Removal of Surface Contaminants Using Ionic Liquids
Rajiv Kohli, The Aerospace Corporation, Houston, TX, USA
Outline
3.2. Abbreviations and Nomenclature
3.2.3. Alkyl Groups (Rn where n = 1, 2, 3, 4, …)
3.6. Solubility Considerations
3.7. Modeling and Predictions of Thermodynamic Properties
3.9. Electrical Conductance and High Vacuum Analytical Applications
4. Principles of Cleaning with ILs
5. Advantages and Disadvantages of ILs
6.5. Cleaning with ILs and Supercritical Gases
6.6. Cleaning of Oil-Contaminated Sands and Particulate Matter
6.7. Decontamination of Hazardous Materials
6.11.1. Hydrocarbon Production
1 Introduction
Conventional solvents, such as chlorinated compounds, hydrochlorofluorocarbons (HCFCs), trichloroethane and other ozone-depleting solvents (ODCs), are commonly used for industrial and precision cleaning in a variety of applications. Many of these solvents are deemed detrimental to the environment [1,2]. Concerns about ozone depletion, global warming, and air pollution have led to new regulations and mandates for the reduction in the use of these solvents. In fact, there is a specific schedule for the United States to phase out its production and consumption of HCFCs in accordance with the terms of the Montreal Protocol as shown in Table 1.1 [3]. The search for alternate cleaning methods to replace these solvents has led to the consideration of various alternative cleaning substances and technologies.
TABLE 1.1
Comparison of the Montreal Protocol and United States Phase Out Schedules for HCFCs

∗The cap for developed countries is set at 2.8% of that country’s 1989 chlorofluorocarbon consumption plus 100% of that country’s 1989 HCFC consumption.
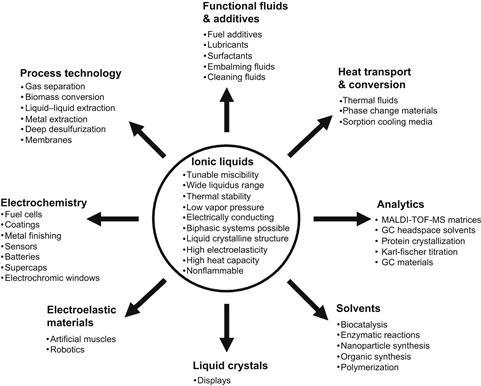
Ionic liquids (ILs)1 are a new class of materials with unusual and unique properties that make them attractive as process and performance chemicals for a wide range of applications [4–49]. These applications include electrodeposition, electrosynthesis, electrocatalysis, electrochemical capacitor, lubricants, embalming fluids, biocatalysis, plasticizers, solvents, lithium ion batteries, fuel cells, solvents to manufacture nanomaterials, extraction, gas absorption agents, energetic materials for propulsion, and other applications. Figure 1.1 summarizes important properties of ILs and their current and potential applications. Recently, cleaning applications have been proposed and demonstrated. The focus of this chapter is on developments in ILs for removal of surface contaminants.
2 Surface Cleanliness Levels
Surface contamination can be in many forms and may be present in a variety of states on the surface. The most common categories include the following:
Other contaminant categories include metals, toxic and hazardous chemicals, radioactive materials, and biological substances that are identified for surfaces employed in specific industries, such as semiconductor, metals processing, chemical production, nuclear industry, pharmaceutical manufacture, and food processing, handling, and delivery.
Common contamination sources can include machining oils and greases, hydraulic and cleaning fluids, adhesives, waxes, human contamination, and particulates. In addition, a whole host of other chemical contaminants from a variety of sources may soil a surface. Typical cleaning specifications are based on the amount of specific or characteristic contaminant remaining on the surface after it has been cleaned.
Most precision technology applications require characterization of particles, as well as nonvolatile residue (NVR). For example, civilian and defense space agencies in the United States (NASA, National Aeronautics and Space Administration; DoD, Department of Defense) and Europe (ESA, European Space Agency) specify surface cleanliness levels for space hardware in the microparticle size range [50,51]. The cleanliness levels are based on contamination levels established in the industry standard IEST-STD-CC1246D for particles from Level 1 to Level 1000 and for NVR from Level AA5 (10 ng/0.1 m2) to Level J (25 mg/0.1 m2) [52].
The cleanliness levels commonly used by NASA to specify particle and NVR contamination for space hardware are 50 A, 100 A and 300 A (A = 1 mg/0.1 m2) [50], although for other applications stricter cleanliness levels may be specified, such as Level 10 for particles and Level A/5 (200 µg/0.1 m2) or A/10 (100 µg/0.1 m2) for NVR [53]. In many other commercial applications, the precision cleanliness level is defined as an organic contaminant level less than 10 µg/cm2, although many applications are setting the requirement at 1 µg/cm2 [50]. These cleanliness levels are either very desirable or required by the function of parts such as metal devices, machined parts, electronic assemblies, optical and laser components, precision mechanical parts, and computer parts.
3 Ionic Liquids
There is a vast amount of published scientific literature on the synthesis, characterization, properties and applications of ILs, represented by Refs [4–49] and the citations therein. No attempt is made to review and summarize the extensive available information except as it relates to surface cleaning. In the following sections, we provide a brief overview of ILs and their characteristics conducive to surface cleaning.
3.1 Background
ILs refer here to purely ionic, salt-like materials that are in liquid form at unusually low temperatures. Broadly defined, ILs are compounds which are liquid below 373 K. More commonly, ILs have melting points below room temperature; some of them even have melting points below 273 K. In general, large and bulky organic cations are combined with weakly coordinating organic or inorganic anions with low-symmetry structures to form the IL. These factors tend to reduce the lattice energy of the crystalline form of the salt and prevent efficient ion lattice packing, resulting in weak coulombic interactions that lower the melting point to give room temperature liquids rather than high-melting solids. Upon mixing, these components turn into liquid at about 313 K or less, and the mixture behaves like an IL.
Some of these salts may have a nitrogen-containing aromatic moiety as the cationic component. Other salts may have a phosphorous-containing cationic component. Typical anionic components of these salts include, but are not limited to, methylsulfate, PF6−, BF4−, or halides. Table 1.2 lists the cations and anions that have been used to make ILs. Figure 1.2 shows the structure of more common cations and anions.
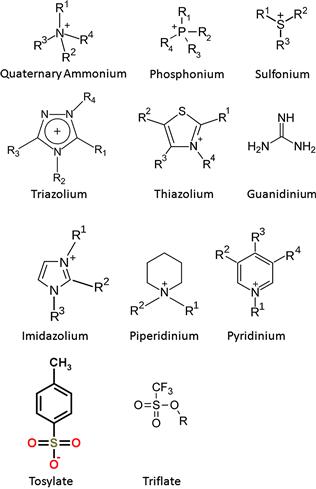
FIGURE 1.2 Structures of typical cations and anions used for making ionic liquids. R is an alkyl group or an aryl group.
The cation, anion, and alkyl chain moieties can be adjusted and mixed such that the desired solvating properties, viscosity, melting point, and other properties can be customized for the intended application. These customized ILs are often referred to as “designer solvents”.
The combination of a wide range of cations and anions leads to a very large number of possible single-component ILs that could be synthesized, with 1012 binary and 1018 ternary systems possible. In fact, the number is essentially infinite if we consider quaternary and higher combinations. In reality, the number is of the order of a few thousand ILs that have been described, of which a few hundred are commercially available from suppliers worldwide [54–67].
3.2 Abbreviations and Nomenclature
For the purposes of this chapter, the common cations and anions, and the side chain alkyl groups that constitute ILs are represented as follows [34,68].
3.2.2 Anions
• Halides: bromide, Br−; chloride, Cl−
• Alkylcarboxylate, [RCO2]−. Acetate [CH3CO2]− is written as [OAc]
• Trifluoromethanesulfonate, [CF3SO3]−: triflate is written as [OTf]
• Toluenesulfonate, [CH3C6H4SO3]−: tosylate is written as [OTs]
• Trifluoromethansulfonylimide, [N(SO2CF3)2]: triflimide is written as [NTf2]
3.2.3 Alkyl Groups (Rn where n = 1, 2, 3, 4, …)
Alkyl methyl IM is a very common cation and is written as [Cnmim] or [CnMIM] where n is the number of carbon atoms in the linear or branched, substituted or unsubstituted, alkyl, aryl, alkoxyalkyl, alkylenearyl hydroxyalkyl, or haloalkyl groups. As an example, 1-butyl-3-methyl imidazolium cation is denoted as [C4mim]+ or [BMIM]+. Similarly, [C6py]+ denotes 1-hexylpyridinium.
Quaternary ammonium compounds are derivatives of ammonium compounds in which all four of the hydrogens bonded to nitrogen have been replaced with hydrocarbyl groups. Here the symbol C denoting the carbon atom is replaced with N. For example, [N1,8,8,8]+ denotes the methyltrioctylammonium cation. Similarly, for tetraalkylphosphonium cations C is replaced with P as in, for example, [P2,2,2,1]+ which denotes the triethylmethylphosphonium cation. The free electron pairs of one of the two nitrogen atoms in the five-membered imidazoline ring and of the sole nitrogen atom in the five-membered pyrrolidine or six-membered pyridine ring are donated to univalent alkyl groups to produce an N+ cation.
Trifluoromethanesulfonate, also known by the trivial name triflate, is a functional group. The triflate group is usually represented by [OTf]. For example, 1-triazolium triflate is abbreviated as [Tz1][OTf].
Sulfonium compounds have the structure R3S+ cation and an associated anion. As an example, trimethylsulfonium bromide is written [(CH3)3S] Br. Generally, but not necessarily, all three R groups are hydrocarbyl.
Polyatomic anions are written with square brackets, but there are no brackets around monoatomic anions. For example, bromide is written as Br− while dicyanamide is written as [N(CN)2]− or abbreviated as [DCA] or [dca].
To represent ILs, the charge signs are deleted when a cation is paired with an anion. Thus, [C4mim][PF6] represents 1-butyl-3-methylimidiazolium hexafluorophosphate.
3.3 General Characteristics
Some of the properties that make ILs attractive alternatives to conventional solvents include the following key features.
1. ILs have a broad liquid range as low as 173 K to as high as 473 K. This feature permits effective process control over a wide range of applications.
2. With a few exceptions, ILs have no measurable vapor pressure; thus, they are easy to handle and they reduce safety concerns where volatility (pollution via an air pathway) could be an issue. This feature also enables vacuum applications.
3. ILs are effective solvents for a broad range of organic, inorganic, and organometallic materials due to their high polarity. This implies low volumes used in cleaning and other process applications.
4. ILs can be tuned to the specific application and chemistry desired. For example, they can be selectively made to have properties ranging from hydrophilic to hydrophobic.
5. ILs are effective Brønsted/Lewis/Franklin acids.
6. ILs are nonflammable below the decomposition temperature.
7. ILs exhibit high thermal stability. Decomposition temperatures above 573 K are not rare.
8. High electrical conductivity of ILs prevents electrostatic charging.
9. High stability of the ILs against oxidation and reduction can be realized.
Typical properties of ILs are compared with the properties of organic solvents in Table 1.3 [23].
TABLE 1.3
Comparison of Typical Characteristics of Representative Organic Solvents with Ionic Liquids

Not all ILs are actually liquid at room temperature as shown in Fig. 1.3 [69]. A solid IL, methyl-tri-n-butyl-ammonium dioctyl sulfosuccinate [MeBu3N][DOSS] with a melting point around 313 K is shown on the left, while on the right is 1-butyl-3-methyl imidazolium (diethylene glycol monomethyl ether) sulfate [BMIM][DEGMME SO4] which is a room temperature ionic liquid (RTIL). Figure 1.4 shows an RTIL, [BMIM][NTf2], compared with common table salt. Some ILs are colorless, while others are pale yellow to orange to dark amber, or they exhibit a rainbow of colors for metal-based ILs (Fig. 1.5) [70–84].

FIGURE 1.3 Physical appearance of ionic liquids. On the left is methyl-tri-n-butylammonium dioctyl sulfosuccinate with a melting point around 313 K. On the right is 1-butyl-3-methyl imidazolium (diethylene glycol monomethyl ether) sulfate which is liquid at room temperature [69]. A color version of this figure appears in the color plate section.

FIGURE 1.4 A room temperature ionic liquid compared with common table salt [47]. A color version of this figure appears in the color plate section.

FIGURE 1.5 Metal-based ionic liquids exhibit a wide range of colors. The liquids are from left to right: copper-based compound, cobalt-based compound, manganese-based compound, iron-based compound, nickel-based compound, and vanadium-based compound [84]. Source: Courtesy of Sandia National Laboratories, Albuquerque, NM. A color version of this figure appears in the color plate section.
ILs can be divided into two broad categories: aprotic ionic liquids (AILs) and protic ionic liquids (PILs). AILs generally consist solely of cations with substituents other than a proton (typically an alkyl group) at the site occupied by the proton in an analogous PIL, and an anion. Examples of AILs are [Cnmim][NTf2] and [Cn mpyrr][NTf2] families. PILs are produced by proton transfer from a Brønsted acid to a Brønsted base, and are capable of hydrogen bonding, including proton acceptance and proton donation [22,85]. Examples include imidazolium or alkylammonium cations combined with fluorinated or carboxylate anions [86]. There has been increasing interest in PILs for their beneficial characteristics, including low cost, simple synthesis and purification methods, low toxicity and high biodegradability, which tend to outweigh their potentially negative characteristics of nonnegligible vapor pressures and slightly lower conductivity than AILs. The first reported room temperature PIL was ethanolammonium nitrate (melting point of 325–328 K) in 1888 [87], followed by hydrazinium azide (melting point of 348 K) and several low melting organic salts in 1891 [88,89], and ethylammonium nitrate (melting point of 285 K) synthesized in 1914 [90].
The key to the unique properties and outstanding flexibility of PILs lies in their chemical nature. PILs are intermediate between fully ILs, such as the widely available dialkylimidazolium salts, and molecular liquids such as hydrocarbons, alcohols and water (Fig. 1.6).

FIGURE 1.6 Protic ionic liquids are located within a “solvent spectrum” from pure ionic liquids to molecular liquids.
PILs can vary enormously in terms of their properties depending on their position within this “solvent spectrum”. PILs formed from the reaction of a strong base with a strong acid are effectively entirely ionic in character and lie toward the right-hand side. These materials characteristically exhibit very low vapor pressures and high ionic conductivities. By contrast, PILs prepared from a weaker base and acid combination exist in a state of constant equilibrium between ionic and molecular species. These tend to exhibit much lower viscosities than fully ILs as well as appreciable vapor pressures and volatility.
The strong ionic (coulombic) interaction within ILs is intentionally weakened to given low melting liquids; nevertheless, these interactions remain strong enough to result in negligible vapor pressure at room temperature in these substances (unless decomposition occurs). The low vapor pressure makes them combustion-resistant, evaporation-proof, and nonflammable, resulting in a highly thermally, mechanically as well as electrochemically stable product, suitable for vacuum applications. In addition, ILs offer other favorable properties: unique and very appealing solvating properties by virtue of their high polarity and charge density, and their immiscibility with water or organic solvents that results in multiphasic systems.
The specific properties of an IL can be almost selected ad hoc, in order to have a compound with the most appropriate characteristics for a specific application. The choice of the cation has a strong impact on the physical properties (melting point, viscosity, conductivity, density, refractive index, etc.) of the IL and will often define its stability, while the chemistry and functionality of the IL is, in general, controlled by the choice of the anion. The anions and cations can be independently selected and combined to design and fine-tune the physicochemical properties of the IL, while at the same time introducing specific features such as controlling solute solubility, hydrophobicity vs hydrophilicity, and other functionalities for a given application. Thus, tailor-made IL materials and solutions are possible.
Although [Cnmim][PF6] and [Cnmim][BF4] were the first ILs with specific functionalities—so-called “designer solvents”—and are still dominant in many applications, they have a serious drawback. The [PF6]− and the [BF4]− anions will degrade in aqueous media, resulting in the formation of toxic and corrosive HF or fluorides. Several non-fluorine-containing ILs have been developed that are stable toward hydrolysis, and are better choices with respect to performance and handling. One example is methyltrioctylammonium thiosalicylate, [N1,8,8,8][TOS] with a melting point below 263 K that has been used to decontaminate various materials [91,92]. This compound is completely stable toward hydrolysis, it is not corrosive, and its constituent ions are nontoxic. Another example is 1,2,3-triazolium salts with various anions (tosylate, triflate, halides, etc.) that exhibit high thermal and chemical stability under alkaline conditions [93,94].
Many ILs are immiscible with water and also do not dissolve alkanes and heavy aromatic compounds. In this case, biphasic or triphasic solutions are formed with the IL (Fig. 1.7(a) and (b)). For example, [Cnmim][BF4] salts are miscible with water at room temperature for alkyl chain length less than six, but at or above six carbon atoms, they form a separate phase when mixed with water [4]. This behavior can be of substantial benefit when carrying out solvent extractions or product separations.
This multiphasic behavior has important implications for clean synthesis and for cleaning with supercritical fluids such as supercritical CO2 (SC-CO2) [96]. The relative solubilities of the ionic and extraction phases can be adjusted to make the separation as easy as possible. Furthermore, since the IL has effectively no vapor pressure and therefore cannot be lost, volatile products can be separated from the IL by distillation. SC-CO2 is largely soluble in most ILs, but the solubility of ILs in SC-CO2 is negligible [97–99]. The combination of the advantages of ILs and SC-CO2 is a new and interesting application for removal of contaminants. A recent example is the use of [BMIM][PF4] and SC-CO2 to clean soils contaminated with naphthalene [100,101].
3.4 Thermal Properties
A typical IL such as [EMIM][EtSO4] (m.p. 253 K), compared with a typical inorganic salt such as NaCl (m.p. 1074 K), has a significantly lower symmetry, making it more difficult to form a crystal [5]. Furthermore, the charge of the cation as well as the charge of the anion is distributed over a larger volume of the molecule by resonance. As a consequence, the solidification of the IL will take place at lower temperatures. A binary IL system may contain several different ionic species whose melting point and properties depend on the mole fractions of each component. For example, the melting point dependence on composition for the binary IL [EMIM]Cl-AlCl3 is shown in Fig. 1.8 [102,103]. More complex phase behavior has been observed in other IL systems such as [EMIM][Tf2]-AlCl3, alkyl IM, and alkylpyridinium chlorometallate systems [71,73,74,104–111].
By increasing the chain length, the lattice energy of the compound is further reduced and it lowers the melting point (Fig. 1.9). However, there is a maximum chain length before other forms of bonding begin to dominate and a glass transition is observed instead of a melting point (Figs 1.10 and 1.11). In fact, all the temperatures below 273 K are glass transition temperatures rather than true melting points.

