Myocardial tissue engineering
Q.Z. Chen, Monash University, Australia
S.E. Harding, Imperial College London, UK
R. Rai and A.R. Boccaccini, University of Erlangen-Nuremberg, Germany
Abstract:
In the pursuit of engineering a functional myocardium, studies integrating highly innovative research in stem cells and biomaterials development have opened new frontiers. Stringent properties like biocompatibility, ability to foster cells, biodegradability and mechanical compliance have been the norm for material selection. Fabrication techniques implemented individually or in conjunction have been used to fabricate scaffolds that best mimic the anisotropic nature of the cardiac extracellular matrix (ECM). Such biomimetic scaffolds provide topographical cues for contact guidance to align cells required to achieve synchronous electrophysiological coupling of the engineered myocardium to the native heart. Various functionalization strategies in tandem with scaffold fabrication designs and bioreactor studies have been undertaken to achieve vascularization in the engineered cardiac construct.
Key words
myocardial tissue engineering (MTE); stem cells; biomaterials; biocompatibility; vascularization
12.1 Introduction
Heart disease is the leading cause of death and disability worldwide, accounting for nearly 40% of human mortality (Fig. 12.1). Heart tissue engineering, however, is one of the least explored areas in the rapidly evolving field of tissue engineering at least according to the number of research publications in the field (Fig. 12.2). Myocardial tissue engineering (MTE) for regeneration of the heart represents one of the most exciting areas of development, but it is also an undertaking involving multiple challenges.

Heart failure is caused by a variety of underlying diseases. The single most common cause of heart failure is a heart attack, which typically results in extensive and permanent cell loss. The cell death results in heart wall thinning and then ventricular dilation. The enlargement in ventricular volume leads to progressive structural and functional changes (called ventricular remodelling). Ventricular remodelling is initially compensatory, but subsequently adds further inefficiency to the compromised mechanical pumping of the ventricular muscles. Such inefficiency predisposes the subject to end stage heart failure, a condition in which the heart cannot pump sufficient amounts of blood to meet the metabolic requirements of the body (Burns and Kumar, 2003).
Pharmacological therapy represents the standard treatment for patients with heart disease. Interventional therapies, such as implantation of pacing devices, are receiving widespread application for patients with marked symptoms despite pharmacological therapy (Young and Mills, 2004). However, neither drug nor interventional therapies can adequately prevent disease progression to end stages (Packer, 2002). Eventually, heart transplantation is the final treatment option for end stage heart failure. Owing to the lack of organ donors and issues associated with immune rejection, new strategies to repair the injured heart are needed (Zammaretti and Jaconi, 2004; Vunjak-Novakovic et al., 2010, 2011; Eschenhagen et al., 2012). Among several tissue engineering approaches, ventricle restrain and cell therapy are currently being investigated to address the above-mentioned pathological consequences of heart attack, i.e. ventricle expansion and massive cell death. The important benefit of ventricle restraint is that a cardiac support device reduces the stress in heart muscle, thus preventing ventricular expansion, one of the more important pathophysiological mechanisms underlying the clinical cause of progressive heart failure. Cell therapy, on the other hand, aims to compensate the cell loss in the injured or diseased heart muscle.
In this chapter, we present a review of the achievements of myocardial tissue engineering, focusing on biomaterials-based strategies. A number of excellent reviews focusing on cell-based therapies for myocardial regeneration, have been published (Eschenhagen, 2005; Leor et al., 2005; Gerecht-Nir et al., 2006; Zimmermann et al., 2006; Wang and Guan, 2010; Huu et al., 2012; Soler-Botija et al., 2012).
12.2 Cell sources
While acute mortality from myocardial infarction is still a primary cause of death, faster treatment is improving death rates. However, this treatment has created a larger population with damaged heart muscle, and this is in addition to those who have cardiac compromise from drugs (such as chemotherapeutic agents), hypertension, diabetes or long-standing genetic conditions. The burden of chronic heart failure is therefore increasing even as acute cardiac death declines (Khatibzadeh et al., 2012). True cardiac regeneration will be achieved only if new contracting myocardial muscle is added to the heart and this has not been convincingly attained by current clinical strategies. The modest benefits observed after bone marrow or mesenchymal stem cell manipulations have been ascribed largely to paracrine mechanisms (Mirotsou et al., 2011), since neither pre-clinical nor clinical studies show evidence of new cardiac muscle forming to replace the scar (Martin-Rendon et al., 2008; Sussman and Murry, 2008). Final modes of benefit appear to be improved revascularization or protection of compromised myocardium from further deterioration (Tse et al., 2003; Lee et al., 2009). In this, they represent extensions of current surgical and pharmacological treatments which replace blocked blood vessels to rescue vulnerable muscle and use neurohormonal blockers to prevent further deterioration of the viable myocardium.
