on a the steam Rankine cycle using fossil fuels other than coal (e.g., natural gas or fuel oil) do exist. However, due to economic advantages and efficiency improvements, oil- and gas-fired power plants are combined types, including a combustion turbine and a steam turbine. These types of combined plants will be discussed later in this chapter, after the introduction of gas turbine power plants below.
5.2.6 Organic Rankine Cycles
Unlike the more common steam Rankine cycles, an organic working fluid is used in the ORCs. Local and small-scale power generation as well as renewable energy systems and low-grade heat recovery systems are the best applications for ORC technology. Some examples of low-temperature heat sources from renewable sources are solar radiation, biomass combustion systems, heat recovery systems from engine exhaust gases, geothermal energy, and ocean thermal energy.
ORC technology has received increased attention in the last decades. The manufacturers of ORC provide a wide range of solutions, based on the temperature level and sources. Table 5.23 gives a list of some existing ORC manufacturers and briefly describes their technology.
Table 5.23
List of some ORC Manufacturers and Technology Descriptions
| Manufacturer | Power range | Heat source temperature | Technology description | Applications |
| ORMAT, US | 200 KW–72 MW | 150°–300 °C | Fluid : n-pentane | Geothermal, WHR, solar |
| Turboden, Italy | 200 KW–2 MW | 100°–300°C | Fluids: OMTS, Solkatherm Axial turbines | Geothermal, CHP |
| Adoratec, Germany | 350 KW–1600 KW | 300 °C | Fluid : OMTS | CHP |
| GMK, Germany | 50 K–2 MW | 120°–350 °C | Fluid: GL160 (GMK proprietary) 3000 rpm multistage axial turbines | Geothermal, CHP, WHR |
| Koehler-Ziegler, Germany | 70–200 KW | 150–270 °C | Fluid: Hydrocarbons, screw expander | CHP |
| UTC, US | 280 KW | > 93 °C | N/A | Geothermal, WHR |
| Cryostar | N/A | 100–400 °C | Fluids: R245fa, R134a Radial inflow turbine | WHR Geothermal |
| Freepower, UK | 6 KW–120 KW | 180–225 °C | N/A | WHR |
| Tri-o-gen, NL | 160 KW | > 350 °C | Turbo-expander | WHR |
| 50 KW | > 93 °C | Twin screw expander | WHR | |
| Infinity turbine | 250 KW | > 80 °C | Fluid: R134a Radial turbo expander | WHR |

WHR, waste heat recovery; CHP, combined heating and power.
Working fluid characteristics have great influence in determining the cycle configuration. Selection of the working fluid must be done in compliance with the ORC application, which is influenced by the type of heat source, level of temperature, modes of heat transfer, and scale of the application. Pure fluids and fluid mixtures can both be considered. Also, the retrograde characteristic of the working fluid plays a crucial role in determining the cycle configuration. For example, if a retrograde fluid is used, then the expansion of saturated vapor occurs in the superheated region. This is due to the fact that the slope of the vapor saturation curve on a T–s diagram for retrograde fluids is always positive (e.g., toluene). On the other hand, if the fluid is regular then the expansion of saturated vapor occurs in the two-phase region. The slope of the vapor saturation curve of regular fluids is negative in the T–s diagram (e.g., R134a).
One important feature related to organic working fluid refers to the fact that a large pressure ratio over the turbine can be obtained for relatively small pressure differences. For example, with the organic fluid R123 the ORC operates at a boiling pressure of 5.87 bar and a condensation pressure of 0.85 bar. The pressure ratio is thus 6.9 and the pressure difference is 5.02 bar. This point signifies the advantage of using organic fluids as the working fluid in Rankine cycles. The most important fluid properties for organic working fluid selection are:
• Thermodynamic and physical properties such as density, boiling enthalpy, liquid heat capacity, viscosity, thermal conductivity, melting point temperature, normal boiling point, boiling latent heat (must be high), critical temperature, critical pressure, retrograde or regular behavior, and zeotropic or azeotropic characteristic in the case of mixtures.
