Al-Naseri S.K, Abbas T.R. Predicting NOM removal by fixed-bed GAC adsorbers. Jordan J. Civ. Eng. 2009;3:172–183.
Álvarez-Uriarte J.I, Iriarte-Velasco U, Chimeno-Alanís N, González-Velasco J.R. The effect of mixed oxidants and powdered activated carbon on the removal of natural organic matter. J. Hazard. Mater. 2010;181:426–431.
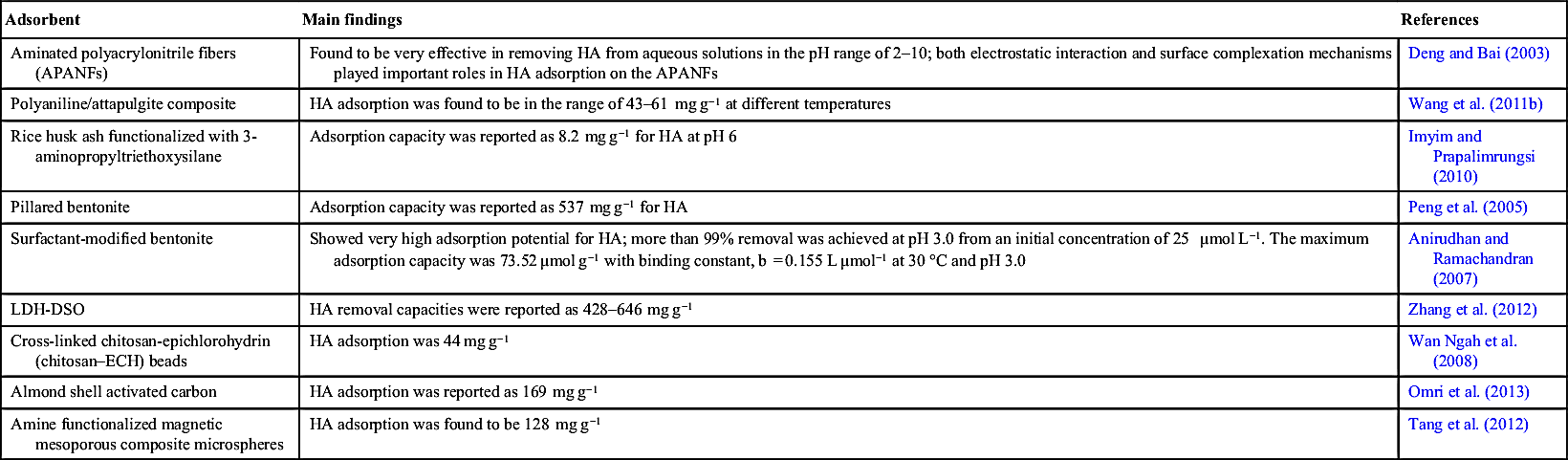
Anirudhan T.S, Ramachandran M. Surfactant-modified bentonite as adsorbent for the removal of humic acid from wastewaters. Appl. Clay Sci. 2007;35:276–281.
Bai R, Zhang X. Polypyrrole-coated granules for humic acid removal. J. Colloid Interface Sci. 2001;243:52–60.
Bansal R.C, Goyal M. Activated Carbon Adsorption. Boca Raton: CRC Press; 2005.
Bell-Ajy K, Abbaszadegan M, Ibrahim E, Verges LeChevallier D. Conventional and optimized coagulation for NOM removal. J. Am. Water Works Assoc. 2000;92:44–58.
Bhatnagar A, Hogland W, Marques M, Sillanpää M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013;219:499–511.
Bhattacharya P, Conroy N, Rao A.M, Powell B.A, Ladner D.A, Ke P.C. PAMAM dendrimer for mitigating humic foulant. RSC Adv. 2012;2:7997–8001.
Bjelopavlic M, Newcombe G, Hayes R. Adsorption of NOM onto activated carbon: effect of surface charge, ionic strength, and pore volume distribution. J. Colloid Interface Sci. 1999;210:271–280.
Bolto B, Dixon D, Eldridge R, King S. Cationic polymer and clay or metal oxide combinations for natural organic matter removal. Water Res. 2001;35:2669–2676.
Bond T, Goslan E.H, Parsons S.A, Jefferson B. Treatment of disinfection by‐product precursors. Environ. Technol. 2011;32:1–25.
Brunauer S, Emmett P.H, Teller E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938;60:309–319.