Production of new myocardium has clearly been a goal of the cardiovascular regenerative medicine field from the beginning: we know from left ventricular assist device experience that reversal of overloading of the failing heart produces a contractile improvement in remaining cardiomyocytes (Soppa et al., 2008), so that any additional muscle created will stimulate a virtuous circle for cardiac function. Recent evidence suggests that there is some capacity of the heart for endogenous repair from intrinsic adult cardiac stem cells (CSCs) (Kajstura et al., 2012). Generation of new cardiomyocytes from explanted and cultured CSCs is at an earlier stage, with consensus largely reached on the presence of regenerating cells within the adult myocardium but with information still needed on their defining characteristics and path to adult cardiomyocytes (Mercola et al., 2011). As candidates for exogenous delivery of cardiac muscle, autologous CSCs have the most immediate clinical potential, with CSC populations in at least four human trials in the US and Japan in 2012 (SCIPIO, CADUSEUS, ALCADIA, TICAP). Differentiation of cardiomyocytes from human embryonic and induced pluripotent stem cells (hESC, hiPSC) has been successfully achieved (Yang et al., 2008; Dixon et al., 2011) and the issues now are of maturation, scale-up, safety and delivery. While there are still concerns about the potential for undifferentiated cells within the preparation to produce tumours, the hESC/hiPSC-derived cardiomyocytes have the most clear-cut (though immature) cardiac phenotype (Zhang et al., 2009; Itzhaki et al., 2011) and are another viable candidate for heart muscle repair. For the patient-derived iPSC cells, there is also the hope that there might be an advantage in delivering these cells back to the same patient, overcoming immune barriers. Experiments comparing hESC and iPSC in mouse, however, suggest that the manipulation necessary to obtain iPSC might produce an immune response even in the same subject, so caution is again required (Okita et al., 2011).
However, for all exogenous stem cell derivatives, salutary lessons have been learned about the difficulties of delivering and retaining cells within the highly kinetic and (during disease) inflammatory milieu of the beating heart. Disappearance of introduced cells has been a highly frequent finding, with estimates of 90–99% of cells lost both rapidly after injection and then more slowly over the following days and weeks (Fukushima et al., 2007). This has prompted the widespread efforts to provide a tissue engineering solution, in order to retain the delivered cells and to support them during integration to the host. Below we will address the advances in the materials available for cardiac cell delivery, and their additional potential beneficial effects on cardiac remodelling.
12.3 Biomaterials-based strategies in myocardial tissue engineering (MTE)
In tissue engineering strategies based on the use of scaffolds, the regenerative ability of the host body should be increased through a designed construct that is populated with isolated cells and signalling molecules, aiming at regenerating functional tissue as an alternative to conventional organ transplantation and tissue reconstruction. In the following paragraphs, we review and discuss the design criteria, and potential biomaterials used in MTE approaches involving the use of scaffolds.
12.3.1 Design criteria of MTE constructs
One of the major challenges in tissue engineering is the design and fabrication of a tissue-like material to mimic extracellular matrix (ECM) of the tissue that is to be engineered. Hence, biomaterial constructs must meet several stringent criteria. Specific requirements for biomaterials used for MTE are:
• Ability to deliver cells. The material should not only be biocompatible, but also foster cell attachment, survival, proliferation and differentiation.
• Biodegradability. The composition of the material, combined with the porous structure of the scaffold, should lead to biodegradation in vivo at a rate that matches the tissue regeneration rate. In other words, a synthetic scaffold should remain in the body for as short a period as possible, at the same time it must maintain its viability long enough for the cells to make their own matrix.
• Mechanical properties. The principle in the mechanical design of the biomaterial is that it should not interrupt the normal beating process of cardiomyocytes, while providing mechanical support for the cells to attach and to secrete their own matrix.
Among these criteria, biocompability and cell-supporting and fostering abilities are of highest importance for tissue engineering. So far, numerous biocompatible and biodegradable materials, especially polymers, have been developed to support and foster cells, as described in different chapters of this book. However, a number of issues remain to be addressed, including non-linearly elastic mechanical properties, of relevance for MTE, which are discussed in the following sections.
Ideally, a construct or scaffold material should display the mechanical and functional properties of the native myocardium, such as coherent contractions, low diastolic tension, and syncytial propagation of action potentials. While natural collagens are an immediate option, their variable physical properties, including mechanical properties, with different sources of the protein matrices have hampered their application. Concerns have also arisen regarding immunogenic problems associated with the introduction of foreign collagen. As such, it is not surprising that much attention has been paid to synthetic polymers, especially elastomers, which have reproducible properties and are considered highly reliable materials for tissue engineering.
The stress–strain curves of synthetic elastomers are linear at low strains (15% is the maximal strain of living tissues), whereas those of biological tissues (such as heart muscle) are non-linear (J-shaped, as shown in Fig. 12.3). The structural mechanism behind these two different elastic behaviours is that the polymer chains are randomly tangled in a synthetic elastomer (Fig. 12.4a), whereas protein nanofibres are aligned in the muscular tissue (Fig. 12.4b). In fact cardiac muscle fibres are highly branched and hierarchically surrounded and embedded in a 3D collagen network comprising distinct endomysial, perimysial and epimysial levels of organization. This complex structure with non-linear elasticity resembles a honey-comb structure and imparts cardiac anisotropy, i.e. direction-dependent electrical and mechanical properties (Freed et al., 2009). Therefore to mimic this cardiac-like ECM in the fabricated scaffolds, various approaches of scaffold development are currently being investigated.

