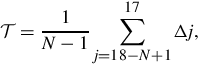An Application of HDR in Medical Imaging
G. Ramponi*; A. Badano†; S. Bonfiglio‡; L. Albani‡; G. Guarnieri* * University of Trieste, Trieste, Italy
† US Food and Drug Administration, Silver Spring, MD, United States
‡ Barco FIMI, Saronno (VA), Italy
Abstract
In medical diagnostic imaging applications a high dynamic range (HDR) display is a key issue: if properly implemented, it might enable the physician to detect a disease rapidly and in a highly reliable way. The display device should be able to faithfully reproduce the faintest changes in image data associated with tissue properties. The characteristics of the human visual system should also be taken into account in the design of the display. In this chapter, we describe a solution for an HDR medical display. We first analyze the specific requirements for an HDR display device that operates in the medical context and review procedures that can be followed to evaluate its performance. We then describe in some detail a “dual-layer” LCD, composed of two stacked liquid crystal panels, a prototype of which is presently being developed.
Keywords
High dynamic range display; Dual-layer LCD; Medical imaging; Diagnostic display; Medical display evaluation
Acknowledgments
This work was supported by the CHIRON European project, co-funded by the EU-ARTEMIS Joint Undertaking program under grant agreement no. 100228. Badano acknowledges partial support from Cooperative Research and Development Agreement between FIMI/Barco and Center for Devices and Radiological Health.
The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
19.1 Introduction
The “slow revolution” that brought the field of diagnostic radiology from analog film to digital imaging paved the way to important benefits that were progressively achieved in the last few decades: above all, the possibility for medical personnel to rapidly share huge amounts of patient-related information. However, some of the consequences of the analog-to-digital shift were problematic. One issue, whose importance is often underestimated, was the visual quality of the images presented to the physician. As will be described in detail herein, the requirements of the different specialties with regard to medical images span a wide range; the weakest link in the processing chain, which determines the overall quality, is frequently the display equipment.
In particular, the dynamic range of the luminance that the device can emit and its relation to the typical illumination of the environment in which the device operates should be considered with care. Old analog films placed on a lightbox could yield peaks of 4000 cd/m2, and could span a luminance range of several log units: this made details well visible even in a highly illuminated room. The best medical CRT displays at the time of the digital transition, instead, had a maximum luminance in the several hundred candelas per square meter (cd/m2) range. Only later did LCDs reach 1000 cd/m2, at the price of an undesired higher luminance for “black” pixels.
In this chapter we will describe a solution aimed at overcoming dynamic range problems in the visualization of medical images. We will first analyze the specific requirements for a high dynamic range (HDR) display that should operate in the medical (diagnostic) context, and the procedures that are followed in a laboratory of the US Food and Drug Administration to evaluate such a display. We will then describe in some detail an LCD composed of two stacked liquid crystal panels, a prototype of which is presently being developed at Barco.
19.2 Requirements of HDR Visualization in the Medical Field
19.2.1 General Requirements for Medical Imaging Displays
In critical tasks such as diagnostic procedures, medical images should provide sufficient information to allow clinicians to detect diseases and to make medical decisions with the highest possible degree of accuracy (Martin et al., 1999). Here an important role is played by the visualization part of the medical imaging chain; a suitable display permits one to achieve the optimal compromise between productivity (short image interpretation time) and accuracy (low false positive and false negative rates) of the diagnosis. According to Reiner et al. (2003) the frequency of false positive readings is on the order of 2–15%, while false negative readings are much more frequent — that is, on the order of 20–30%. Approximately 50% of the total errors are due to perceptive issues. A good display also has other advantages; for example, it can permit a reduction of the radiation dosage (Kubo et al., 2014).
Characteristics such as image accuracy, spatial resolution, grayscale resolution (bit depth), color discrimination, black level and dynamic range, viewing angle, video response time, and absence of artifacts (eg, noise, Moiré effect) have different impacts on the outcome of the medical task depending on the specific application.
Another important requirement for medical applications is the consistency of the image rendering across different displays and therefore the need for a standardization. For grayscale radiographic images used for primary diagnosis, the Digital Imaging and Communications in Medicine (DICOM) grayscale standard display function (GSDF) has been universally accepted by regulatory authorities throughout the world as a standard (ACR and NEMA).
Moreover, much attention has to be devoted to perceptual issues (eg, visual acuity, contrast sensitivity), without forgetting that they show a significant amount of interobserver and intraobserver variability: different observers may have quite different performance because of different vision capabilities, age, and experience level; the same observer can show a lack of consistency in the assessment of the same image (“reader jitter”).