Capar G, Yetis U. Removal of natural organic matter and trihalomethanes from the drinking water of Ankara City. Turk. J. Eng. Environ. Sci. 2001;25:527–535.
Cheng W, Dastgheib S.A, Karanfil T. Adsorption of dissolved natural organic matter by modified activated carbons. Water Res. 2005;39:2281–2290.
Choo K.-H, Kang S.-K. Removal of residual organic matter from secondary effluent by iron oxides adsorption. Desalination. 2003;154:139–146.
Chow C.W.K, Majewski P, Bauer S, Fabris R, Drikas M. Removal of natural organic matter using self-assembled monolayer technology. Desalin. Water Treat. 2009;12:344–351.
Crozes G, White P, Marshall M. Enhanced coagulation: its effect on NOM removal and chemical costs. J. Am. Water Works Assoc. 1995;87:78–89.
Dąbrowski A. Adsorption — from theory to practice. Adv. Colloid Interface Sci. 2001;93:135–224.
Dastgheib S.A, Karanfil T, Cheng W. Tailoring activated carbons for enhanced removal of natural organic matter from natural waters. Carbon. 2004;42:547–557.
Davis J.A. Adsorption of natural dissolved organic matter at the oxide/water interface. Geochim. Cosmochim. Acta. 1982;46:2381–2393.
Day G.M, Hart B.T, McKelvie I.D, Beckett R. Adsorption of natural organic matter onto goethite. Colloids Surf. A Physicochem. Eng. Aspects. 1994;89:1–13.
Deng S. Sorbent technology. In: Encyclopedia of Chemical Processing. New York: Taylor & Francis; 2006.
Deng S, Bai R.B. Aminated polyacrylonitrile fibers for humic acid adsorption: behaviors and mechanisms. Environ. Sci. Technol. 2003;37:5799–5805.
Ding C, Shang C. Mechanisms controlling adsorption of natural organic matter on surfactant-modified iron oxide-coated sand. Water Res. 2010;44:3651–3658.
Ding C, Yang X, Liu W, Chang Y, Shang C. Removal of natural organic matter using surfactant-modified iron oxide-coated sand. J. Hazard. Mater. 2010;174:567–572.
Dong C, Chen W, Liu C. Preparation of novel magnetic chitosan nanoparticle and its application for removal of humic acid from aqueous solution. Appl. Surf. Sci. 2014;292:1067–1076.
Doulia D, Leodopoulos C, Gimouhopoulos K, Rigas F. Adsorption of humic acid on acid-activated Greek bentonite. J. Colloid Interface Sci. 2009;340:131–141.
Eikebrokk B, Fabris R, Drikas M, Chow C. NOM characteristics and treatability by coagulation. In: Hahn H.H, Hoffman E, Ødegaard H, eds. Chemical Water and Wastewater Treatment IX. London: IWA Publishing; 2007.
Fabris R, Chow C.W.K, Drikas M. Practical application of a combined treatment process for removal of recalcitrant NOM – alum and PAC. Water Sci. Technol. Water Supply. 2004;4:89–94.
Fabris R, Chow C.W.K, Drikas M, Eikebrokk B. Comparison of NOM character in selected Australian and Norwegian drinking waters. Water Res. 2008;42:4188–4196.
Fettig J. Modelling the uptake of natural organic matter (NOM) by different granular sorbent media. J. Water Supply Res. Technol. Aqua. 2005;54:83–93.
Freundlich H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906;57:385–471.
Genz A, Baumgarten B, Goernitz M, Jekel M. NOM removal by adsorption onto granular ferric hydroxide: equilibrium, kinetics, filter and regeneration studies. Water Res. 2008;42:238–248.
Grieco S.A, Ramarao B.V. Removal of TCEP from aqueous solutions by adsorption with zeolites. Colloids Surf. A Physicochem. Eng. Aspects. 2013;434:329–338.
Gu B, Schmitt J, Chen Z, Liang L, McCarthy J.F. Adsorption and desorption of natural organic matter on iron oxide: mechanisms and models. Environ. Sci. Technol. 1994;28:38–46.
Heijman S.G.J, van Paassen A.M, van der Meer W.G.J, Hopman R. Adsorptive removal of natural organic matter during drinking water treatment. Water Sci. Technol. 1999;40:183–190.
Humbert H, Gallard H, Suty H, Croué J.-P. Natural organic matter (NOM) and pesticides removal using a combination of ion exchange resin and powdered activated carbon (PAC). Water Res. 2008;42:1635–1643.