12.3.2 Fabrication of scaffolds for MTE
Micropatterning and microfabrication techniques have been used on soft substrates to develop scaffolds with anisotropic features and the ability to provide contact guidance to the seeded cells to achieve cell alignment (Engelmayr et al., 2008; Cimetta et al., 2009; Guillemette et al., 2010; Patel et al., 2011; Feinberg et al., 2012; Kim et al., 2012; Zhang et al., 2012). In the studies carried out by Engelmayr et al. (2008) using excimer laser microablation, 3D scaffolds of poly(glycerol sebacate) (PGS) resembling cardiac accordion-like honeycomb microstructure with anisotropic features was fabricated. The scaffold was able to promote the formation of grafts with aligned heart neonatal rat heart cells and mechanical properties closely resembling that of the native myocardium. Similar observation of cell alignment was observed when C2C12 muscle cells were seeded on PGS scaffolds with designed parallel array of grooves (Guillemette et al., 2010). In 2009, Cimetta et al. also demonstrated production of arrays of cardiac muscle myofibers on soft substrate of micro patterned hydrogel using the microcontact printing (μCP) technique. The cardiomyocytes expressed correct phenotypic markers and also exhibited spontaneous contractile activity. Nanotopographically defined hydrogel mimicking the native myocardial matrix has been also developed using autologous cardiosphere-derived cells as construct for cardiac patch application (Kim et al., 2012). The control of contractile strength in engineered cardiac muscle by hierarchical tissue architecture, enabled by the development of micropatterned scaffold, was demonstrated in the studies carried out by Feinberg et al. (2012). Increasing contractile strength with cellular alignment but with an even greater increase (of > 1000%) between isotropic and anisotropic organization was observed.
The process of electrospinning has been increasingly used for the fabrication of scaffolds for MTE applications (Kai et al., 2011a; Ravichandran et al., 2011, 2012a, b). It is a cost-effective technique, able to rapidly create nanofibrous structures resembling the architecture of native cardiac ECM using a plethora of polymeric systems. The scaffold produce are highly porous, with easy avenues for producing aligned fibers to orient cell alignment, and possessing high surface to volume ratio amenable for supporting cell growth (Ramakrishna et al., 2005; Sell et al., 2010; Kai et al., 2011a). For example in the study carried out by Kai et al. (2011a) poly(ε-caprolactone)/gelatine-aligned nanofibrous composite scaffold showed anisotropic physicochemical characteristics, providing topographical and biological cues for cardiomyocytes alignment. In addition co-axial fibers can also be fabricated where the ‘core’ and the ‘shell’ of the fibres are composed of different polymeric systems and also loaded with different active molecules like growth factors or signalling molecules to provide chemical cues for cell material interactions (Ravichandran et al., 2011, 2012a). The large number of variable parameters in electrospinning allows to tune the mechanical properties of the product to closely resemble those of any soft tissue to be engineered and to adjust its degradation kinetics to meet the specific requirements of a clinical application.
12.4 Potential scaffolding biomaterials
So far, a number of polymeric biomaterials, natural, synthetic and composites have been developed or are under development for myocardial tissue engineering. An overview of biomaterials applied in myocardial tissue engineering is given in Table 12.1. In this section, the studies conducted on each of these polymers are briefly discussed.
Table 12.1
Overview of biomaterials used in myocardium tissue engineering
| Biomaterial | Physical state | Ref |
| Natural | ||
| Collagen | Liquid/gel | Eschenhagen et al. (1997); Zimmermann et al. (2000, 2006) |
| Collagen mesh | Solid | Kofidis et al. (2003); Gonnerman et al. (2012) |
| Gelatine mesh | Solid | Li et al. (1999); Prabhakaran et al. (2011a) |
| Alginate mesh | Solid | Leor et al. (2000); Shachar et al. (2011) |
| Hyaluronan | Solid | Gallina et al. (2012); Lionetti et al. (2012) |
| Synthetic | ||
| PGA and copolymer with PLA | Solid | Radisic and Novakovic (2005) |
| PLLA | Solid | Radisic and Novakovic (2005) |
| PCL | Solid | Shin et al. (2004); Prabhakaran et al. (2011b) |
| PGS, PPS | Solid | Wang et al. (2002); Ravichandran et al. (2011, 2012a, b); Chen et al. (2010) |

12.4.1 Collagen gel matrix
In 1997, Eschenhagen et al. reported, for the first time, an artificial heart tissue, which was termed engineered heart tissue (EHT). In this work, embryonic chick cardiomyocytes were mixed with collagen solution and allowed to gel between two Velcro-coated glass tubes. By culturing the cardiomyocytes in the collagen matrix, they produced a spontaneously and coherently contracting 3D heart tissue in vitro. Immunohistochemistry and electron microscopy revealed a highly organized myocardium-like structure exhibiting typical cross-striation, sarcomeric myofilaments, intercalated discs, desmosomes, and tight junctions.
More recently, large (thickness 1–4 mm and diameter 15 mm), mechanically supportive EHTs were produced with neonatal rat heart cells, and were implanted on hearts with myocardial infarction in immune-suppressed rats (Zimmermann et al., 2006). When evaluated 28 days later, EHTs showed several beneficial effects:
• developing electrical coupling to the native myocardium without evidence of arrhythmia induction;
• preventing further dilation;
• inducing systolic wall thickening of infarcted myocardial segments; and
• faster fractional area shortening of infarcted hearts, compared with controls (sham operation and noncontractile constructs).
In summary, the research has confirmed that EHTs have many structural, functional, and physiological characteristics of cardiac tissue, and that EHTs can be implanted to both healthy and infarcted hearts and can survive in vivo in both situations. The potential for expansion of this work to the construction of large implants remain to be established.