Finally — considering the intensive use of images in some clinical procedures — ergonomic requirements such as viewing comfort and absence of aftereffects of the vision experience should be taken into account.
19.2.2 The Added Value of HDR Displays in Specific Medical Applications
The display’s weaknesses in terms of dynamic range and maximum luminance may be unacceptable in medical applications where a large number of luminance levels and fine details with very small luminance differences need to be discriminated. The display of a high-quality diagnostic image depends on at least three factors: (i) the capability of the detector to provide a large number of levels (bit depth of the acquired data set), (ii) a sophisticated mapping that converts source data into suitable driving levels, and (iii) the visualization of each of them as a distinctly perceived luminance value on the display screen.
A joint and harmonized advance in the whole image chain (acquisition, ie, sensors, image processing/mapping, and image visualization, ie, display) is needed. With regard to the visualization part, even if source data are represented by a large number of bits, the detectability of distinct luminance levels associated with each grayscale step depends on the luminance range of the device. Indeed, if the grayscale steps are too closely spaced, some may fall below the threshold of human perception.
In consumer applications, dynamic range compression or tone mapping techniques allow HDR images to be visualized on conventional displays (Ashikhmin, 2002; Meylan and Suesstrunk, 2006; Mantiuk et al., 2006), but their application on medical images poses serious concerns because the photometric distortion that is intrinsically introduced by the processing can cause the loss of clinically relevant details (Guarnieri et al., 2008b). In the medical field window-and-level adjustment techniques are used, at the price of a long analysis time and with the risk of missing details in the search phase (Yeganeh et al., 2012). Alternatively, an approach was proposed still based on the standard eight-bit gray level resolution but supported by eye-tracking techniques that dynamically process the display image by optimizing the luminance and contrast of the inspected area (Cheng and Badano, 2010).
Recent advances in the display industry have contributed to the development of HDR LCDs with extended luminance range, capable of effectively generating perceivable scales that extend to beyond 14 bits and of reproducing the original image with no theoretical distortion and no loss of information with respect to grayscale values. They will allow radiologists and physicians to perceive subtle and medically significant details in images where there is a large variation of the dynamic range in the data. Various technologies for HDR displays have been proposed (see Part IV of this book and Section 19.4). Regardless of the technological option adopted for the display, much attention has to be devoted to the implementation of the solution because an increased luminance range may come at a price with respect to other image quality parameters, such as increased veiling glare, optical crosstalk, and visual adaptation.
19.2.3 Gray Level Mapping in HDR Medical Images
Medical images evidence and translate into luminance levels some properties of the patient’s tissues (eg, density in the case of radiography and computed tomography, or water concentration in the case of magnetic resonance imaging). Some sort of mapping is needed at the display stage to convert the source data into luminance values. One desirable property the mapping should have is that equal changes in the input values should produce equal changes in the perceived luminance regardless of the background to ensure that parts with the same density have the same visibility on any background. Because of the intrinsically nonlinear behavior of the human visual system, a nonlinear mapping that takes into account the properties of the human visual system and possibly adapts to the luminance range of the individual display device is needed (Albani et al., 2013). As mentioned earlier, the solution of choice for standard medical displays is the DICOM GSDF (ACR and NEMA). The function is based on the model of the contrast sensitivity of the human eye developed by Barten (1999) and uses the concept of just noticeable difference (JND), which represents the minimum variation of luminance that an average observer is able to detect in specified conditions. Unfortunately, the DICOM GSDF was developed for regular dynamic range displays (currently found in clinical use) and it is inadequate for the newly developed HDR equipment featuring a black level below 0.01 cd/m2 — a value that lies outside the interval in which the DICOM GSDF is defined (0.05–4000 cd/m2). In particular, if the DICOM GSDF is used, the details in the dark portions of the image are less visible than the details in the bright portions. This suggests that Barten’s model overestimates the sensitivity of the eye at very low luminance levels.
It is expedient to propose an extension of the DICOM GSDF. To do so, perceptual issues should be reconsidered; the DICOM curve is indeed based on a theoretical approach that is often not applicable to the case of radiological images having as a background a mixture of white and dark areas that produce a changed perception (McCann and Rizzi, 2011).
The extension of the DICOM GSDF is still an open problem; a possible solution will be presented in Section 19.4.3.