• Environmental impact and safety factors such as ozone depletion potential (ODP), global warming potential (GWP), atmospheric lifetime (e.g., R11 45 years, R152 0.6 years), toxicity, flammability.
• Availability, stability at high temperature, and cost effectiveness.
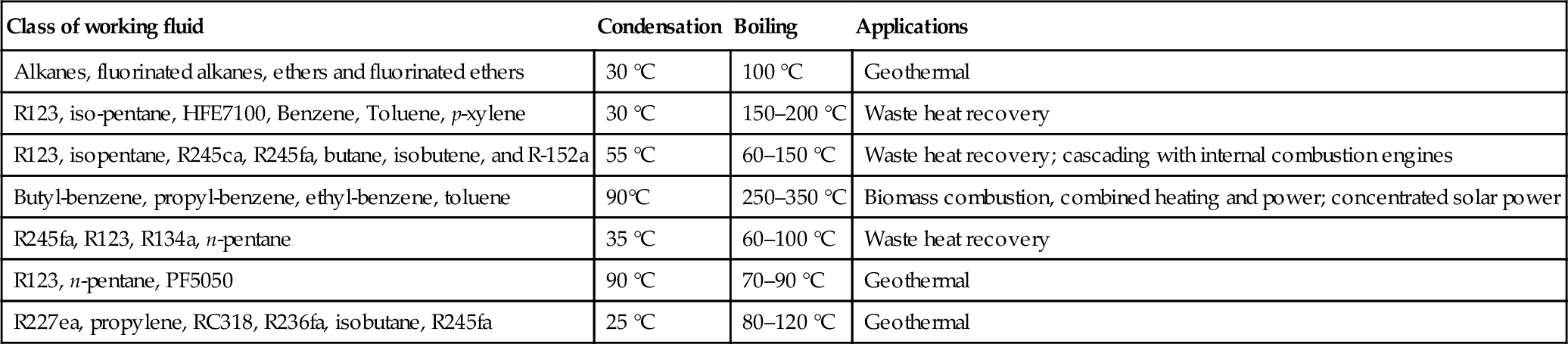
Table 5.24 gives the main categories of working fluids for ORCs and the typical applications for each category. Some proposed working fluids are propylene, propane, R227ea, R236fa, RC318, cyclohexane, R245fa, R141b, R365mfc, R11, R113, R114, toluene, fluorinol (CF3CH2OH), R41, R123, R245ca, isobutene, propane–ethane, and siloxanes (MM, MD4M, D4, D5). Table 5.24 lists the working fluids in order of their molecular masses and gives some main parameters, namely normal boiling point, critical pressure and temperature, ODP, and GWP.
Table 5.24
Classes of Working Fluids for ORC and Typical ORC Applications
| Class of working fluid | Condensation | Boiling | Applications |
| Alkanes, fluorinated alkanes, ethers and fluorinated ethers | 30 °C | 100 °C | Geothermal |
| R123, iso-pentane, HFE7100, Benzene, Toluene, p-xylene | 30 °C | 150–200 °C | Waste heat recovery |
| R123, isopentane, R245ca, R245fa, butane, isobutene, and R-152a | 55 °C | 60–150 °C | Waste heat recovery; cascading with internal combustion engines |
| Butyl-benzene, propyl-benzene, ethyl-benzene, toluene | 90°C | 250–350 °C | Biomass combustion, combined heating and power; concentrated solar power |
| R245fa, R123, R134a, n-pentane | 35 °C | 60–100 °C | Waste heat recovery |
| R123, n-pentane, PF5050 | 90 °C | 70–90 °C | Geothermal |
| R227ea, propylene, RC318, R236fa, isobutane, R245fa | 25 °C | 80–120 °C | Geothermal |
Both turbo-expanders and positive displacement expanders are considered prime movers for ORCs. The choice depends on the scale of the application, the working fluid, pressure differentials, and pressure and volume ratios. For large-scale applications turbo-expanders will be the preferred choice because of their higher efficiency. At lower-scale applications, when pressure drop across the prime mover must be high, the preferred choice is a positive displacement expander because it has contact seals which reduce the bypass leakages. When pressure difference per stage is high, turbo-expanders (turbines) have bypass leakage flow at blade tips which is accentuated for small-scale applications.
