8
Carbon Nanomaterial Sensors for Cancer and Disease Diagnosis
Tran T. Tung1,2 Kumud M. Tripathi3 TaeYoung Kim3 Melinda Krebsz1,4 Tibor Pasinszki 4 and Dusan Losic1,2
1 School of Chemical Engineering, University of Adelaide, South Australia
2 ARC Research Hub for Graphene Enabled Industry Transformation, University of Adelaide, South Australia
3 Department of Bionanotechnology, Gachon University, South Korea
4 Department of Chemistry, College of Engineering, Science and Technology, Fiji National University, Fiji
8.1 Introduction
Nanomaterials, particularly nanocarbon allotropes, provide numerous advantages for designing new and high‐performance sensing platforms with high sensitivity, rapid response, and fast recovery for multianalyte detection due to their outstanding structures, remarkable properties, low‐cost, and scalable production [1–5]. Sensors based on carbon nanomaterials including carbon nanoparticles (NPs), carbon nanotubes (CNTs), carbon nanodots (CNDs), and graphene derivatives have been extensively used for different sensing applications such as gas/vapor sensing, optical sensing, and electromechanical sensing. Based on these sensing materials, research efforts are largely focused on the exploration of new detection concepts and improvement of sensor performances in terms of sensitivity, selectivity, detection limit, response time, and reversibility. Their practical application is also broad ranging from environmental monitoring, public safety, food packaging, biomedical tracking, bioimaging, and bioanalysis and therapy. Particularly, the use of sensors in biometrics and human health monitoring was attractive offering a capability to monitor and diagnose debilitating diseases.
There are two main sensing approaches for human health monitoring; one is based on VOCs, and another is based on detection of biomarkers. The first method involves the analysis of VOCs generated in the human body by alteration of metabolic pathways related to liver enzymes, carbohydrate metabolism, oxidative stress, lipid metabolism, and cytochrome P450 [6]. The VOC family is composed of saturated and unsaturated hydrocarbons, aromatic compounds, alcohols, aldehydes, ketones and nitriles, and is generated via various biochemical pathways [1, 6, 7]. A disease can be recognized accurately by its specific pattern of VOCs, with low possibility of interference by other diseases [1, 6, 8, 9]. In fact, VOCs, after their generation, are emitted in body fluids such as exhaled breath, urine headspace, saliva, blood headspace, milk skin headspace, or the headspace of disease‐related cells [1, 10, 11]. Thus, a collection of exhaled breath and skin headspace are frequently used as patient‐friendly sources of VOCs and their chemical fingerprinting for early detection and monitoring of diseases [12, 13]. Therefore, the detection of VOC is a new frontier in rapid, sensitive, selective and noninvasive analysis and medical diagnosis of human diseases [3]. The second method involves the detection of abnormal levels or fluctuation of biomarkers in the human body. These biomarkers include DNAs, peptides, proteins, hormones, antigens, glucose, dopamine (DA), and uric acid (UA), and have been extensively studied in biomedical applications. For example, antigens are well‐known cancer biomarkers that present in tumor tissue and serum. Similarly, the detection of disease specific proteins, peptides, DNAs, and their fragments in serum provides valuable information for disease diagnosis and monitoring. Glucose is a key compound for the monitoring of diabetes mellitus. DA levels are observed to fluctuate/decrease in individuals who suffer from neurological diseases such as Parkinson's, Schizophrenia, and attention deficit hyperactivity disorder (ADHD). Furthermore, unusually low and high levels of UA in the body leads to many diseases, including gout, cardiovascular disease, and hypertension [9]. Therefore, direct molecular detection of biomarkers is a promising approach for diagnosis and monitoring of numerous diseases, as well as a cornerstone of modern molecular medicine. Their point‐of‐care sensing devices are of crucial importance for clinical therapy.
Current principle methods for VOC detection are based on measurement of changes in the electrical, optical, chemical, and chromic properties of the sensing materials after interaction with VOC molecules. Among different techniques, the combination of gas chromatography–mass spectrometry (GC–MS) is generally used for the detection of disease biomarkers in breath [14]. However, high costs of these instruments, sample preparation requirements, limited portability, lack of real‐time diagnosis, and complicated analysis limit their applications. Therefore, development of simple, low cost, portable, and efficient sensing devices are needed to address clinical requirements for fast medical diagnosis based on VOC biomarkers.
In parallel, current commercially available detection methods for the detection of biomarkers are still based on meters, monitoring systems, tests kits, biosensors that utilize mediator‐enzyme interactions, and medical imaging. However, due to enzymatic activity dependence of these sensors, various issues arise regarding cost, analysis time, or complex and easy susceptibility to environmental changes [9]. Furthermore, some techniques are limited by the sensitivity, complexity, and low‐selectivity challenges of these detection methods. As a result, further development of precise, sensitive, and selective measurements of biomarkers at a low level inside or outside of living systems (predominantly humans) is required and can greatly contribute to the monitoring and diagnosis of diseases.