FIGURE 1.9 Melting points for the [Cnmim]Cl ionic liquids as a function of the alkyl chain length. [5]
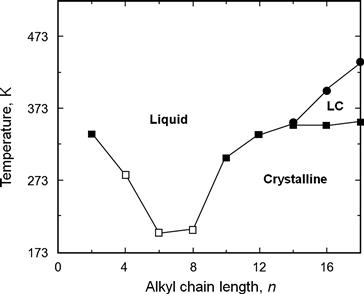
FIGURE 1.10 Melting point phase diagram for the [Cnmim][PF6] ionic liquids as a function of the alkyl chain length. The melting transitions are shown from the crystalline phase (closed squares), glassy materials (open squares) and the clearing transition (closed circles)2. LC is the liquid crystal state. [5]

FIGURE 1.11 Melting point phase diagram for the [Cnmim][BF4] ionic liquids as a function of the alkyl chain length. The melting transitions are shown from the crystalline phase (closed squares), glassy materials (open squares) and the clearing transition (closed circles). LC is the liquid crystal state. [5]
The effect of the anion on the melting point is significant. For example, changing from [C4mim]Cl to [C4mim][PF6] or [C4mim][BF4] can change the melting point from 353 K to 278 K or to 202 K, respectively (Figs 1.10 and 1.11), making these lower melting point liquids more fluid and easier to handle.
The lack of a boiling point means that many ILs are liquid over very wide temperature ranges from 300° to 400° from the melting point to the decomposition temperature of the IL.
3.5 Volatility
Volatility of ILs is a key characteristic in the application of ILs for removal of contaminants. Most AILs have nonnegligible vapor pressures and can be distilled under reduced pressures and moderate temperatures without decomposition [112–117], although the vapor pressure under ambient conditions is nonmeasurable [118]. For most processes operating at room temperature, the benefits of nonvolatility of the IL apply. The cohesive energy density of most ILs is very large at room temperature, which is the reason for their low volatility under ambient conditions [119–124]. For higher temperature operations, volatility is a nontrivial consideration and the vapor pressure of the IL is required. Several measurements of the vapor pressure and the enthalpy of vaporization have been reported [38,118,125–140] and various models and methods have been used with varying degrees of success to correlate the data and to predict the vaporization enthalpy [118,129,131,136–149]. Most of the theoretical models are based on atomistic simulations which allow analysis of different contributions to the vaporization enthalpy and also enable parameterization of new force fields [118,144–149]. Recently, quantum chemical methods, such as COnductor-like Screening MOdel for Real Solvents (COSMO-RS), have been successfully employed to obtain reliable predictions of the enthalpy of vaporization of a few imidazolium-based ILs [150], but these calculations are time-consuming and expensive, and usually not straightforward [118]. Even so, the available data are very limited and predictions based on atomistic simulations for new ILs may not be sufficiently validated with experimental data.
3.6 Solubility Considerations
Many organic, inorganic and organometallic materials exhibit high solubility in ILs, which is the basic principle of removal of surface contaminants by ILs. An effective screening tool for selecting the most preferable solvent is the solubility parameter which works by the rule of thumb of “like dissolves like” [151]. Thus, the smaller the difference in solubility parameters between the solute and solvent, the higher the solubility of the solute in the solvent. Thermodynamically, in order for the solvent to be effective in dissolving the solute, the total Gibbs free energy of mixing, ΔGmix, must be zero or negative.
![]() (1.1)
(1.1)
Here ΔHmix, ΔSmix, and T are the enthalpy of mixing, entropy of mixing, and the absolute temperature, respectively. The solubility parameter, δ, is related to the enthalpy of mixing by the following relationship.
![]() (1.2)
(1.2)
Here v, X, and ϕ are the molar volume, mole fraction, and volume fraction, respectively; the subscripts 1 and 2 refer, respectively, to the solute and solvent in the mixture.
The solubility parameter is, in turn, defined as the square root of the cohesive energy density, ED, which is related to the enthalpy of vaporization, ![]() , by Eqn (1.3).
, by Eqn (1.3).
![]() (1.3)
(1.3)
where R is the gas constant.
The δ values for volatile solvents can be obtained directly from ![]() or from vapor pressure–temperature data. However, the extremely low vapor pressure of ILs makes it difficult to experimentally measure the
or from vapor pressure–temperature data. However, the extremely low vapor pressure of ILs makes it difficult to experimentally measure the ![]() values. Thus, both indirect and direct methods have been used for the estimation of solubility parameters from experimental data, including calorimetry, melting temperatures of ILs, inverse gas chromatography, intrinsic viscosity measurements, the activation energy of viscosity, and surface tension measurements [120,121,123,124,152–163].
values. Thus, both indirect and direct methods have been used for the estimation of solubility parameters from experimental data, including calorimetry, melting temperatures of ILs, inverse gas chromatography, intrinsic viscosity measurements, the activation energy of viscosity, and surface tension measurements [120,121,123,124,152–163].
Various theoretical methods have also been employed to estimate the δ values for ILs, including the Kamlet–Taft equation, nonrandom hydrogen bonding models, statistical associating fluid theory (SAFT), regular solution theory, lattice energy density, molecular dynamics simulations, and group contribution methods [119,144–148,156–166]. Good agreement can be found with the data even though different methods were used to determine the solubility parameters [162,164,165]. However, the available experimental database is still limited to validate estimations of the δ values for new or improved ILs. A shortcoming of most theoretical models is the limited capability to account for strong long-range and directional interactions such as coulombic and hydrogen bonding which can influence the entropy of mixing through various orientational degrees of freedom. However, molecular dynamics simulations can account for most interactions, and have been applied successfully to a few IL systems, including imidazolium, pyridinium, ammonium, phosphonium and guanidinium cations and fluorophosphates, fluoroborate, triflate, and fluoroacetate anions. Still, the best and most accurate predictions of the solubility parameters have been obtained by trial and error [164,167].
3.7 Modeling and Predictions of Thermodynamic Properties
Given the large number of ILs that can be formed, accurate models for the appropriate description of thermodynamic properties of ILs are needed for engineering process applications, in particular the solubility of gases such as CO2 for cleaning applications. This is important since the solubility of CO2 in ILs can vary from very low to more than 80% by mole fraction [168,169].
A rigorous molecular thermodynamic model must take into account all the interactions in the system, including hydrogen bonding, electrostatic attraction, ionic and polar interactions, as well as hard chain repulsion and dispersion–attraction forces. For associating and polar molecules, the dimensionless residual Helmholtz energy Ares of the system is composed of the sum of the individual contributions of these interactions:
![]() (1.4)
(1.4)
where the superscripts denote, respectively, the hard chain contribution; the association term due to hydrogen bonding among polar molecules and electrostatic interactions between solvent and solute molecules; coulombic ion–ion interaction; the dispersion term; and the polar term. Each term in Eqn (1.4) is represented by one or more parameters characteristic of each pure component.
Several different theoretical and empirical approaches, correlations and equations of state (EoS) have been used to reproduce experimental data and to predict the thermodynamic properties of new IL systems [144–149,152–162,165,166,170–178]. These approaches include classical cubic EoS; activity coefficient models; simple and elaborate group contribution methods; square-well chain fluids EoS; lattice models; various SAFT-based models (Soft SAFT, Perturbed Chain (PC)-SAFT, variable potential range SAFT, truncated PC-SAFT (tPC-SAFT), electrolyte (ePC-SAFT), and heterosegmented SAFT). More sophisticated computational techniques include molecular dynamics and Monte Carlo simulations, as well as quantum chemistry calculations (COSMO-RS). The latter approaches are based on well-established theoretical foundations and provide realistic molecular models for pure ILs and their mixtures, and have been applied successfully to several IL systems [144–149,170,172–175]. However, their use requires a lot of experience with specialized software, and the calculations are time-consuming, and expensive. For practical applications of these modeling approaches, tradeoffs must be made among simplicity, accuracy, reliability, time and cost considerations. A simpler approach, such as an EoS, may be warranted, although the lack of a sufficiently large database of measured data tends to limit the usefulness of the EoS approach to reliably predict the thermodynamic properties for new and improved ILs. On the other hand, SAFT-based models have a physical basis for modeling the IL molecules and they fulfill most of the requirements. These models have been the most prevalent among the different modeling approaches to represent gas solubilities in IL systems [98,99,179–189]. Successful examples of their application include the solubility of CO2, CO, O2, CHF3, H2S, SO2 and NH3 in imidazolium-based ILs, H2 and Xe solubility in [Tf2N]-imidazolium-based ILs, density and molar volumes of [BF4]−, [PF6]−, [NO3]−, and [Tf2N]-imidazolium-based ILs, CO2 solubility and binary vapor–liquid, liquid–liquid, and solid–liquid equilibria in other homologous series of ILs [166,169,172,176–178].
3.8 Viscosity
Generally, ILs are much more viscous than conventional organic solvents, and the viscosity values of most ILs are 2–3 orders of magnitude larger than even heavy organic solvents. For example, the viscosity is only 0.6076 mPa.s for benzene [190], 0.575 mPa.s for toluene [191], 0.42 mPa.s for methyl ethyl ketone [190], or 0.894 mPa.s for cyclohexane [192] at room temperature, whereas it is 70 mPa.s for 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [193–195] and even as high as 2945 mPa.s and 5647 mPa.s for ethylenediamine di-n-butylphosphate and diethanolamine acetate, respectively [196]. For most cleaning applications with ILs, it is desirable to have low viscosities from a process perspective including reduced power requirements, ease of handling and disposal (dissolution, decantation, filtration, and separation), and high heat and/or mass transfer rates.
For a given cation, the viscosity of the RTILs is strongly determined by the nature of the anion. The viscosity is the lowest for RTILs containing the large [NTf2]− anion and the highest for RTILs containing nonplanar highly symmetric or nearly spherical anions. The most viscous ILs are the [PF6]−-containing salts. In addition, increasing the length of alkyl chains results in higher viscosities because of stronger van der Waals interactions between the larger cations. A significant decrease of viscosity of ILs is generally observed as the temperature increases, represented typically by an Arrhenius-type relationship. The experimental and theoretical information on the viscosity of ILs has been recently reviewed in several publications [20,22,144,147–149,197–204].
The viscosity of ILs is highly sensitive to trace amounts of water and other impurities; even small concentrations of impurities can have a large impact on the viscosity [204–214]. For example, the viscosity of [P6,6,6,14][dca] with water content of 300 ppm is around 378 mPa.s compared with around 379 mPa.s for a sample with 249 ppm water [204], indicating that a small increase of 50 ppm water could reduce the viscosity by about 0.4%. The decrease in viscosity is much greater for higher water contents. For example, the viscosity of [C4mim][NTf2] with water content of 19 ppm is 51 mPa.s [211], whereas it is 27 mPa.s with a high water content of 3280 ppm [206], a reduction of nearly 90%. In a few ILs, however, water (5–40% w/w) can increase the viscosity resulting in gel formation in [Cnmim]+ cations (n = 8 or 10) with [BF4]−, halide, nitrate, and other anions [215,216]. This is one reason why it is critical to use high-purity starting materials for making ILs and for their use in cleaning and other process applications. In addition, it is also desirable to develop methods for separation and purification of ILs for recycling and reuse.
Some applications, such as electropolishing, require high ionic conductivities. The high viscosity of the RTILs has a major impact on their conductivities because conductivity is inversely linked to the viscosity. Increasing the length of the alkyl chains generally results in higher viscosity and lower conductivity. The Walden plot of the equivalent molar conductivity (Λ) against the log of the fluidity (inverse viscosity, η−1) is a qualitative way to represent the iconicity of the ILs [14,22,121,199,201,217–224]. Figure 1.12 shows a Walden plot for a series of AILs and for lithium ILs for comparison [221]. The straight line represents the ideal Walden rule, Λη = constant, and is calibrated using the data for a 0.01 M KCl aqueous solution, where the ions are known to be fully dissociated and to have equal mobility. However, the average slope of the “ideal” KCl line may not be unity, but is closer 0.87 [222]. Deviations from the reference line in the Walden plot have been used to classify specific ILs as either good or poor ILs, or as nonionic (molecular) liquids [14]. Those ILs having good ionicity are likely to have other related good properties, such as high ionic conductivity. The viscosity and conductivity are often reflected in the glass transition temperature with low values leading to other favorable properties [22]. This can be achieved by modification of the cationic or anionic component of the IL, such as reducing the size of the cation or increasing its symmetry.
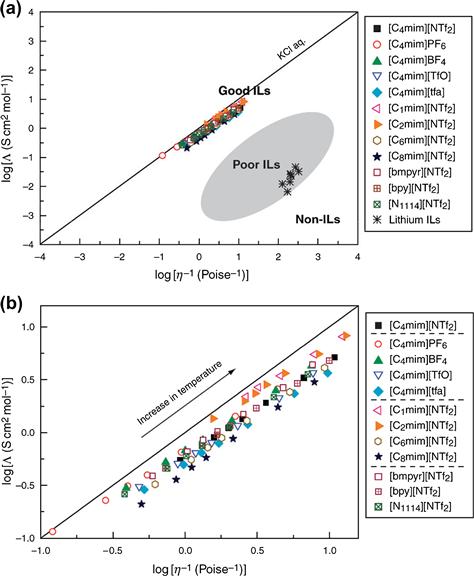
FIGURE 1.12 Walden plot of log (molar conductivity, Λ) against log (reciprocal viscosity η−1) for ionic liquids. The upper figure (a) includes a classification of good and poor ionic liquids, as well as nonionic liquids [14]. The lower figure (b) is a close-up view of the region occupied by typical aprotic ionic liquids. The solid line indicates the ideal line for a completely dissociated strong electrolyte aqueous solution (KCl aq.) [221]. A color version of this figure appears in the color plate section.
3.9 Electrical Conductance and High Vacuum Analytical Applications
ILs can behave as electrically conducting materials. This characteristic along with their extremely low volatility has application in analyses under vacuum conditions. Surface analysis techniques are routinely used to characterize contaminants and the cleanliness of surfaces in precision cleaning applications [225].
3.9.1 Electron Microscopy
Electron beam techniques, such as scanning electron microscopy (SEM) and transmission electron microscopy (TEM), require high vacuum conditions, and often the samples are insulating, or they may be liquid-containing environmental samples. The first direct SEM observation of an IL was a drop of [BMIM][PF6] (Fig. 1.13) [226]. The dark contrast image indicates that the IL behaves as an electrically conducting material. Furthermore, the IL shows no evidence of evaporation and maintains its liquid droplet shape under high vacuum conditions and electron beam irradiation. These characteristics are very useful in making insulating materials electrically conducting by coating the specimen with the IL [226,227], or for preparing biological contaminant samples by rehydrating them with ILs [228–232]. Figure 1.14 shows the SEM image of a grain of star sand shell (a type of foraminifer) which was dipped in [EMIM][TFSI] (left image), compared with an untreated grain (right image) [226]. The porous shell absorbs and retains the viscous IL. The difference between the images is obvious: the IL-treated grain is a clear image showing details of the surface, whereas the untreated grain gives a highly charged white image due to accumulation of electron charges. Similar experiments have been conducted on different IL-treated materials in a TEM instrument [32,233–237].

FIGURE 1.13 SEM image of drops of ionic liquid [BMIM][PF6]. [226]

FIGURE 1.14 SEM images of two grains of star sand shell. Left grain was dipped in [EMIM][TFSI] and right one was subject to no treatment before observation. [226]
3.9.2 Surface Analysis
Surface analysis techniques, such as Auger electron spectroscopy, time-of-flight secondary ion mass spectrometry (TOF-SIMS) and X-ray photoelectron spectroscopy (XPS), can provide detailed information on the chemical structure of surface contaminants [225], but the analysis is performed under high vacuum which is not conducive to wet or liquid samples. The negligible vapor pressure of ILs makes them suitable for investigation by ultrahigh vacuum techniques. A number of studies have been reported using individual and combined surface analysis techniques to investigate ILs [32,238–249]. A major advantage of these techniques, XPS in particular, is that elemental identification is possible and can be used to detect the presence of surface-active contaminants, such as silicone, as well as contaminants that may be dissolved in the IL. Since ILs can be distilled under reduced vacuum (Section 3.5), ultrahigh vacuum technique with line of sight mass spectrometry enables determination of the enthalpy of vaporization of ILs [114,138]. Recently, TOF-SIMS experiments have revealed that a charge pattern could be created on a frozen IL sample surface, and the pattern could be erased by melting the sample [244]. This observation may be applicable to a rewritable data storage system.
Scanning probe techniques are also increasingly being used to investigate various surface and interface phenomena in ILs. Techniques such as atomic force microscopy (AFM) and scanning tunneling microscopy have been successfully applied to probe the structure and image the surfaces in IL/solid systems with atomic level resolution [250–257]. The use of AFM has recently proved to be of major importance in monitoring and characterizing the effectiveness of new enzyme and IL ([BMIM][BF4] and [EMIM][EtSO4]) formulations for removing protein-based materials from painted and polychrome works of art [257]. This is a demonstration that AFM monitoring protocol can be applied to everyday situations in conservation practice.
3.10 Toxicity Concerns
Toxicity is a major consideration in commercial applications of ILs. If ILs are to be employed on a wide scale in industrial applications in place of volatile organic solvents for removal of surface contaminants, they should possess broader “green” properties including low toxicity and biodegradability. One of the appealing characteristics of ILs for cleaning applications is their nontoxicity in the air environment. However, this is limited mainly to their low vapor pressure under ambient conditions (Section 3.5) which can reduce the risk of air pollution through evaporation or sublimation. On the other hand, most ILs do have finite solubility in water [258–266], and could be released into the aquatic environment via this path through accidental spills, effluents, and other such mechanisms, as well as microbial degradation, sorption and desorption, and similar mechanisms in the terrestrial environment [267–277]. Many of the precursors to ILs are toxic and environmentally hazardous, and many of the more common ILs have toxicities that vary considerably across organisms and trophic levels [37,267–277]. The toxicity does depend on the specific cations and anions. For example, [EMIM]Cl is nontoxic, whereas a close derivative [BMIM]Cl is toxic [278]. This has spurred the development of degradable and biorenewable ILs, as well as the development of many approaches for recycling and recovery of ILs, effluent and wastewater treatments, and other related processes [270,272].
As noted above, many studies have been conducted to assess the toxicity and biodegradation of ILs. However, given the nearly infinite number of possible IL systems and the varied ecosystems, it is infeasible to adequately assess the toxicity of all untested ILs [272]. The current assessment method, quantitative structure–activity relationships (QSARs), is labor-intensive and time-consuming. To overcome this limitation, there has been growing effort to develop QSAR-based models to predict the toxicity of unknown ILs [261,272,279–281]. Recently, a new approach has been devised to screen the toxicity of unknown ILs toward Vibrio fischeri, a standard bacterial assay for IL toxicity [282]. This method uses toxicity data of 30 known anions and 64 known cations and the toxicity of toluene or chloroform as a threshold value and applies partial least squares-discriminant analysis (PLS-DA) to screen the 1920 ILs that can be formed by their combinations for ecological toxicity. The accuracy of the model was validated with a test set of 147 samples and achieved a nonerror rate of 93%. The successful results suggest that this model can be used as a screening tool to assist the design of aquatic environmentally friendly ILs and also in development of prediction methods for other organisms and environments.