19.3 Evaluation of Medical HDR Displays
Tisdall et al. (2008) compared signal detectability in an HDR display and a standard medical LCD using a low-resolution eight-bit rear panel for the backlight and a high-resolution eight-bit front panel to obtain a 16-bit grayscale. They quantified the performance for a detection task with the display at both settings (standard LCD and HDR display). The results showed that subjects performed similarly in a detection task on the standard LCD and on an HDR display. Tisdall et al. concluded that further experiments were needed to verify this claim and that careful characterization of HDR devices is required to understand the benefits and drawbacks of HDR display technology.
In this section, we summarize the current understanding of how to evaluate medical HDR displays. Areas of image quality that are of particular relevance to HDR technologies for medical displays include the luminance and color response and uniformity, spatial noise and resolution, and veiling glare. For a more complete treatment, the reader is encouraged to read the literature on medical display characterization (Samei et al., 2005; Saha et al., 2006; Vogel et al., 2007; VESA, 2003).
19.3.1 Luminance
Luminance response is typically measured with a luminance meter connected to a computer that automatically sweeps the range of digital driving levels and record the output. Although 18 measurement points have been recommended in the past, it is advisable to record the response to 256 levels to accommodate nonlinear intrinsic behavior — for instance, in the case of LCDs. In HDR displays, the complete set of available grayscale combinations can include 65,536 measurements, making the entire set difficult to acquire. In this situation, a subset of graylevels can be measured and then analyzed to develop a full model of the output luminance. One area that needs further research is the appropriate mapping of the luminance output in the range below 0.1 cd/m2. Even if the viewing is performed in a dark room, the reflected luminance will increase the minimum luminance of the model applied. Thus, it is uncertain at the moment how to calibrate HDR displays. In addition, as already mentioned, the well-known and widely adopted GSDF (ACR and NEMA) is not defined for very low luminance, and even the underlying Barten model that constitutes the foundation of the GSDF model was not developed for that range.
19.3.2 Uniformity
Luminance uniformity is another area of concern and is measured according to American Association of Physicists in Medicine (AAPM) Task Group 18 (TG18) (Samei et al., 2005) using a high-gain photodiode with the tip of the probe 1 mm from the center of each display. To determine changes with respect to viewing angle, measurements are taken at 0°, 15°, 30°, and 45°. The luminance nonuniformity metric is calculated as ![]() , where
, where ![]() and
and ![]() are the maximum and minimum luminance, respectively.
are the maximum and minimum luminance, respectively.
Color display systems are becoming commoner in medical imaging. Color monitors are replacing grayscale monitors to accommodate more imaging modalities as well as to increase the functionality of the visualization. Color coordinates (u′,v′) are measured following the 1976 CIE color scheme. The color nonuniformity is defined as the maximum distance between the color coordinates (u′,v′) for the various points on the display screen:
The maximum change in (u′,v′) is the reported color nonuniformity parameter, which should be less than 0.01 for an acceptable clinical workstation according to AAPM TG18. Measurements of luminance and color coordinates are taken at five screen locations (four corners and the center), for uniform backgrounds at gray levels of 10, 50, 128, 200, and 255 on an eight-bit scale. Recently, a number of reports have highlighted the relevance of performing colorimetric measurements on medical displays (Fan et al., 2007; Krupinski et al., 2011; Roehrig et al., 2010; Silverstein et al., 2012; Cheng and Badano, 2011).
19.3.3 Grayscale Tracking
A complementary metric often used in the display industry is grayscale tracking, which is defined as the variation in color coordinates of the grayscale gradation.
Grayscale tracking can be characterized by measurement of color coordinates (u′,v′) with a color probe at all graylevels. A recent approach being considered by AAPM Task Group 196 to determine the variation in color of a grayscale gradation is summarized in the following. Assuming a display is calibrated to the GSDF, use TG18-LN test patterns (TG18-LNi , i = 1, 2, …, 18) and record the luminance and color coordinates (u′,v′). For the analysis, it is expedient to consider only measurements from patterns with a recorded luminance higher than 5 cd/m2 or 1% of the luminance measured for TG18-LN18 (full white). Denote the remaining measurements as N. One possible definition of a quantity of interest (![]() ) is the average distance in the u′,v′ plane between the measurement of full white (TG18-LN18) and the nondiscarded measurements:
) is the average distance in the u′,v′ plane between the measurement of full white (TG18-LN18) and the nondiscarded measurements:
where
This metric ![]() quantifies how close the grayscale chromaticity is to the chromaticity of full white, with a lower value representing an improved grayscale tracking performance.
quantifies how close the grayscale chromaticity is to the chromaticity of full white, with a lower value representing an improved grayscale tracking performance.
