Chapter 3
Conversion and Storage
Energy storage is a growing issue in our society. Fossil fuel resources such as petroleum are becoming not only more scarce in some areas but also increasingly inaccessible and costly. Most nations are now addressing the possibilities of providing energy in as many forms possible from sources other than fossil fuel. Petroleum products have largely been responsible for the immense progress made in Western society. Since the beginning of the 20th century, petroleum products have enabled the development of railroads, aircraft propulsion, and large ocean-traversing vessels, as well as the automobile. As far as we know now, there is no other equivalent source of energy in the form of combustible fuel, except perhaps for alcohol (ethanol). However, we are rapidly realizing that alcohol is not a practical solution to the greatly increasing demands for portable world energy.
Here, we will briefly review the role and merits of many competitive mechanisms available for secondary energy sources, ranging from compressed air and fly-wheels to electrochemical cells. Electrochemical cells still appear to be the most promising method of storing energy, which is probably the principal reason for its attractiveness. There are many practical considerations involved in selecting one means or another to store energy for use over time. Among these factors, perhaps cost is the most critical. Considerations such as safety, availability, life, dependability, cost, etc., would follow next.
Unfortunately, nature doesn’t provide unlimited choices to achieve an inexpensive reliable, safe, and readily available means of storing useful energy.
To focus our attention, we should establish two general categories of energy sources and identify them as primary or secondary. In reality, there is only one source in the strictest sense of the origin of energy. Secondary sources are actually intermediary places or devices where energy, from one of the few primary sources, is stored until needed at a later time.
We will describe and compare the various mechanisms offered by nature and made practical by present-day technology to store energy, followed by a section on electrochemical systems that concentrate on the redox type of devices that now seem to offer the most promise for solving long-term storage problems. Subsequently, details of performance, electrochemistry, and methods of constructing and testing these systems are presented.
First, in order to establish a firmer base, a physics background has been presented, along with the development of an argument for redox cells as practical devices.
This book describes a particular class of approaches to large-scale energy storage. Large-scale is defined here as amounts of energy exceeding 1 kWh that is stored in a single unit or group of energy storing units. The class of main interest here is that of reversible electrochemical cells. Since most cells or batteries with which we are familiar in our daily lives, such as the dry cell, alkaline cells, lithium ion batteries, and the ubiquitous lead acid battery, are not likely candidates, they will not be covered here. Only the approaches that have a chance to succeed as secondary batteries for solar, wind, load leveling, and emergency power applications will be discussed.
Storing energy in its many forms in nature is a vital part of all processes and life itself on Earth. As we explore these processes (Table 3.1) and their importance to us, we can gradually make some observations that lead to revealing and important conclusions.
Table 3.1 Energy sources accumulated by “natural” processes over recent and remote past.
| Energy source | Process of formation |
| Wind | Form of delayed solar, non-uniform heating of earth |
| Hydropower | Form of delayed solar evaporation and condensation, rain |
| Incident solar | Promptly available energy when converted to thermal or Photovoltaic |
| Tides | Gravity of moon and relative motion of Earth & moon |
| Geo-thermal | Residual thermal energy (compression) of earth formation |
| Nuclear | Remnants of initial matter formation processes |
| Fossil fuels | Accumulated conversion of organic matter deposits (solar) |
| Organic fuels | Wood, hydrocarbon gasses, (delayed solar) |
| Metal stressing | Steel springs |
| Elastic deformation | Rubber bands |
| Elevated weights | Impact devices, clocks |
| Compressed gases | CO2 or compressed air powered devices |
| Chemical reactions | Gun powder and explosive mixtures |
| Masses in motion | Fly wheels, rotating masses and linear, high-speed impact |
| Electrochemical | Batteries and fuel cells |
Unfortunately, nature is not very cooperative when it comes to providing a multitude of materials with the totality of desirable and needed properties, such as low-cost, safety, availability, electrochemically well-behaved in aqueous solutions, and a large enough energy and power density potential to make simple, practical storage systems.