Hyung H, Kim J.-H. Natural organic matter (NOM) adsorption to multi-walled carbon nanotubes: effect of NOM characteristics and water quality parameters. Environ. Sci. Technol. 2008;42:4416–4421.
Imyim A, Prapalimrungsi E. Humic acids removal from water by aminopropyl functionalized rice husk ash. J. Hazard. Mater. 2010;184:775–781.
Iriarte-Velasco U, Álvarez-Uriarte J.I, Chimeno-Alanís N, González-Velasco J.R. Natural organic matter adsorption onto granular activated carbons: implications in the molecular weight and disinfection byproducts formation. Ind. Eng. Chem. Res. 2008;47:7868–7876.
Joseph L, Flora J.R.V, Park Y.-G, Badawy M, Saleh H, Yoon Y. Removal of natural organic matter from potential drinking water sources by combined coagulation and adsorption using carbon nanomaterials. Sep. Purif. Technol. 2012;95:64–72.
Jung H.R, Seo S.D, Choi H.C. Preparation of mesostructured carbon (MSC) for removal of natural organic matter (NOM) in aqueous solution. Mater. Sci. Forum. 2007;544–545:123–126.
Kaneco S, Itoh K, Katsumata H, Suzuki T, Masuyama K, Funasaka K, Hatano K, Ohta K. Removal of natural organic polyelectrolytes by adsorption onto tobermorite. Environ. Sci. Technol. 2003;37:1448–1451.
Karanfil T, Kitis M, Kilduff J.E, Wigton A. Role of granular activated carbon surface chemistry on the adsorption of organic compounds. 2. Natural organic matter. Environ. Sci. Technol. 1999;33:3225–3233.
Kasprzyk-Hordern B. Chemistry of alumina, reactions in aqueous solution and its application in water treatment. Adv. Colloid Interface Sci. 2004;110:19–48.
Kim S, Kim J, Seo G. Iron oxide nanoparticle-impregnated powder-activated carbon (IPAC) for NOM removal in MF membrane water treatment system. Desalin. Water Treat. 2013;51:1–9.
Kitis M, Kaplan S.S, Karakaya E, Yigit N.O, Civelekoglu G. Adsorption of natural organic matter from waters by iron coated pumice. Chemosphere. 2007;66:130–138.
Korshin G.V, Benjamin M.M, Sletten R.S. Adsorption of natural organic matter (NOM) on iron oxide: effects on NOM composition and formation of organo-halide compounds during chlorination. Water Res. 1997;31:1643–1650.
Kumar E, Bhatnagar A, Hogland W, Marques M, Sillanpää M. Interaction of anionic pollutants with Al-based adsorbents in aqueous media – a review. Chem. Eng. J. 2014;241:443–456.
Lambert S.D, Graham N.J.D. Removal of non-specific dissolved organic matter from upland potable water supplies—I. Adsorption. Water Res. 1995;29:2421–2426.
Langmuir I. The adsorption of gases on plane surface of glass, mica and platinum. J. Am. Chem. Soc. 1916;40:1361–1368.
Lin J, Zhan Y. Adsorption of humic acid from aqueous solution onto unmodified and surfactant-modified chitosan/zeolite composites. Chem. Eng. J. 2012;200–202:202–213.
Lin L, Lei Z, Wang L, Liu X, Zhang Y, Wan C, Lee D.-J, Tay J.H. Adsorption mechanisms of high-levels of ammonium onto natural and NaCl-modified zeolites. Sep. Purif. Technol. 2013;103:15–20.
Liu F, Xu Z, Wan H, Wan Y, Zheng S, Zhu D. Enhanced adsorption of humic acids on ordered mesoporous carbon compared with microporous activated carbon. Environ. Toxicol. Chem. 2011;30:793–800.
Liu F.-F, Fan J.-L, Wang S.-G, Ma G.-H. Adsorption of natural organic matter analogues by multi-walled carbon nanotubes: comparison with powdered activated carbon. Chem. Eng. J. 2013;219:450–458.
Liu Z, Zu Y, Meng R, Xing Z, Tan S, Zhao L, Sun T, Zhou Z. Adsorption of humic acid onto carbonaceous surfaces: atomic force microscopy study. Microsc. Microanal. 2011;17:1015–1021.
Lu C, Su F. Adsorption of natural organic matter by carbon nanotubes. Sep. Purif. Technol. 2007;58:113–121.