19.3.4 Spatial Noise and Resolution
The analyses of subpixel structures and noise in HDR displays involve the acquisition of uniform patterns with a CCD camera. The camera should be cooled to reduce dark counts caused by thermal noise. A macro lens is needed to get magnified images on the CCD chip. A single line set at gray level 128 is placed both vertically and horizontally (Badano et al., 2004). It is useful to test different conditions with HDR displays to see the effects of different gray levels on the pixel.
19.3.5 Temporal Response
Of relevance for image browsing typical in cross-sectional medical imaging, the temporal response of the display device needs to be characterized. Temporal response measurements are typically performed with gray level transitions relying on a measurement system composed of a display driver for pattern generation and a photomultiplier tube for light detection (Liang and Badano, 2006, 2007). Gray level transition time is measured by the capturing of the optical pulse corresponding to a luminance change. The rise time is usually defined as the time required for the luminance to change from 10% to 90% of the luminance difference, while the fall time is from 90% to 10%. One must take care when measuring all the components associated with the time response of an HDR display particularly when different components of the device might have different time constants (eg, modulated backlight and primary shutter display component). To date, this topic has not been investigated in the literature.
19.3.6 Veiling Glare
HDR technologies with a large luminance range and a deep bit depth allow more information to be offered to a reader. However, a major limitation of HDR is the long-range light scattering from bright areas in the image that can reduce perceived contrast. This phenomenon is known as veiling glare. Operating over too wide a luminance range may cause readers to miss contrast in dark regions due to adaptation to bright areas and veiling glare effects in the human eye, or, alternatively, miss edges in bright regions due to short-range scattering or blurring (Tisdall et al., 2008).
Glare in human vision is caused by scattering of light in the cornea, lens, and retina and diffraction in the coherent cell structures on the outer radial areas of the lens. These effects result in “bloom” and “flare lines” perceived by readers in the vicinity of bright objects. Many attempts have been made to empirically model the veiling glare effect in the human visual system in terms of the equivalent veiling luminance (Vos, 1984; Stiles, 1929; Holladay, 1929). Investigations into a subject’s visual threshold under different conditions of glare found it to be dependent on the angular separation between the source and the object and to increase with increased illumination of the glare source. Other studies, summarized in Vos (1984), give different values for thresholds depending on the validity domain. One cause of this variability was reported to be the age of the subjects in each experiment. Vos (2003a,b) introduced age into the CIE general disability glare equation:
This equation does not include individual variations due to other factors, such as disease and ocular pigmentation, and was used to analyze disability glare particularly in traffic. Veiling glare is prominent in other components of the imaging chain, including acquisition (Raskar et al., 2008) and display (Flynn and Badano, 1999). Hardware glare can significantly degrade the quality of an image on different displays as characterized by the veiling glare response function by Flynn and Badano (1999). They developed an experimental technique to measure the degradation in image quality due to veiling glare in display devices such as radiographic film, monochrome monitors, and color monitors with antireflective surface coating. In HDR medical displays, the combined effect of veiling glare in the display and in the human visual system can negatively influence the detectability of lesions, as represented in Fig. 19.1.

19.3.7 Quantization
Among the many differences between reading a digital mammogram on transilluminated film and utilizing electronic display devices is the quantization of the image data. Quantization is defined as a lossy data compression technique by which intervals of data are grouped or binned into a single value (or quantum). Quantization processes are used in image acquisition, transmission, archiving, and display. Most display systems are limited to eight-bit luminance scales, but several approaches have been implemented to present extended grayscales. The chief examples of these technologies are the temporal and subpixel (Flynn et al., 1995) modulation techniques common in modern medical imaging workstation displays. Such techniques have successfully expanded the grayscale from eight bits, which is the commonest resolution for current LCDs, to 10–11 bits. However, these techniques have drawbacks with regard to other display quality characteristics, including spatial and temporal resolution.
While medical images are acquired with grayscales of 12–16 bits, most imaging systems quantize the data to eight bits for display. It is assumed that this loss of information does not translate into a degradation of visual task performance. This assumption relies primarily on literature reports that state that the human visual system is not able to perceive more than a small number of distinct luminance levels (Barten, 1999). These claims are sometimes based on human visual system internal noise considerations or on the effects of a wide luminance range on optimal perceptual adaptation. However, these claims have not been verified for the case of medical image interpretation. Previous attempts to study similar questions in the area of image quantization and observer performance have not been conclusive (Krupinski et al., 2007; Reiner et al., 2001). Burgess (1985) studied the effect of image quantization in the presence of white (uncorrelated) noise and found that performance decreased below 0.9 for quantization steps larger than the noise variance. His findings have not been extended to correlated noise images. Krupinski et al. (2007) studied the effect of 8-bit and 11-bit display of chest radiographs, comparing a conventional LCD with a similar device but with extended grayscale mapping of 11 bits. Both devices were calibrated to the same luminance range, forcing the luminance increase between grayscale steps to be reduced in the 11-bit device. Krupinski et al. found no statistical significance between the two devices but reported decreased reading times for the 11-bit presentation.