A simplified approach to visualizing the data from various toxicological tests on ILs has been proposed [283]. In this method, data from each test are given a score ranging from 1 for nontoxic to 3 for very toxic. The scores from the different toxicity tests are then combined to give an overall score for the IL structure. The combined scores are used to visually represent the ILs using a dendrogram (tree diagram) where a score of 1 (nontoxic) is green, 2 (intermediate toxicity) is yellow or amber and 3 (toxic) is red. Each IL is color coded according to its toxicity score and arranged by cation class with increasing alkyl chain length, and by the type of anion. Figure 1.15 shows toxicity dendrograms for selected imidazolium and ammonium-based ILs [276,283]. The dendrogram enables easy visualization of the data to identify toxicity trends and select (nontoxic biocompatible) or rule out (too toxic) ILs for further evaluation.
3.11 Data Compilations
As noted in the previous sections, there is a large body of published experimental data on the thermodynamic and thermophysical properties of ILs. And a variety of empirical and physical correlations and models have been developed to reproduce and predict these properties. Several compilations of the properties of ILs have been published in review articles and in printed books [147,202,284–286], but many of them are for specific properties or for families of ILs. There are a few general databases, such as Reaxys [287] and SciFinder [288], or handbooks, such as Beilstein [289] and Gmelin [290], where a wide range of properties of ILs can be found among a vast array of chemical reaction and substance information in inorganic, organometallic and organic chemistry research. There are also IL databases developed by commercial companies such as Merck, Ionic Liquids Technologies (IoLiTec), and Sigma–Aldrich but these are not complete and most of them are for products of the company; some of these databases are not available anymore. Nearly 2000 ILs are included in these compilations for which 29 different physicochemical properties are collected. A large amount of data on the properties of ILs is now contained in web-based searchable comprehensive databases maintained at the National Institute of Science and Technology, Boulder, Colorado, USA (212 ions and 339 ILs as of 2010) [291] and at the Dortmund Data Bank, Dortmund, Germany (766 ILs as of 2011) [292]. Delph-IL is a new web-based IL property database that contains data and synthesis procedures for more than 1000 ILs and it is continuously updated with new property data [293]. One shortcoming of these databases is limited information on IL purity, experimental or simulated determination method, or the source of the ILs (purchased or synthesized). These factors can significantly impact their thermophysical and thermodynamic properties.
Toxicity information on more than 600 ILs is available in the UFT/Merck IL database maintained at the University of Bremen in Bremen, Germany [278].
3.12 Deep Eutectic Solvents
Deep eutectic solvents (DES) are a new generation of solvents that can offset the major drawbacks of common ILs, namely high toxicity, nonbiodegradability, complex synthesis requiring purification, and high cost of the starting materials [294–307]. DES are derived simply by mixing together two safe components (cheap, renewable and biodegradable), which are capable of forming an eutectic mixture. The term DES has been coined mainly to differentiate them from true ILs, and also to reflect the large depression of several hundred degrees in the freezing point of the eutectic mixture (Table 1.4).
TABLE 1.4
Molar Ratio and Temperature at the Eutectic Point in Several Choline Chloride-Based Deep Eutectic Solvents
| Deep Eutectic Solvent | Molar Ratio | Eutectic Temperature, K |
| Choline chloride with urea (Reline) | 1:2 | 285 |
| Choline chloride with ethylene glycol (Ethaline) | 1:2 | 373 |
| Choline chloride with glycerol (Glyceline) | 1:2 | 327 |
| Choline chloride with phenylacetic acid | 1:2 | 298 |
| Choline chloride with citric acid | 1:1 | 342 |
| Choline chloride with succinic acid | 1:1 | 344 |
| Choline chloride with malonic acid (Maline) | 1:1 | 283 |
| Choline chloride with oxalic acid (Oxaline) | 1:1 | 307 |
| Choline chloride with m-cresol | 1:2 | 238 |
| Choline chloride with p-cresol | 1:2 | 263 |
| Choline chloride with phenol | 1:2 | 243 |
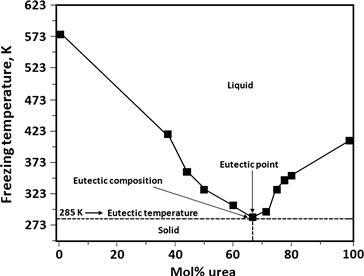
The freezing points of the DES are also affected by the organic salt (ammonium or phosphonium salts). For example, urea mixed with ammonium salts in a molar ratio of 2:1 (urea:salt) results in freezing points ranging from 235 K to 386 K for the corresponding DES [307]. The deep freezing point depression of DES is illustrated by the phase diagram of urea–choline chloride system (Fig. 1.16). The melting point of choline chloride is 575 K and of urea is 406 K. But when they are mixed together in a 2:1 (urea:choline chloride) molar ratio, they form an eutectic mixture at 285 K. Typically, the freezing point of a urea–choline salt-derived DES decreases in the order F− > [NO3]− > Cl− > [BF4]−, suggesting a correlation with the hydrogen bond strength [307].
For their formation DES need a hydrogen bond donor, such as urea, glycerols, renewable carboxylic acids (for example, malonic, oxalic, citric, succinic or amino acids) or renewable polyols (for example, glycerol, and carbohydrates), in addition to anions and cations. The hydrogen bond donor results in a weaker anion/cation interaction, thereby achieving the low melting temperatures of the DES. One of the most common cations is choline chloride, which is a cheap, biodegradable and nontoxic quaternary ammonium salt. Although DES are made up of cations and anions, they can also be obtained from nonionic species, and are not considered true ILs. Table 1.5 compares the general characteristics of DES with traditional ILs.
TABLE 1.5
Comparison of the Characteristics of Ionic Liquids with Deep Eutectic Solvents
| Ionic Liquids | Deep Eutectic Solvents |
| Low-melting point ionic compounds | Low-melting eutectic mixture of compounds |
| Not always environmental friendly—can be toxic | Biodegradable and nontoxic starting materials |
| Solution conductivity—moderate to high | Highly conductive |
| Expensive—recycling is critical | Cheaper than ILs |
| Highly viscous | Viscosity can be lowered by mixing with suitable ionic solvents |
| Complex synthesis and purification required | Simple synthesis by mixing inexpensive starting components and no subsequent purification required |
| Moisture-sensitive ILs must be handled under dry or inert conditions | Generally not moisture-sensitive and convenient storage |
The physicochemical properties (density, viscosity, refractive index, conductivity, surface tension, chemical inertness, etc.) of DES, particularly choline chloride-based solvents, are similar to traditional IM-based ILs, and they can be attractive substitutes in many applications. As discussed in Section 6, DES have recently been successfully employed for back-end-of-the-line (BEOL) cleaning in semiconductor applications [308–312] and for other cleaning applications [313,314].
4 Principles of Cleaning with ILs
4.1 Basic Principles
For cleaning applications, the key attributes of ILs are thermal and chemical stability, melting point, viscosity, solubility, biodegradability, and toxicity, all of which offer advantages compared with other volatile solutions and organic solvents. Their solubility in other solvents and their viscosities can be modified by suitable cation/anion combinations. Solubility control of the ILs is important for the cleaning process, allowing the choice of the specific solvent for removing the contaminant and the IL from the surface. Controlling their viscosity is also important. For example, more viscous ILs will be less penetrative and less disruptive of weathering products during cleaning of sensitive surfaces, such as stained glass in historically significant medieval structures.
One concern regarding the more common ILs is that their balance of intermolecular forces does not match well with the target contaminants in industrial cleaning, making ILs less useful as cleaning solvents [315]. However, this limitation can be overcome by suitable combinations of cations and anions to yield ILs designed for the specific contaminant of interest. Unfortunately, this design feature, in itself, may be a limitation due to limited availability, high cost of synthesis, and lack of toxicological and biodegradability information for new formulations.
4.2 Cost Considerations
Cost is an issue central to any commercial use of ILs [33,62,316,317]. The major cost component in cleaning with ILs is the cost of the IL itself. Pure ILs as available today are manufactured only in kilogram quantities and are not cheap. This high cost stems from (1) the high cost of the components and (2) purification required in the synthesis. Materials to make the dialkylimidazolium cation and fluorine-containing anions are expensive. The price is also influenced by specific requirements on the specification parameters for a particular IL formulation if this makes additional manufacturing steps necessary. Realistically, ILs may never be cheap when compared with other common organic solvents. However, in any cost analysis of a cleaning process the purchase price is only one component. With increasing regulation of volatile organic solvents, ILs with their negligible volatility offer definite cost advantages. As an example, most common solvents such as acetonitrile or benzene can be purchased for less than $1 per kilogram in bulk. Although ILs are unlikely to approach $1 per kilogram, it is reasonable to expect based on economies of scale that price targets of $10–20 per kilogram level could be achieved. This expectation is based on initial production runs carried out at a commercial producer BASF (Ludwigshafen, Germany) on a ton scale.
In general, there is little difference in prices between various ILs. Based on internal access to a very broad range of IL precursors and backward process integration, BASF has been able to perform cost calculations with a high degree of accuracy, indicating only small differences between imidazolium salts and pyridinium, pyrrolidinium, ammonium, and phosphonium salts [61]. The technical performance of the IL will always decide which compound is best suited for the application.
What this means is that ILs would have to be used in applications where they can be recovered and reused. Used solvents are rarely disposed if they have commercial value. For high-priced ILs, recovery and reuse will be essential to economic viability of the process.
Equipment costs for IL cleaning applications are expected to be low, since the ILs are generally used as replacements for existing cleaning solutions or they are additives to the cleaning solutions. New or additional equipment should not be required for an existing process. The chemical inertness of ILs toward most materials means that equipment costs will be comparable to a conventional process even for a new dedicated IL process application. The cost of more conventional solvent process applications also reflects the expenses and capital costs for personnel protection and emission control hardware and monitoring equipment, which may not be required for IL applications. Even if required, they will not be added costs if existing equipment can be used.
The relatively benign nature of ILs suggests that operating costs will be generally low. Most cleaning applications can be performed at ambient temperature and pressure, so energy costs should be low. The major component of the operating cost is IL purification for reuse.
Waste disposal costs for cleaning with ILs are likely to be lower than other solvent cleaning technologies since the waste residue is 100% contaminant. The IL can be recovered and recycled. And if the contaminant can also be recovered, recycled, or reclaimed, there is no cost associated with disposal of waste.
For many cleaning applications, the IL is applied in diluted form. Thus, the incremental cost of the IL itself may not be significant if higher cleaning efficiencies are achieved than competing cleaning processes. On the other hand, undiluted IL used as the cleaning medium will be significantly more expensive in terms of material costs to render the IL cleaning application uncompetitive with other solvent cleaning processes.
The high costs of ILs may be reduced to be competitive with other solvents if DES can be used as an effective replacement for a given cleaning application. As mentioned in Section 3.12, DES can be produced in ton quantities from inexpensive precursors. Also, DES do not require purification, since preparation requires only stirring the components with gentle warming. The purity of the starting materials determines the final purity.
The very limited number of cleaning applications demonstrated with ILs and DES makes it difficult to perform meaningful life cycle cost assessments and comparisons with other solvent cleaning processes at this time. One direct cost comparison has been performed with a water-based cleaning system processing 14,700 kg garments per month [308].
• Water-based system: $0.074/kg per month, which includes costs for water (10%), energy (44%) and cleaning chemicals (46%).
• IL-based system: $0.061/kg per month, which includes costs for the IL solvent (32%), energy (13%) and cleaning chemicals (55%).
The initial high cost of the IL solvent is ameliorated because the solvent is recovered and recycled. Energy costs are lower because no drying is required. Cost of cleaning chemicals is assumed to be the same as in the water-based system, but smaller quantities of chemicals need to be added since the solvent is recycled. In reality the cost of chemicals will be lower. This comparison shows IL-based cleaning processes can offer competitive advantages compared with conventional solvent-based processes.
5 Advantages and Disadvantages of ILs
ILs are being considered and employed for cleaning and related applications. The advantages and disadvantages of ILs for these applications are listed here.
5.1 Advantages
1. ILs offer tunable physicochemical properties for adaptability for a given cleaning application.
2. ILs have high solubility for a very wide range of target contaminants, including organic, inorganic and microbial substances, as well as biomaterials.
3. The tunable properties and high solubility of ILs allow for process intensification and improved cleaning regimes by using low liquid volumes to achieve a given cleanliness level.
4. Chemical inertness toward plant infrastructure makes ILs easy replacements for conventional solvents in existing processes.
5. ILs are largely nonflammable, which is a significant safety advantage in cleaning.
6. Contaminants are the sole waste product. Hence, the waste disposal costs are low. In fact, waste disposal costs may be eliminated if the contaminants can be recovered, reclaimed or recycled.
7. The ILs can be recovered and reused, thus offsetting their high costs.
8. This is a noncorrosive, environmentally friendly process. No hazardous wastes and emissions are generated. ILs and DES can be tuned to be compatible with virtually all materials.
9. Dissolution kinetics is high and cleaning process times are relatively short with ILs, which leads to reduced process operating costs.
10. Energy consumption is expected to be low since there is no heat input to the cleaning process.
11. Cleaning with ILs and DES can be performed at ambient temperatures and pressures which is a significant advantage for cleaning temperature-sensitive parts.
5.2 Disadvantages
1. ILs are expensive, but the prices will be lower as demand increase for large quantities. The high cost makes recovery an economic imperative. The material cost can be offset if cheaper DES of equivalent cleaning effectiveness can be substituted.
3. Process complexity is high especially if the IL formulation has to be tailored for unknown contaminants. This also requires high level of technical skill.
4. New formulations must be tested to generate information on the chemical and physical properties, biodegradability, and toxicity of the ILs.
5. Many ILs are toxic and require special handling for operation and disposal. Recovery and reuse are also an environmental imperative.
6. There is a very small experience base for cleaning applications with ILs, so overall process operating and life cycle costs are difficult to assess at this time.
7. The cost of purification of used solvents must be considered for recycling and reuse of the IL.
6 Applications
ILs and DES have only recently been considered for cleaning applications. Several IL and DES formulations have been proposed and developed for cleaning applications [318–330]. Some examples of IL and DES cleaning applications are discussed below.
6.1 Semiconductor Cleaning
Semiconductor device fabrication involves etching of the photoresist layer to obtain the necessary features on the substrate. Conventional cleaning methods employ strong reactive chemicals to remove the post-etch residues, followed by multiple process steps for rinsing and drying the parts. This is not an environmentally friendly process, requiring large volumes of toxic and corrosive chemicals that must be disposed after use. A new method of cleaning semiconductor substrates has been proposed that overcomes these disadvantages [331]. It uses IL-based cleaning solution compositions for stripping photoresists and cleaning organic and inorganic compounds, including post-etch and post-ash residues, from substrate. High solubility of these residues in ILs allows for process intensification, since the low liquid volumes permit substantial reduction in the amount of chemicals required to achieve the specified cleanliness levels. The cleaning method involves contacting (by immersion or spraying) the contaminated surface of a semiconductor substrate, in the form of a wafer or an integrated circuit, with an IL consisting of an imidazolium, pyridinium, pyrrolidinium, ammonium or phosphonium cation with various halides ([Cl]−), organic ([OTf], and [OTs]), and inorganic ([BF4] and [PF6]) anions. Exposure times are as short as 30 s to 30 min at 293–343 K, depending on the composition of the cleaning solution. The IL can be used undiluted or it can be diluted with other polar solvents. The process results in trace residual contamination below 4 nm and <50 ppb. Because the chemical concentrations and application time can be significantly reduced, more aggressive chemistries can be used for precise process control, resulting in reduced chemical consumption, application of new chemistries for newer semiconductor materials, and significantly reduce or eliminate certain final rinsing and drying steps. The process and chemistries may also have application in nanotechnology device fabrication and in the biotechnology sector.
Recently, DES have been employed for removing post-etch residues formed by CF4/O2 etching of resist films on copper in BEOL cleaning in semiconductor processing [309,311,312]. Eutectic mixtures of urea–choline chloride and choline chloride–malonic acid were effective in removing residue films at rates of ∼11–17 Å/min and ∼30 Å/min, respectively, by immersion cleaning in the temperature range 313–343 K. The higher rate with the choline chloride–malonic acid system can be attributed to the higher solubility of copper oxides in this system [297]. Malonic acid also has high solubility for other metal oxides and is used for decontaminating nickel alloys and stainless steels [313,314].
6.2 Brush Cleaning
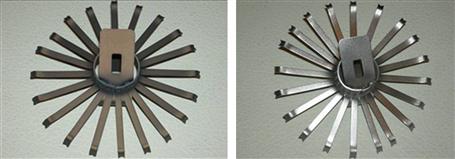
Brush cleaning is an established method of removing contaminants (particles, fibers, and other substances) safely and gently from high-value and sensitive surfaces (Fig. 1.17). Unfortunately, electrostatic charging can significantly affect the efficiency of the cleaning process. The process is efficient only if the brush bristles are moistened by a solvent that can also neutralize the charge. Conventionally, dilute NaCl solution has been used that acts as a wetting and antistatic agent to overcome the problem of electrostatic charging. In practice, the brush filaments are moistened by a fine spray of the cleaning solution through a Venturi nozzle. Unfortunately, crystallization of the NaCl from the spray leads to encrustation and blockage of the nozzle and frequent maintenance is required at short intervals. To overcome this problem, quaternary ammonium-based ILs have been used as antistatic additives in the cleaning solutions replacing NaCl [332–335]. The micro-moistened brush filaments effectively bind the contaminants and transport them to the suction system. The effect of replacement on the nozzle is clearly evident (Fig. 1.18) without affecting the performance of the cleaning process.

FIGURE 1.17 The removal of contaminant particles by brush cleaning (a and b) is much more efficient if the brush filaments are coated with a conducting ionic liquid film, applied by a spray from a fine nozzle (c, d and e). The adhering particles are removed by a rotor at the end of the process [334,335]. Source: Courtesy of IoLiTec GmbH and Wandres Micro-Cleaning, Germany. A color version of this figure appears in the color plate section.
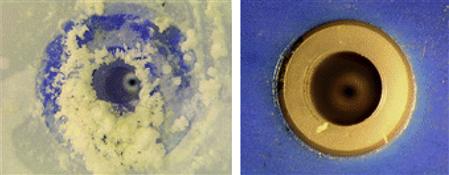
FIGURE 1.18 A spray nozzle for aqueous solutions of sodium chloride (left) and a hydrophilic ionic liquid (right), each after 10 h of operation [334]. Source: Courtesy of IoLiTec GmbH, Germany. A color version of this figure appears in the color plate section.
Another innovative example of the use of ILs (ammonium, oxonium, sulfonium or phosphonium-based salts) as antistatic wetting agents is production of flat structures with antistatic properties [336,337]. Such surfaces can prevent attraction and adherence of dust and other undesirable contaminants. The IL is incorporated in the polymer matrix before curing.