A sensor, so‐called signal transducer, is a device that transforms information from stimuli such as gas, liquids, light, temperature, pressure, strain, and so on into analytically useful signals (e.g. electrical signals) that can be easily monitored [15]. Chemical vapor sensors mainly operate on the basis of chemoresistive and piezoelectric technology. The sensing mechanism for their detection by sensing nanomaterials is based on their conductance changes upon the vapor molecules adsorb on the surface, which allows changing the carrier concentration or number of density in different ways. The different responses of sensing materials toward chemical vapors are due to electron‐withdrawing vapor molecules (acceptors) level or electro‐donating vapor molecules (donors) level. These devices can detect the presence of specific chemical vapors or gases in an area, often as part of a safety system, and they are widely used in public safety and industry. A biosensor mainly operates based on electrochemical or optoelectronic technology. The device is generally defined as an analytical tool that converts biological responses into qualifiable and processable signals [16]. Biosensors are used in various industries, including agriculture, medicine, public safety, and environmental monitoring.
There is a continuous effort to increase sensor performances, and carbonaceus nanomaterials of zero‐dimensional (0D), one‐dimensional (1D), and two‐dimensional (2D) structures, namely CNDs, CNTs, and graphene, respectively, provide novel routes to this end. Based on their unique properties, these materials offer the development of sensing platforms for simple, fast, and ultrasensitive detection of biomolecular adsorption/binding. Versatile methods have been developed for simultaneous functionalization and passivation of sensor surfaces in order to allow for enhanced detection and improved selectivity of these sensing devices. In this chapter, we present two sensor approaches for VOCs and biomarkers detection by using chemical vapor sensors (breath analysis) and biosensors (biomarkers analysis), respectively, as well as recent advances in carbon nanomaterials‐based sensors for cancer detection and disease diagnosis.
8.2 Detection of VOC by Using Gas/Vapor Sensors for Cancer and Disease Diagnosis
Diagnosis of diseases at an early stage and further follow‐up of disease conditions provide better chance of recovery and public health protection, as suitable treatment can be applied in time [17]. However, most of the clinical diagnostic techniques are invasive, time consuming, and expensive, and require well‐trained manpower. Additionally, the antemortem diagnosis of various diseases, including cancer, relies mostly on the occurrence of associated clinical symptoms and proficiency of the treating physician [18]. For the diagnosis of a particular disease, one of the most promising approaches is to develop new diagnostic tool to detect disease‐specific biomarkers in a noninvasive manner [17]. The detection of VOCs has overcome many constraints of the invasive diagnostic techniques over the past two decades and relies on the noninvasive rapid diagnosis of linked diseases. Appropriate biomarkers analysis could enable early diagnosis before the development of diseases [18]. VOCs are emitted from biological specimens in human body into the environment in various ways; exhaled breath, skin and bodily fluids, and feces contain biomarkers that can be used for the assessment of diseases. Characteristic alteration in associated biomarkers reflect the physiochemical, metabolic, and neuropathologic changes in the human body, thus biomarkers emerge as reliable analytes [18]. Among the choice of human samples (sweat, urine, saliva, and sputum) breath analysis provides a rich and diverse source of cues for the noninvasive and long‐term monitoring [19]. Breath analysis offers the diverse advantage of a less‐complicated mixture than blood/urine. Correlation between VOCs present in exhaled breath and physical condition of a human has been extensively studied [20, 21]. Specific VOCs are released constantly by the cellular biochemical processes in their microenvironment and are exhaled in breath after alveolar exchange from the blood. Metabolites reflecting different disease, especially cancer, conditions can be analyzed by the detection of particular VOCs in exhaled breath. For example, alterations in hydrogen sulfide (H2S), toluene (C6H5CH3), acetone (CH3COCH3), ammonia (NH3), pentane, and nitrogen monoxide (NO) concentration in exhaled breath are recognized as indicators for diagnosing halitosis, lung cancer, diabetes, kidney malfunction, heart diseases, and asthma, respectively [22, 23]. Specifically, 22 VOCs, including benzene, styrene (ethenylbenzene), hexanal, 2.2.4.6.6‐pentamethyl heptanes, propyl benzene, 2‐methyl heptanes, decane, 1,4‐dimethyl benzene, 3‐methyl nonane, 1‐hexene, hepatanal, undecane, 1‐methyl‐2‐pentylcyclopropane, methyl cyclopentane, trichlorofluoro benzene, 1‐methylethenyl benzene, cyclohexane, 1‐heptene, 3‐methyl octane, 1,2,4‐trimethyl benzene, 2‐methyl‐(isoprene)‐1,3‐butadiene, and 2,4‐dimethyl heptanes are listed as biomarkers for lung cancers [24]. Isoprene and sulfur‐containing compounds in exhaled breath are associated with cholesterol metabolism disorders and liver disfunction, respectively [25]. Disease‐specific breath prints rely on the significant advantage as robust and easily accessible biomarkers before the onset of clinical symptoms or signs [1]. Consequently, breath analysis exhibits considerable potential as a clinical reality for the diagnosis of various diseases at their early stage. However, technical difficulties associated with the wide occurrence of VOCs at the relatively trace level of concentration in complex exhaled breath sample are major obstacles for clinical analysis.