Mantell C.L. Adsorption. New York: McGraw-Hill Book Company; 1951.
Marsh H, Rodríguez-Reinoso F. Activated Carbon. Oxford: Elsevier; 2006.
Martucci A, Pasti L, Marchetti N, Cavazzini A, Dondi F, Alberti A. Adsorption of pharmaceuticals from aqueous solutions on synthetic zeolites. Microporous Mesoporous Mater. 2012;148:174–183.
Matilainen A, Sillanpää M. Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere. 2010;80:351–365.
Matilainen A, Vepsäläinen M, Sillanpää M. Natural organic matter removal by coagulation during drinking water treatment: a review. Adv. Colloid Interface Sci. 2010;159:189–197.
Matilainen A, Vieno N, Tuhkanen T. Efficiency of the activated carbon filtration in the natural organic matter removal. Environ. Int. 2006;32:324–331.
Matsui Y, Murase R, Sanogawa T, Aoki N, Mima S, Inoue T, Matsushita T. Micro-ground powdered activated carbon for effective removal of natural organic matter during water treatment. Water Sci. Technol. Water Supply. 2004;4:155–163.
Matsui Y, Yuasa A, Li F. Overall adsorption isotherm of natural organic matter. J. Environ. Eng. 1998;124:1099–1107.
McBain J.W. The mechanism of the adsorption (“sorption”) of hydrogen by carbon. Philos. Mag. Ser. 1909;6:916–935.
McMeen C.R, Benjamin M.M. NOM removal by slow sand filtration through iron oxide-coated olivine. J. Am. Water Works Assoc. 1997;89:57–71.
Metsämuuronen S, Sillanpää M, Bhatnagar A, Mänttäri M. Natural organic matter removal from drinking water by membrane technology. Sep. Purif. Rev. 2014;43:1–61.
Moussavi S.P, Ehrampoush M.H, Mahvi A.H, Ahmadian M, Rahimi S. Adsorption of humic acid from aqueous solution on single-walled carbon nanotubes. Asian J. Chem. 2013;25:5319–5324.
Naghizadeh A, Nasseri S, Rashidi A.M, Kalantary R.R, Nabizadeh R, Mahvi A.H. Adsorption kinetics and thermodynamics of hydrophobic natural organic matter (NOM) removal from aqueous solution by multi-wall carbon nanotubes. Water Sci. Technol. Water Supply. 2013;13:273–285.
Najafi M, Rostamian R, Rafati A.A. Chemically modified silica gel with thiol group as an adsorbent for retention of some toxic soft metal ions from water and industrial effluent. Chem. Eng. J. 2011;168:426–432.
Ng M, Kho E, Liu S, Lim M, Amal R. Highly adsorptive and regenerative magnetic TiO2 for natural organic matter (NOM) removal in water. Chem. Eng. J. 2014;246:196–203.
Omri A, Benzina M, Trabelsi W, Ammar N. Adsorptive removal of humic acid on activated carbon prepared from almond shell: approach for the treatment of industrial phosphoric acid solution. Desalin. Water Treat. 2013:1–12.
Owen D.M, Amy G.L, Chowdhury Z.K, Paode R, McCoy G, Viscosil K. NOM characterization and treatability. J. Am. Water Works Assoc. 1985;87:46–63.
Peng X, Luan Z, Chen F, Tian B, Jia Z. Adsorption of humic acid onto pillared bentonite. Desalination. 2005;174:135–143.
Pürschel M, Ender V. Sorption of natural organic matter by adsorber and ion exchange resins – investigations with starch and phenylalanine as model substances. In: 15th International Conference on the Properties of Water and Steam (15th ICPWS), Radisson SAS Hotel Berlin. 2008.
Qi S, Schideman L.C. An overall isotherm for activated carbon adsorption of dissolved natural organic matter in water. Water Res. 2008;42:3353–3360.
Richardson S.D, Plewa M.J, Wagner E.D, Schoeny R, DeMarini D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat. Res. 2007;636:178–242.
Shaker A.M, Komy Z.R, Heggy S.E.M, El-Sayed M.E.A. Kinetic study for adsorption humic acid on soil minerals. J. Phys. Chem. A. 2012;116:10889–10896.
Shi D, Xie S, Wen D, Xi D. Removal of bromate and natural organic matter by using biologically activated carbon. Int. J. Environ. Pollut. 2009;38:180–192.