12.4.2 Collagen fibrous mesh (or collagen sponge)
The application of collagen gel matrix is limited by insufficient mechanical strength. This has led researchers to look for new approaches based on solid scaffolds. Kofidis et al. (2003) seeded neonatal rat cardiomyocytes in vitro into a 3D solid collagen mesh and called the product artificial myocardial tissue (AMT). The artificial myocardial tissue was shown to possess structural, mechanical, physiological and biologic characteristics similar to native cardiac tissue (Kofidis et al., 2003). Kofidis et al. (2005) utilized undifferentiated embryonic stem cells as the substrate of artificial myocardial tissue. The bioartificial mixtures were implanted in the infarcted area of the rat hearts. Studies revealed that embryonic stem cells formed stable intramyocardial grafts that were incorporated into the surrounding area without distorting myocardial geometry, thereby preventing ventricular wall thinning (Kofidis et al., 2005). Very recently interaction between HL-1 cardiomyocytes and a series of geometrically anisotropic collagen–glycosaminoglycan (CG) scaffolds with aligned tracks of ellipsoidal pores designed to mimic elements of the native geometric cardiac anisotropy was investigated (Gonnerman et al., 2012). The study showed the role of scaffold geometric anisotropy and pore size on cardiomyocyte bioactivity, with Hl-1 cells exhibiting significantly elevated 3D alignment and earlier spontaneous beating within anisotropic CG scaffolds, relative to isotropic scaffold controls. Also the spontaneous beating occurred at significantly higher instances for larger pore size anisotropic variants. In fact the anisotropic scaffold with sufficiently large pore size (> 150 mm) provided the most suitable microenvironment to induce cardiomyocyte alignment, beating, and bioactivity for cardiac tissue engineering applications. Similarly, in the studies carried out by Zhang et al. (2012) chitosan–collagen scaffolds with micropores and array of parallel channels (~ 200 μm in diameter) were developed for MTE application using mechanical stimulation. The fabricated scaffold designed to mimic native cardiac ECM, when seeded with neonatal rat heart cells began to contract synchronously after 3 days of culture. Mechanical stimulation promoted cell alignment, elongation and expression of cardiac marker, connexin-43. Thus, the incorporated channels in the scaffold enhanced oxygen transport and facilitated the establishment of cell connections within the construct.
12.4.3 Gelatine mesh
Gelatine mesh is also widely investigated for cardiac muscle engineering. Li et al. (1999) seeded foetal rat ventricular muscle (not isolated cells) into a gelatine-foam to form grafts. The substrates were cultured in vitro for 7 days, forming a beating cardiac graft. The grafts implanted into subcutaneous tissue contracted regularly and spontaneously. When implanted onto myocardial scar tissue, cells within the grafts survived and formed junctions with the recipient heart cells. Fibrous composites of gelatine with synthetic polymers such as poly(DL-lactide-co-glycolide) have also been developed as potential biomimetic cardiac patch. When seeded with cardiomyocytes, the composite fibrous scaffolds were found to support cell growth, with the cells expressing cardiac specific proteins such as alpha-actinin and troponin I indicating structural maturation and cytoskeletal development of the cardiomyocytes (Prabhakaran et al., 2011a).
12.4.4 Alginate mesh
In addition to protein-based materials, there is intensive activity in the area of natural polysaccharides. Alginate, a negatively charged polysaccharide from seaweed that forms hydrogels in the presence of calcium ions, was initially developed for drug delivery and it is under development for tissue engineering scaffolds. The group of Cohen (Zmora et al., 2002) produced an alginate sponge using a freeze-drying technique, with porosity being 90% and pore size 50–150 μm. Moreover, Leor et al. (2000) seeded foetal rat myocardial cells into this sponge to form an engineered heart construct. After 4 days of culture in vitro, the engineered constructs were implanted into rat hearts with myocardium infarct. Hearts were harvested 9 weeks after implantation. A large number of blood vessels were found in the grafted area, indicating intensive neovascularization. The specimens showed almost complete disappearance of the scaffold and good integration into the host. In contrast to the control animals which developed significant left ventricular dilatation accompanied by progressive deterioration in left ventricular contractility, the graft-treated rats showed attenuation of left ventricular dilatation and unchanged contractility in left ventricle.
As alginate molecules lack the intrinsic ability for cell adherence, functionalization of alginate by immobilizing arginine-glycine-aspartate (RGD) peptides within the alginate matrix has also been carried out (Shachar et al., 2011). In this study, the RGD peptide was immobilized in the alginate scaffold using carbodiimide chemistry. When seeded with neonatal rat cardiac cells, the RGD functionalized scaffold promoted cell attachment to the matrix, increased cell survival and recovery and induced the organization of a cardiac muscle tissue. The cardiomyocytes reorganized their myofibrils and reconstructed myofibres composed of multiple cardiomyocytes in a typical myofiber bundle. On the other hand, in the unmodified control scaffolds no such structural organization of the cardiomyocytes was observed. In another study the effect of combining two matrix-attached peptides, the adhesion peptide G4RGDY (target for integrin binding) and heparin binding peptide G4SPPRRARVTY (HBP) (target for cell syndecan interactions) into alginate scaffold for promoting cardiac tissue regeneration was investigated (Sapir et al., 2011). The cardiac tissue developed in the HBP/RGD-attached scaffolds revealed the best features of a functional muscle tissue as opposed to the HBP/RGD and unfunctionalized scaffold. By day 7, well-developed myocardial fibres were observed in these cell constructs, but at day 14 only the HBP/RGD-attached constructs presented an isotropic myofibre arrangement with expression of a-actinin, N-cadherin and Connexin-43. The studies therefore demonstrated that a combinatorial peptide approach representing different signalling in ECM–cell interactions play a key role, contributing to the formation of functional cardiac muscle tissue, in vitro.