The concentration of VOC biomarkers in the exhaled breath of a patient is significantly higher than the normal one. A variety of analytical techniques are used to recognize the correlation between alteration in concentration of VOCs and existence of diseases [19]. GC‐MS, infrared spectroscopy, and optical spectroscopy are typically used for VOCs analysis [19]. However, the sizable and immobile equipment, relatively slow measuring procedures, trained manpower requirements, and complexity of their manipulation hamper their application in real‐time diagnosis. Despite of recent advances in GC‐MS technique for compound identification, like solid‐phase microextraction (SPME), GC‐MS still does not account for all VOCs in exhaled breath and reveals only a fraction of extracted analytes [26]. The requirements of high operating temperature (200–280 °C) and costly equipment are further drawbacks for SPME [27]. The colorimetric technique, polymer‐coated surface acoustic wave (SAW) sensors, piezoresistive sensors, and coated quartz crystal microbalance (QCM) have been also applied for VOCs analysis. QCM‐based VOCs sensors exhibited high sensitivity and can be easily integrated with various micro‐devices [28]. However, materials requisite for QCM technique, the highly exposed surface area and sufficient adsorbing sites toward VOCs, are limited. QCM also suffers from the disadvantage of instability during analysis because of the absorbance of foreign molecules such as moisture [28]. Recently, demand for portable and real‐time monitoring has led to the miniaturization of VOCs sensors. Therefore, researchers' attention has been focused on the colorimetric technique to obtain portable and efficient VOCs sensors. Selectivity, sensitivity, response time, and stability can be simultaneously addressed by optical sensors [29]. Optical sensing devices offer remote sensing capability and the elimination of explosion risk [29]. Nevertheless, the synthesis of optical sensing materials and fabrication of optical sensors are laborious and time‐consuming.
As an alternative, chemiresistive sensors for exhaled breath analysis have attracted a great deal of attention recently, because they offer several technological advantages due to their significant potential for miniaturization, low‐cost, portability, and real‐time diagnosis. The unique properties of nanomaterials, their facile incorporation into chemiresistive sensors, and the new emerging technologies for nanomaterial fabrication boosted the development of VOC sensors in the past decade [30]. Charge transfer between adsorbed VOCs and nanomaterials led to alteration in surface charge density and consequently in conductance of these sensors. Rapid progress has been made in the incorporation of NPs into clinical and laboratory diagnostic tools toward the standardization of VOCs analysis in exhaled breath. In particular, NP‐based sensors for breath analysis exhibited high sensitivity and quick response toward trace level and wide varieties of VOCs in the highly humid atmosphere [31]. The application of nanoscale structures in sensors, especially nanocarbons such as CNTs, CNDs, graphene, and graphene quantum dots (GQDs), opened the door for the construction of the next generation point‐of‐care diagnostic devices [1, 5, 32, 33]. Nanocarbons having a high aspect ratio offer highly active interfaces, and nanocarbon‐based sensors have been shown to reach a detection limit of parts‐per‐billion (ppb). Several research breakthroughs have been achieved recently on an exploration of nanocarbon‐based VOC sensors for disease diagnosis [1]. Nanocarbons have also been incorporated into chemiresistor arrays on a solid substrate in order to construct electronic nose (e‐nose) for the detection of VOCs and hence disease diagnosis in exhaled breath based on a pattern‐recognition technique. Their application is extended for the diagnosis of both cancerous and noncancerous diseases, such as lung, prostate, breast, gastric, colon, ovarian, and hepatic cancers, Parkinson's, Alzheimer's, and acute kidney diseases, chronic renal failure (CRF), tuberculosis, and pulmonary hypertension type noncancerous diseases [31]. Nanocarbon‐based VOC sensors are also attractive for “on‐site” monitoring and are promising alternatives for rapid estimation. From a practical point of view, nanoelectronic sensors based on nanocarbons offer high carrier mobility, compatibility with modern technologies, low power consumption, and easy miniaturization.