6.3 Parts Cleaning
Several studies have been performed to evaluate the effectiveness of ILs for parts cleaning as replacement cleaners for conventional solvents that are considered ODCs or hazardous air pollutants with high global warming potentials. One study for NASA evaluated 2-ethylhexyl lactate for its efficiency in removing various contaminants (such as hydraulic fluids) that are representative of those encountered in processing oxygen system components. Average cleaning efficiencies of 85% or higher were obtained with stainless steel substrates contaminated with each individual contaminant and their mixtures [338,339]. This cleaning performance was superior to deionized water and was comparable to HFE-7100, which is used for vapor degreasing operations.
Several PIL solutions have been developed for cleaning a variety of contaminated surfaces of metals, ceramics, glasses, semiconductors, and plastic materials [340]. The ILs are obtained by mixing an imidazole diamine with an acid alone, such as acetic or propionic acid, or in combination with acids containing at least one carboxylic function, such as maleic, lactic, succinic or oxalic acids. The cleaning solutions are used undiluted, although they can be diluted with water up to 30% by weight. Cleaning is accomplished by immersion in the cleaning tank at an ultrasonic frequency between 30 and 80 kHz in the temperature range 303–343 K. Cleaning efficiencies of 100% were achieved with various combined IL-acid cleaning solution compositions for diverse contaminants such as resins, bituminous inks, and cutting oils on silicon wafers, aluminum, copper, stainless steel and molybdenum parts.
In a study for the US Air Force, cleaning efficiency tests using two ILs, [EMIM][Ac] and [EMIM][EtSO4], were conducted on medium carbon, low-alloy steel (Grade 4130 aircraft quality steel) and 2024-T3 aluminum alloy panels coated with MoS2 grease [341,342]. These ILs were selected based on a literature search and vendor survey. In general, [EMIM][Ac] achieved high cleaning efficiencies with both materials, while [EMIM][EtSO4] showed comparable efficiency on the aluminum alloy panels, but lower efficiency on the steel panels. Some chemical etching was observed on the surface of the panels. Chemical properties evaluations and materials compatibility testing showed satisfactory performance. These ILs show potential for use in wipe cleaning applications, although more extensive testing is needed to optimize cleaning performance to ensure they meet Air Force requirements.
Hot processing of metals into finished shapes, such as strip and wire, results in the formation of oxide scale on the surface. These oxides must be removed from the finished metal product which has to be bright metallic and clean with no oxide residues on the metal surface. Conventional scale removal methods involve chemical, mechanical, or thermal treatments which have serious disadvantages, including use of corrosive acids and hazardous chemicals, dust and fine particulate generation, loss of metal, and high temperatures. A new IL-based method has been developed and successfully demonstrated for single-step removal of oxide scale on parts (sheets, coils, strips, wires, and rods) made from various grades of steels [324]. This method employs an IL composition that can perform conditioning and pickling of the oxide scale on metal parts, followed by removal of the scale. The IL is composed of (1) at least one organic salt as a source of inorganic Lewis-basic anions (such as halides or pseudohalides of organic cations) and (2) one or more Lewis-acidic inorganic metal salts of which at least one metal salt comprises a metal cation with an oxidation state more positive than the lowest positive oxidation state of the metallic element itself (such as FeCl3). The molar ratio of components a:b is less than 1:1. Removal of the scale is accomplished by contacting the part with the IL composition for a short time (typically 30 min) at 373 K for open-treatment baths or at lower temperatures under vacuum.
An intriguing observation that may have application to removal of surface contaminants is that an IL droplet rolling off on an inclined solid surface collects all surface impurities along its path, leaving no microscopically visible traces behind [343,344]. Since ILs are powerful solvents, it is expected that such cleaning action will also remove grease spots from the surface.
6.4 Electropolishing
A large variety of metallic substrates, such as stainless steels, molybdenum and titanium alloys, aluminum, and nickel–cobalt alloys, can be electropolished in ILs and DES to yield a clean, shiny, and smooth surface (Fig. 1.19) [320,345–347]. The high solubility of oxides in ILs and DES allows for process intensification [297,348]. The operating parameters are comparable to those in existing acid-based electrolytes, but significantly higher current efficiency can be achieved. Typical operating parameters include a process temperature of 303–323 K and 3–5 V for 10 min. The polishing process can be improved significantly by additives such as oxalic acid, enabling the process to be extended to other systems. The major advantages of the process include use of noncorrosive electrolyte solution, improved surface finish, reduced and simplified waste management, recycling and reuse of the electrolyte, and safer operating conditions.
6.5 Cleaning with ILs and Supercritical Gases
A novel application of IL and SC-CO2 is to serially clean contaminated soils [100,101]. The IL is used to dissolve soil contaminants under ambient conditions and SC-CO2 is used to recover the contaminants from the IL extracts. The efficacy of the process has been demonstrated by extracting naphthalene from soil with [BMIM][PF6] IL. The amount of naphthalene remaining in the soil (∼21–44 µg) was below the contamination limit of 50 µg. Subsequently, SC-CO2 was used to recover the naphthalene dissolved in the IL. At 313 K and 14 MPa, naphthalene recovery was nearly 84% for an extraction time of 4 h. A process flow sheet has been developed for IL extraction of contaminated soils and continuous SC-CO2 extraction of the contaminants from IL extracts for recovery and reuse of the IL.
Recently, ILs have been proposed as detergents to improve the effectiveness of the dry-cleaning process using SC-CO2 [349]. The IL additive helps remove hydrophilic and/or polar impurities more effectively than conventional detergents that are ineffective in removing such impurities.
6.6 Cleaning of Oil-Contaminated Sands and Particulate Matter
A new IL-based method of separating hydrocarbons from tar and beach sands and particulate matter has been developed [350,351]. This method uses IM-based ILs, or other ILs that are soluble in water and insoluble in nonpolar organic solvents, to separate the heavy viscous oil from the sand, as well as separating oil from drill cuttings. By simply mixing the components together at room temperature, three-phase separation is achieved into minerals, IL, and hydrocarbons layers without generating wastewater. Standard solid–liquid and liquid–liquid extraction techniques can be used to separate the components. Bitumen is easily separated from the tar sands, but separation of the residual oil from the drill cuttings requires the addition of a volatile solvent to the mixture. The solvent can be subsequently separated from the oil by vacuum distillation. This method uses very little energy and water, and all solvents are recycled and reused. The method has also been demonstrated on the beach sand contaminated by the Deepwater Horizon oil spill in the Gulf of Mexico. Complete separation of the tar from the sand could be achieved with a separation solution containing [EMIM]Cl.
6.7 Decontamination of Hazardous Materials
Hazardous materials in the form of wastes are a toxic by-product of industrial activity in the public and private sectors. Conventional methods of destroying chlorinated hydrocarbon waste include thermal incineration, supercritical water oxidation, direct chemical oxidation, photochemical oxidation, and solvent extraction among many different treatment techniques [352]. Each of these techniques has disadvantages and limitations, including high capital, installation, energy, and operating costs, public acceptability, handling and disposal of the process wastes, and not environmentally benign. A new process has been proposed for the destruction of halogenated hydrocarbons using a superoxide ion in DES [353,354]. The superoxide, such as H2O2, can be generated electrochemically by reduction of oxygen in the DES or dissolving alkali or alkaline-earth superoxides in the DES. A wide variety of halogenated hydrocarbons and chemical warfare agents were destroyed by reacting them with the DES at ambient pressure and temperature. No toxic by-products were produced.
An alternative treatment for decontamination and detoxification of chemical warfare agents is based on surfactant microemulsions that incorporate various ILs with tailored properties [355,356].
6.8 Microbial Contamination
Microbial contamination is a subject of growing concern in the health and food sectors. The risk of transmission of diseases, chronic plant, animal and human infections, failure of medical implants and allograft tissue, and microbial corrosion and biofouling are some of the disastrous consequences caused by microbial contamination. Many ILs display strong activity against clinically significant Gram-positive and Gram-negative bacteria, algae, and fungi [268–277,357–363]. They are also effective in breaking down microbial biofilms that cause infections in hospitals and other medical facilities [361]. By altering the cation and anion pairings and the alkyl chain lengths, the toxicity and other properties of the ILs can be tuned, thereby facilitating their use as surface biocides for disinfecting contaminated surfaces. For example, methyl and hydroxyethyl-substituted IM salts show significantly improved antimicrobial potency of these ILs [269,357]. ILs are being proposed as biocides, disinfectants, antiseptics, or sterilization agents for medical devices and instruments, or as preservatives. The ILs could be applied to a surface that is already contaminated, or the surface could be coated to prevent biofilms from forming, such as in anti-infective medical devices. ILs may also have application as antifouling agents in various industrial and marine applications where biofilms can clog pipes, cause surface corrosion, and affect the performance of the systems.
6.9 Cleaning in Place
The contamination of equipment by both products and by-products is a major problem in pharmaceutical manufacturing. The ability of ILs to dissolve large concentrations of pharmaceutical compounds and intermediates that are otherwise intractable to removal by conventional solvents is a benefit to the pharmaceutical process industry. For example, ammonium-based ILs have been shown to dissolve 300–500 g/L of amoxicillin [364], a contaminant in pharmaceutical manufacturing. The solubility of amoxicillin in conventional organic solvents is low, making the cleaning process less effective and requiring handling and disposal of large volumes of the used solvents. Cleaning with ILs offers the ability to selectively dissolve specific compounds and residues, high solubility of the target contaminants, noncorrosive solvent exposure of the equipment, reduced waste disposal costs, and user safety and convenience. Cleaning can be effected in place by spraying or immersing the contaminated equipment in the cleaning solution.
6.10 Cleaning of Artworks
Medieval stained glass has relatively high content of alkali and alkaline-earth ions (potassium, sodium and calcium) and low percentage of silica. This composition contributes to its deterioration due to relative humidity fluctuations, pollutants, and biological activity. In the presence of atmospheric gases such as CO2 and SO2, corrosion crusts are formed on the surface, composed mainly of calcium salts (CaCO3 and CaSO4, as well as CaC2O4) that form from the interaction between the glass and oxalic acid produced by microorganisms at high humidity levels (>85%) [365]. Conventional mechanical and chemical cleaning methods used by conservators can be aggressive to the glass, or they have low efficiency in the removal of the corrosion crusts. They can also damage the weathering products that are considered part of the historical record of the glass.
In a recent study [366,367], the use of ILs was assessed as alternative to conventional mechanical and chemical cleaning methods on the fifteenth century stained-glass panel S07c, Figura Aureolada, from the Monastery of Santa Maria da Vitória, in Batalha, Portugal. The ILs were selected from quaternary imidazolium, phosphonium, and quaternary ammonium cations, combined with chloride, dicyanamide and ethyl sulfate anions. [EMIM][EtSO4] and [OMIM][DCA] revealed the best results and were used for cleaning the two stained-glass fragments from the panel S07c. Their main action was to soften the corrosion layer. In the case of the two fragments in question the results were successful, proving that it was possible to remove the crusts in a controlled way. The experiments suggest that ILs are a good alternative to current cleaning methods applied to stained glass, but further testing is needed before ILs are considered safe for cleaning stained glass.
In another recent example, new formulations of enzymes (proteases) and ILs [BMIM][BF4] and [EMIM][EtSO4] have been used to successfully remove proteinaceous varnish layers (egg white, animal glue, isinglass and casein) from painted and polychrome works of art [368]. The treatment was applied on documented reconstruction of paintings and gold leaf gilding. These innovative IL + enzyme formulations suggest the use of ILs as an alternative solvent to enzymes alone (proteases, amylases, lipases, and cellulases) [369,370]. A key factor in enzyme cleaning of paintings is the outdoor temperature which can significantly slow the cleaning process. The cost of the enzymes is also a reason for the lack of their widespread use. ILs can be designed (cation–anion combinations) to meet different requirements such as improvement of enzyme cleaning rate and effectiveness, surface compatibility, and safety.
6.11 Industrial Applications
Some industrial contamination-related applications are discussed below.
6.11.1 Hydrocarbon Production
Scale formation is a common reason for a decline in hydrocarbon production in or on the wellbore and in the near-wellbore region of the hydrocarbon-bearing formation. The scale is composed of minerals, such as BaSO4, CaCO3, and CaSO4, which may build up to eventually choke off the wellbore. Common methods for scale removal are mechanical or chemical techniques, which are often ineffective or damaging to the steel lining of the wellbore. A new method [371] for scale removal in wellbores exploits one or more attributes of ILs.
1. Dissolution of the scale due to the high solubility of a wide range of organic and inorganic materials in the selected IL. Figure 1.20 compares the dissolution rate of BaSO4 in trimethylamine dialuminum heptachloride (TMAHIL-67) with a conventional ethylenediaminetetraacetic acid-based solvent.

FIGURE 1.20 Dissolution of BaSO4 scale in an ionic liquid TMAHIL-67 vs an EDTA-based solvent. [371]
2. Substantial heat is generated on IL formation from the precursors pumped downwell separately and allowed to react in the wellbore or near-wellbore area. The heat generated can melt the contaminants (paraffin, wax, and sludge) coating the wellbore. The IL is then directly in contact with the scale and can dissolve it. The precursors can be encapsulated in a permeable membrane for delayed or controlled release.
3. ILs can act as a carrier solvent to transport a variety of agents or materials, including scale-removal agents, such as strong or super acids to the scale deposit(s).
4. Reaction of the selected IL with an aqueous liquid to generate acids or super acids that can react with the scale. The rate of acid generation can be controlled by controlling the rate of water addition. The efficiency of acid generation by the selected IL composed of an [AlCl4−] anion is much higher than organic acids, allowing for process intensification.
The results from laboratory testing demonstrate that ILs are suitable candidates for the dissolution of multiple types of scale common in oil field environments, particularly BaSO4, CaSO4, and CaCO3 [371].
Drilling fluids, commonly used in drill pipes for hydrocarbon recovery or for geothermal energy, serve multiple functions, including cooling the rotary drilling bit, suspend and remove the borehole cuttings, act as barrier to prevent entrance of the fluids into the surrounding earth, and counteract the pressure from the surrounding earth formation. Additives such as CaCO3, Fe2O3 or BaSO4 are incorporated in the fluid to enhance many of these functions. Unfortunately, these additives could separate precipitate from the fluid, exacerbating scale formation and leading to interruption or stoppage of drilling operations. Various compositions of ILs have been proposed as replacements for the conventional oil-based drilling fluids to mitigate these shortcomings [372]. The IL-based fluids are composed of a single IL or a mixture of ILs, and are designed to meet specific fluid property requirements for the given drilling application. Furthermore, the IL fluid can be recovered, cleaned, and reused.
Fracturing is a new technology for enhanced recovery of hydrocarbons. In this process, cracks are formed by drilling in the subterranean strata. Cellulosic materials are frequently added to thicken the drilling or fracturing fluid for enhanced drilling performance, but in the presence of water, these materials hydrolyze to revert back to cellulose. However, cellulose is sparingly soluble in water and most organic solvents, requiring frequent cleaning of the wellbore and resulting fractures to remove deposited cellulosic material that can impede or stop the flow of hydrocarbons through the fractures. On the other hand, RTILs such as [C4mim]Cl can dissolve up to 25 wt% cellulose, which is considerably higher than organic solvents [373–376]. As noted previously, however, ILs are expensive and many salts are toxic, and require special handling. By comparison, DES are produced from inexpensive, nontoxic precursors, and can be handled safely (Section 3.12). A new method using DES has been developed for solubilizing/removing cellulose or chemically modified cellulosic materials utilized in subterranean drilling operations [328]. DES useful as cellulose solvents include quaternary ammonium compounds, including choline chloride and chlorocholine chloride, reacted with a compound selected from amides, amines, carboxylic acids, alcohols, and metal halides. The method involves pumping the DES downhole after fracturing operations to remove cellulosic material left behind in the fractures, on the face of the formation, along the wellbore, or elsewhere within the subterranean region. The DES can be used alone, or in sequential treatment, whereby DES treatment is followed by flushing with water, caustic or acid solutions, or anhydride substances. The tested DES were capable of solubilizing up to 50 wt% cellulosic material such as xanthan gum, cellulose fibers, modified guar gum, carboxymethyl tamarind, and sodium carboxymethyl cellulose. By comparison, the [C4mim]Cl IL dissolved a maximum of 25 wt% cellulosic material.
6.11.2 Fuel Desulfurization
Another cleaning-related application is desulfurization of fuels using various classes of ILs. Several variations of the process have been reported and have been reviewed recently [377,378]. This involves direct liquid–liquid extraction of sulfur-containing compounds in a single step, or oxidative desulfurization in which the sulfur compounds are extracted and oxidized to sulfoxides and sulfones in the extractant, followed by liquid–liquid extraction to more efficiently remove the compounds. IL desulfurization technologies have several limitations that must be overcome before commercial implementation is feasible. Most of the reported studies involved model fuels under laboratory conditions that are not representative of real-world conditions. Although the reported extraction efficiencies were high, preparation of efficient catalysts is still a complicated process and not commercially available. Other limitations relate to the partitioning of the components, need for multiple extraction cycles, loss of hydrocarbons, and decomposition of the ILs on exposure to water.
6.12 Consumer Product Applications
Many ILs are biodegradable and nontoxic liquids, which can be used in consumer product applications. They also exhibit the ability to dissolve a wide variety of materials and contaminants including salts (lime scale, bleaches, and metal tarnishes); fats; proteins and amino acids; anionic, cationic and nonionic surfactants that can then be used to solubilize oils; sugars and polysaccharides; and metal oxides and a wide range of solutes. For many of these ILs, the production costs are low associated with the inexpensive raw materials used for their production. These attributes make them suitable for use as cleaners (liquid or aerosol sprays, pour-on liquids, and liquid additives), both in the household and in an industrial environment, as well as for other consumer applications [317,319,322,325–327,349,379–381]. The IL-containing compositions can be formulated in the form of liquid, gel, paste, foam, or solid. Some examples of consumer product applications are detergent formulations for laundry, dish washing, and hard surface cleaning; biofilm removal; waterless garment cleaning; textile, yarn, and leather cleaning with liquid or supercritical CO2; fabric care products; interior and exterior car care products; personal care products such as hair coloring and conditioning and baby shampoo formulations; and jewelry cleaning.
7 Summary and Conclusions
ILs and DES are a new class of low melting point solvents with attractive characteristics for cleaning applications. These characteristics include high solubility for a wide range of contaminants, thermal and chemical stability, low melting point (even below 273 K), low volatility, and high conductivity. IL solvents do have some disadvantages including high cost, complex synthesis, need for purification for reuse, and high toxicity and nonbiodegradability of several formulations. Several DES formulations have been developed to overcome some of these limitations. Cleaning applications have been successfully demonstrated although many of these are largely still at the laboratory stage. Applications range from semiconductor wafer and integrated circuit cleaning, brush cleaning for microcontaminants, precision cleaning of parts for aerospace applications, oxide-scale removal on metals, electropolishing of metals, microbial decontamination, cleaning of artworks, cleaning of wellbores in oil and gas recovery, soil decontamination, and consumer product applications.
Acknowledgment
The author would like to thank the staff of the STI Library at the Johnson Space Center for help with locating obscure reference articles.
Disclaimer
Mention of commercial products in this chapter is for information only and does not imply recommendation or endorsement by The Aerospace Corporation. All trademarks, service marks, and trade names are the property of their respective owners.
