Case 3. Improving Stanford Blood Center’s Platelet Supply Chain1
1 This case was prepared by Yenho Thomas Chung (LG CNS Entrue Consulting Partners), Professor Feryal Erhun (Stanford University), and Professor Tim Kraft (University of Virginia) based on field research of an actual business situation. Some names, dates, and data are disguised, and some material is fictionalized for pedagogical reasons. It was written as a basis for class discussion rather than to illustrate effective or ineffective handling of an administrative situation. The authors would like to thank their collaborators at Stanford Blood Center and Stanford University Medical Center for their efforts.
† LG CNS Entrue Consulting Partners, Seoul, South Korea; [email protected]
‡ Stanford University, Stanford, California, USA; [email protected]
* University of Virginia, Charlottesville, Virginia, USA; [email protected]
William leaned back in his chair and looked at the platelet outdate numbers for April again. One-third of the platelets outdated—how could this be? William’s boss and the platelet donors would not be pleased. As the project manager for Stanford Blood Center’s (SBC) process improvement initiative, William knew that he and his team had to do something fast; otherwise, SBC would start to lose valuable donors and incur platelet shortages. Due to the short shelf-life for platelets (five days) and volatile demands, it was critical that SBC carefully manage the platelet supply process, which included distributing platelets to local hospitals as well as collecting and redistributing unused platelet inventory among those hospitals. However, as April’s numbers demonstrated, there were significant flaws in SBC’s platelet supply process.
Because his team was mainly composed of medical personnel and staff, William decided to seek outside support for SBC’s platelet process problem. A few months earlier, he had read an article in the Stanford Report on risk management in supply chains by Professor Feryal Erhun from Stanford University’s Department of Management Science & Engineering (MS&E). William recognized many of the symptoms that Prof. Erhun discussed in her article in the SBC supply process. Now he just hoped this afternoon’s meeting with Prof. Erhun and one of her Ph.D. students would provide insights into SBC’s supply problem.
Stanford Blood Center: “Give Blood for Life”2
Located in Palo Alto, CA, Stanford Blood Center (SBC) is a not-for-profit organization, which was established in 1978 to meet the increasing needs of Stanford University Medical Center (SUMC), which comprised Stanford University Hospital and Lucile Salter Packard Children’s Hospital. Since then SBC has expanded its scope to serve El Camino Hospital, the Palo Alto VA, and O’Connor Hospital. In addition to providing blood testing and transfusion services, SBC also acts as a teaching and research setting for Stanford medical students and faculty. It is the second-largest transfusion facility in the U.S with approximately 60,000 blood donations and 100,000 blood products for medical use per year.2
SBC has always been on the forefront of transfusion medicine. For example, in 1983, two years before the AIDS virus antibody test was developed, SBC became the first blood center to screen for AIDS-contaminated blood. In 1987, SBC became the first blood center in the U.S. to screen donors for HTLV-I, a virus believed to cause a form of adult leukemia. Additionally, SBC was the first blood center in the world to routinely test for cytomegalovirus (CMV) and provide CMV-negative blood for immuno-compromised transfusion recipients, and SBC was among the first in the U.S. to provide human leukocyte antigen (HLA) compatible platelets.2
Although most medical institutions are known to be conservative and reluctant to share their information with outsiders, SBC has always fostered a collaborative environment. This is why William sought out the help of the MS&E team. However, even with the support of the MS&E team, William knew that the sweeping improvements SBC desired could not be achieved without the cooperation of SBC’s largest customer, SUMC. William had unsuccessfully approached SUMC staff previously about working together to improve the supply process. Without solid evidence that there were inefficiencies in the supply chain between SBC and SUMC, it was difficult to convince SUMC staff that collaboration between SBC and SUMC would improve the system efficiency. To get the SUMC staffs’ attention this time, William would need tangible evidence of the inefficiencies in the current supply chain.
Platelets: Perishable Inventory with Five-Day Shelf Life
Platelets are small cell fragments found in the blood plasma of mammals. Platelets are responsible for starting the formation of blood clots when bleeding occurs and thus are often transfused to patients to treat or prevent bleeding during surgeries. There are no artificial substitutes for platelets; platelets transfused to a patient must be collected from another human being. In addition, the donor pool for platelets is limited due to restrictions on the number of times a donor can donate per year and the often lengthy donation process.