There are many configurations of the ORC which differ from the typical reheat-regenerative steam cycle configuration that normally uses steam extraction. For example, in ORC with retrograde fluid it is possible to use a counter-current heat exchanger as a regenerator. In addition, ORC with retrograde fluids can operate without superheating, which makes them better suited for applications that involve heat recovery or solar energy. Other configurations are the reheat-regeneration cycle and supercritical cycle.
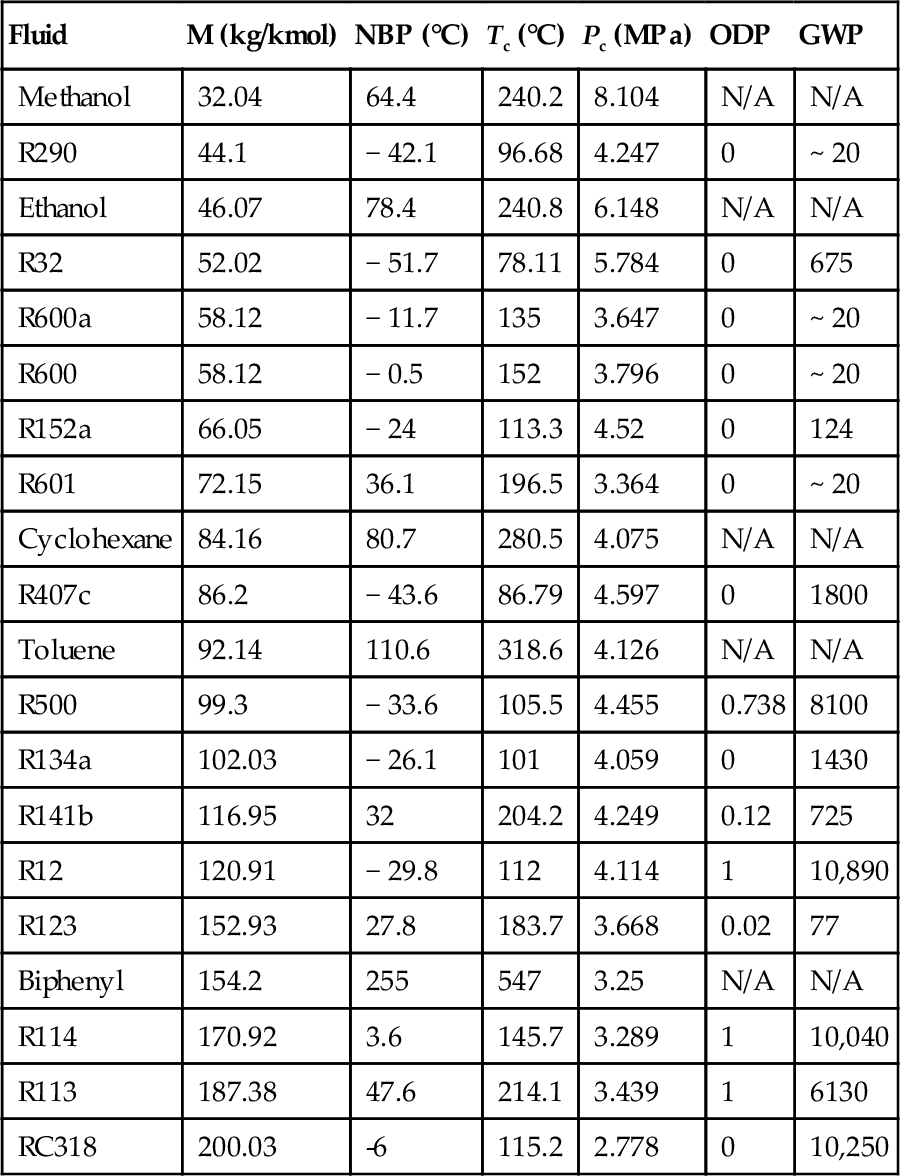
An ORC of simple configuration is shown in Figure 5.34. The cycle operates with the organic fluid R134a and is heated with a hot fluid. This cycle configuration can be applied for waste heat recovery when the heat source is low grade. Notice that superheating is not required because the moisture at the end of the expansion process is very low when saturated vapors are expanded (Table 5.25).