References
1. U.S. EPA. The U.S Solvent Cleaning Industry and the Transition to Non Ozone Depleting Substances. EPA Report Washington, D.C.,: U.S. Environmental Protection Agency (EPA); 2004; www.epa.gov/ozone/snap/solvents/EPASolventMarketReport.pdf; 2004.
2. Durkee JB. Cleaning with Solvents. In: Kohli R, Mittal KL, eds. Developments in Surface Contamination and Cleaning. Norwich, NY: William Andrew Publishing; 2008;759–871. (Chapter 15).
3. U.S. EPA. HCFC Phaseout Schedule. Washington, D.C.,: U.S. Environmental Protection Agency; 2012; In: http://www.epa.gov/ozone/title6/phaseout/hcfc.html; 2012.
4. Earle MJ, Seddon KR. Ionic liquids Green solvents for the future. Pure Appl Chem. 2000;72:1391.
5. Rooney DW, Seddon KR. Ionic Liquids. In: Wypych G, ed. Handbook of Solvents. Toronto, Canada: ChemTec Publishing; 2001;1459–1484. (Chapter 21.2).
6. Lempert RJ, Norling P, Pernin C, Resetar S, Mahnovski S. Next Generation Environmental Technologies Benefits and Barriers, Appendix A17 Room Temperature Ionic Liquids. Santa Monica, CA: Rand Corporation; 2003; Rand Monograph Report MR-1682-OSTP www.rand.org; 2003.
7. Mu Z, Liu W, Zhang S, Zhou F. Functional room-temperature ionic liquids as lubricants for an aluminum-on-steel system. Chem Lett. 2004;33:524.
8. Majewski P, Pernak A, Grzymisławski M, Iwanik K, Pernak J. Ionic liquids in embalming and tissue preservation: can traditional formalin-fixation be replaced safely? Acta Histochem. 2005;105:135.
9. Pernak A, Iwanik K, Majewski P, Grzymisławski M, Pernak J. Ionic liquids as an alternative to formalin in histopathological diagnosis. Acta Histochem. 2005;107:149.
10. Pernak J, Stefaniak F, Węglewski J. Phosphonium acesulfamate based ionic liquids. Eur J Org Chem. 2005;4:650.
11. Liotta CL, Pollet P, Blecher MA, Aronson JB, Samanata S, Griffith KN. Ionic liquid energetic materials. U.S. Patent Application 2005/0269001 2005.
12. Sigma-Aldrich. Enabling Technologies – Ionic Liquids. St. Louis, MO: Company publication, Sigma-Aldrich; 2005; ChemFiles, vol. 5, No. 6, pp. 1-23 http://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Aldrich/Brochure/al_chemfile_v5_n6.pdf; 2005.
13. Sigma-Aldrich. Ionic Liquids. St. Louis, MO: Company publication, Sigma-Aldrich; 2006; ChemFiles, vol. 6, No. 9, pp. 1-18 http://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Aldrich/Brochure/al_chemfile_v6_n9.pdf; 2006.
14. Angell CA, Byrne N, Belieres J-P. Parallel developments in aprotic and protic ionic liquids: physical chemistry and applications. Acc Chem Res. 2007;40:1228.
15. ACS Symposium Series vol. 975. Brennecke JF, Rogers RD, Seddon KR, eds. Ionic Liquids IV Not Just Solvents Anymore. Washington, D.C.,: American Chemical Society; 2007.
16. Wasserscheid P, Welton T. Ionic Liquids in Synthesis. second ed. Weinheim, Germany: Wiley-VCH; 2007.
17. Abbott AP, Davies DL, Capper G, Rasheed RK, Tambyrajah V. Ionic liquids and their use. U.S. Patent 7,196,221 2007.
18. Shreeve JM. Ionic Liquids as Energetic Materials. Report AFRL-SR-AR-TR-07-0094 Arlington, VA: Air Force Research Laboratory, Air Force Office of Scientific Research; 2007.
19. Stasiewicz M, Mulkiewicz E, Tomczak-Wandzel R, et al. Assessing toxicity and biodegradation of novel, environmentally benign ionic liquids (1-alkoxymethyl-3-hydroxypyridinium chloride, saccharinate and acesulfamates) on cellular and molecular level. Ecotoxicol Environ Saf. 2008;71:157.
20. Pensado AS, Comuñas MJP, Fernańdez J. The pressure–viscosity coefficient of several ionic liquids. Tribol Lett. 2008;31:107.
21. Koel M, ed. Ionic Liquids in Chemical Analysis. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2008.
22. Greaves TL, Drummond CJ. Protic ionic liquids: properties and applications. Chem Rev. 2008;108:206.
23. Plechkova NV, Seddon KR. Applications of ionic liquids in the chemical industry. Chem Soc Rev. 2008;37:123.
24. Wishart JF. Energy applications of ionic liquids. Energy Environ Sci. 2009;2:956.
25. Minami I. Ionic liquids in tribology. Molecules. 2009;14:2286.
26. Bermúdez MD, Jiménez AE, Sanes J, Carrión FJ. Ionic liquids as advanced lubricant fluids. Molecules. 2009;14:2888.
27. ACS Symposium Series vol. 1030. Plechkova NV, Rogers RD, Seddon KR, eds. Ionic Liquids: From Knowledge to Application. Washington, D.C.,: American Chemical Society; 2009.
28. Kirchner B, ed. Ionic Liquids, Topics in Current Chemistry 290. Berlin and Heidelberg, Germany: Springer Verlag; 2009.
29. Kerton FM. Room Temperature Ionic Liquids and Eutectic Mixtures. Alternative Solvents for Green Chemistry Oxford, UK: RSC Publishing; 2009; pp. 118–142. (Chapter 6).
30. Giernoth R. Task-specific ionic liquids. Angew Chem Int Ed. 2010;49:2834.
31. Chiappe C, Malvaldi M. Highly concentrated ‘‘solutions’’ of metal cations in ionic liquids: current status and future challenges. Phys Chem Chem Phys. 2010;12:11191.
32. Torimoto T, Tsuda T, Okazaki K-I, Kuwabata S. New frontiers in materials science opened by ionic liquids. Adv Mater. 2010;22:1196.
33. Gorke J, Srienc F, Kazlauskas R. Toward advanced ionic liquids Polar, enzyme-friendly solvents for biocatalysis. Biotechnol Bioprocess Eng. 2010;15:40.
34. Freemantle M. An Introduction to Ionic Liquids. Cambridge, UK: Royal Society of Chemistry; 2010.
35. Zhang Y, Gao H, Joo Y-H, Shreeve JM. Ionic liquids as hypergolic fuels. Angew Chem Int Ed. 2011;50:9554.
36. Hawkins T. R&D of Energetic Ionic Liquids. Washington, D.C.: Presentation at Partners in Environmental Technology; December 2011; In: http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA554403; December 2011.
37. Gao H, Shreeve JM. Azole-based energetic salts. Chem Rev. 2011;111:7377.
38. Street Jr KW, Morales W, Koch VR, Valco DJ, Richard RM, Hanks N. Evaluation of vapor pressure and ultra-high vacuum tribological properties of ionic liquids. Tribol Trans. 2011;54:911.
39. Kokorin A, ed. Ionic Liquids: Theory, Properties, New Approaches. Rijeka, Croatia: InTech Publishing –Open Access Company; 2011; In: www.intechopen.com/books/ionic-liquids-theory-properties-new-approaches; 2011.
40. Handy ST, ed. Ionic Liquids – Classes and Properties. Rijeka, Croatia: InTech Publishing – Open Access Company; 2011; In: http://www.intechopen.com/books/ionic-liquids-classes-and-properties; 2011.
41. Torriero AAJ, Shiddiky MJA, eds. Electrochemical Properties and Applications of Ionic Liquids. Hauppauge, NY: Nova Science Publishers; 2011.
42. Reichardt Ch, Welton T. Solvents and Green Chemistry. In: Electrochemical Properties and Applications of Ionic Liquids. Weinheim, Germany: Wiley-VCH Verlag; 2011;509–548. (Chapter 8).
43. Mohammad A, Inamuddin, eds. Green Solvents II: Properties and Applications of Ionic Liquids. Berlin and Heidelberg, Germany: Springer Verlag; 2012.
44. Singh VV, Nigam AK, Batra A, Boopathi M, Singh B, Vijayaraghavan R. Applications of ionic liquids in electrochemical sensors and biosensors. Int J Electrochem. 2012. doi 10.1155/2012/165683 Article # 165683.
45. Ionic Liquids, Themed Issue, Faraday Discuss. 154 (2012) 1.
46. Arnaud CH. Ionic liquids improve separations. Chem Eng News April 2, 2012;36–37.
47. Ionic Liquid, Wikipedia Article, 2012. http://en.wikipedia.org/wiki/Ionic_liquid.
48. Meindersma GW, Maase M, De Haan AB. Ionic liquids. Ullmann’s Encyclopedia of Industrial Chemistry. vol. 19 seventh ed. Weinheim, Germany: Wiley-VCH; 2012; pp. 548–575.
49. Plechkova NV, Seddon KR, eds. Ionic Liquids UnCOILed: Critical Expert Overviews. New York, NY: John Wiley & Sons; 2013.
50. NASA Document JPR 5322.1. Contamination Control Requirements Manual. Houston, TX: National Aeronautics and Space Administration, Johnson Space Center; 2009.
51. ESA Standard ECSS-Q-70-01B. Space Product Assurance – Cleanliness and Contamination Control. Noordwijk, The Netherlands: European Space Agency; 2008.
52. IEST Standard IEST-STD-CC1246D. Product Cleanliness Levels and Contamination Control Program. Rolling Meadows, IL: Institute for Environmental Science and Technology (IEST); 2002.
53. Stansbery EK. Genesis Discovery Mission Contamination Control Plan. NASA Document GN-460000–100 Houston, TX: National Aeronautics and Space Administration, Johnson Space Center; 1998.
54. Sigma-Aldrich Corporation, St. Louis, MO. www.sigma-aldrich.com.
55. Phosphonium Ionic Liquids, Cytec Industries, Woodland Park, NJ. www.cytec.com.
56. Ionic Liquids, Covalent Associates, Corvallis, OR. www.covalentassociates.com.
57. DuPont FluoroIntermediates. www.dupont.com/fluoroIntermediates.
58. Proionic GmbH, Grambach, Austria. http://www.proionic.com/en/.
59. Ionic Liquids Handbook. ACROS Organics, Geel, Belgium. www.acros.com.
60. Ionic Liquids, Solvionic, Toulouse, France. www.solvionic.com.
61. Ionic Liquids. IoLiTec Ionic Liquids Technologies GmbH, Heilbronn, Germany. www.iolitec.com.
62. Ionic Liquids – Solutions for Your Success. BASF, Ludwigshafen, Germany. http://www.intermediates.basf.com/chemicals/ionic-liquids/index.
63. Ionic Liquids, Solchemar, Lisbon, Portugal. www.solchemar.com.
64. Ionic Liquids, Scionix Ltd, London, UK. www.scionix.co.uk.
65. Kanto Chemical, Tokyo, Japan. www.kanto.co.jp.
66. Imidazole Derivatives, Nippon Gohsei, Osaka, Japan. http://www.nichigo.co.jp/english/product/index02.html.
67. Ionic Liquids, Chemada Fine Chemicals, HaNegev, Israel. www.chemada.com.
68. McNaught AD, Wilkinson A, eds. Compendium of Chemical Terminology, IUPAC Recommendations The “Gold Book”. second ed. Oxford, UK: Blackwell Scientific Publications; 1997; Electronic interactive version http://goldbook.iupac.org/; 1997.
69. Davis Jr JH, Fox PA. From curiosities to commodities: ionic liquids begin the transition. Chem Commun. 2003;1209–1212.
70. Bolkan SA, Yoke JT. Room temperature fused salts based on copper(I) chloride-1-methyl-3-ethylimidazolium chloride mixtures 1 Physical properties. J Chem Eng Data. 1986;31:194.
71. Wicelinski SP, Gale RJ, Wilkes JS. Differential scanning calorimetric study of low melting organic chlorogallate systems. Thermochim Acta. 1988;26:255.
72. Schreiter ER, Stevens JE, Ortwerth MF, Freeman RG. A room-temperature molten salt prepared from AuCl3 and 1-ethyl-3-methylimidazolium chloride. Inorg Chem. 1999;38:3935.
73. Yang J-Z, Tian P, He L-L, Xu W-G. Studies on room temperature ionic liquid InCl3-EMIC. Fluid Phase Equilib. 2003;204:295.
74. Yang J-Z, Tian P, Xu W-G, Liu S-Z. Studies on an ionic liquid prepared from InCl3 and 1-methyl-3-butylimidazolium chloride. Thermochim Acta. 2004;412:1.
75. Hayashi S, Yamaguchi H. Discovery of a magnetic ionic liquid [bmim]FeCl4. Chem Lett. 2004;33:1590.
76. Yoshida Y, Fujii J, Muroi K, et al. Highly conducting ionic liquids based on 1-ethyl-3-methylimidazolium cation. Synth Met. 2005;153:421.
77. Rickert PG, Antonio MR, Firestone MA, et al. Tetraalkylphosphonium polyoxometalate ionic liquids: novel, organic–inorganic hybrid materials. J Phys Chem B. 2007;111:4685.
78. Tang S-F, Babai A, Mudring A-V. Low melting europium ionic liquids as luminescent soft materials. Angew Chem Int Ed. 2008;47:7631.
79. Mallick B, Balke B, Felser C, Mudring A-V. Dysprosium room-temperature ionic liquids with strong luminescence and response to magnetic fields. Angew Chem Int Ed. 2008;47:7635.
80. Yoshida Y, Tanaka H, Saito G, Ouahab L, Yoshida H, Sato N. Valence-tautomeric ionic liquid composed of a cobalt bis(dioxolene) complex dianion. Inorg Chem. 2009;48:9989.
81. Pratt III HD, Rose AJ, Staiger CL, Ingersoll D, Anderson TM. Synthesis and characterization of ionic liquids containing copper, manganese, or zinc coordination cations. Dalton Trans. 2011;40:11396.
82. Santos FM, Brandão P, Félix V, et al. Organic–inorganic hybrid materials based on iron(III)-polyoxotungstates and 1-butyl-3-methylimidazolium cations. Dalton Trans. 2012;41:12145.
83. Mallick B, Metlen A, Nieuwenhuyzen M, Rogers RD, Mudring A-V. Mercuric ionic liquids: [Cnmim][HgX3], where n = 3, 4 and X = Cl, Br. Inorg Chem. 2012;51:193.
84. Hobby S. Sandia National Laboratories Researchers Find Energy Storage “Solutions” in MetILs. Albuquerque, NM: Sandia Labs News Releases; February 17, 2012.
85. Greaves TL, Weerawardena A, Fong C, Krodkiewska I, Drummond CJ. Protic ionic liquids: solvents with tunable phase behavior and physicochemical properties. J Phys Chem B. 2006;110:22479.
86. Marsh KN, Boxall JA, Lichtenthaler R. Room temperature ionic liquids and their mixtures. Fluid Phase Equilib. 2004;219:93.
87. Gabriel S, Weiner J. Ueber einige Abkömmlinge des Propylamins. Ber Dtsch Chem Ges. 1888;21:2669.
88. Curtius Th. Neues vom Stickstoffwasserstoff. Ber Dtsch Chem Ges. 1891;24:3341.
89. Schall C. Über organische und geschmolzene Salze. Zeitschr Elektrochem. 1908;14:397.
90. Walden P. Über die Molekulargrösse und elektrische Leitfähigkeit einiger geschmolzenen Salze. Bull Acad Sci St Petersburg 1914;405–422.
91. Kalb RS, Kotschan MJ. Trioctylmethylammonium thiosalicylate (TOMATS) A novel, high-performance, task-specific ionic liquid for the extraction of heavy metals from aqueous solutions. Aldrich ChemFiles. 2006;6:13.
92. Egorov VM, Djigailo DI, Momotenko DS, et al. Task-specific ionic liquid trioctylmethylammonium salicylate as extraction solvent for transition metal ions. Talanta. 2010;80:1177.
93. Yacob Z, Liebscher J. 1,2,3-Triazolium Salts as a Versatile New Class of Ionic Liquids. In: Handy ST, ed. Ionic Liquids – Classes and Properties. Rijeka, Croatia: InTech Publishing – Open Access Company; 2011;1–22. In: http://www.intechopen.com/books/ionic-liquids-classes-and-properties/1-2-3-triazolium-salts-as-a-versatile-new-class-of-ionic-liquids; 2011.
94. Sanghi S, Willett E, Versek C, Tuominen M, Coughlin EB. Physicochemical properties of 1,2,3-triazolium ionic liquids. RSC Adv. 2012;2:848.
95. Lago S, Rodríguez H, Khoshkbarchi MK, Soto A, Arce A. Enhanced oil recovery using the ionic liquid trihexyl(tetradecyl)phosphonium chloride: phase behaviour and properties. RSC Adv. 2012;2:9392.
96. Kohli R. Surface contamination removal using dense-phase fluids: liquid and supercritical carbon dioxide. In: Kohli R, Mittal KL, eds. Oxford, UK: Elsevier; 2013;1–54. Developments in Surface Contamination and Cleaning. vol. 5 (Chapter 1).
97. Keskin S, Kayrak-Talay D, Akman U, Hortaçsu O. A review of ionic liquids towards supercritical fluid applications. J Supercrit Fluids. 2007;43:150.
98. Carvalho PJ, Álvarez VH, Marrucho IM, Aznar M, Coutinho JAP. High carbon dioxide solubilities in trihexyltetradecylphosphonium-based ionic liquids. J Supercrit Fluids. 2010;52:258.
99. Sedláková Z, Wagner Z. High pressure phase equilibria in systems containing CO2 and ionic liquid of the [Cnmim][Tf2N] type. Chem Biochem Eng Q. 2012;26:55.
100. S. Keskin, U. Akman, O. Hortaçsu, Continuous cleaning of contaminated soils using ionic liquids and supercritical CO2, in: Proceedings of the Tenth European Meeting on Supercritical Fluids: Reactions, Materials and Natural Products, Colmar, France, 2005, Pi3.1–Pi3.6.
101. Keskin S, Akman U, Hortaçsu O. Soil remediation via an ionic liquid and supercritical CO2. Chem Eng Proc. 2008;47:1693.
102. Fannin Jr AA, Floreani DA, King LA, et al. Properties of 1,3-dialkylimidazolium chloride-aluminum chloride ionic liquids 2 Phase transitions, densities, electrical conductivities, and viscosities. J Phys Chem. 1984;88:2614.
103. Hussey CL, Scheffler TB, Wilkes JS, Fannin Jr AA. Chloroaluminate equilibria in the aluminum chloride‐1‐methyl‐3‐ethylimidazolium chloride ionic liquid. J Electrochem Soc. 1986;133:1389.
104. Gordon CM, Holbrey JD, Kennedy AR, Seddon KR. Ionic liquid crystals: hexafluorophosphate salts. J Mater Chem. 1998;8:2627.