8.2.1 Carbon Nanodots (CNDs) and Graphene Quantum Dots (GQDs) for VOC Sensors
CNDs and GQDs are emerging ultra‐small carbon nanostructures with unique physiochemical properties due to pronounced quantum confinement and edge effects [34–36]. Novel structural, electronic, and morphological characteristics of these 0D nanocarbons have captured intensive attention for myriad applications [37–40]. CNDs are spherical nanocarbons, composed of both sp3 and sp2 carbon domains. Chemical stability and environmental sensitivity of CNDs emissions in a broader range provide possibilities for their use in VOCs sensing applications. There have been a number of researches devoted to developing CNDs‐based sensors for VOCs sensing and disease diagnosis. For example, amphiphilic CNDs with strong solvatochromic properties and high quantum yield were synthesized from (Z)‐4‐(2‐cyano‐2‐(4′‐(diphenylamino)‐[1,1′‐biphenyl]‐4‐yl)‐vinyl)benzonitrile (pCN‐TPA) and PEG2K (molecular weight 2000) using the solvothermal technique [41]. Solid sensors as optic nose were fabricated by casting these CNDs' solutions on test strips to instantly sniff out VOCs for on‐site detection. These sensors could discriminate between wide ranges of VOCs recognized by the change in fluorescence color by naked eyes. Dolai et al. used a composite of CNDs‐aerogel (silica) matrix to fabricate a sensing platform for aromatic VOCs detection [42]. CNDs‐aerogel was fabricated via in situ one‐step process using D‐glucose as a carbon precursor. These aerogels were reported to discriminate between aromatic VOCs, like isomers of phenylenediamine, based on fluorescence shifts and degrees of quenching. In another report, sensing of NO2 was realized using carbon quantum dots (CQDs) functionalized silica aerogels based on fluorescence quenching technique [43]. Bhattacharyya et al. reported the detection of tuberculosis specific volatile organic biomarkers (VOBs) using CdSe quantum dots (QDs) and sugar‐drink‐derived CNDs [44]. A schematic representation for the detection of VOBs based on changes in optical response is shown in Figure 8.1. Emission intensity of CNDs was reported to decrease upon exposure to VOBs (Figure 8.1b,c).

Figure 8.1 (a) A diagram (schematic) showing a proposed method of TB biomarker detection using quantum dots. The methodology involves the collection of breath into a Tedlar bag and subsequent mixing into a QDs (or C‐dot) solution. The spectral analysis will be performed for estimation of VOB concentration and prediction of patient health. (b) Absorbance and (c) emission spectra of pristine carbon dots and with different concentrations of VOBs. It can be seen that nicotinate‐CDs solution can be easily distinguished from pristine CDs solution using UV irradiation [44]. (See color plate section for the color representation of this figure.)
Source: Reproduced with permission from Elsevier.
On the other side, GQDs as small disks of graphene nanosheets (2–20 nm) with better surface grafting properties than CNDs, owing to π−π conjugated network, zigzag edges, crystalline nature, and excellent biocompatibility, could meet the requisite of ideal VOCs sensor [45]. Although GQDs have attracted significant research interests and progress has been made in the design of novel photoluminescence (PL), electrochemical, and electrochemiluminescence sensors, research on GQDs for VOC sensor applications is still in its early state. An important example of hybrid materials of GQDs with conducting polymers for chemoresistive VOC sensors has been reported recently by Gavgani et al. [45]. Sensors were fabricated by drop casting N‐doped GQDs/poly(3,4‐ethylenedioxythiophene) (PEDOT)–poly(styrenesulfonate) (PSS) composite solutions on interdigitated Au electrodes over a large area of the silicon substrate. These sensors were reported to exhibit high response toward methanol in the 1–1000 ppm range at room temperature due to direct charge transfer and swelling process. Sensing of ammonia using S and N co‐doped GQDs/polyaniline (PANI) flexible sensors with fast response and recovery at room temperature was reported [46].
8.2.2 Carbon Nanotubes (CNTs) for VOC Sensors
CNT‐based VOCs sensors have been extensively explored as one of pioneering sensing components owing to their super sensitivity at room temperature and fast response time due to their unique physical and chemical properties [47]. Besides nano size and high aspect ratio, CNTs have exhibited distinctive hollow tubular structure, which offers high efficiency for breath analysis−based disease diagnosis [27]. Both multi‐walled carbon nanotubes (MWCNTs)‐ and single‐walled carbon nanotubes (SWCNTs)‐based VOCs sensors have enabled disruptive technology for wireless sensors and feasibility to integrate them into wearable devices [47, 48]. Despite such advantages, CNT‐based sensors faced the problems of relatively long recovery time, poor selectivity, and complex synthesis procedure, which limited their application in real‐world diagnostic technologies. Therefore, composite fabrication was adopted to enhance chemisorptions of VOCs on CNTs surface, and surface functionalization of CNTs were used to tune their properties [49].