12.4.5 Other natural polymeric systems
Among the natural polymers, hyaluronan-based materials are also being studied to develop scaffolds for MTE applications, because of their important biocompatibility characteristic (Gallina et al., 2012). This is because when compared with other polymeric systems, where the degradation products may be toxic, the primary degradation products of hyaluronan are hyaluronic acid that occurs naturally in the ECM of the heart.
Therefore, in this context of MTE application, Gallina et al. (2012) investigated the performance of neonatal mouse ventricular myocytes (NMVM) cultured on a hyaluronan based polymer scaffold. They found progressive morphological organization of the cells inside the biopolymer, with both clear sarcomeric arrangement along the scaffold fibers and gap junctions development between adjacent cells. In addition contractile activity of NMVMs adherent onto HYALONECT® up to 48 h from seeding, indicated a progressive differentiation of the cells toward the adult phenotype. Furthermore, in vivo intracellular calcium measurements performed using calcium fluorimetric confocal imaging revealed the presence of spontaneous calcium transients. Lionetti et al. (2012) also showed that the injection of hyaluronan mixed esters of retinoic acid and butyric acid alone was able to rescue infarcted rat hearts by improving by improving vascularization, cardiomyocytes survival and tissue function without the need of stem cell transplantation.
12.4.6 Poly(glycolic acid) (PGA) and its copolymer with poly(lactic acid) (PLA)
The biodegradable synthetic polymers most often utilized for scaffolds in tissue engineering are the poly(α-hydroxy acids), including poly(lactic acid) (PLA) and poly(glycolic acid) (PGA), as well as poly(lactic-co-glycolide) (PLGA) copolymers. Freed and Vunjak Novakovic in 1997 demonstrated that cultivation of primary neonatal rat cardiomyocytes on highly porous (porosity being 97%) PGA scaffolds in bioreactors could result in contractile 3D cardiac-like tissues, which consisted of cardiomyocytes with cardiac-specific structural and electrophysiological properties, contracting spontaneously and synchronously. In the subsequent studies, the group invested great efforts to overcome the limitation on the thickness of engineered tissue through improving cell seeding and tissue culturing conditions (bioreactor). A construct thickness of 1–2 mm was reported (Carrier et al., 2002).
12.4.7 Poly(ε-caprolactone) (PCL)-based polymeric platforms
Poly(ε-caprolactone) (PCL) is another important member of the aliphatic polyester family. It is a thermoplastic elastomer and has been used to effectively entrap antibiotic drugs. A construct made with PCL has been considered as a drug delivery system. The degradation of PCL and its copolymers involves similar mechanisms to PLA, proceeding in two stages: random hydrolytic ester cleavage and weight loss through the diffusion of oligometric species from the bulk. Shin et al. (2004) demonstrated the formation of contractile cardiac grafts in vitro using a nanofibrous PCL mesh. After neonatal rat cardiomyocytes were seeded in the nanofibrous mesh, the construct was cultured, while being suspended across a wire ring that acted as a passive load to contracting cardiomyocytes. The cardiomyocytes started beating after 3 days and were cultured in vitro for 14 days. The cardiomyocytes attached well on the PCL meshes and expressed cardiac-specific proteins such as alpha-myosin heavy chain, connexion-43 and cardiac troponin I. This work indicated that using this technique, cardiac grafts can be matured in vitro to obtain sufficient function prior to implantation. Fibrous composite scaffolds containing poly(L-lactic acid)-co-poly(ε-caprolactone) (PLCL) blended with another synthetic polymer poly(1,8-octanediol-co-citrate) (POC) was also investigated for cardiac patch application (Prabhakaran et al., 2011b). The composite scaffolds with POC : PLCL weight ratio of 40:60 was found to have a tensile strength of 1.04 ± 0.11 MPa and Young’s modulus of 0.51 ± 0.10 MPa, comparable to the native cardiac tissue. The proliferation of cardiac myoblast cells on the electrospun POC/PLCL scaffolds was found to increase from days 2 to 8, with the increasing concentration of POC in the composite. The study therefore demonstrated that the composite fibrous scaffolds developed had potential to be explored as matrix for MTE application.
12.4.8 Soft elastomers: poly(polyol-sebacate) (PPS)
To engineer the tissue of heart, which beats cyclically and constantly throughout life, the biomaterial should be ideally as soft and elastic as heart muscle. These mechanical characteristics are impossible with polyester-based thermosetting polymers, such as PGA, and PLA, and their copolymers, because they undergo plastic deformation and they are prone to failure when exposed to long-term cyclic strains. The limitation of their use in engineering flexible tissues has made biomaterial scientists turn to elastomers for cardiac tissue engineering. Poly(polyol-sebacate) (PPS) is one such family of cross-linked elastomers that was developed for medical applications (Sestoft, 1985; Ellwood, 1995; Natah et al., 1997; Wang et al., 2002). Amongst this family of synthetic elastomers, poly(glycerol sebacate) (PGS) has been widely investigated as a suitable biomaterial for MTE (Wang et al., 2002; Engelmayr et al., 2008; Q. Z. Chen et al., 2010, 2011; Kenar et al., 2010; Park et al., 2011; Ravichandran et al., 2011, 2012a; Rai et al., 2012).