105. Holbrey JD, Seddon KR. The phase behaviour of 1-alkyl-3-methylimidazolium tetrafluoroborates; ionic liquids and ionic crystals. J Chem Soc Dalton Trans. 1999;2133–2139.
106. Neve F, Francescangeli O, Crispini A. Crystal architecture and mesophase structure of long-chain n-alkylpyridinium tetrachlorometallates. Inorg Chim Acta. 2002;338:51.
107. Kärkkäinen J. Preparation and characterization of some ionic liquids and their use in the dimerization reaction of 2-methylpropene. Ph.D. Dissertation Oulu, Finland: University of Oulu; 2007.
108. Solà Cervera JL, König A. Recycling concept for aluminum electrodeposition from the ionic liquid system emim [Tf2N]-AlCl3. Chem Eng Technol. 2010;33:1979.
109. Apperley DC, Hardacre C, Licence P, et al. Speciation of chloroindate(III) ionic liquids. Dalton Trans. 2010;39:8679.
110. Currie M, Estager J, Licence P, et al. Chlorostannate(II) ionic liquids: speciation, lewis acidity, and oxidative stability. Inorg Chem. 2013;52:1710.
111. Estager J, Nockemann P, Seddon KR, Swadźba-Kwaśny M, Tyrrell S. Validation of speciation techniques: a study of chlorozincate(II) ionic liquids. Inorg Chem. 2011;50:5258.
112. Earle MJ, Esperança JMSS, Gilea MA, et al. The distillation and volatility of ionic liquids. Nature. 2006;439:831.
113. Wasserscheid P. Chemistry: volatile times for ionic liquids. Nature. 2006;439:797.
114. Armstrong JP, Hurst C, Jones RG, et al. Vapourisation of ionic liquids. Phys Chem Chem Phys. 2007;9:982.
115. Taylor AW, Lovelock KRJ, Deyko A, Licence P, Jones RG. High vacuum distillation of ionic liquids and separation of ionic liquid mixtures. Phys Chem Chem Phys. 2010;12:1772.
116. Maase M. Distillation of ionic liquids. U.S. Patent 7,754,053 2010.
117. Masonne K, Siemer M, Mormann W, Leng W. Distillation of ionic liquids. U.S. Patent Application 2010/0300870 2010.
118. Esperança JMSS, Lopes JNC, Tariq M, Santos LMNBF, Magee JW, Rebelo LPN. Volatility of aprotic ionic liquids—a review. J Chem Eng Data. 2010;55:3.
119. Swiderski K, McLean A, Gordon CM, Vaughan DH. Estimates of internal energies of vaporisation of some room temperature ionic liquids. Chem Commun. 2004;2178–2179.
120. Lee SH, Lee SB. The Hildebrand solubility parameters, cohesive energy densities and internal energies of 1-alkyl-3-methylimidazolium-based room temperature ionic liquids. Chem Commun. 2005;3469–3471.
121. Santos LMNBF, Lopes JNC, Coutinho JAP, et al. Ionic liquids: first direct determination of their cohesive energy. J Am Chem Soc. 2007;129:284.
122. Kelkar MS, Maginn EJ. Calculating the enthalpy of vaporization for ionic liquid clusters. J Phys Chem B. 2007;111:9424.
123. Marciniak A. The solubility parameters of ionic liquids. Int J Mol Sci. 2010;11:1973.
124. Marciniak A. The Hildebrand solubility parameters of ionic liquids—part 2. Int J Mol Sci. 2011;12:3553.
125. Øye HA, Jagtoyen M, Oksefjell T, Wilkes JS. Vapour pressure and thermodynamics of the system 1-methyl-3-ethyl-imidazolium chloride – aluminium chloride. Mater Sci Forum. 1991;73–75:183.
126. Paulechka YU, Kabo GJ, Blokhin AV, Vydrov OA, Magee JW, Frenkel M. Thermodynamic properties of 1-butyl-3-methylimidazolium hexafluorophosphate in the ideal gas state. J Chem Eng Data. 2003;48:457.
127. Paulechka YU, Zaitsau Dz H, Kabo GJ, Strechan AA. Vapor pressure and thermal stability of ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide. Thermochim Acta. 2005;439:158.
128. Rebelo LPN, Canongia Lopes JN, Esperança JMSS, Filipe E. On the critical temperature, normal boiling point, and vapor pressure of ionic liquids. J Phys Chem B. 2005;109:6040.
129. Zaitsau Dz H, Kabo GJ, Strechan AA, et al. Experimental vapor pressures of 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imides and a correlation scheme for estimation of vaporization enthalpies of ionic liquids. J Phys Chem A. 2006;110:7303.
130. Widegren JA, Wang Y-M, Henderson WA, Magee JW. Relative volatilities of ionic liquids by vacuum distillation of mixtures. J Phys Chem B. 2007;111:8959.
131. Emel’yanenko VN, Verevkin SP, Heintz A. The gaseous enthalpy of formation of the ionic liquid 1-butyl-3-methylimidazolium dicyanamide from combustion calorimetry, vapor pressure measurements, and ab initio calculations. J Am Chem Soc. 2007;129:3930.
132. Luo H, Baker GA, Dai S. Isothermogravimetric determination of the enthalpies of vaporization of 1-alkyl-3-methylimidazolium ionic liquids. J Phys Chem B. 2008;112:10077.
133. Aschenbrenner O, Supasitmongkol S, Taylor M, Styring P. Measurement of vapour pressures of ionic liquids and other low vapour pressure solvents. Green Chem. 2009;11:1217.
134. Chambreau SD, Vaghjiani GL, To A, et al. Heats of vaporization of room temperature ionic liquids by tunable vacuum ultraviolet photoionization. J Phys Chem B. 2010;114:1361.
135. Wang C, Luo H, Li H, Dai S. Direct UV-spectroscopic measurement of selected ionic-liquid vapors. Phys Chem Chem Phys. 2010;12:7246.
136. Rocha MAA, Lima CFRAC, Gomes LR, et al. High-accuracy vapor pressure data of the extended [CnC1im][Ntf2] ionic liquid series: trend changes and structural shifts. J Phys Chem B. 2011;115:10919.
137. Deyko A, Lovelock KRJ, Corfield J-A, et al. Measuring and predicting ΔvapH298 values of ionic liquids. Phys Chem Chem Phys. 2009;11:8544.
138. Lovelock KRJ, Deyko A, Licence P, Jones RG. Vaporisation of an ionic liquid near room temperature. Phys Chem Chem Phys. 2010;12:8893.
139. Deyko A, Hessey SG, Licence P, et al. The enthalpies of vaporisation of ionic liquids: new measurements and predictions. Phys Chem Chem Phys. 2012;14:3181.
140. Zaitsau Dz H, Fumino K, Emel’yanenko VN, Yermalayeu AV, Ludwig R, Verevkin SP. Structure–property relationships in ionic liquids: a study of the anion dependence in vaporization enthalpies of imidazolium-based ionic liquids. ChemPhysChem. 2012;13:1868.
141. Verevkin SP. Predicting the enthalpy of vaporization of ionic liquids: a simple rule for a complex property. Angew Chem Int Ed. 2008;47:5071.
142. Bier M, Dietrich S. Vapor pressure of ionic liquids. Mol Phys. 2010;108:211.
143. Valderrama JO, Forero LA. An analytical expression for the vapor pressure of ionic liquids based on an equation of state. Fluid Phase Equilib. 2012;317:77.
144. Maginn EJ. Molecular simulation of ionic liquids: current status and future opportunities. J Phys.: Condens Matter. 2009;21:373101.
145. Maginn EJ, Elliott JR. Historical perspective and current outlook for molecular dynamics as a chemical engineering tool. Ind Eng Chem Res. 2010;49:3059.
146. Dommert F, Wendler K, Berger R, Delle Site L, Holm Ch. Force fields for studying the structure and dynamics of ionic liquids: a critical review of recent developments. Chem Phys Chem. 2012;13:1625.
147. Aparicio S, Atilhan M, Karadas F. Thermophysical properties of pure ionic liquids: review of present situation. Ind Eng Chem Res. 2010;49:9580.
148. Coutinho JAP, Carvalho PJ, Oliveira NMC. Predictive methods for the estimation of thermophysical properties of ionic liquids. RSC Adv. 2012;2:7322.
149. Izgorodina EI. Theoretical Approaches to Ionic Liquids: From Past History to Future Directions. In: Plechkova NV, Seddon KR, eds. Ionic Liquids UnCOILed: Critical Expert Oveviews. New York, NY: John Wiley & Sons; 2013;181–230. (Chapter 6).
150. Diedenhofen M, Klamt A, Marsh KN, Schäfer A. Prediction of the vapor pressure and vaporization enthalpy of 1-n-alkyl-3-methylimidazolium-bis-(trifluoromethanesulfonyl) amide ionic liquids. Phys Chem Chem Phys. 2007;9:4653.
151. Hansen CM. Hansen Solubility Parameters: A User’s Handbook. second ed. Boca Raton, FL: CRC Press; 2007.
152. Camper D, Scovazzo P, Koval C, Noble R. Gas solubilities in room-temperature ionic liquids. Ind Eng Chem Res. 2004;43:3049.
153. Jin H, O’Hare B, Dong J, et al. Physical properties of ionic liquids consisting of the 1-butyl-3-ethylimidazolium cation with various anions and the bis(trifluoromethylsulfonyl)imide anion with various cations. J Phys Chem B. 2008;112:81.
154. Kilaru PK, Scovazzo P. Correlations of low-pressure carbon dioxide and hydrocarbon solubilities in imidazolium-, phosphonium-, and ammonium-based room-temperature ionic liquids Part 2 Using activation energy of viscosity. Ind Eng Chem Res. 2008;47:910.
155. Moganty SS, Baltus RE. Regular solution theory for low pressure carbon dioxide solubility in room temperature ionic liquids: ionic liquid solubility parameter from activation energy of viscosity. Ind Eng Chem Res. 2010;49:5846.
156. Kamlet MJ, Abboud J-LM, Abraham MH, Taft RW. Linear solvation energy relationships 23 A comprehensive collection of the solvatochromic parameters, π∗, α, and β, and some methods for simplifying the generalized solvatochromic equation. J Org Chem. 1983;48:2877.
157. Scovazzo P, Camper D, Kieft J, Poshusta J, Koval C, Noble R. Regular solution theory and CO2 gas solubility in room-temperature ionic liquids. Ind Eng Chem Res. 2004;43:6855.
158. Camper D, Becker C, Koval C, Noble RD. Low pressure hydrocarbon solubility in room temperature ionic liquids containing imidazolium rings interpreted using regular solution theory. Ind Eng Chem Res. 2005;44:1928.
159. Finotello A, Bara JE, Camper D, Noble RD. Room-temperature ionic liquids: temperature dependence of gas solubility selectivity. Ind Eng Chem Res. 2008;47:3453.
160. Gupta J, Nunes C, Vyas S, Jonnalagadda S. Prediction of solubility parameters and miscibility of pharmaceutical compounds by molecular dynamics simulations. J Phys Chem B. 2011;115:2014.
161. Paduszyński K, Chiyen J, Ramjugernath D, Letcher TM, Domańska U. Liquid–liquid phase equilibrium of (piperidinium-based ionic liquid + an alcohol) binary systems and modelling with NRHB and PCP-SAFT. Fluid Phase Equilib. 2011;305:43.
162. Batista MLS, Neves CMSS, Carvalho PJ, Gani R, Coutinho JAP. Chameleonic behavior of ionic liquids and its impact on the estimation of solubility parameters. J Phys Chem B. 2011;115:12879.
163. Verevkin SP, Emel’yanenko VN, Zaitsau Dz H, Ralys RV. Ionic liquids: differential scanning calorimetry as a new indirect method for determination of vaporization enthalpies. J Phys Chem B. 2012;116:4276.
164. Ueki T, Watanabe M. Polymers in ionic liquids: dawn of neoteric solvents and innovative materials. Bull Chem Soc Jpn. 2012;85:33.
165. Sistla YS, Jain L, Khanna A. Validation and prediction of solubility parameters of ionic liquids for CO2 capture. Sep Purif Technol. 2012;97:51.
166. Paduszyński K, Domańska U. Thermodynamic modeling of ionic liquid systems: development and detailed overview of novel methodology based on the PC-SAFT. J Phys Chem B. 2012;116:5002.
167. B. Iliev, M. Smiglak, A. Świerczyńska, T.J.S. Schubert, Miscibility and phase separation of various ionic liquids with common organic solvents and water, ILSEPT First International Conference on Ionic Liquids in Separation and Purification Technology, Stiges, Spain, 2011. http://www.iolitec.de/en/Download-document/669-2011-ILSEPT-Solubility.html.
168. Bermejo MD, Martín A. Solubility of gases in ionic liquids. Global J Phys Chem. 2011;2:324.
169. Ji X, Held C, Sadowski G. Modeling imidazolium-based ionic liquids with ePC-SAFT. Fluid Phase Equilib. 2012;335:64.
170. Ludwig R. Thermodynamic properties of ionic liquids – a cluster approach. Phys Chem Chem Phys. 2008;10:4333.
171. Roth M. Partitioning behaviour of organic compounds between ionic liquids and supercritical fluids. J Chromatogr A. 2009;1216:1861.
172. Vega LF, Vilaseca O, Llovell F, Andreu JS. Modeling ionic liquids and the solubility of gases in them: recent advances and perspectives. Fluid Phase Equilib. 2010;294:15.
173. Ferreira AR, Freire MG, Ribeiro JC, Lopes FM, Crespo JG, Coutinho JAP. An overview of the liquid−liquid equilibria of (ionic liquid + hydrocarbon) binary systems and their modeling by the conductor-like screening model for real solvents. Ind Eng Chem Res. 2011;50:5279.
174. Annat G, Forsyth M, MacFarlane DR. Ionic liquid mixtures – variations in physical properties and their origins in molecular structure. J Phys Chem B. 2012;116:8251.
175. Bernales VS, Marenich AV, Contreras R, Cramer CJ, Truhlar DG. Quantum mechanical continuum solvation models for ionic liquids. J Phys Chem B. 2012;116:9122.
176. Llovell F, Valente E, Vilaseca O, Vega LF. Modeling complex associating mixtures with [Cn-mim][Tf2N] ionic liquids: predictions from the soft-SAFT equation. J Phys Chem B. 2011;115:4387.
177. Oliveira MB, Llovell F, Coutinho JAP, Vega LF. Modeling the [NTf2] pyridinium ionic liquids family and their mixtures with the soft statistical associating fluid theory equation of state. J Phys Chem B. 2012;116:9089.
178. F. Llovell, M. Belo, O. Vilaseca, J.A.P. Coutinho, L.F. Vega, Thermodynamic modeling of the solubility of supercritical CO2 and other gases on ionic liquids with the soft-SAFT equation of state, in: Proceedings of the Tenth International Symposium on Supercritical Fluids, ISSF, 2012, J. Supercrit. Fluids (2013). http://issf2012.com/handouts/documents/333_004.pdf.
179. Blanchard LA, Hancu D, Beckman EJ, Brennecke JF. Green processing using ionic liquids and CO2. Nature. 1999;399:28.
180. Brennecke JF, Maginn EJ. Ionic liquids: innovative fluids for chemical processing. AIChE J. 2001;47:2384.
181. Blanchard LA, Gu Z, Brennecke JF. High-pressure phase behavior of ionic liquid/CO2 systems. J Phys Chem B. 2001;105:2437.
182. Scurto AM, Aki SNVK, Brennecke JF. CO2 as a separation switch for ionic liquid/organic mixtures. J Am Chem Soc. 2002;124:10276.
183. Cadena C, Anthony JL, Shah JK, Morrow TI, Brennecke JF, Maginn EJ. Why is CO2 so soluble in imidazolium-based ionic liquids? J Am Chem Soc. 2004;126:5300.
184. Shariati A, Peters CJ. High pressure phase equilibria of systems with ionic liquids. J Supercrit Fluids. 2005;34:171.
185. A. Adamou, J.-J. Letourneau, E. Rodier, R. David, P. Guiraud, Characterization of the ionic liquid bmimPF6 in supercritical conditions, in: Proceedings of the Tenth European Meeting on Supercritical Fluids: Reactions, Materials and Natural Products, Colmar, France, 2005 Pi5.1-Pi5.6.
186. K.I. Gutkowski, A. Shariati, B. Breure, S.B. Bottini, E.A. Brignole, C.J. Peters, Experiments and modelling of systems with ionic liquids, in: Proceedings of the Tenth European Meeting on Supercritical Fluids: Reactions, Materials and Natural Products, Colmar, France, 2005 Pi1.1-Pi1.6.
187. Gutkowski KI, Shariati A, Peters CJ. High-pressure phase behavior of the binary ionic liquid system 1-octyl-3-methylimidazolium tetrafluoroborate + carbon dioxide. J Supercrit Fluids. 2006;39:187.
188. Shariati A, Raeissi S, Peters CJ. CO2 Solubility in Alkylimidazolium-Based Ionic Liquids. In: Letcher TM, ed. Developments and Applications in Solubility, RSC Publishing, Cambridge. Cambridge, UK: RSC Publishing; 2007;131–152.
189. Mattedi S, Carvalho PJ, Coutinho JAP, Alvarez VH, Iglesias M. High pressure CO2 solubility in n-methyl-2-hydroxyethylammonium protic ionic liquids. J Supercrit Fluids. 2011;56:224.
190. Pure Component Properties, Queriable Database. Seoul, South Korea: CHERIC Chemical Engineering Research Information Center; 2012; In: http://www.cheric.org/research/kdb/hcprop/cmpsrch.php; 2012.
191. Santos FJV, de Castro CAN, Dymond JH, Dalaouti NK, Assael MJ, Nagashima A. Standard reference data for the viscosity of toluene. J Phys Chem Ref Data. 2006;35:1.
192. Cyclohexane Data Sheet. Philadelphia, PA: Sunoco Chemicals; 2012.
193. Widegren JA, Magee JW. Density, viscosity, speed of sound, and electrolytic conductivity for the ionic liquid 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide and its mixtures with water. J Chem Eng Data. 2007;52:2331.
194. Marsh KN, Brennecke JF, Chirico RD, et al. Thermodynamic and thermophysical properties of the reference ionic liquid: 1-hexyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]amide (including mixtures) Part 1 Experimental methods and results (IUPAC technical report). Pure Appl Chem. 2009;81:781.
195. Chirico RD, Diky V, Magee JW, Frenkel M, Marsh KN. Thermodynamic and thermophysical properties of the reference ionic liquid: 1-hexyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]amide (including mixtures) Part 2 Critical evaluation and recommended property values (IUPAC technical report). Pure Appl Chem. 2009;81:791.
196. Burrell GL, Burgar IM, Separovic F, Dunlop NF. Preparation of protic ionic liquids with minimal water content and 16N NMR study of proton transfer. Phys Chem Chem Phys. 2010;12:1571.