All applications or manifestations of energy storage can be put into one or more of these categories. Certainly, if we wish to power a portable power tool or an electric automobile, a hydroelectric plant is hardly useable. However, if we use the energy produced by the station for storage in an electric battery, it becomes a practical situation. That’s an example of the portability or mobility reason for storage. In the second instance we might have the need to store solar energy during the daylight hours for use after sunset to power light, etc. There are many cases where the convenience factor is not met – where the generation of energy is occurring at a time that coincides with the need or application time.
It must be pointed out here that when we refer to energy we are essentially referring to the “capacity to do work” in the classic sense of Force × Distance. It is this capacity that is being transferred from one source to another device that is capable of storing this capacity for work.
Storage of energy in one form or another to be used at a later time has been extremely important not only to mankind, but also to every form of life. Storage of energy in the form of chemical structures such as carbohydrates enables life forms to survive for periods of time between food intake activities.
Other, simpler forms of energy storage that are familiar to all of us are listed below. These forms of energy are categorized for the purpose of distinguishing basic differences in the origin and the physical processes involved.
Since all of the above are commonly known and familiar to the reader, there is little need to give many examples of each of these processes or mechanisms. It is interesting to note here that energy sources that are derived from “natural” sources provide, by far, the largest amount of the energy consumed by industry and commerce. Especially in the case of fossil fuels, the available energy density is much greater than any other competing man-made source for most common applications – nuclear power not included.
Returning to a less esoteric domain, the outline of the presentation is as follows, listing the primary topics that will be covered:
- Primary energy sources – On a grand cosmic scale, primary sources are limited to nuclear and gravitational. All subsequent forms of energy are consequences, as far as we know at present, of these two principal sources. These are sources over which humans have no control.
- Secondary sources of energy – It is necessary to define what we mean by “secondary.” Usually the definition involves some process of storing, and is associated with those resultant processes over which we do have control.
- Conversion processes – Those devices that enable us to transform one form of energy into another more.
3.1 Availability of Solar Energy
Indicative to this explanation is a comparison between the solar energy that is available from photovoltaic conversion mechanisms and the solar energy that is available via plants’ conversion into combustible fuels, such as methanol.
The total available energy via combustion from 1 gallon of gasoline in an internal combustion engine is about 36kWh. The conversion efficiency of an automobile engine to mechanical output is at most about 20%. Thus, if we had very efficient electric motors instead, we would need to have at least 1/5 of the 36kWh × 500 million gallons per day. That number is 3,500 million kilowatt-hours of energy from sunlight per day.
Again, if the conversion efficiency were 100%, dividing the numbers would reveal that we need about 5,000 square miles of area to provide the equivalent of gasoline. Unfortunately, we must multiply that figure by 10 to account for conversion losses, giving us a total of about 50,000 square miles. However, the following problem remains: How does one use solar energy to operate cars without storage?
The practicality and cost of solar energy for consumer use to power industry and residential homes has become a widespread concern that stems from questioning energy sources and their limits. A simple estimate of available solar energy per unit land area, especially if it is to be collected and transformed directly into electric power, reveals that immense land areas are required to provide even modest amounts of energy for commercial use as illustrated below.
The following calculations present a rather dismal prospect for widespread use of solar energy as a substitute for the more “conventional” sources presently in use. Regardless, the problem of providing energy to meet the increasing demands of the future is very serious. Other than oil and coal, there are no realistic answers. Nuclear, along with cheap and reliable energy storage (such as batteries), could be an answer to well-designed, plug-in hybrid vehicles, giving a range of 200 or more miles on a single charge.
As an example, consider a middle region of the North American Continent and use simple approximations. At high noon (normal incidence) on a cloudless day at the Equator, incident solar power surface density is in the range of 0.12 watts per square cm. Since sunlight is available only half the day (about 10 hours of useful daylight), the energy density is reduced to approximately 1.2 watt-hours per square cm per day.
And, due to Earth’s rotation on its axis, the sun’s radiation makes a changing angle to the Earth’s surface perpendicular. Therefore, we can simply approximate that by another 50% factor. Hence, the energy density over a 24-hour period is further reduced to 0.6 watt-hours per square cm.