19.4 The Dual-Layer Approach
One possible method of increasing the dynamic range of an LCD is to stack a strong backlight and two liquid crystal panels, one in front of the other (Visser et al., 2005). The transmittance of this “dual-layer LCD” is equal to the pointwise product of the transmittances of the two individual layers, and the theoretical dynamic range is squared for equal liquid crystal panels. More precisely, in ideal conditions (which will later be discussed), the output luminance Lout(x,y) at pixel location (x,y) is given by
where B is the backlight intensity (cd/m2) and Tb(x,t) and Tf(x,y) are the adimensional transmittances of the two panels. Dual-layer displays, calibrated for a white level of 500 cd/m2, are able to achieve a measured black level of around 0.003 cd/m2. Moreover, the bit depth also increases, because the light is modulated by two eight-bit values,1 although the overall bit depth of the dual-layer display is less than the theoretical 16 bits because output levels corresponding to the 216 possible inputs are partially overlapped and nonuniformly spaced.
A drawback of this approach is that the backlight intensity must be greater than that of a standard display, because a medical-grade grayscale liquid crystal panel transmits around 30% of the light when fully on, and this value is reduced to 10% for color panels because of the additional presence of the color filters. For this reason, only grayscale displays are currently practical.
19.4.1 Image Splitting and the Problem of the Parallax Error
A dual-layer LCD requires the use of dedicated “image splitting” algorithms to generate the two images which drive the back panel and the front panel (Guarnieri et al., 2008a). Designing a splitting algorithm is a complex task that involves an accurate study of the behavior of the individual panels and the human visual system, and requires the use of advanced mathematical techniques for an efficient implementation.
A splitting algorithm also has the additional objective of minimizing the parallax error that is caused by the unavoidable slight misalignment of the two panels and that is described in the following.
Fig. 19.2 shows the effect on spatial resolution from the use a dual-layer approach to achieve HDR. Fig. 19.2A–D depicts a horizontal line image on a uniform background as visualized in different implementations of the display. In the synchronized mode, both layers of the display present the same image data. The same data when shown only by the front (back) layer are presented in Fig. 19.2B and C. For comparison, Fig. 19.2D shows the same image on a single-layer display. These photographs of the display emissions directly describe how the spatial resolution properties of the HDR display change depending on the addressing modes. While these images should all contain similar representations of the data, it is clear that the spatial resolution achieved in the image in Fig. 19.2C is lower than that achieved in the other images. The same effect but for a vertical line can be seen in Fig. 19.2E–H. The data shown in Fig. 19.2 can also be analyzed by Fourier analysis to show the change of the modulation transfer function for different addressing modes. The effect of parallax is added to this effect if one looks at the line images from a nonperpendicular direction. Fig. 19.2I–N shows images of the same patterns as in Fig. 19.2A–H but taken at 5 degree in the orthogonal direction from the line. The same degradation effect described above is seen at an angle but compounded with the addition effect of parallax. The images in Fig. 19.2 have been scaled down from their actual dynamic range to allow for reproduction in this media. The analysis of these images with the methods described in Yamasaki et al. (2013) would provide a quantitative characterization of the importance of this effect in HDR displays that rely on dual emission layers.

Parallax effects on spatial resolution are problematic for medical image visualization for two reasons. Firstly, parallax affects the spatial resolution of the display device in a way that makes it variable across the screen. This is problematic because detail in medical images will appear with different content in spatial frequencies depending on the location of the features examined. In addition, this variability will make clinical decisions become inconsistent between readers looking at the same image on a device. Secondly, the effect of parallax also incorporates a dependency on the driving level used. This implies that the spatial frequency content of an image displayed on the HDR display will depend on the gray level, which is not an absolute, characteristic feature of the image data and can therefore lead to interpretation errors. An example of this effect can be appreciated in that the sharpness of a microcalcification cluster in a mammogram would be seen differently depending on the baseline gray level of the surrounding area. This characteristic is not desired in medical imaging visualization because it adds variability to the reading process that is difficult to control or compensate for through image processing.