Composite fabrication is clearly desirable to harness the unique properties of CNTs through the incorporation of diverse functional materials [50]. To date, various materials such as inorganic nanostructures, macromolecules, metal nanoparticles, biomaterials, organic crystals, metal‐organic frameworks, polymers, and nanocarbons are extensively explored for composite fabrication with CNTs and construction of ideal vapor sensors [51–53]. Salavagione et al. have also been overviewed conductive polymer composites (CPC) of CNTs and different type of polymers for chemical sensors [54]. The conductive architecture of different polyhedral oligomeric silsesquioxanes (POSS) covalently and noncovalently bonded to the surface of functionalized CNT offer transducers in the 3D surface structure for the detection of VOCs [55]. Fabrication of electronic nose (e‐nose) with CPC‐based quantum resistive sensor (QRS) arrays were reported by Chatterjee et al. [56]. CNTs were dispersed in the matrix of poly(styrene) (PS), polycarbonate (PC), poly(lactic acid) (PLA), polycaprolactone (PCL), and poly(methyl methacrylate) (PMMA) for CPC synthesis and sensors were fabricated by spraying the layers of CPC on electrodes. Initial properties of CNT‐based VOC sensors such as controlled nano‐architecture and conductivity were tailored by varying the number of sprayed layers using the spray layer‐by‐layer (sLbL) technique. Such fabricated e‐nose was reported to discriminate between 18 polar and nonpolar lung cancer biomarkers with ppm level sensitivity. Nag et al. reported the fabrication of 3D architecture of sulfonated poly(oxy‐1,4‐phenylene‐oxy‐1,4‐phenylene‐carbonyl‐1,4‐phenylene) [SPEEK]‐carboxylic acid functionalized MWCNTs‐buckminsterfullerene (C60) nanocomposite sensors for the discrimination between both single and binary mixtures of lung cancer VOCs [57]. Sensors were fabricated by using sLbL technique and integrated into an e‐nose for the analysis of complex volatolome. Selectivity was tailored by varying the degrees of sulfonation rather than changing the nature of matrices. Sulfonated poly(ether ether ketone) (SPEEK) functionalized sensors exhibited higher selectivity and sensitivity toward methanol among a set of eight lung cancer VOCs even at the sub‐ppm level. The high content of water vapor in exhaled breath compared to those of VOCs biomarkers is one of the crucial challenges of screening the small signal of non‐polar VOCs. To overcome this problem Haick et al. fabricated VOCs sensors based on bilayers of self‐assembled hexa‐peri‐hexabenzocoronene (HBC‐C12 ) caplayers and a random network of CNTs for the discrimination of nonpolar VOC biomarkers of cancer from water in exhaled breath [58]. Selectivity of these sensors were attributed to dissolution of nonpolar VOCs into the hydrophobic HBC‐C12 layer.
Swager et al. [25] functionalized MWCNTs with different molecular recognition elements exhibiting different binding interactions with different VOCs using zwitter‐ionic and post‐transformation synthetic techniques. These sensors led to the accurate detection of 20 diverse VOCs. In a similar report Haick et al. used HBC derivatives in self‐assembled spongelike form for CNTs functionalization to boost up sensitivity and selectivity for nonpolar cancer biomarkers [59]. HBC discotic liquid crystal functionalization offered π‐π interactions between aromatic cores and almost negligible attraction toward water vapors, therefore enhanced selectivity toward nonpolar VOCs. Figure 8.2a shows the multiple conductive paths of CNTs. Self‐assembled structures of HBC‐C6,2 and HBC‐C12 are shown in Figure 8.2b,c, respectively. HBC‐C6,2 functionalized CNT‐based sensors exhibited higher sensitivity enhancement than HBC‐C12 toward water, methanol, octane, and decane, as shown in Figure 8.2d. These sensors showed improved sensitivity at all tested concentration (Figure 8.2e–h).

Figure 8.2 SEM images of (a) random network (RN)‐CNT cast from DMF solution; (b) HBC‐C6,2 structures cast from 10−3 M solution in xylene; and (c) HBC‐C12 structures cast from 104 M solution in toluene. (d) The response to two kinds of HBC‐functionalized RN‐CNT sensors, normalized to the response of the corresponding pristine sensor, to water, methanol, decane, and octane at p a /p o = 1. Calibration curves: ΔR/R b versus analyte concentration (pa/po = 0.05−1). Normalized resistance, ΔR/R b , of an RN‐CNT sensor before and after the functionalization with a discontinuous HBC‐C12 or HBC‐C6,2 layer upon exposure to (e) decane, (f) octane, (g) water, and (h) methanol in the vapor phase. All presented values of ΔR/R b have a signal‐to‐noise ratio greater than 3 [59]. (See color plate section for the color representation of this figure.)
Source: Reproduced with permission from the American Chemical Society.
SWCNTs‐based VOCs sensors are recognized for their ultra‐sensitivity owing to high electrical mobility, Schottky barrier changes, and unique charge transfer mechanism [25]. In particular, 1D structure of SWCNTs and conductance change on adsorption of gas molecules enable them to be suitable candidates for the development of low power microelectronics like a miniaturized high‐density nanosensors array [60]. Similar to MWCNTs, functionalization of SWCNTs was highly explored to enhance their sensing efficiency. Shirsat et al. reported the surface functionalization of SWCNTs with free‐base‐ and metallo‐porphyrins and further used them for sensors fabrication to monitor VOCs concentration in air [60]. Swager et al. [61] used meso‐Tetraphenylporphyrin (tppH2 ) functionalized SWCNTs for the fabrication of chemiresistive VOCs sensors. These sensors were reported to discriminate between five different types of VOCs. Discrimination of lung cancer VOCs between patient and healthy person was reported using SWCNTs and sensors based on nonpolymeric organic materials [24]. E‐nose composed of nanocomposites of SWCNTs with five different polymers, including poly(styrenecomaleic acid) partial isobutyl/methyl mixed ester (PSE), polystyrene sulfonic acid, PVP, polyvinyl chloride (PVC), and poly(vinyl alcohol) (PVA), were fabricated by Kerdcharoen et al. [62]. This e‐nose was explored to discriminate between hepatocellular carcinoma (HCC) patients and healthy persons via exhaled breath analysis. Sensitivity toward a broad range of VOCs including acetone, methyl‐ethyl‐ketone, ammonia, and toluene with 95% confidence level in principal component analysis (PCA) showed the potential of this sensor for clinical diagnosis.