Siddiqui M, Amy G, Ryan J, Odem W. Membranes for the control of natural organic matter from surface waters. Water Res. 2000;34:3355–3370.
Smith B, Yang J, Bitter J.L, Ball W.P, Fairbrother D.H. Influence of surface oxygen on the interactions of carbon nanotubes with natural organic matter. Environ. Sci. Technol. 2012;46:12839–12847.
Smith E.H. Bench-scale tests and modeling of adsorption of natural organic matter by activated carbon. Water Res. 1994;28:1693–1702.
Tang Y, Liang S, Yu S, Gao N, Zhang J, Guo H, Wang Y. Enhanced adsorption of humic acid on amine functionalized magnetic mesoporous composite microspheres. Colloids Surf. A Physicochem. Eng. Aspects. 2012;406:61–67.
Velten S, Knappe D.R.U, Traber J, Kaiser H.-P, von Gunten U, Boller M, Meylan S. Characterization of natural organic matter adsorption in granular activated carbon adsorbers. Water Res. 2011;45:3951–3959.
Vidic R.D, Suidan M.T. Role of dissolved oxygen on the adsorptive capacity of activated carbon for synthetic and natural organic matter. Environ. Sci. Technol. 1991;25:1612–1618.
Wan Ngah W.S, Hanafiah M.A.K.M, Yong S.S. Adsorption of humic acid from aqueous solutions on crosslinked chitosan–epichlorohydrin beads: kinetics and isotherm studies. Colloids Surf. B Biointerfaces. 2008;65:18–24.
Wang H, Kang J, Liu H, Qu J. Preparation of organically functionalized silica gel as adsorbent for copper ion adsorption. J. Environ. Sci. 2009;21:1473–1479.
Wang H, Keller A, Li F. Natural organic matter removal by adsorption onto carbonaceous nanoparticles and coagulation. J. Environ. Eng. 2010;136:1075–1081.
Wang H, Keller A.A, Clark K.K. Natural organic matter removal by adsorption onto magnetic permanently confined micelle arrays. J. Hazard. Mater. 2011;194:156–161.
Wang J, Han X, Ma H, Ji Y, Bi L. Adsorptive removal of humic acid from aqueous solution on polyaniline/attapulgite composite. Chem. Eng. J. 2011;173:171–177.
Wang M, Liao L, Zhang Z, Li Z. Adsorption of low concentration humic acid from water by palygorskite. Appl. Clay Sci. 2012;67–68:164–168.
Wang S, Peng Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010;156:11–24.
Wu C.N, Wang L, Ling Q, Tang Y.C, Huang J, Pan F.K. Removal of natural organic matter of artificial lake by modified fly ash. Adv. Mater. Res. 2013;690–693:1020–1023.
Xie Q, Xie J, Wang Z, Wu D, Zhang Z, Kong H. Adsorption of organic pollutants by surfactant modified zeolite as controlled by surfactant chain length. Microporous Mesoporous Mater. 2013;179:144–150.
Zaitseva N, Zaitsev V, Walcarius A. Chromium(VI) removal via reduction–sorption on bi-functional silica adsorbents. J. Hazard. Mater. 2013;250–251:454–461.
Zhan Y, Lin J, Qiu Y, Gao N, Zhu Z. Adsorption of humic acid from aqueous solution on bilayer hexadecyltrimethyl ammonium bromide-modified zeolite. Front. Environ. Sci. Eng. China. 2011;5:65–75.
Zhan Y, Zhu Z, Lin J, Qiu Y, Zhao J. Removal of humic acid from aqueous solution by cetylpyridinium bromide modified zeolite. J. Environ. Sci. 2010;22:1327–1334.
Zhang G, Wu T, Li Y, Huang X, Wang Y, Wang G. Sorption of humic acid to organo layered double hydroxides in aqueous solution. Chem. Eng. J. 2012;191:306–313.
Zhang J, Liu F. Adsorption of natural organic matter onto a composite adsorbent prepared with chitosan and powdered activated carbon. Desalin. Water Treat. 2010;20:291–296.
Zhang X, Bai R. Mechanisms and kinetics of humic acid adsorption onto chitosan-coated granules. J. Colloid Interface Sci. 2003;264:30–38.
Zhao L, Luo F, Wasikiewicz J.M, Mitomo H, Nagasawa N, Yagi T, Tamada M, Yoshii F. Adsorption of humic acid from aqueous solution onto irradiation-crosslinked carboxymethylchitosan. Bioresour. Technol. 2008;99:1911–1917.