We will now describe different splitting algorithms, point out their drawbacks, and use this knowledge to design a more advanced technique. Only the conceptual approach to these algorithms is described in this chapter; in Guarnieri et al. (2008a,b,c), the interested reader can find the whole mathematical formulation, together with the details of its numerical implementation and a feasible solution of the problem.
19.4.2 Techniques for Image Splitting
The simplest possible technique is to perform the splitting on a pixel-by-pixel basis. More precisely, if we indicate with Lin(x,y) the luminance of the input image at pixel location (x,y) and follow the notation used in Eq. (19.4), the splitting algorithm takes the form
In other words, we compute the transmittance of the back panel by mapping the input luminance with a suitable nonlinear function F(⋅), and we subsequently compute the transmittance of the front panel by division in order to guarantee that the product of the two images reproduces the input. The splitting is computed on linear data; the nonlinear encoding of the source image (if present) and the response of the liquid crystal panels are compensated appropriately by the mapping of the data before and after the processing. An intuitive choice for F(⋅) is a square root (Penz, 1982): in this way, each panel displays the same image.
If this simple technique is used on an actual dual-layer display, a problem immediately becomes visible. There is a small but not negligible distance between the liquid crystal cells of the two panels, due to the presence of glass sheets and polarizing filters in between. Therefore, if the observer looks at the display from an oblique angle, the two images appear misaligned. More precisely, instead of seeing the correct image ![]() , an observer focusing on position (x,y) on the display surface sees a distorted image
, an observer focusing on position (x,y) on the display surface sees a distorted image ![]() , where the displacements Δx and Δy depend on the viewing angle. This form of distortion is the parallax error that was described in Section 19.4.1. It can be proved that in order to minimize the parallax error, a splitting algorithm should minimize the norm of the relative gradient of the back panel (ie, the back panel should be smooth).
, where the displacements Δx and Δy depend on the viewing angle. This form of distortion is the parallax error that was described in Section 19.4.1. It can be proved that in order to minimize the parallax error, a splitting algorithm should minimize the norm of the relative gradient of the back panel (ie, the back panel should be smooth).
However, the back-panel values cannot be generated by a simple low-pass filter, because of limitations in the dynamic range of the panels and the need to obtain a perfect or quasi-perfect reconstruction of the output image (Guarnieri et al., 2008a). One possible solution is to generate, by means of a constrained optimization algorithm, a back panel that minimizes the parallax error and from which a front panel with suitable black-to-white range can be generated. To do so, it is computationally advantageous to minimize the mean squared value of the parallax error, subject to constraints that can be easily derived by the panel specifications. If perfect reconstruction is required, the transmittance of the front panel can be computed by division, as in Eq. (19.5).
An example of splitting of an actual medical image is shown in Fig, 19.3.

Fig. 19.3 shows that the back panel still contains sharp edges. This happens when the input image contains edges which have a greater magnitude than the dynamic range of the panels. In this case, the front panel alone is not able to completely reproduce the edge, and a fraction of its magnitude must be transferred onto the back panel.
One possible solution is to allow some distortion in the visualized image. The properties of the visual system suggest that the details on the dark side of a high-contrast edge are less visible; this can be used to relax the requirement of perfect reconstruction in those portions. As a consequence, the back-panel transmittance Tb(x,y) and the front-panel transmittance Tf(x,y) are no longer linked by Eq. (19.5), and are both treated as unknowns in the optimization problem. The constraints are simple bounds, constant over the entire image:
where ![]() and
and ![]() are the black and white levels of the panels.
are the black and white levels of the panels.
The reconstruction error metric to be used in this case must satisfy two strongly conflicting requirements. It should be kept as simple as possible, because it is being used inside an optimization algorithm; this excludes advanced methods such as visible difference predictors (Daly, 1993; Mantiuk et al., 2004). At the same time, the expression should be spatially adaptive because, as mentioned above, the eye sensitivity depends on the context.
We can obtain a pointwise estimate of the visibility of the reconstruction error by taking the ratio between the luminance error and the JND. We obtain the JND by mapping the adaptation level Lad with a threshold versus intensity (TVI) function (Ashikhmin, 2002); possible choices for the latter are discussed in Section 19.4.3. In turn, we compute Lad by filtering the input image with a low-pass filter; in this sense the metric is spatially adaptive (in particular, it correctly predicts the lower visibility of the details near brighter portions), but at the same time the computational cost is low. The function to be minimized takes the following form:
where kp is a user-adjustable scalar parameter that balances the relative weight of the reconstruction and the parallax errors.