Besides cancer detection, VOCs sensors are recognized as efficient tools for the early level diagnosis of diverse neurodegenerative diseases via the detection and discrimination of specific biomarkers. Lack of symptoms in the early stage of the development of neurodegenerative diseases is mostly associated with critical neurodegenerative disorder prior to clinical assessment [19]. VOCs‐based diagnostic tool should enable noninvasive diagnosis of neurodegenerative diseases at an earlier stage for the current drug treatments prior to neuronal damage [19]. Tisch et al. proposed a proof‐of‐concept for the discrimination of breath print of presymptomatic Parkinson's disease (PD) in rats with organically functionalized SWCNTs sensors array [63]. Ionescu et al. reported the diagnosis of multiple sclerosis (MS), a common chronic neurological disease of youths (20–40 years old), via breath analysis using polycyclic aromatic hydrocarbons (PAHs) and SWCNTs bilayers [26]. A random network of SWCNTs and PAHs eliminated conductivity variations and added the advantage of detection of VOCs in the highly humid atmosphere. These sensors exhibited high sensitivity of ~85.3%, specificity of ~70.6%, and 80.4% accuracy. Hexanal and 5‐methyl‐undecane as typical biomarkers of MS were differentiated between patients with MS relative to healthy controls using a combination of gas chromatography, GC‐MS and SPME techniques. Haick et al. tested the exhaled breath from rats using organic‐material‐coated SWCNTs‐based sensors and a pattern‐recognition technique to discriminate between healthy rats and those with chronic renal failure (CRF) [21]. Zhang et al. [64] fabricated wireless sensor array from single‐stranded DNA (ssDNA)‐decorated SWCNTs using photolithography and E‐beam deposition technique to analyze the pathophysiological conditions of human by VOCs analysis in exhaled breath. These sensors array exhibited reversible and repeatable responses with high sensitivity and selectivity. Tang et al. [27] proposed sol‐gel technology and microwave induced plasma‐based fabrication of MWCNTs‐coated stainless steel fibers as a new kind of fiber for SPME to enable in‐house fabrication technique. These fibers were further used for the analysis of alkanes in human breath. Bayn et al. [65] profiled the VOCs with Au NPs and SWCNTs‐based sensors array for the identification of brucellosis in exhaled breath. Brucellosis, a zoonotic disease in domestic cattle and other mammalian is of considerable economic and health importance worldwide. Sensors, when combined with a statistical algorithm were capable of discriminating between five VOCs biomarkers and were independent of animal's environment.
8.2.3 Graphene for VOC Sensors
Recent years have witnessed a tremendous development of research on graphene and its composites for VOC sensing applications [15, 19]. Due to graphene's superior properties, this one‐atom‐thick fabric of carbon has attracted much attention of scientists for making electronic sensors. Sensitivity, selectivity, rapid response, reversibility, durability, and reliable detection are the main driving forces for any sensing technology. Graphene is the most innovative nanomaterial owing to its remarkable biocompatibility, high electrical conductivity, high aspect ratio, and low intrinsic noises [66–68]. Graphene assumed as potential candidates for ultra‐sensitive VOCs sensors because of the extreme sensitivity of graphene conductance to the local electrical and chemical perturbations, since almost every atom is surfacialy exposed to surroundings [19]. Planar nanostructures of graphene facilitate facile device integration in contrast to other forms of nanocarbons. In addition, the exceptional band structure of graphene (zero gap semiconductors) can be easily tuned by heteroatom doping, cutting its dimensions, surface functionalization, or via composite fabrication. Working principle for the majority of graphene‐based sensors for VOCs detection is based on a change in conductivity [69]. Ultra‐high surface area and electronic properties of graphene show altered carrier density, which strongly depends on surface adsorbents acting as donors or acceptors, as in the case of solid‐state sensors [69]. Graphene oxide (GO) and reduced GO (rGO) offer oxygenated functional groups for further functionalization with the added advantage of low cost and mass fabrication [70]. Graphene and related nanostructures respond sensitively to the interaction of trace amounts of divers VOC molecules [71].