The above energy per unit area per day becomes 0.6Wh/cm2 = 3.6 Wh/in2, or 3.6 Wh/in2 = 500 Wh/ft2.
Since there are about 5,300square feet per square mile, there are 5300×5300×500 = 28 million × 500WH from 1 square mile, or 15,000 megawatt hours per square mile in any one clear day at the equator.
At latitudes of the mid-United States region, we might need to divide that number by two, making the total sunlight energy equal to about 7,000 megawatt-hours per day per square mile of area.
Now let’s take a brief look at what the order of magnitude of the needs are for domestic energy per day or per year in the United States. According to the website Globilis, the amount of energy produced and consumed per year in 2002 was 4 million kilowatt-hours. That number reduces to 10,000 million kWh per day consumption.
So, if we want to produce (assuming 100% conversion efficiency of electromagnetic energy from the Sun) that amount of energy, we would need an area of about 1400 square miles. Present-day efficiencies of solar photovoltaic cells can be in the order of 10% to 20%. Using these lower efficiency figures, we need 7,000 to 14,000 square miles of accessible area to generate the electrical energy presently being used by the United States. Imagine the maintenance and access roads required for such a solar collector field. Simply the problem of keeping the surfaces of semi-conductors and collectors clean and repaired would be monumental.
Now, consider the gasoline situation. The United States used 21 million barrels of oil per day in 2007. The yield at the refineries was about 20 gallons of gasoline per barrel of oil, which means the nation actually used over 400 million gallons of gasoline per day – an astronomical figure.
The problem remains unsolved. In addition, we must also have available storage to make the energy portable and useable whatever peak demands arise.
Now, consider corn as a source of methanol. The data from the US Department of Agriculture shows that the average yield of corn per growing period of at least six months is about 150 bushels per acre. A bushel of corn will yield 2.5 to 3 gallons of ethanol. Ethanol has less than half the energy content per gallon than gasoline. Thus, we would obtain about 200 gallons of gasoline equivalent per acre of cornfield.
Returning to the gasoline consumption rate above, if the United States consumes about 400 million gallons per day multiplied by 300 days per year, then the country consumes about 12,000 million gallons per year. After dividing the numbers for corn yield, close to 100 million acres of farmland would be required.
A square mile is equal to 700 acres. After dividing again, it appears that about 150,000 square miles of farmland is needed under ideal circumstances – no provision is made here for roads, buildings, fertilizer, machinery, etc., – which is not very encouraging.
3.2 Conversion Processes
The following are non-mechanical methods for converting from one form of energy to another. A simple mechanical example would be the generation of electric energy by mechanically moving an electric conductor through a magnetic field as is done in a dynamo.
This is intended only as a brief review of a few well-known means employed to convert energy from one form directly into an electrical output.
3.2.1 Photovoltaic Conversion Process
The photovoltaic process (Figure 3.1) converts direct conversion from light energy (photons) to electricity (volts and amps) via p-n solid-state devices. Electrons are raised to the necessary high levels of kinetic energy and cross over the junctions with a corresponding voltage.

Figure 3.1 Photovoltaic semi-conductor cross-section.
Efficiencies are generally above 20% and thin film device can be less expensive, but at lower efficiencies.
3.2.2 Thermoelectric Effects: Seebeck and Peltier
The search continues for materials with lower thermal conductivity, higher electrical conductivity, and higher Seebeck coefficients. Some materials that are used as a thermoelectric heat pump (cooling device), such as bismuth telluride, would give higher conversion efficiencies if they could be operated at higher sustained temperatures. Unfortunately, electrical contact deterioration, materials diffusion within the structure, and melting points exclude them from such application.
The amount of heat loss due to heat conduction along the paths from the hot to the cooler junctions significantly contributes to conversion efficiency degradation. Joule heating, due to ohmic resistance of these paths, adds to the inefficiencies. The attractive- ness of no mechanically moving parts does not quite make these devices practical, except in special applications where inertness and long life are important. In addition, these types of devices are subject to the ever-present Carnot efficiency limitations encountered by all heat engines. Heat to electrical efficiency is 12 to 18%.