These polymers are biocompatible and inexpensive (Wang et al., 2002) and they have already shown potential applications in nerve (Sundback et al., 2005) and vascular tissue engineering (Bettinger et al., 2005, 2006; Motlagh et al., 2006; Kemppainen and Hollister, 2010). A polyol is a sugar alcohol containing multiple hydroxyl groups (e.g. glycerol, mannitol, sorbitol and xylitol). Sebacic acid, a dicarboxylic acid with structure (HOOC) (CH2)8(COOH), is a naturally occurring chemical derivative of castor oil which has been proven safe in vivo (Tamada and Langer, 1992; Grego and Mingrone, 1995). Polyol and sebacic acid are both endogenous monomers found in human metabolism.
PPSs contain hydrolysable bonds (e.g., ester bonds) that can be degraded by hydrolysis, a reaction that can be catalysed by biological enzymes. In an in vitro study (Wang et al., 2002), PGS was reported to degrade up to 11–23% (in terms of weight loss) after 60 days’ agitation in PBS at 37 °C. Similar degradation kinetics was also reported by Liang et al. (2010), who found that the weight loss of PGS and PGS/Bioglass® composites was around 10–25% after 60 days’ incubation in a standard tissue culture medium. However, the above in vitro degradation rates of PGS-based materials are inconsistent with the degradation kinetics of PGS in vivo. Wang et al. (2003) reported that PGS is completely resorbed after 60 days’ implantation in rats. This comparatively faster degradation rate of PGS in vivo was also reported by Stuckey et al. (2010), who used PGS sheets as a pericardial heart patch. They found that the PGS patch was completely resorbed in 6 weeks.
These examples of in vivo degradation indicate that aqueous enzymatic action, combined with dynamic tissue movements and vascular perfusion, might enhance the enzymatic breakdown of ester bonds in PGS and thus facilitate the hydrolytic weakening of this material in vivo. The enzym-mediated degradation of PGS and PXS (poly xylitol sebcate) has been simulated and studied in vitro, using a protocol that is able to quantitatively capture the features of in vivo degradation of PPS-based materials (Liang et al., 2011; Chen et al., 2012). In brief, PPS are rapidly degrading polymers. The rapid degradation is believed to limit their application as a scaffold material in engineering tissues that have a healing rate of several months or years (e.g. cardiac muscle). Hence innovative strategies are needed.
The typical mechanical properties of PGS are listed in Table 12.2 (Wang et al., 2002; Q. Z. Chen et al., 2010). The mechanical properties of PGS can be tuned by varying curing temperature and time, which influence the crosslink density of the materials (Jaafer et al., 2010).
Table 12.2
Mechanical properties of poly(polyol sebacate) (PPS) and their copolymers
| Polymer | Young’s modulus (MPa) | UTS (MPa) | Elongation at beak (%) | Resilience | Ref. |
| PGS | 0.05–1.5 | 0.3–1.5 | 40–500 | > 98% | Chen Q. Z. et al. (2010); Liang et al. (2010) |
| PXS | 0.8–5.3 | 0.5–1.5 | 30–200 | N/A | Bruggeman et al. (2008, 2009) |
| PMS | 0.4–2.7 | 0.6–1.2 | 66–200 | Bruggeman et al. (2008) | |
| PSS | 02–380 | 0.8–18 | 10–50 | Bruggeman et al. (2008) | |
| PGSA | 0.05–1.38 | 0.05–0.5 | 42–189 | N/A | Nijst et al. (2007) |
| PGS-co-LA | 6–21 | N/A | N/A | N/A | Sun et al. (2009) |
| APS* | 1.45–4.34 | 0.24–1.69 | 21–92 | N/A | Bettinger et al. (2008) |
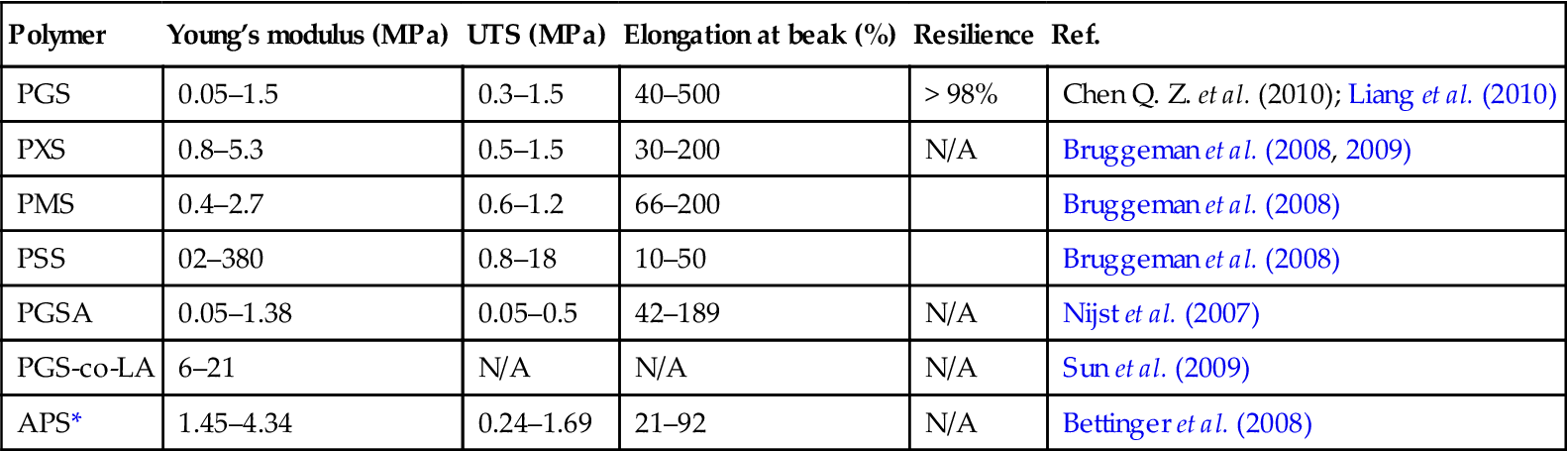
*APS = poly(1, 3-diamino-2-hydroxypropane-co-polyol-sebacate); PMS = poly(mannitol sebacate); PSS = poly(sorbitol sebacate); PGSA = poly(glycerol-co-sebacate) acrylate; PGS-co-LA = poly(glycerol sebacate)-co-lactic acid.