197. Hapiot P, Lagrost C. Electrochemical reactivity in room-temperature ionic liquids. Chem Rev. 2008;108:2238.
198. Borodin O, Smith GD, Kim H. Viscosity of a room temperature ionic liquid: predictions from nonequilibrium and equilibrium molecular dynamics simulations. J Phys Chem B. 2009;113:4771.
199. Castiglione F, Raos G, Appetecchi GB, et al. Blending ionic liquids: how physico-chemical properties change. Phys Chem Chem Phys. 2010;12:1784.
200. de Castro CAN. Thermophysical properties of ionic liquids: do we know how to measure them accurately? J Mol Liq. 2010;156:10.
201. Lopes JNC, Costa Gomes MF, Husson P, et al. Polarity, viscosity, and ionic conductivity of liquid mixtures containing [C4C1im][Ntf2] and a molecular component. J Phys Chem B. 2011;115:6988.
202. Yu G, Zhao D, Wen L, Yang S, Chen X. Viscosity of ionic liquids: database, observation, and quantitative structure–property relationship analysis. AIChE J. 2012;58:2885.
203. Butler SN, Müller-Plathe F. A molecular dynamics study of viscosity in ionic liquids directed by quantitative structure–property relationships. ChemPhysChem. 2012;13:1791.
204. Diogo JCF, Caetano FJP, Fareleira JMNA, Wakeham WA, Afonso CAM, Marques CS. Viscosity measurements of the ionic liquid trihexyl(tetradecyl)phosphonium dicyanamide [P6,6,6,14][dca] using the vibrating wire technique. J Chem Eng Data. 2012;57:1015.
205. Seddon KR, Stark A, Torres MJ. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl Chem. 2000;72:2275.
206. Huddleston JG, Visser AE, Reichert WM, Willauer HD, Broker GA, Rogers RD. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001;3:156.
207. Villagrán C, Deetlefs M, Pitner WR, Hardacre C. Quantification of halide in ionic liquids using ion chromatography. Anal Chem. 2004;76:2118.
208. Widegren JA, Laesecke A, Magee JW. The effect of dissolved water on the viscosities of hydrophobic room-temperature ionic liquids. Chem Commun. 2005;1610–1612.
209. Silvester DS, Compton RG. Electrochemistry in room temperature ionic liquids: a review and some possible applications. Z Phys Chem. 2006;220:1247.
210. Burrell AK, Del Sesto RE, Baker SN, McCleskey TM, Baker GA. The large scale synthesis of pure imidazolium and pyrrolidinium ionic liquids. Green Chem. 2007;9:449.
211. Harris KR, Kanakubo M, Woolf LA. Temperature and pressure dependence of the viscosity of the ionic liquids 1-hexyl-3-methylimidazolium hexafluorophosphate and 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. J Chem Eng Data. 2007;52:1080.
212. Randström S, Montanino M, Appetecchi GB, Lagergren C, Moreno A, Passerini S. Effect of water and oxygen traces on the cathodic stability of n-alkyl-n-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide. Electrochim Acta. 2008;53:6397.
213. Carvalho PJ, Regueira T, Santos LMNBF, Fernandez J, Coutinho JAP. Effect of water on the viscosities and densities of 1-butyl-3-methylimidazolium dicyanamide and 1-butyl-3-methylimidazolium tricyanomethane at atmospheric pressure. J Chem Eng Data. 2010;55:645.
214. Spohr HV, Patey GN. The influence of water on the structural and transport properties of model ionic liquids. J Chem Phys. 2010;132:234510.
215. Firestone MA, Dzielawa JA, Zapol P, Curtiss LA, Seifert S, Dietz ML. Lyotropic liquid–crystalline gel formation in a room-temperature ionic liquid. Langmuir. 2002;18:7258.
216. Green O, Grubjesic S, Lee S, Firestone MA. The design of polymeric ionic liquids for the preparation of functional materials. Polym Rev. 2009;49:339.
217. Yoshizawa M, Xu W, Angell CA. Ionic liquids by proton transfer: vapor pressure, conductivity, and the relevance of ΔpKa from aqueous solutions. J Am Chem Soc. 2003;125:15411.
218. Xu W, Cooper EI, Angell CA. Ionic liquids: ion mobilities, glass temperatures, and fragilities. J Phys Chem B. 2003;107:6170.
219. Tokuda H, Tsuzuki S, Susan MAH, Hayamizu K, Watanabe M. How ionic are room-temperature ionic liquids? An indicator of the physicochemical properties. J Phys Chem B. 2006;110:19593.
220. Hayes R, Warr GG, Atkin R. At the interface: solvation and designing ionic liquids. Phys Chem Chem Phys. 2010;12:1709.
221. Ueno K, Tokuda H, Watanabe M. Ionicity in ionic liquids: correlation with ionic structure and physicochemical properties. Phys Chem Chem Phys. 2010;12:1649.
222. Schreiner C, Zugmann S, Hartl R, Gores HJ. Fractional Walden rule for ionic liquids: examples from recent measurements and a critique of the so-called ideal KCl line for the Walden plot. J Chem Eng Data. 2010;55:1784.
223. MacFarlane DR, Forsyth M, Izgorodina EI, Abbott AP, Annata G, Fraser K. On the concept of ionicity in ionic liquids. Phys Chem Chem Phys. 2009;11:4962.
224. Liu H, Maginn E. An MD study of the applicability of the Walden rule and the Nernst–Einstein model for ionic liquids. Chem Phys Chem. 2012;13:1701.
225. Kohli R. Methods for monitoring and measuring cleanliness of surfaces. In: Kohli R, Mittal KL, eds. Oxford, UK: Elsevier; 2012;107–178. Developments in Surface Contamination and Cleaning. vol. 4 (Chapter 3).
226. Kuwabata S, Kongkanand A, Oyamatsu D, Torimoto T. Observation of ionic liquid by scanning electron microscope. Chem Lett. 2006;35:600.
227. Arimoto S, Sugimura M, Kageyama H, Torimoto T, Kuwabata S. Development of new techniques for scanning electron microscope observation using ionic liquid. Electrochim Acta. 2008;53:6228.
228. Kuwabata S, Tsuda T, Torimoto T. Room-temperature ionic liquid as new medium for material production and analyses under vacuum conditions. J Phys Chem Lett. 2010;1:3177.
229. Ishigaki Y, Nakamura Y, Takehara T, et al. Ionic liquid enables simple and rapid sample preparation of human culturing cells for scanning electron microscope analysis. Microsc Res Tech. 2011;74:415.
230. Ishigaki Y, Nakamura Y, Takehara T, et al. Comparative study of hydrophilic and hydrophobic ionic liquids for observing cultured human cells by scanning electron microscopy. Microsc Res Tech. 2011;74:1104.
231. Tsuda T, Nemoto N, Kawakami K, et al. SEM observation of wet biological specimens pretreated with room-temperature ionic liquid. Chembiochem. 2011;12:2547.
232. Dwiranti A, Lin L, Mochizuki E, et al. Chromosome observation by scanning electron microscopy using ionic liquid. Microsc Res Tech. 2012;75:1113.
233. Torimoto T, Okazaki K, Kiyama T, Hirahara K, Tanaka N, Kuwabata S. Sputter deposition onto ionic liquids: simple and clean synthesis of highly dispersed ultrafine metal nanoparticles. Appl Phys Lett. 2006;89:243117.
234. Okazaki K, Kiyama T, Hirahara K, Tanaka N, Kuwabata S, Torimoto T. Single-step synthesis of gold–silver alloy nanoparticles in ionic liquids by a sputter deposition technique. Chem Commun. 2008;691–693.
235. Ueno K, Hata K, Katakabe T, Kondoh M, Watanabe M. Nanocomposite ion gels based on silica nanoparticles and an ionic liquid: ionic transport, viscoelastic properties, and microstructure. J Phys Chem B. 2008;112:9013.
236. Ueno K, Inaba A, Kondoh M, Watanabe M. Colloidal stability of bare and polymer-grafted silica nanoparticles in ionic liquids. Langmuir. 2008;24:5253.
237. Ueno K, Inaba A, Sano Y, Kondoh M, Watanabe M. A soft glassy colloidal array in ionic liquid, which exhibits homogeneous, non-brilliant and angle-independent structural colours. Chem Commun. 2009;3603–3605.
238. Yoshimura D, Yokoyama T, Nishi T, et al. Electronic structure of ionic liquids at the surface studied by UV photoemission. J Electron Spectrosc Relat Phenom. 2005;144–147:319.
239. Smith EF, Rutten FJM, Villar-Garcia IJ, Briggs D, Licence P. Ionic liquids in vacuo: analysis of liquid surfaces using ultra-high-vacuum techniques. Langmuir. 2006;22:9386.
240. Höfft O, Bahr S, Himmerlich M, Krischok S, Schaefer JA, Kempter V. Electronic structure of the surface of the ionic liquid [EMIM][Tf2N] studied by metastable impact electron spectroscopy (MIES), UPS, and XPS. Langmuir. 2006;22:7120.
241. Aliaga C, Santos CS, Baldelli S. Surface chemistry of room-temperature ionic liquids. Phys Chem Chem Phys. 2007;9:3683.
242. Nakajima K, Ohno A, Suzuki M, Kimura K. Observation of molecular ordering at the surface of trimethylpropylammonium bis(trifluoromethanesulfonyl)imide using high-resolution Rutherford backscattering spectroscopy. Langmuir. 2008;24:2282.
243. Lovelock KRJ, Kolbeck C, Cremer T, et al. Influence of different substituents on the surface composition of ionic liquids studied using ARXPS. J Phys Chem B. 2009;113:2854.
244. Rutten FJM, Tadesse H, Licence P. Rewritable imaging on the surface of frozen ionic liquids. Angew Chem Int Ed. 2007;46:4163.
245. Dyson PJ, Henderson MA, McIndoe JS. Ionic liquids: solutions for electrospray ionisation mass spectrometry. In: Plechkova NV, Rogers RD, Seddon KR, eds. Ionic Liquids: From Knowledge to Application. Washington, D.C: American Chemical Society; 2009;135–146. ACS Symposium Series vol. 1030.
246. Kanai K, Nishi T, Iwahashi T, et al. Electronic structures of imidazolium-based ionic liquids. J Electron Spectrosc Relat Phenom. 2009;174:110.
247. Holzweber M, Pittenauer E, Hutter H. Investigation of ionic liquids under Bi-ion and Bi-cluster ions bombardment by ToF-SIMS. J Mass Spectrom. 2010;45:1104.
248. Fujiwara Y, Saito N, Nonaka H, et al. Time-of-flight secondary ion mass spectrometry (TOF-SIMS) using the metal-cluster-complex primary ion of Ir4(CO)7+. Surf Interface Anal. 2011;43:245.
249. Niedermaier I, Kolbeck C, Taccardi N, et al. Organic reactions in ionic liquids studied by in situ XPS. ChemPhysChem. 2012;13:1725.
250. Bovio S, Podestà A, Milani P. Investigation of Interfacial Properties of Supported [C4mim][NTf2] Thin Films by Atomic Force Microscopy. In: Plechkova NV, Rogers RD, Seddon KR, eds. Ionic Liquids: From Knowledge to Application. Washington, D.C: American Chemical Society; 2009;273–290. ACS Symposium Series vol. 1030.
251. Atkin R, Warr GG. Bulk and interfacial nanostructure in protic room temperature ionic liquids. In: Plechkova NV, Rogers RD, Seddon KR, eds. Ionic Liquids: From Knowledge to Application. Washington, D.C: American Chemical Society; 2009;317–333. ACS Symposium Series vol. 1030.
252. Atkin R, El Abedin SZ, Hayes R, Gasparotto LHS, Borisenko N, Endres F. AFM and STM studies on the surface interaction of [BMP]TFSA and [EMIM]TFSA ionic liquids with Au(111). J Phys Chem C. 2009;113:13266.
253. Yokota Y, Harada T, Fukui K. Direct observation of layered structures at ionic liquid/solid interfaces by using frequency-modulation atomic force microscopy. Chem Commun. 2010;46:8627.
254. Labuda A, Grütter P. Atomic force microscopy in viscous ionic liquids. Langmuir. 2012;28:5319.
255. Ichii T, Fujimura M, Negami M, Murase K, Sugimura H. Frequency modulation atomic force microscopy in ionic liquid using quartz tuning fork sensors. Jpn J Appl Phys. 2012;51:08KB08.
256. Borisenko N, El Abedin SZ, Endres F. An in-situ STM and DTS study of the extremely pure [EMIM]FAP/Au(111) interface. Chemphyschem. 2012;13:1736.
257. C. Pereira, I. Ferreira, L.C. Branco, I.C.A. Sandu, T. Busani, Atomic Force Microscopy as a Valuable Tool in an Innovative Multi-Scale and Multi-Technique Non-Invasive Approach to Surface Cleaning Monitoring, Youth in the Conservation of Cultural Heritage, YOCOCU 2012, Antwerpen, Belgium, June 2012. www.yococu.com/6_Paintings/A-08.doc.
258. Anthony JL, Maginn EJ, Brennecke JF. Solution thermodynamics of imidazolium-based ionic liquids and water. J Phys Chem B. 2001;105:10942.
259. Wong DSH, Chen JP, Chang JM, Chou CH. Phase equilibria of water and ionic liquids [emim][PF6] and [bmim][PF6]. Fluid Phase Equilib. 2002;194–197:1089.
260. McFarlane J, Ridenour WB, Luo H, Hunt RD, DePaoli DW, Ren RX. Room temperature ionic liquids for separating organics from produced water. Sep Sci Technol. 2005;40:1245.
261. Couling DJ, Bernot RJ, Docherty KM, Dixon JK, Maginn EJ. Assessing the factors responsible for ionic liquid toxicity to aquatic organisms via quantitative structure–property relationship modeling. Green Chem. 2006;8:82.
262. Freire MG, Santos LMNBF, Fernandes AM, Coutinho JAP, Marrucho IM. An overview of the mutual solubilities of water–imidazolium-based ionic liquids systems. Fluid Phase Equilib. 2007;261:449.
263. Kakiuchi T. Mutual solubility of hydrophobic ionic liquids and water in liquid–liquid two-phase systems for analytical chemistry. Anal Sci. 2008;24:1221.
264. Ranke J, Othman A, Fan P, Müller A. Explaining ionic liquid water solubility in terms of cation and anion hydrophobicity. Int J Mol Sci. 2009;10:1271.
265. Řehák K, Morávek P, Strejc M. Determination of mutual solubilities of ionic liquids and water. Fluid Phase Equilib. 2012;316:17.
266. Zhou T, Chen L, Ye Y, et al. An overview of mutual solubility of ionic liquids and water predicted by COSMO-RS. Ind Eng Chem Res. 2012;51:6256.
267. Integrated Laboratory Systems, Inc., Ionic Liquids 1-butyl-3-methylimidazolium chloride (CAS No. 79917-90-1), 1-butyl-1-methylpyrrolidinium chloride (CAS No. 479500-35-1) n-butylpyridinium chloride (CAS No. 1124-64-7). Review of Toxicological Literature, Report for National Toxicology Program (NTP)/National Institute of Environmental Health Sciences (NIEHS), Research Triangle Park, NC, 2004. http://ntp.niehs.nih.gov/ntp/htdocs/Chem_Background/ExSumPdf/Ionic_liquids.pdf.
268. Scammells PJ, Scott JL, Singer RD. Ionic liquids: the neglected issues. Aust J Chem. 2005;58:155.
269. Docherty KM, Kulpa Jr CF. Toxicity and Antimicrobial Activity of Imidazolium and Pyridinium Ionic Liquids. Green Chem. 2005;7:185.
270. Zhao D, Liao Y, Zhang Z. Toxicity of ionic liquids. CLEAN: Soil, Air, Water. 2007;35:42.
271. Ranke J, Müller A, Bottin-Weber U, et al. Lipophilicity parameters for ionic liquid cations and their correlation to in vitro cytotoxicity. Ecotoxicol Environ Saf. 2007;67:430.
272. Ranke J, Stolte S, Störmann R, Arning J, Jastorff B. Design of sustainable chemical products – the example of ionic liquids. Chem Rev. 2007;107:2183.
273. Pham TPT, Cho CW, Yun YS. Environmental fate and toxicity of ionic liquids: a review. Water Res. 2010;44:352.
274. Frade RFM, Afonso CAM. Impact of ionic liquids in environment and humans: an overview. Hum Exp Toxicol. 2010;29:1038.
275. Coleman D, Gathergood N. Biodegradation studies of ionic liquids. Chem Soc Rev. 2010;39:600.
276. Wood N. Toxicity of Ionic Liquids and Organic Solvents towards Escherichia coli and Pseudomonas putida. Manchester, UK: The University of Manchester; 2011; Ph.D. Dissertation.
277. Liwarska-Bizukojc E, Gendaszewska D. Removal of imidazolium ionic liquids by microbial associations: study of the biodegradability and kinetics. J Biosci Bioeng. 2013;115:71.
278. The UFT/Merck Ionic Liquids Biological Effects Database. Bremen, Germany: University of Bremen; 2012; In: http://www.il-eco.uft.uni-bremen.de; 2012.
279. Arning J, Stolte S, Böschen A, et al. Qualitative and quantitative structure activity relationships for the inhibitory effects of cationic head groups, functionalised side chains and anions of ionic liquids on acetylcholinesterase. Green Chem. 2008;10:47.
280. García-Lorenzo A, Tojo E, Tojo J, et al. Cytotoxicity of selected imidazolium-derived ionic liquids in the human caco-2 cell line Sub-structural toxicological interpretation through a QSAR study. Green Chem. 2008;10:508.
281. Torrecilla JS, García J, Rojo E, Rodríguez F. Estimation of toxicity of ionic liquids in leukemia rat cell line and acetylcholinesterase enzyme by principal component analysis, neural networks and multiple lineal regressions. J Hazard Mater. 2009;164:182.
282. Alvarez-Guerra M, Irabien A. Design of ionic liquids: an ecotoxicity (Vibrio fischeri) discrimination approach. Green Chem. 2011;13:1507.
283. Wood N, Stephens G. Accelerating the discovery of biocompatible ionic liquids. Phys Chem Chem Phys. 2010;12:1670.
284. Zhang S, Sun N, He X, Lu X, Zhang X. Physical properties of ionic liquids: database and evaluation. J Phys Chem Ref Data. 2006;35:1475.
285. Zhang S, Lu X, Zhou Q, Li X, Zhang X, Li S, eds. Ionic Liquids: Physicochemical Properties. Oxford, UK: Elsevier; 2009.
286. Stark A, van den Berg J-A. The Handbook of Ionic Liquids: Data. New York, NY: Wiley-Interscience; 2011.
287. Reaxys Overview. Amsterdam, The Netherlands: Reed Elsevier Properties SA; 2012; In: www.reaxys.com; 2012.
288. SciFinder, Chemical Abstracts Service, American Chemical Society, Columbus, OH, 2012. www.cas.org/products/scifinder.
289. Beilstein Handbuch der organischen Chemie. Hamburg and Leipzig, Germany: Leopold Voss Verlag; 1998.
290. Gmelin Handbook of Inorganic and Organometallic Chemistry. Berlin and Heidelberg, Germany: Springer Verlag; 1997.
291. IL THERMO Ionic Liquids Database. Boulder, CO: National Institute of Science and Technology; 2012; NIST Standard Reference Database #147 http://ilthermo.boulder.nist.gov/ILThermo/mainmenu.uix; 2012.