Thermal energy, Q, pumped into or out of a thermoelectric junction per unit time is simply expressed as
(3.1)
The Seebeck effect is utilized in many ways, but it is most commonly used in the well-known thermocouple used to measure temperature. The materials (usually metal alloys) are selected for this purpose on the basis of their thermoelectric coefficients and stability at high temperatures. The alumel-chromel, or iron- constantan materials couples, is very common. The Seebeck coefficient is very dependent on both operating temperature ranges and materials choice.
3.2.3 Multiple P-N Cell Structure Shown with Heat
It is necessary to conduct heat to and from the hot and cold junctions, respectively, in parallel for an array of junctions while still providing electrical conduction in series (Figure 3.2). At times this can be a somewhat formidable engineering and materials application problem.

Figure 3.2 Multiple themoelectric junctions – thermopile.
From: Introduction to Energy Technology, Ann Arbor Science, 1976.
In general, semiconductor materials with large Peltier potentials, low thermal conductivity, and high electronic conductivity are desirable. Unfortunately, these properties can become mutually exclusive because of the physics of the processes.
For example, at 300°C temperature differential, output is about 0.05 volts per junction.
3.2.4 Early Examples of Thermoelectric Generators
Thermoelectric energy conversion systems were devised in the mid-19th century and employed for limited practical purposes to operate communications equipment. There are records of Edison experimenting with very large arrays of metal alloy wire junctions wrapped around Franklin stoves to generate sensible electric power.
In more recent times, numerous similar devices were constructed in Russia and utilized to broadcast news and propaganda to remote areas in that country that had no other sources of electrical power to operate radios.
3.2.5 Thermionic Converter
For a thermionic converter, theoretical efficiency is about 75%, practical efficiency can be about 15%, and the hot electrode comes to about 1600–2000°C.
These devices, with a perhaps misleading name, convert heat energy into electrical output primarily by “boiling” conduction band electrons from metallic surfaces placed in a vacuum (Figure 3.3). The drawbacks to this approach seem to be largely associated with their operating life, particularly with the emitter.

Figure 3.3 Illustration of an operating thermionic converter.
3.2.6 Thermogalvanic Conversion
Thermogalvanic conversion is a process going directly from a temperature differential to an electrochemical potential. An example of a symmetrical cell employing the phenomenon described by the Gibbs-Helmholtz relationship is shown in Figure 3.4. For thermogalvanic conversion, dE/dT ~ 0.01 volts per deg C, efficiency can be about 15%, and temperature range is from 140° on the cold side to 350° on the hot side.

Figure 3.4 Thermogalvanic cell depiction employing silver iodide electrolyte.
There is another mechanism for transforming heat energy directly into electrical form without the necessity of mechanically moving parts or any other such complexities. As in the case of thermoelectric effects, there is an analogous one in chemistry. It is known as the thermogalvanic effect, as expressed by the Gibbs-Helmholtz equation. At first glance, this could be mistaken as another colligate property of matter. However, upon closer examination we see that the electric potential/temperature behavior of substances is quantitatively dependent upon the material property itself, namely, the free energy of formation as a function of temperature.
The equation relating the enthalpy, H, and the free energy change ΔF, to the rate of change of free energy with temperature is
(3.2)
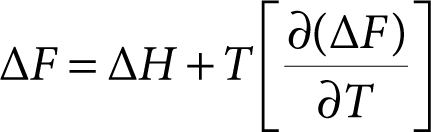
where pressure is assumed to be constant.
The equivalent of free energy (in watt-second) can be put into terms of voltages and the quantity of electric charge, e.g.,
(3.3)

where E is in volts, Ff is Faraday’s number of 96,500 coulombs per equivalent, and n is the electric charge on the ion. Thus, we then have the following equation as a net expression for the change in electrochemical voltage as a function of temperature:
(3.4)

or
(3.5)

By applying this last relationship to a symmetrical “thermo-galvanic cell” that employs the silver/silver chloride electrodes, as shown in Figure 3.4, the value of ∂E/∂T at an average temperature of 300°C can be estimated from enthalpy data that is readily obtained from sources such as the Handbook of Chemistry and Physics, published by The Chemical Rubber Publishing Co.