Choi et al. reported the detection of diabetes by the analysis of acetone in exhaled breath using WO3 nanofibers (NFs) functionalized with non‐oxidized graphene (NOGR) [22]. Analysis of exhaled breath for disease diagnosis with graphene‐WO3 composite using Kelvin probe force microscopy technique was reported by Choi et al. [72]. WO3 hemitubes fabricated by oxygen plasma treatment and electrospinning was homogenously mixed with graphene for composite fabrication. Sensors were fabricated by drop casting and were able to respond in highly humid conditions at various temperatures from 200 °C to 350 °C. Highly sensitive detection of acetone (R air /R gas = 1.7 at 5 ppm) and H2S (R air /R gas = 3.3 at 5 ppm) were reported for the diagnosis of diabetes and halitosis, respectively, and were attributed to the electronic sensitization between p‐type graphene‐based materials and n‐type WO3 hemitubes. Tung et al. [73] fabricated GO and ionic liquid polymers (PILs)−based sensing skin using the sLbL technique for the detection of alteration of VOCs biomarkers. QRS based on Fe3O4–rGO, PEDOT, and PIL hybrid materials were reported to show enhanced sensitivity, reduced response time, and reversible behavior toward both polar and nonpolar VOCs [74]. In a similar report, QRS based on Ag‐rGO‐PIL hybrid material were used to detect a set of VOCs [75]. Saxena et al. [76] reported the detection of toluene in exhaled breath using GO‐based sensors for the diagnosis of lung cancer. Detection of decane, one of the significant biomarkers of lung cancer, with GO thin film coated SAW sensors was reported by Zhang et al. [77]. This sensor is capable of detecting 0.2 ppm of decane. Chen et al. studied the diagnosis and distinguishing of early gastric cancer (EGC, stages I and II) and advanced gastric cancer (AGC, stages III and IV) via the detection of 14 VOC biomarkers with the combined use of GC‐MS‐SPME techniques [23]. They further fabricated surface‐enhanced Raman scattering (SERS) sensors by drop‐casting GO solution on glass surface sputtered with 300 nm thick Au film. These sensors were then reduced with hydrazine vapor to fabricate a uniform film of rGO on Au film to adsorb VOCs as shown in Figure 8.3a. SERS sensors were reported to analyze both real and simulated breath samples (Figure 8.3b,d). PCA analysis (PC1 and PC2) for three groups are shown in Figure 8.3c, which reveal the clear discrimination between EGC and AGC patients from healthy persons. PCA analysis of real and simulated breath patterns are illustrated in Figure 8.3e.

Figure 8.3 (a) Schematic diagrams of SERS sensor and overview of the processes involved in the breath. (b) Processed Raman spectra of VOCs biomarker patterns. The biomarker patterns had the same components in breath samples of healthy persons (c) and EGC (b) and AGC (a) patients. (c) PCA of the data set of biomarker patterns of healthy persons (area c) and EGC (area b) and AGC (area a) patients. Each data point in the PCA corresponds to the area of 14 bands in the processed Raman spectra. (d) SERS spectra of simulated and real breath samples of healthy persons (c, c′) and EGC (b, b′) and AGC (a, a′) patients. (e) PCA of the data set of simulated and real breath samples of healthy persons (c, c′) and EGC (b, b′) and AGC (a, a′) patients. Each data point in the PCA corresponds to the area of 14 bands in the SERS spectra [23]. (See color plate section for the color representation of this figure.)
Source: Reproduced with permission from the American Chemical Society.
8.3 Detection of Biomarkers Using Biosensors for Cancer and Disease Diagnosis
Diseases continuously maintain threat to human health and life; therefore, their recognition and monitoring in clinical treatment is of crucial importance. Cancer is one of the leading causes of human death. Many diseases and cancers have a high chance of cure if diagnosed early. Therefore, screening both healthy and infected patients is essential [78]. The abnormal levels or fluctuation of biomarkers in the human body, such as glucose, DA, UA, peptides, proteins, DNAs, enzymes, hormones, and antigens, can be associated with diseases. For example, abnormal glucose level in the body may cause diabetes mellitus, which is recognized as one of the leading causes of morbidity and mortality worldwide and becoming a major health problem for many developed societies [79]. DA is a neurotransmitter that is crucially important in normal brain functions including movement and emotional responses due to its involvement in brain‐body integration signals. Low levels or complete depletion of DA in the central nervous system is a major cause of several neurological diseases including schizophrenia, Parkinson's disease and attention deficit hyperactivity disorder (ADHD/ADD) [80]. UA is an end product of purine derivatives in human metabolism. Under normal conditions, it is excreted by the kidneys and intestinal tract. Abnormal levels of UA in biological fluids serves as markers for several disorders including renal disease and Lesch‐Nyhan syndrome [81]. Additionally, excessive amounts of UA in serum (a condition known as hyperuricemia) is associated with hypertension, metabolic syndrome, and cardiovascular disease. Overtime, deposition of urate microcrystals in various tissues leads to gout. Furthermore, abnormally low levels of UA leads to MS and oxidative stress. Consequently, rapid, reliable, and cost effective determination of biomakers in biological fluids is routinely required for the diagnosis and therapy of various diseases. However, monitoring many of biomarkers is challenging due to their low concentration in biological samples. Requirements for a biosensor therefore include high sensitivity and selectivity, as well as fast response, low cost, and portability. Carbonaceus nanomaterials (GQDs, CNT, and graphene) offer novel routes to design new biosensors due to their remarkable properties and easy functionalization or hybridization for synergistic effects. In biosensing devices, these materials are generally used: (i) in the recognition element of the sensor, where they provide binding sites for target analytes or molecules capturing target analytes, (ii) in the transducer component that converts the detected molecular interaction on the electrode surface into a measurable signal, and (iii) labels for target molecules in signal amplification. These sensing components strongly determine the performance of the sensor in the recognition process of analytes.