A full analysis of the variational problem (Eq. 19.7) is beyond the scope of this chapter; it can be found in Guarnieri et al. (2008c) together with a discrete formulation of the problem itself and its numerical solution.
19.4.3 Development of an HDR Display Function
The DICOM GSDF described in Section 19.2.3 has been universally adopted to convert the original image data into luminance values, but has some drawbacks which limit its use on HDR displays. One issue is that the black level of HDR displays can be lower than 0.05 cd/m2, which is the lower limit of the DICOM GSDF. It is possible to extrapolate the low-luminance values with the original equations of Barten’s model, but this “extended” DICOM curve does not produce satisfactory results. Indeed, experiments performed on a dual-layer LCD showed that DICOM-mapped images appear too dark and that the details in the dark portions are less visible than those in the bright portions, therefore failing the requirement of perceptual uniformity. Examination showed that Barten’s model overestimates the eye sensitivity at low luminance levels, because it predicts JNDs that tend to zero as the luminance decreases. This implies that the eye has infinite sensitivity and is able to detect arbitrarily small luminance differences. Other models of visual JNDs have been introduced — for instance, by Blackwell CIE (1981) and Ferwerda et al. (1996), and the predicted JNDs tend to a constant nonzero limit as the luminance decreases. Display functions computed with these models produce a more uniform visibility of the details but result in compatibility issues, because the experiments used to derive the models are different from Barten’s: Barten measured the contrast sensitivity by means of static sinusoidal gratings, whereas Blackwell and Ferwerda et al. measured the temporal sensitivity by means of brief flashing dots. This motivated a series of psychophysical experiments, aiming to measure the visual JND for a wide range of luminance levels and using a test pattern as similar as possible to the one used for the DICOM GSDF.
The experiments were performed on volunteers at the University of Trieste. The recently proposed two-alternative forced-choice method with denoising (García-Pérez, 2010) was used to collect JND values at different background levels throughout the dynamic range of the dual-layer display. The experiments were conducted in a dark room, with use of a chin rest to ensure a constant viewing distance; although unrealistic, these conditions were essential to produce consistent results.
To compute a display function, it is necessary to interpolate the JNDs, measured at specific background levels, with a continuous TVI function. For this purpose, Blackwell’s model was taken as a reference, because it is simple and provides good results in practice (the units are candelas per square meter):
The model of Ferwerda et al., instead, has a piecewise definition which makes it more difficult to handle. The analytical form was kept the same and the constants were adjusted by means of a maximum-likelihood fit to the experimental data. One important property is that the image obtained with a display function is not influenced by multiplicative scale factors in the display function itself or in the TVI function used to derive it, and this suggested a procedure to “average” the measurements of different observers: in the maximum-likelihood fit, a different leading scale factor was allowed for each observer, but the same value was enforced for all the other constants. The family of curves obtained with this procedure is as follows:
19.4.4 A Dual-Layer LCD Prototype
Barco demonstrated a prototype of a dual-layer HDR display (Albani et al., 2013); its schematic structure and a photograph are shown in Figs. 19.4 and 19.5.


The prototype is based on 19-inch SXGA (1280×1024) monochrome liquid crystal panels with an in-plane switching pixel design to ensure consistency of image quality over a wide viewing angle. As already mentioned, a dual-layer LCD requires a backlight solution with high brightness; to achieve this, a novel LED direct backlight with a high number of LEDs was designed. This is not common, for cost reasons, in conventional LCDs. The form factor of the LEDs and their matrix structure result in efficient heat sinking and a uniform temperature distribution, solving in this way the problem of the spatial nonuniformity of the output luminance evidenced in previous dual-layer LCDs using a backlight with multiple cold cathode fluorescent lamps.
Special care and dedicated tools were used to get a proper alignment of the two liquid crystal layers and reduce the parallax error; nevertheless, a small displacement between the two panels is unavoidable. The industrialization of the product will require minimization of the gap between the two liquid crystal layers by use of panels with thin glass. To avoid a visible change in luminance, it was necessary to blur the image that is displayed on the back panel, and to process the image on the front panel to compensate for this blurring (see Section 19.4.1).
Image processing in the dual-layer LCD requires a powerful computational platform to achieve real-time behavior; for this reason a graphics processing unit running on a proprietary graphics board was used (Szydzik et al., 2013). It creates the opportunity of using HDR displays in real-time medical procedures such as interventional X-ray.