Table 5.25
Working Fluids Commonly Used in ORC
| Fluid | M (kg/kmol) | NBP (°C) | Tc (°C) | Pc (MPa) | ODP | GWP |
| Methanol | 32.04 | 64.4 | 240.2 | 8.104 | N/A | N/A |
| R290 | 44.1 | − 42.1 | 96.68 | 4.247 | 0 | ~ 20 |
| Ethanol | 46.07 | 78.4 | 240.8 | 6.148 | N/A | N/A |
| R32 | 52.02 | − 51.7 | 78.11 | 5.784 | 0 | 675 |
| R600a | 58.12 | − 11.7 | 135 | 3.647 | 0 | ~ 20 |
| R600 | 58.12 | − 0.5 | 152 | 3.796 | 0 | ~ 20 |
| R152a | 66.05 | − 24 | 113.3 | 4.52 | 0 | 124 |
| R601 | 72.15 | 36.1 | 196.5 | 3.364 | 0 | ~ 20 |
| Cyclohexane | 84.16 | 80.7 | 280.5 | 4.075 | N/A | N/A |
| R407c | 86.2 | − 43.6 | 86.79 | 4.597 | 0 | 1800 |
| Toluene | 92.14 | 110.6 | 318.6 | 4.126 | N/A | N/A |
| R500 | 99.3 | − 33.6 | 105.5 | 4.455 | 0.738 | 8100 |
| R134a | 102.03 | − 26.1 | 101 | 4.059 | 0 | 1430 |
| R141b | 116.95 | 32 | 204.2 | 4.249 | 0.12 | 725 |
| R12 | 120.91 | − 29.8 | 112 | 4.114 | 1 | 10,890 |
| R123 | 152.93 | 27.8 | 183.7 | 3.668 | 0.02 | 77 |
| Biphenyl | 154.2 | 255 | 547 | 3.25 | N/A | N/A |
| R114 | 170.92 | 3.6 | 145.7 | 3.289 | 1 | 10,040 |
| R113 | 187.38 | 47.6 | 214.1 | 3.439 | 1 | 6130 |
| RC318 | 200.03 | -6 | 115.2 | 2.778 | 0 | 10,250 |
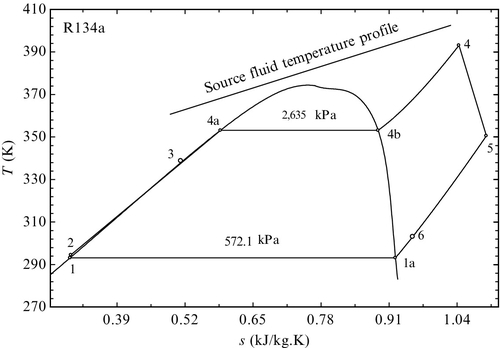
The regenerative schemes for ORC do not require (in general) vapor extraction (as in the case of steam cycles). Rather an additional heat exchanger is inserted between the turbine exit and the pump exit, which allows for a transfer of heat between the hotter working fluid at the turbine exhaust and the colder liquid at the pump discharge. The configuration of a regenerative ORC is presented in Figure 5.35. An example of ORC with regeneration that uses R134a as working fluid is presented in Figure 5.36. In this cycle, boiling occurs at 353 K; it starts with saturated liquid in state 4a and reaches saturated vapor in state 4b (pressure drop is neglected). In the condenser, there is a de-superheating process according to line 6–1a, where 1a represents saturated vapor (see Figure 5.36). Further, the condensation process continues until reaching saturated liquid in state 1.