Most recently, non-linear elastic nanofibres of PGS have been successfully fabricated using the co-axial (core/shell) electrospinning technique (Chen and Xu, 2011; Ravichandran et al., 2011, 2012a; Xu and Chen, 2012). In all these studies, PGS formed the core whereas natural polymers such as gelatine (Ravichandran et al., 2011) and fibrinogen (Ravichandran et al., 2012a) were used for fabricating the shells of the electrospun fibres. Ravichandran et al. (2012b) used this co-axial electrospinning approach to synthesize minimally invasive short injectable nanofibres of PGS for MTE. The synthesis was carried out by first fabricating PGS/PLLA core/shell fibres, following which PLLA shell was selectively removed by dissolution using solvent system of dichloromethane and hexane in a 2:1 ratio.
12.4.9 Electroactive systems and composites
The cumulative effect of incorporating fillers such as bioactive glass particles, TiO2, particles, carbon nanotubes (CNT) into polymeric matrices has been investigated to develop composite systems for MTE applications (Chen et al., 2010a; Liang et al., 2010; Stuckey et al., 2010; Jawad et al., 2011; Mooney et al., 2012). Additionally, fillers like CNT, carbon nanofibers (Stout et al., 2011), gold nanoparticles (You et al., 2011) along with conductive polymers like polypyrrole (Kai et al., 2011b) and polyaniline (Jun et al., 2009; Booriello et al., 2011) have also been used for the development of electroactive matrices for MTE applications.
The pumping action of the heart is brought about by synchronous contractile activity of the cardiomyocytes, which are further dependent on continuous electrical conductivity to function (Stout et al., 2011). However, after an episode of myocardial infarction, the heart pumps irregularly and chaotically, as the electrical signals and action potential impulses generated by the sinoatrial cells and atrioventricular cells lack the capability to spread over the entire cardiac muscle (Kai et al., 2011b). One of the major limiting factors in the advancement of MTE is the inability to achieve synchronous contractility in the engineered cardiac construct in vitro. Upon implantation, this asynchrony may inhibit electrophysiological coupling between the cardiac construct and the native myocardium. Furthermore, the scaffold should be capable of conducting electrical impulses in order to synchronize with the native heart. Traditional polymeric biomaterials are unable to carry out this function and hence contribute to the irregular beating of the myocardium leading to conditions like arryhthymia. Therefore, to overcome this limitation of conventional nonconductive polymeric biomaterials, increasing numbers of studies are being carried out to develop electroactive conductive matrices as scaffolds for MTE applications.
In addition to providing cues for synchronous contractility of the seeded cardiomyocytes, i.e. by upregulating expression of the gap junction protein connexin-43 (You et al., 2011), the conductive matrices are also being investigated to provide cues for stem cell differentiation into cardiomyocyte lineage. For instance, a composite fibrous matrix of PLA and electroactive carbon nanotubes was shown to provide cardiomimetic cue for directing mesenchymal stem cells (MSC) differentiation into cardiomyocyte lineage by harnessing the electrical properties of the CNT (Mooney et al., 2012). In this study, when electrical stimulation of the MSCs seeded on the fibrous conductive matrix was carried out using an electrophysiological bioreactor, the cells reoriented perpendicularly to the direction of the current and adopted an elongated morphology. The stimulated cells also exhibited an upregulation for the cardiac markers like myosin heavy chain, Nkx2.5, GATA-4, cardiac troponin t (CTT) and connexion 43 (C43).
In studies carried out by Stout et al. (2011) the density of seeded human cardiomyocytes increased with increasing amounts of carbon nanofibers (CNF) incorporated in a PLGA : CNF system. The authors attributed this increased growth of cardiomocytes observed to the conductivity of the system, topography of the PLGA : CNF composites and/or to the increased presence of CNF in the composites which controlled the initial protein adsorption through altered surface energies.