In the prototype, a field-programmable gate array-based platform drives the hardware components of the dual-layer LCD (the two panels and the backlight controlling the color and light emitted by the LED matrix), while the image processing algorithm was implemented with OpenCL for multitask processing on a multicore architecture. The choice of the OpenCL implementation ensures a high level of hardware and platform independency, which provides flexibility toward future hardware platforms.
The performances of the dual-layer display were analyzed with the procedures described in Section 19.3, and satisfactory results were obtained: the black level was 0.007 cd/m2, the maximum luminance was 1000 cd/m2, and the static contrast was 140,000:1.
The prototype permitted the initiation of clinical validations of HDR visualization; a first observers-based study was performed and related to the comparison of the performance of low dynamic range and HDR displays in the detection of nodules in X-ray chest images. The study evidenced a shorter time for the execution of the diagnostic task when the HDR display was used (20.52 s per case on average for the HDR display, 23.32 s per case on average for the low dynamic range display) and a statistically significant difference (2.65%) in favor of the HDR display in the accuracy of the detection of nodules mainly with difficult or very difficult degree of detection (small size, very low contrast).
19.5 Conclusions
An HDR display is beneficial in all medical applications requiring the visualization of images with a wide range of gray levels. Examples are as follows:
• Analysis of chest radiographs: Chest radiography shows low-density details, such as nodules, blood vessels, or lesions in the soft tissues, which are located over a background that can contain very wide density variations because of the presence of the bones of the rib cage.
• Mammography-based diagnosis: This task involves the detection of masses and microcalcifications of very small size and low density; mammographic images are typically rich in details, with very fine luminance differences. Furthermore, the improved visibility of HDR displays in the darker areas of the image permits better delineation of the anatomical structures.
• Ophthalmologic applications: Tests performed in Trieste (Italy) evidenced how an HDR display allows better recognition of different retinal layers.
• Endoscopic and minimally invasive applications: Color and moving images are involved here; endoscopes currently available on the market already have HDR. In addition, often the light source of the endoscope generates a dark area around the light spot, and an HDR display will allow the detection of details there.
In all the above-mentioned applications the benefits of HDR displays will consist in improved detection accuracy and/or faster execution of search and decision tasks. Some first evidence was already gathered in studies reported in the literature and comparing low dynamic range and HDR displays (Badano et al., 2009).
Other critical issues need to be considered in the design of an HDR imaging chain for medical applications.
In the area of luminance mapping, future developments may involve the personalization of the display function for the individual observer, taking into account in particular the loss of visual acuity, especially in the dark portions, which occurs with aging. One difficulty comes from the length and complexity of the psychophysical experiments that are needed to build an individual vision model, which may render the observers unwilling to take the test. For this reason, faster but still accurate tests should be developed. A second issue concerns the influence of ambient light. It is well known that the ambient light reflected off the screen surface reduces the contrast of the visualized image, and recommendations exist which relate the display black level to the maximum allowable ambient light (American Association of Physicists in Medicine, 2005). However, for an HDR display, this would require a very dark reading room, which is impractical. Moreover, a bright display visualized in a dark room is fatiguing for the eye, and indeed some experiments showed an improved visibility (ie, a lower JND) with the lights on. For this reason, it is advisable to design reading rooms with an ambient light that illuminates the visual field around the display, in order to produce comfortable visual surroundings, without illuminating the surface of the displays (Xthona, 2003).
An additional problem is represented by the presence of very bright and very dark regions in the same image; this can introduce a further loss of detail visibility because of various causes:
• Veiling glare, which was described in Section 19.3.6 (Choi et al., 2012, 2014).
• The underadaptation condition: The DICOM GSDF and its alternatives are derived from JND models that measure the eye sensitivity by means of low-contrast test patterns under the assumption that the observer’s eye is adapted to a uniform luminance level; this kind of model does not describe accurately the perception of an image that contains wide luminance variations (Pattanaik et al., 2000).
• Different backgrounds generate different perceptions of the same gray value: areas shown on a black background are perceived to be lighter than the same observed areas on a bright background and a drastic reduction of the perceived luminance range is produced by the context (Rizzi and McCann, 2009).
Performance characterization merits more work too. First, further resolution and noise measurements need to be performed to determine the effect of different gray levels on the magnitude of display interpixel and intrapixel noise. In the same area, more work is needed to determine a metric for the influence of the parallax effect on resolution and noise in the context of a medical image where a set of edges can be superimposed on a slowly varying anatomical background field.
Finally, for a further large and long-term performance improvement, a technological discontinuity is needed: future HDR medical displays will probably use more efficient and chromatically richer quantum dot backlights, or they will switch from LCD technology to organic LED technology.