The exergy destruction in the regenerator is accounted for with the help of regenerator effectiveness. This parameter is defined based on the sketch in Figure 5.37, which illustrates a regenerator in the form of a heat exchanger that facilitates a heat transfer process between a hotter stream and a colder stream. Both streams exchange sensible heat. The effectiveness is defined by the ratio between the heat transfer rate that ideally can be extracted from the hotter stream (![]() ) and the heat which is actually received by the colder stream (
) and the heat which is actually received by the colder stream (![]() . Note that the ideal, maximum heat rate that the hotter fluid can transfer occurs when T2 = T3 (see Figure 5.37); therefore the ideal maximum heat transfer in the regenerator occurs when h2 = h2(T3) and is given by
. Note that the ideal, maximum heat rate that the hotter fluid can transfer occurs when T2 = T3 (see Figure 5.37); therefore the ideal maximum heat transfer in the regenerator occurs when h2 = h2(T3) and is given by ![]() . According to this definition, the regenerator effectiveness is
. According to this definition, the regenerator effectiveness is

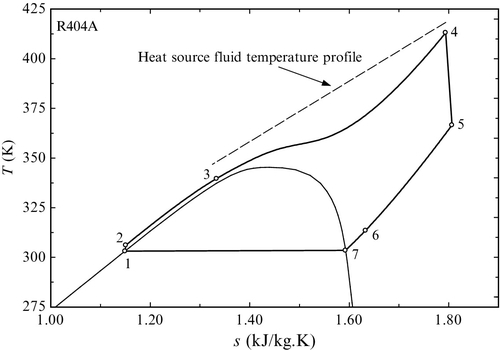
An interesting type of regenerative ORC is that using retrograde working fluids where saturated vapor is fed into the turbine while the expanded fluid is in superheated state. An example of such a cycle is shown in Figure 5.38, with toluene as the working fluid. As seen, the boiling temperature is 573 K and the boiling pressure is 1665 kPa. For a condensation at 303 K the saturation pressure is 2.911 kP (vacuum). Therefore the pressure ratio over the turbine is 395; at the same time the volumetric expansion ratio is 285. Figure 5.38 is suggested the temperature profile at the source when a hot fluid which exchanges sensible heat is used as the heat source (e.g., hot combustion gases, high-temperature geothermal brine, etc.).

An emerging type of ORC is the supercritical cycle. This cycle works generally based on a scheme with regeneration such as that presented in Figure 5.35. In supercritical cycles the working fluid is pressurized to supercritical pressure and heated. As the pressure is supercritical, there is no boiling during heating. Rather, when the temperature becomes superior to critical point the working fluid enters into a supercritical state where the thermodynamic properties are neither of a liquid nor of a gas but a combination of these. Given that there is no boiling, the typical pinch point problem that occurs in vapor generators for Rankine cycles is minimized. Therefore, a gain on exergy efficiency can be obtained with respect to cycles with boiling. However, due to the high pressures associated with supercritical cycles, one must exercise care when selecting a working fluid.
An example of a supercritical ORC operating with R404A working fluid (which is a blend of refrigerants) is shown in Figure 5.39. The high pressure in this cycle is 44.82 bar while the low pressure is 14.28 bar, making a pressure ratio of 3.1, which is applicable to positive displacement expanders. The temperature profiles match very well, proving that there is reduced exergy destruction in a supercritical ORC. In the regenerative heat exchanger a pinch point appears due to the nonlinear temperature profile of the supercritical fluid.