Solid silver iodide, or AgI, was explored some years ago as a possible electrolyte for a galvanic version of thermoelectricity. This compound has properties that lend themselves to such application because its electrical ionic resistivity as an electrolyte is a usable range, and the gradient of voltage versus temperature has unusually high values. Some of this data is presented in Figure 3.4.
3.3 Storage Processes
3.3.1 Redox Full-Flow Electrolyte Systems
The storage of electrical energy in chemical form by reversible electrochemical processes is in widespread use. The main limitations to all present electrochemical couples (batteries) are their shelf and cycle (operational) life as well as their energy density and power delivery capabilities. For stationary applications, energy and power densities are not of primary importance. Their energy turnaround efficiencies, cost, and operational life are more significant.
This technical exhibit describes an electrochemical cell, which stores energy on the basis of concentration differences at opposite electrodes and between the same chemical species. The low electric potentials at which these cells operate eliminate the possibilities of water electrolysis and the formation of hydrogen or oxygen gas at the electrodes during charging. Thus, a sealed system can be constructed that requires no maintenance. Since the cell is chemically symmetrical, its cycle life is indefinitely great.
One of the few basic electrochemical processes that can be employed in an energy cell and also has little deleterious effects upon either electrode or cell structure materials is
(3.6)
This energy storage system is described and compared with other methods as candidates for numerous space applications.
This technical exhibit is about simple energy storage for applications where reliability and life are among the primary considerations. In those cases where the primary energy is generated from solar, wind, tides, and even I.C. engines, there is a need for storage for some period of time. This energy is then used at a later time when the demand requires it. The electrochemical system that is proposed here and identified for reference convenience as a symmetric cell appears to be an attractive process.
A brief review of a number of more likely competitive systems in terms of performance is also presented. Cell electrochemistry, transport processes, and cell performance are also presented here.
3.3.2 Full Flow and Static Electrolyte System Comparisons
Redox cells with two electrolytes and all liquid reagents can be designed and operated as either static electrolyte systems or full flow electrolytes. In the first case the electrolytes remain in their respective compartments (negative and positive sides of a cell with a barrier or membrane separating the two compartments) as in conventional batteries. The drawing in Figure 3.5 shows such a basic design.
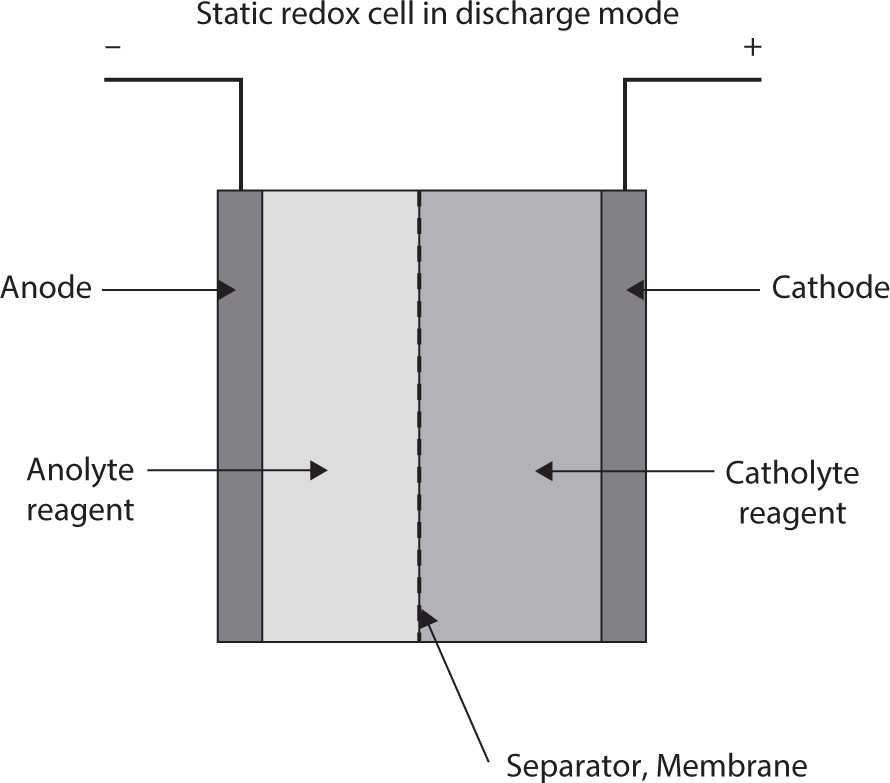
Figure 3.5 Fixed electrolyte redox cell.