292. DDBSP 2011 – Ionic Liquids, Dortmund Data Bank Software & Separation Technology GmbH, Oldenburg, Germany, 2012. www.ddbst.com.
293. Delph-IL Ionic Liquids Database. Dailin, China: Novionic Inc; 2012; In: http://delphil.net/web/html/features.html; 2012.
294. Deep Eutectic Solvent, Wikipedia Article, 2012. http://en.wikipedia.org/w/index.php?oldid=493816010.
295. Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V. Novel solvent properties of choline chloride/urea mixtures. Chem Commun. 2003;70–71.
296. Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK. Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc. 2004;126:9142.
297. Abbott AP, Capper G, Davies DL, McKenzie KJ, Obi SU. Solubility of metal oxides in deep eutectic solvents based on choline chloride. J Chem Eng Data. 2006;51:1280.
298. Abbott AP, Barron JC, Ryder KS, Wilson D. Eutectic-based ionic liquids with metal-containing anions and cations. Chem Eur J. 2007;13:6495.
299. Morrison HG, Sun CC, Neervannan S. Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles. Int J Pharm. 2009;378:136.
300. Haerens K, Matthijs E, Chmielarz A, Van der Bruggen B. The use of ionic liquids based on choline chloride for metal deposition: a green alternative? J Environ Manage. 2009;90:3245.
301. Kareem MA, Mjalli FS, Ali Hashim M, Al-Nashef IM. Phosphonium-based ionic liquids analogues and their physical properties. J Chem Eng Data. 2010;55:4632.
302. Al Nashef IM, Al Zahrani SM. Process for the destruction of halogenated hydrocarbons and their homologous/analogous in deep eutectic solvents at ambient conditions. U.S. Patent No. 7,812,211 2010.
303. Al Nashef IM, Al Zahrani SM. Method for the preparation of reactive hydrogen peroxide in deep eutectic solvents. U.S. Patent No. 7,763,768 2010.
304. Ciocirlan O, Iulian O, Croitoru O. Effect of temperature on the physico-chemical properties of three ionic liquids containing choline chloride. Rev Chim (Bucharest). 2010;61:721.
305. Miller RF. Deep eutectic solvents and applications. U.S. Patent No. 8,022,014 2011.
306. Hall M, Bansal P, Lee JH, Realff MJ, Bommarius AS. Biological pretreatment of cellulose: enhancing enzymatic hydrolysis rate using cellulose-binding domains from cellulases. Bioresour Technol. 2011;102:2910.
307. Zhang Q, De Oliveira Vigier K, Royer S, Jérôme F. Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev. 2012;41:7108.
308. Thanu DPR, Raghavan S. Benign Deep Eutectic Solvents for Replacement of Organic Solvents Based Cleaning Formulations in BEOL Cleaning. TECHCON Proceedings Semiconductor Research Corporation 2010; pp. 1–4.
309. Thanu DPR, Raghavan S, Keswani M. Use of urea-choline chloride eutectic solvent for back end of line cleaning applications. Electrochem Solid-State Lett. 2011;14:H358.
310. Thanu DPR. Use of Dilute Hydrofluoric Acid and Deep Eutectic Solvent Systems for Back End of Line Cleaning in Integrated Circuit Fabrication. Tucson, AZ: University of Arizona; 2011; Ph.D. Dissertation.
311. Thanu DPR, Raghavan S, Keswani M. Effect of water addition to choline chloride-urea deep eutectic solvent (DES) on the removal of post-etch residues formed on copper. IEEE Trans Semicond Manuf. 2012;25:516.
312. Taubert J, Keswani M, Raghavan S. Post-etch residue removal using choline chloride–malonic acid deep eutectic solvent (DES). Microelectron Eng. 2013;102:81.
313. Borghi EB, Ali SP, Morando PJ, Blesa MA. Cleaning of stainless steel surfaces and oxide dissolution by malonic and oxalic acids. J Nucl Mater. 1996;229:115.
314. García D, Bruyère VIE, Bordoni R, Olmedo AM, Morando PJ. Malonic acid: a potential reagent in decontamination processes for Ni-rich alloy surfaces. J Nucl Mater. 2011;412:72.
315. Durkee JB. Will ionic liquids be useful cleaning chemicals? Controlled Environ Mag. January 2008;11:29.
316. Rakita PE. Challenges to the Commercial Production of Ionic Liquids. In: Rogers RD, Seddon KR, eds. Ionic Liquids as Green Solvents. Washington, D.C: American Chemical Society; 2003;32–40. ACS Symposium Series vol. 856 (Chapter 3).
317. S.S. Seelig, A. O’Lenick, Green solvents and ionic liquids: formulating for the sustainable future, in: Proceedings of the 101st American Oil Chemists’ Society Annual Meeting and Exposition, Phoenix, AZ, 2010. http://www.aocs.org/files/ampresentation/35844_fulltext.pdf.
318. Koch VR, Nanjundiah C, Carlin RT. Hydrophobic ionic liquids. U.S. Patent 5,827,602 1998.
319. Mertens B, Mondin M, Andries N, Massaux J. All purpose liquid cleaning composition comprising anionic, amine oxide and EO-BO nonionic surfactant. U.S. Patent 6,020,296 2000.
320. Abbott AP, Davies DL, Capper G, Rasheed RK, Tambyrajah V. Ionic liquids and their use as solvents. WIPO Patent WO 2002/026701 2002.
321. McEwen AB. Cyclic delocalized cations connected by spacer groups. U.S. Patent 6,513,241 2003.
322. Price KN, Hartshorn RT, Rohrbaugh RH, et al. Ionic liquid based products and method of using the same. U. S. Patent Application 20060240728 2006.
323. Binnemans K, Görller-Walrand AC, Nockemann P, Thijs B. Novel ionic liquids. WIPO Patent WO 2007/147222 2007.
324. Bacardit R, Giordani P, Rigamonti M, Bankmann D, Del Mar Combarros Gracia M. Ionic liquid composition for the removal of oxide scale. WIPO Patent Application WO 2010/052123 2010.
325. Hecht SE, Cron SL, Scheibel JJ, et al. Ionic liquids derived from surfactants. U.S. Patent Application 2010/0099314 2010.
326. Hecht SE, Miracle GS, Cron SL, Showell MS. Ionic liquids derived from peracid anions. U.S. Patent 7,786,065 2010.
327. Hecht SE, Price KN, Berger PS, et al. Multiphase cleaning compositions having ionic liquid phase. U.S. Patent 7,928,053 2011.
328. Miller RF. Deep eutectic solvents and their applications. U.S. Patent 8,022,014 2011.
329. Chauvin Y, Magna L, Niccolai GP, Basset J-M. Imidazolium salts and the use of these ionic liquids as a solvent. U.S. Patent 7,915,426 2011.
330. Dai S, Luo H. Synthesis of ionic liquids. U.S. Patent 8,049,026 2011.
331. Small RJ. Semiconductor cleaning using superacids. U.S. Patent 7,923,424 2011.
332. T. Beyersdorff, T. Schubert, Ionic liquids as antistatic additives in surface cleaning processes, in: Proceedings of the 44th International Detergency Conference, Düsseldorf, Germany, 2009.
333. Bösmann A, Schubert T. Identification of Industrial Applications for Ionic Liquids: High-Performance-Additives for the Use in Hi-Tech-Cleaning-Solutions. Heilbronn, Germany: IoLiTec Ionic Liquids Technologies GmbH; 2012; Poster www.iolitec.de/en/Poster/Page-4.html; 2012.
334. Beyersdorff T, Schubert T. Ionic Liquids as Antistatic Additives in Cleaning Solutions – The Wandres Process. Heilbronn, Germany: IoLiTec Ionic Liquids Technologies GmbH; 2012; In: www.aails.com/is_ia_wp.asp; 2012.
335. Ingomat-cleaner CF 05 – Micro-Cleaning of Flat Surfaces, Product Information, Wandres Micro-Cleaning GmbH, Buchenbach, Germany, 2012. www.wandres.com.
336. Schwab P, Kempka S, Seiler M, Gloeckler B. Performance additives for improving the wetting properties of ionic liquids on solid surfaces. U.S. Patent Application 2010/0029519 2010.
337. Herzog H, Schwab P, Naumann M. Process for producing antistatically treated artificial stone for flat structures. U.S. Patent Application 2010/0192814 2010.
338. ITB Inc. Precision Cleaning of Oxygen Systems and Components. Final Report NASA/CR-2009–214757 2008.
339. ITB Inc. Precision Cleaning of Oxygen Systems and Components Phase II – Oxygen Cleaning Products Preliminary Testing. Final Report NASA DO27 FR O2Clean RM 09 25 09 F 2009.
340. Malbosc F, Bevon M, Fantini S, Olivier-Bourbigou H. Procédé de nettoyage de surfaces mettant en oeuvre un liquide ionique de type protique. WIPO Patent WO 2010/040917 2009.
341. Yerty J, Klingenberg M, Berman E, Voevodin N. Are ionic liquids right for your parts cleaning Job? Prod Finish Mag. April 2012;76:38.
342. Yerty J, Klingenberg M, Berman E, Voevodin N. Ionic liquids for cleaning operations at Air Force logistics centers: part II. Prod Finish Mag. October 2012;76:38 In: http://www.pfonline.com/articles/ionic-liquids-for-cleaning-operations-at-air-force-air-logistics-centers-part-ii; October 2012.
343. I. Ivanov, B. Soklev, S. Karakashev, Cleaning properties of ionic liquids, Second Workshop on Size-Dependent Effects in Materials for Environmental Protection and Energy application, SIZEMAT2, Nessebar, Bulgaria, 2010. http://sizemat2.igic.bas.bg/abstracts/SizeMat2_Abstract_Ivan_Ivanov_Topic_B.pdf.
344. Krumpfer JW, Bian P, Zheng P, Gao L, McCarthy TJ. Contact angle hysteresis on superhydrophobic surfaces: an ionic liquid probe fluid offers mechanistic insight. Langmuir. 2011;27:2166.
345. K. Shukri, New Ionic Liquid Solvent Technology to Transform Metal Finishing. Products and Processes (IONMET), IONMET Report, Deutsche Gesellschaft für Galvano- und Oberflächentechnik e.V., Hilden, Germany, 2007. www.ionmet.org.
346. Abbott AP, Ryder KS, König U. Electrofinishing of metals using eutectic based ionic liquids. Trans Inst Met Finish. 2008;86:196.
347. Collins J. Electropolishing Using Ionic Liquids. Chester, UK: C-Tech Innovation Ltd; 2008; In: http://www.prosurf-online.eu/fileadmin/documents/training/20080221_Birmingham/8_CTECH_JC_Ionmet_polishing.pdf; 2008.
348. Cherginets V. Oxide Solubilities in Ionic Melts. In: Wypych G, ed. Handbook of Solvents. Toronto, Canada: ChemTec Publishing; 2001;1484–1496. (Chapter 21.3).
349. Schwab P. Use of ionic liquids as an additive for cleaning processes and/or supercritical gas. U.S. Patent Application 2010/0016205 2010.
350. Painter P, Williams P, Mannebach E, Lupinsky A. Systems, methods and compositions for the separation and recovery of hydrocarbons from particulate matter. U.S. Patent Application 2011/0042318 2011.
351. Painter P, Williams P, Mannebach E, Lupinsky A. Analogue ionic liquids for the separation and recovery of hydrocarbons from particulate matter. U.S. Patent Application 2012/0048783 2012.
352. Wastes – Hazardous Waste – Treatment, Storage & Disposal (TSD). Washington, D.C.: U.S. Environmental Protection Agency; 2012; In: www.epa.gov/osw/hazard/tsd/index.htm; 2012.
353. Al Nashef IM, Al Zahrani SM. Method for the preparation of reactive hydrogen peroxide in deep eutectic solvents. U.S. Patent 7,763,768 2010.
354. Al Nashef IM, Al Zahrani SM. Process for the destruction of halogenated hydrocarbons and their homologous/analogous in deep eutectic solvents at ambient conditions. U.S. Patent 7,812,211 2010.
355. Nelson WM. Ionic Liquid as Solvent, Catalyst Support: Chemical Agent Decontamination and Detoxification. Research Triangle Park, NC: Army Research Office; 2004; Report 45571.1-CH, U.S.
356. Zech O, Harrar A, Kunz W. Nonaqueous Microemulsions Containing Ionic Liquids – Properties and Applications. In: Kokorin A, ed. Ionic Liquids: Theory, Properties, New Approaches. Rijeka, Croatia: InTech Publishing – Open Access Company; 2011;245–270. www.intechopen.com/books/ionic-liquids-theory-properties-new-approaches; 2011; (Chapter 11).
357. Carson L, Chau PKW, Earle MJ, et al. Antibiofilm activities of 1-alkyl-3-methylimidazolium chloride ionic liquids. Green Chem. 2009;11:492.
358. Hough-Troutman WL, Smiglak M, Griffin S, et al. Ionic liquids with dual biological function: sweet and anti-microbial, hydrophobic quaternary ammonium-based salts. New J Chem. 2009;33:26.
359. Busetti A, Crawford DE, Earle MJ, et al. Antimicrobial and antibiofilm activities of 1-alkylquinolinium bromide ionic liquids. Green Chem. 2010;12:420.
360. Ismail MH, El-Harbawi M, Noaman YA, et al. Synthesis and anti-microbial activity of hydroxylammonium ionic liquids. Chemosphere. 2011;84:101.
361. Gilmore BF, Earle MJ. Development of ionic liquid biocides against microbial biofilms Designer microbicides for infection control. Chem Today. 2011;29:50.
362. Gilmore BF. Antimicrobial Ionic Liquids. In: Kokorin A, ed. Ionic Liquids: Applications and Perspectives. Rijeka, Croatia: InTech Publishing – Open Access Company; 2011;587–603. (Chapter 26).
363. Sekhon BS. Ionic liquids: pharmaceutical and biotechnological applications. Asian J Pharm Biol Res. 2011;1:395.
364. Walker A. Ionic Liquids for Natural Product Extraction. Heslington, York, UK: White paper, Bioniqs Ltd; 2008; In: http://www.slideserve.com/swaantje/ionic-liquids-for-natural-product-extraction-adam-walker-bioniqs-ltd; 2008.
365. Carmona N, Villegas MA, Fernández Navarro JM. Characterisation of an intermediate decay phenomenon of historical glasses. J Mater Sci. 2006;41:2339.
366. A. Machado, P. Redol, L. Branco, M. Vilarigues, Medieval stained glass cleaning with ionic liquids, in: Proceedings of the IIC 2010 Congress – Conservation and the Eastern Mediterranean, Istanbul, Turkey, 2010. http://www.iiconservation.org/node/2769/c2010machado.pdf.
367. A. Machado, P. Redol, L. Branco, M. Vilarigues, Ionic liquids for medieval stained glass cleaning: a new frontier, ICOM-CC Lisbon 2011: Sixteenth Triennial Conference, International Council of Museums – Committee for Conservation, Preprints CD, ISBN: 978-989-97522-0-7, 2011.
368. C. Pereira, I.C.A. Sandu, L. Branco, T. Busani, I. Ferreira, Innovative Multi-Scale and Multi-Technique Approach to the Study of Enzyme-Based Cleaning of Varnish Layers, Second International Workshop on Physical and Chemical Analytical Techniques in Cultural Heritage, Lisbon, Portugal, 2012. http://cheri.cii.fc.ul.pt/BOOK_Abstracts_2nd_WORKSHOP_P&CATCH.pdf.
369. von Gilsa B. Gemäldereinigung mit Enzymen, Harzseifen und Emulsionen. Zeit Kunsttechnologie Konservierung. 1991;5:48.
370. Todaro C. Gil enzimi: limiti e potenzialità nel campo della pulitura delle pitture murali. In: Biscontin G, Driussi G, eds. XXI International Congress Scienza e Beni Culturali: Sulle Pitture Murali. Venice, Italy: Riflessione, Cconoscenze, Interventi, Arcadia Ricerche; 2005;487–496. In: http://www.arcadiaricerche.it/editoria/2005.htm; 2005.
371. Palmer BJ, Fu D, Card R, Miller MJ. Scale removal. U.S. Patent 6,924,253 2005.
372. Kalb R, Hofstätter H. Method of treating a borehole and drilling fluid. U.S. Patent Application 2012/0103614 2012.
373. Swatloski RP, Spear SK, Holbrey JD, Rogers RD. Dissolution of cellulose with ionic liquids. J Am Chem Soc. 2002;124:4974.
374. Wang H, Gurau G, Rogers RD. Ionic liquid processing of cellulose. Chem Soc Rev. 2012;41:1519.
375. Grävsik J, Raut DG, Mikkola J-P. Challenges and Perspectives of Ionic Liquids vs Traditional Solvents for Cellulose Processing. In: Mun J, Sim H, eds. Handbook of Ionic Liquids: Properties, Applications and Hazards. Hauppauge, NY: Nova Science Publishers; 2012;1–34. (Chapter 1).
376. Processing Cellulose with Ionic Liquids. Ludwigshafen, Germany: White Paper, BASF SE; 2012; In: http://www.intermediates.basf.com/chemicals/web/en/content/products-and-industries/ionic-liquids/applications/cellulose_processing; 2012.
377. Kulkarni PS, Afonso CAM. Deep desulfurization of diesel fuel using ionic liquids: current status and future challenges. Green Chem. 2010;12:1139.
378. Martínez-Palou R, Sánchez PF. Perspectives of Ionic Liquids Applications for Clean Oilfield Technologies. In: Kokorin A, ed. Ionic Liquids: Theory, Properties, New Approaches. Rijeka, Croatia: InTech Publishing – Open Access Company; 2011;567–630. www.intechopen.com/books/ionic-liquids-theory-properties-new-approaches; 2011; (Chapter 24).
379. Davey P. Application study: ionic liquids in consumer products. Perfum and Flavor Mag. April 2008; In: http://www.perfumerflavorist.com/fragrance/application/industrial/16761536.html; April 2008.
380. O’Lenick Jr AJ. Comparatively speaking: simple salt vs ionic liquid. Cosmet Toiletries Mag. November 2010; In: http://www.cosmeticsandtoiletries.com/research/techtransfer/108669424.html; November 2010.
381. Gem Sparkle Ionic Cleaning Liquid, Kassoy LLC, Plainview, NY, 2012. www.kassoy.com/gem-sparkle-ionic-cleaning-liquid.html.
2The liquid crystal state (mesophase) exists within some temperature range, Tm < T < Tc, where Tm is the temperature of melting from solid state into a mesophase, and Tc is the clearing temperature, when the liquid crystal transforms into an isotropic liquid. At the clearing temperature Tc, the mesophase melts into an isotropic liquid with no positional and orientational order.
1Throughout this chapter ionic liquids (ILs) and room temperature ionic liquids (RTILs) will be used interchangeably. Most surface cleaning applications employ RTILs.