Platelets can either be isolated from whole blood donations or collected by an apheresis process that requires sophisticated instrumentation and a highly trained support staff. Whole blood donations are usually collected from random walk-in donors in mobile stations or local blood collection centers. Whole-blood-derived platelets are then split from a unit of whole blood that has not been cooled yet. A whole unit of blood contains not only platelets but also red blood cells, white blood cells, and plasma. Therefore, the amount of whole-blood-derived platelets from a single bag is typically not significant. In contrast, an apheresis device draws blood from a donor and centrifuges the collected blood to separate out platelets and other components. Because the remaining blood is returned to the donor during the process, an apheresis donor can provide more platelets than a whole-blood donor can. Typically, an apheresis donor can donate at least one therapeutic dose, whereas generating a therapeutic dose from whole-blood-derived platelets requires multiple donations and multiple donors. Because apheresis platelets come from a single donor, they are usually less risky in terms of transfusion-transmitted disease, especially since apheresis platelet donors are registered and closely monitored by the blood center staff. In addition, because an apheresis dose does not contain as many red blood cells as a whole-blood-derived dose, apheresis platelets do not have to be cross-matched in terms of blood types (i.e., ABO and Rh+/-).
One of the most challenging aspects regarding platelet inventory management is the extremely short shelf-life for platelets. Whereas some European countries allow 7 days of shelf life, in the U.S., Food and Drug Administration (FDA) regulation requires that every unused platelet unit be discarded after 5 days. In addition, due to the 48-hour testing process that is required right after the donation, the practical shelf life of platelets is actually only 3 days in the U.S. In part due to this short shelf life, 10.9% of apheresis platelet units collected in the U.S. went outdated in 2006; i.e., they expired without being transfused. Therefore, to maintain a reasonably high service level and a low outdate rate, the balance between the supply and the demand for platelets must be closely monitored.
SBC’s Platelet Supply Chain
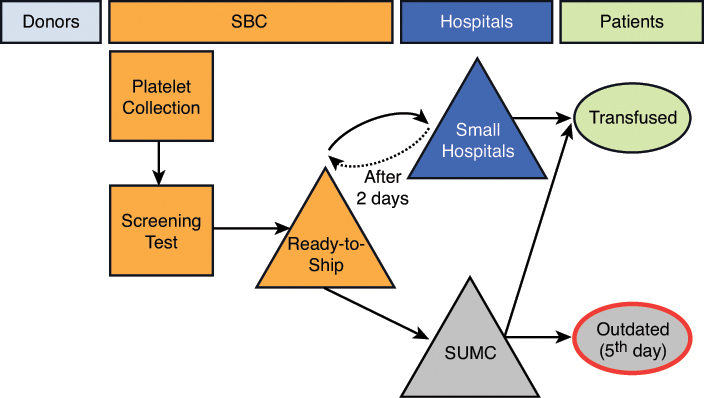
Similar to a typical blood supply chain, SBC’s platelet supply chain starts with the collection of platelets from donors. Once collected, the platelets are then processed, tested, and delivered to the hospitals where they are either transfused to patients or outdated as shown in Figure 3-1. There are three critical processes within this supply chain: the collection process, the rotation process, and the issuing process.
The Collection Process: Platelet Collection and Testing
On March 1, 2004, the AABB3 mandated that each blood center and transfusion service should implement methods to limit and detect bacterial contamination in all platelet components. To comply with this requirement, SBC transitioned to bacterial culture of platelet products and the exclusive use of apheresis platelets. Although SBC is well-equipped and well-staffed, collecting platelets through an apheresis process is a difficult task. While whole-blood-derived collection accommodates random walk-in donors, an apheresis process limits the effective donor pool to altruistic, consistent, and highly committed donors due to instrument immobility and the lengthy 1½–2½ hour collection time.
3 AABB is “an international, not-for-profit association representing [nearly 8,000 individuals and 2,000 institutions] involved in the field of transfusion medicine and cellular therapies” (http://www.aabb.org).