The static electrolyte version of the redox cell offers the advantages of simplicity in design, no mechanically moving parts, and it is easily sealed. However, the energy and power densities of any particular cell design are a compromise because inter electrode cell spacing will affect both coulombic capacity as well as internal cell resistance and reagent availability for discharge. Also, charge retention time is significantly smaller because of ionic and molecular diffusion across the membrane separator.
In the full flow configuration, the two electrolytes are circulated from reservoirs into and out of their respective cell compartments through appropriate manifolds and pumps as shown in the Figures 3.6 and 3.7.
The following are the advantages of this design approach:
- Indefinitely long charge retention times
- No cell imbalance problems with large cell arrays
- System can be electrically shut off by electrolyte draining
- Separation of energy capacity from power delivery parameters in system designs
- Ability to be recharged chemically by replacing electrolytes
- Electrolytes may be electrically recharged in an external device

Figure 3.6 Full flow electrolyte redox battery system.

Figure 3.7 Comparison of power/energy separation to I.C. engine system.
In some applications the advantages or salient characteristics of full flow redox may outweigh the necessary additional complexity and mechanisms.
In striving for the realization of a very long-life secondary battery, one of the developmental paths that can be followed is the use of reagents that remain in solution at all times. In other words, there is no deposition, removal, or change of composition or change in structure of solid reagents at electrode surfaces in the energy storing process. There are very few choices of chemical components that have all the properties necessary to make it a practical electrochemical process. Those that are candidates are listed in Chapter 5. Virtually all of the materials combinations have the singular drawback of having dissimilar materials on opposite electrolytes. Furthermore, since these reagents are in solution, there is the inexorable transport of catholyte materials into the anolyte region and vice versa. In most cases, there is no direct method of returning these unwanted components from one electrolyte to their origin.
An example of such a redox cell is the chromium/iron couple where chromium and iron chlorides are in the negative and positive sides of a two-compartment cell. Since the energy process participants all have positive charges, once any chromium diffuses to the iron side of such a cell it is lost permanently. This type of transfer of ions results in a gradual deterioration of the electrolyte, and the cell will cease functioning until a new electrolyte is introduced from an external source.
Another example of a redox system employs the sulfur/bromine couple and was first developed by TRL, Inc., for National Power, PLC. This is also a materials asymmetric couple whose cycle life is limited by unwanted diffusion of sulfide ions from the negative cell side into the positive, bromine side. Cation membranes are employed (usually NAFION) to maintain effective electrolyte separation, and cycle life can be large, but the electrolytes must be chemically processed periodically in order to sustain performance.
A redox system that employs the same chemical species for energy storage currently in development (University of New South Wales, Australia) is the vanadium redox. In order to recognize an operating cell that meets the conditions of all liquid electrolytes and has the same chemical reagents on both sides, it is necessary to have at least three oxidation states of an anion or cation that are soluble in a polar solvent such as water. Vanadium is the only element with those properties that is reasonably well behaved. The circulating electrolyte design being pursued for load leveling application is illustrated in the drawing of Figure 3.8.

Figure 3.8 Schematic of a vanadium redox cell.
In principle the electrolytes should have an indefinitely long life because vanadium ions are on both sides of the cell, and any unwanted diffusion can be corrected electrically by the transport during the recharging mode. Some limitations, however, are (1) high cost, (2) electrolyte maintenance, (3) hydrogen evolution and an ineffective seal, and (4) energy density limited by materials solubility in water.
