How can a material have both high-tech applications, such as for sports goods, and yet also have the very slippery attribute as the basis of the lead in a pencil? A common misconception is that pencil lead is lead; it is in fact graphite. Graphite, carbon fibre and diamond are all allotropes of carbon – different atomic structures based on the same element.
Both graphite and diamond demonstrate, on an atomic level, the importance of material properties and functionality when structure is altered. The ultimate hardness of diamond is based on a triangular tetrahedral structure, while graphite has a layered structure –imagine layers of chicken wire on top of one another. It is this layering that creates the slipperiness of graphite, three million layers of which would be 1 mm (1/16 in) thick.
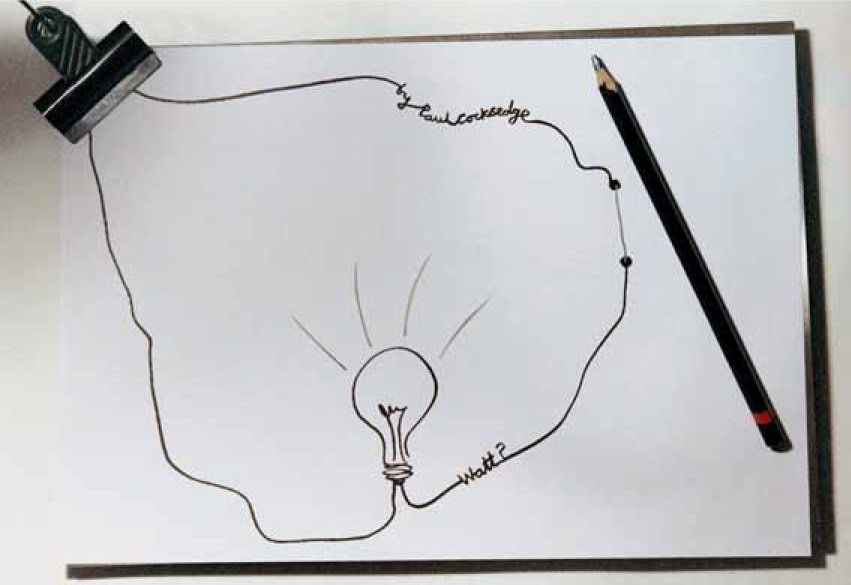
This silvery black, slippery carbon allotrope with a metallic sheen that defines the visual identity of graphite, is marked by three main forms: flake graphite, made up of crystal structures; amorphous graphite, which is soft and non-crystalline; and synthetic graphite, which constitute the largest commercially used form. One of the most interesting characteristics of graphite is its excellent conductive properties; something that can be demonstrated by drawing a pencil line across two electrical contacts. This is the idea behind Paul Cocksedge’s graphite lamp, shown here. A graphite pencil trail completes the circuit and conducts electricity to the lamp.
Image: Paul Cocksedge graphite lamp
Key features
•High strength-to-weight ratio
•Resistant to compression
•Chemically inert
•High resistance to chemicals
•Low friction and self-lubricating
•Non-toxic
•Good stiffness and energy absorption
•Good machinability
•Good electrical conductivity
•Flaky and breaks apart
•High resistance to thermal shock
•Temperature resistance is 3000ºC (5400ºF)
Sources
China is by far the largest producer of graphite. Other producing countries include Ukraine, Brazil, Russia, Canada, India, Zimbabwe, Norway, Mozambique Sri Lanka, Germany and Madagascar.
Cost
This varies depending on grade, but generally ranges from £0.32-2.60 ($0.50-4) per kg.
Sustainability issues
If not responsibly maintained, the environmental impacts of graphite mills can include air pollution and soil contamination from powder spillages leading to heavy metals contaminations of soil. Graphite can be recycled, and it is one of the materials being explored for use in alternative energy applications.
Production
Graphite can be moulded, sintered, used as a lubricant in its powdered form, added to metals and plastics to make them stronger
On a basic level graphite is used in everyday objects, such as pencils, where its soft, slippery qualities are put to good use to create what is essentially a graphite trail on a piece of paper. Its plate-like structure also makes it a good lubricant, especially at high temperatures. But on an advanced level it is a key player in advanced performance sports equipment, where it is used for golf clubs and tennis racquets, and it has an excellent strength-to-weight ratio and high-energy absorption. Graphite is also used in lithium-ion batteries, an increasingly important power source for e-vehicles and other areas of clean-tech.
Derivatives
–Graphene: single layers of graphite are graphene, the material that won Andre Geim and Konstantin Novoselov from Manchester University the Nobel Prize for Physics in 2010.
–Pyrolytic graphite, a material that will float over strong magnets.
| + | – |
|
–Good electrical conductivity –High strength-to-weight ratio –Good chemical resistance –Good resistance to thermal shock |
–Environmental concerns –Prone to flaking and breaking apart |