Amongst the conductive polymers polyaniline (PANi) and polypyrrole are the most studied for MTE owing to their amenable properties of conductivity, reversible oxidation, redox stability, and suitable hydrophobicity for cell adhesion (Kai et al., 2011b). Although potential toxicity of PANi remains a controversial issue, numerous studies have been carried out which have demonstrated the biocompatibility of PANi (Booriello et al., 2011; Humpolicek et al., 2012). In the studies carried out by Borriello et al., for example, PANi doped electroactive composite fibrous PCL substrates were developed as patches for the regeneration of cardiac muscle. Biological investigations revealed that the conductive signals offered by PANi needles promoted the cardiogenic differentiation of hMSC into cardiomyocite-like cells. Furthermore after 3 and 5 days of cell seeding the survival rate of cardiomyocyte-like cells onto PCL/PANi samples was found to be significantly higher than that on the PCL surface, thus demonstrating the effect of conductive signals of PANi on supporting cell proliferation.
To further enhance the biocompatibility of PANi-based platforms for MTE applications, the polymer has also been combined with natural materials, like collagen, to develop a composite scaffold. In the study by Kim et al. (2012) a conductive matrix of PANi and collagen was successfully fabricated with a 7:1 (PANi : collagen) proportion, which showed the highest conductivity (0.27 S/cm). When adult porcine skeletal muscle cells were cultured on the scaffold, the cells showed good attachment and growth on the scaffold. In vivo studies by Kamalesh et al. (2000) showed that when PANi films in the emeraldine oxidation state were implanted into male Sprague-Dawley rats for a period for up to 90 week, the material did not cause any inflammatory response (Kamalesh et al., 2000). However, in the study conducted by Mattioli-Belmonte et al. (2003), PANi implanted subcutaneously in rats induced fibrous encapsulation and inflammatory response. Such discrepancies in the in vivo studies of PANi therefore warrant more research, especially considering the fact that only limited studies have been carried out to understand the in vivo performance of this polymer.
Significant research has also been carried out on the development of composite systems using bioactive glass particles for tissue engineering applications owing to their ability to form tenacious bonds to both hard and soft tissues; bonding is enabled by the formation of a hydroxyapatite (similar to biological apatite) layer on the glass surface on exposure to biological fluids (Misra et al., 2006; Chen et al., 2008). Elastomeric composites of PGS with Bioglass® 45S5 were developed as a matrix for MTE applications by Liang et al. (2010). The incorporated Bioglass® particles were able to counteract the acidic degradation products of PGS, owing to its dissolution products such as Na+, Ca2 +, ![]() and
and ![]() . Additionally, the incorporated Bioglass® particles provided an additional control mechanism for tailoring the mechanical properties and degradation kinetics of the developed composite.
. Additionally, the incorporated Bioglass® particles provided an additional control mechanism for tailoring the mechanical properties and degradation kinetics of the developed composite.
12.5 Conclusions and future trends
Heart muscle engineering aims to regenerate functional myocardium to repair the diseased and injured heart. Besides the development of cell sources for myocardial regeneration, a number of biocompatible polymeric materials have been investigated for cardiac regeneration strategies. This chapter has presented an updated summary of the different biopolymer systems, both natural and synthetic, being considered for the development of matrices (or patches) for CTE. Also fabrication technologies and scaffold designs have been highlighted. Despite some early successes, there are few tissue engineering approaches available for clinical use for the repair of soft, mechanically functional tissues such as the heart. The precise reasons for graft failure in experimental animal studies and preclinical trials are the matter of continuing research. Certainly mechanical dissimilarity between the scaffold and the native tissue that it is replacing is a major concern. Hence, the limitations in cardiac tissue engineering are not only due to cell-related issues (such as scale-up in a rather short period, efficiency of cell seeding or cell survival rate and immune rejection), but are also caused by the properties of the engineered tissue construct.
The engineered heart muscle must develop systolic (contractive) force with appropriate compliance, at the same time it must withstand diastolic (expansive) load. Clearly, materials used to build cardiac tissue constructs have no ability to beat without cells. The contractile movement of the engineered construct is thus completely driven by the seeded myocardial cells that inherently have a beating ability. One can envisage that the transfer of mechanical signals from cells to the scaffold would be jeopardized if the scaffold material is too stiff. Most of the above reviewed biomaterials, including collagen fibres, are much stronger than myocardium. This explains why solid engineered constructs lack a contractile function. On the other hand, collagen gels are too weak to sustain the required mechanical loads. The design of scaffolds with specific porosity structures and topographies can tackle this problem in future MTE strategies.
Vasculature (the formation of blood vessels) is essential for the regeneration of vascularized tissues, such as the heart muscle. Engineering of such tissues relies on the vascularization ability of new grafts. To achieve this, in vitro (i.e. before implantation) priming of tissue constructs for vascularization is desirable. Indeed, angiogenesis and ingrowth of new vessels are necessary to supply the cells with oxygen, nutrients, and growth factors in engineered thick 3D tissues. Achieving vascularization of tissue constructs is the greatest challenge in the field of tissue engineering, including MTE. In this context the increased use of bioreactors to further investigate vascularization strategies of specific scaffolds for MTE is anticipated.