SBC’s marketing department recruits volunteers for regular apheresis platelet donation. It is not easy to recruit a new apheresis platelet donor. The volunteers donate platelets without any compensation; thus, it is very important to maintain a low outdate rate so as not to impair donors’ motivations. For new volunteers, the donor collection staff performs interviews and checks the medical history, vital signs, and physical status of the prospective donor. Once a volunteer is registered and included in a regular donor pool, the marketing department pre-schedules the donor’s visit to the center. The collection staff interviews and examines the prospective donor again to assess his or her eligibility on the day of the donation. A platelet apheresis donor may donate up to 24 times per year.
The apheresis process and platelet shelf life begins once the needle is inserted into the donor’s vein. The platelet product expires five days after the draw date but due to the requisite 48-hour testing process, the usable shelf life is only three days. All tests must comply with the standards established by the FDA. First, sample tubes collected at the time of the platelet donation are submitted for infectious disease testing (e.g., blood-borne agents such as HIV, hepatitis, and syphilis). Second, a sample of the platelet unit is cultured for bacterial growth (i.e., bacterial detection). The infectious disease testing is usually completed within 24 hours but the bacterial detection test requires 48 hours. Finally, platelets suitable for transfusion are shipped to the hospitals with up to 3 days shelf life remaining. Figure 3-2 demonstrates the platelet availability schedule based on draw date.
Currently, SBC schedules donor visits based on the facility capacity, regardless of the incoming demand or the current inventory level. Thus, the number of units collected each day is fairly constant. In order to collect the pre-determined daily amount, registered donors are scheduled several days in advance (Monday through Saturday). Based on historical collection data, the average collection level per day is 40 units Monday through Friday and 60 units on Saturday. Because there is no collection on Sunday and donors are more readily available on Saturdays, SBC collects more platelet units on Saturday.
There are several uncertainties in the platelet collection process. For instance, donors may not show up due to personal emergencies, or some donors may not donate due to their own medical status on the day of the scheduled donation. Thus, to ensure that its needs are met, SBC schedules more donors than needed to protect against unexpected loss in supply as well as unexpected spikes in demand. One additional issue with the collection schedule is that some donors can donate up to three units during a visit due to individual attributes such as higher platelet counts and body surface. In order to cope with supply uncertainties, SBC often collects the maximum number of units from each donor. Consequently, on a given day, SBC may collect a larger amount of platelets than needed; even though this then increases the risk that collected units will go outdated. Despite these precautions, unexpected platelet shortages still occur. When this happens, SBC’s own collection process typically cannot respond quickly enough to demand due to the two-day testing period. In such situations, SBC procures additional platelet units from other regional blood centers.
The Rotation Process: Supply Contracts and Platelet Rotations
SBC’s largest customer, SUMC, is primarily supplied by SBC. SUMC transfuses approximately 9,100 units of platelets per year, which consumes about 80% of SBC’s platelet supply. To utilize its remaining apheresis platelet capacity, SBC also serves small local hospitals. SBC works hard to market to these local hospitals. Although most blood centers are not-for-profit organizations, the blood product market is very competitive and generous contracts are often used to attract new customers. Due to this competition, SBC offers consignment contracts to the small local hospitals. Under a consignment contract, SBC delivers fresh platelets based on the hospital’s daily demand. After two days, the hospitals can then send any leftover or unused units back to SBC. These units, with one day of shelf life remaining, are called “short-dated units.” The small hospitals are not financially responsible for the short-dated units. They only pay for the units that they transfuse and any units that expire while still in their inventory. Consequently, in order to avoid any financial responsibility, the small hospitals return almost all unused, two-day-old units. For the small hospitals, this contract is attractive because they incur very little risk. Conversely, SBC incurs high risk since it does not know how many short-dated units a small hospital might return. When the short-dated units are returned to SBC, these units are rotated to SUMC along with fresh units. Short-dated units are sent to SUMC, because it has the highest demand among the hospitals and, therefore, provides the best opportunity for the short-dated units to be transfused before the end of the day.
SUMC orders platelets from SBC two to four times daily. The ages of platelet units delivered to SUMC differ, ranging from three or four days (fresh units) to five days (short-dated units) old. Although SBC deliveries are triggered by demands at SUMC, not every unit delivered is added to SUMC’s available inventory. On rare occasions, platelets are discarded due to cancelled transfusions, being out of refrigeration too long, or to blood center requests to return questionable products (e.g., donors providing post-donation information that affects eligibility).4 The major concern for SBC and SUMC, however, is outdated units. As there is little consistency in the age of the platelets SUMC receives, there is a high potential for many of the units to become outdated. Due to this high perishable inventory risk, SBC and SUMC have a cost-sharing contract on the outdated units, which is shown in Table 3-1.
4 Since the number of discarded units is negligible, they are not included in the data set provided.
Table 3-1 SBC and SUMC Cost-Sharing Agreement
According to an AABB survey report, U.S. hospitals paid an average of $538.72 for a unit of apheresis platelets. This cost includes procurement costs, operations costs, testing costs, and processing costs. As shown in Table 3-1, SUMC incurs 50% of the costs if it receives the product before the day of expiration (i.e., fresher units) and does not transfuse it. Alternatively, SUMC incurs no costs for outdated units when SBC ships short-dated units. SBC staff believe that this cost-sharing contract reduces the overall system outdate rate, since SUMC receives short-dated units as well as fresh units because of the pricing structure of the cost-sharing contract.
The Issuing Process: Platelet Demand in SUMC
SUMC utilizes platelets for many different types of operations, ranging from day-to-day operations to pre-scheduled or emergency surgeries; therefore, SUMC always has to stock a certain amount of platelet units in its inventory. The platelet supply chain between SBC and SUMC is completely decentralized; i.e., platelet collection and rotation decisions are made solely by SBC and platelet ordering and issuing decisions are made solely by SUMC. From SUMC’s previous ordering and issuing history, William identified that SUMC orders on average 32 platelets per day but transfuses only 25 platelet units on average per day.
A Snapshot of the SBC-SUMC Supply Chain in 2006
For their analysis, William and his team collected data for the number of transfusions and outdates during the last four months, from January 2006 to April 2006. Because obtaining this information from the small local hospitals SBC served would be difficult, the team focused solely on SUMC transactions. The data set includes unit number, draw date by SBC, expiration date, received date by SUMC, issue date by SUMC, and if the unit is outdated instead of transfused, the outdate date. Figure 3-3 demonstrates the outdate rate at SBC and the number of transfusions at SUMC from January 2006 to April 2006. Notice that in April, the outdate rate increased to 30+%, compared to 15% to 20% during the previous months. At the same time, there was a significant decrease in demand in April.
The Problem
After hearing William’s overview of the platelet supply chain, the MS&E team understood the significance of the problem. Although SBC was concerned about increasing costs, the more important issue was the donors. Maintaining a large donor base was extremely important to SBC. Because every donor wants his or her donation to be transfused rather than perish, if SBC continued to maintain a high outdate rate, it risked losing valuable donors. William and the MS&E team agreed to meet in two weeks, after the MS&E team had time to review the data and their notes.
As he led the MS&E team to the lobby of SBC, William thought again about the platelet supply process. What could be causing the high outdate numbers? Could it be the agreements SBC had set up with the hospitals? Was it the collection process itself? Or were there hidden inefficiencies in SUMC’s transfusion process? William hoped that a solution could be found.
Questions
After listening to William’s description of the platelet supply chain, the MS&E team has come up with the following list of questions to answer over the next two weeks:
1. Based on the data provided, are there any potential imbalances between the existing demand and supply patterns?
2. Identify the characteristics of outdated items at SBC/SUMC. Is there any connection between these characteristics and any of the three processes discussed (i.e., collection, rotation, and issuing)?
3. To decrease the outdate rate of platelet units, what recommendations should be made to improve the platelet supply chain between SBC and SUMC? Is there any incentive for either party to follow these recommendations?
4. Identify at least one problem in each processing area (collection, rotation, and issuing), and provide a solution for each of them.