8.3.1 Carbon Nanodot‐ and Graphene Quantum Dot‐Based Biosensors for Disease Biomarkers Detection
Carbon dots (CDs) and graphene GQDs consist of small graphene fragments typically below 20 nm in diameter are classed as a 0D nanostructured material. These materials are superior in terms of chemical inertness, ease of production, biocompatibility, and low toxicity when compared to traditional semiconductor QD materials such as heavy metal containing QDs and organic fluorophores [82]. Both CNDs and GQDs exhibit high PL properties which are of particular interest for the across multiple biomedical fields, especially the development of PL sensors as tools for detection [83]. Utilization of this PL property of GQDs has been investigated for use in biosensors, which is currently undergoing with particular advances in the detection of important targets in conditions such as diabetes mellitus and DA‐related conditions like schizophrenia and Parkinson's disease. Figure 8.4 presents typically using GQDs as a new class of fluorescent nanocarbons for PL sensors [84, 85].
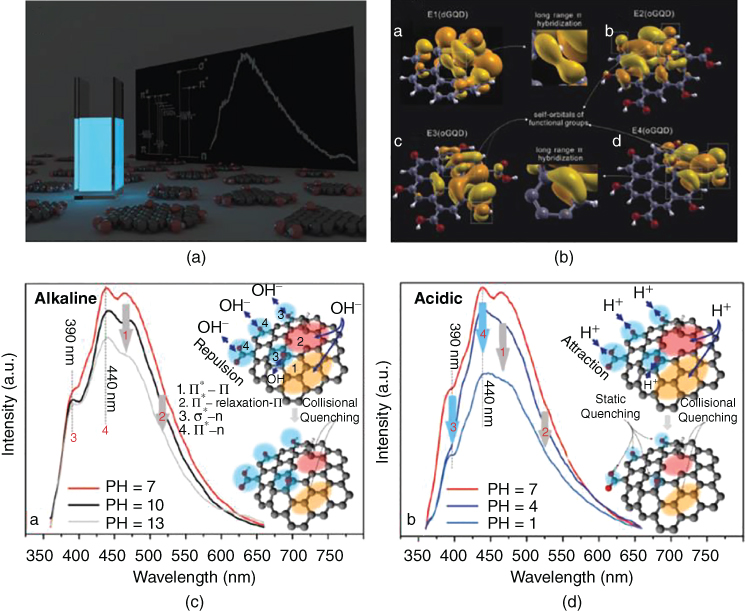
Figure 8.4 (a) Optical PL image of GQDs dispersed in water under 365 nm UV lamp; (b) Simulation model explaining the PL mechanism of QGD containing emission of both disordered aromatic core and emission of functional groups; and (c) The response of GQDs to PH under alkaline and acidic conditions [84].
Source: Copyright 2016. Reproduced with permission from Royal Society of Chemistry.
A novel glucose‐sensing system for operation under physiological conditions was designed by Li et al. based on affinity sensing and electrostatic attraction of anionic fluorescent GQDs with polar carboxyl and hydroxyl groups (acting as fluorescent reporting units) and cationic boronic acid‐substituted bipyridinium salt (BBV) (a fluorescence quencher and glucose reporter) [86]. The attraction between GQDs and BBV ground‐state complex resulted in the transfer of excited‐state electrons from GQDs to BBV, leading to reduced fluorescence intensity in GQDs. When glucose is present, BBVs are converted to tetrahedral anionic glucoboronate esters, allowing neutralization of the net charge of BBV, diminishing its quenching efficiency, and thus allowing fluorescence intensity of GQDs to be recovered. Advantages of this method include simplicity and cost efficiency, as a further chemical modification of GQDs is not required, and environment friendliness due to the elimination of semiconductor QDs and organic dyes and solvents. Razimi et al. fabricated an electrochemical glucose sensor using glucose oxidase GOx‐GQD complex that can detect glucose concentrations in the range of 5–1270 μM, with the detection limit of 1.73 μM, and sensitivity of 0.085 μA μM−1 cm−2 [87]. Interference tests carried out in human plasma samples validated excellent accuracy for glucose determination in real samples, ascertaining the practical application of the developed glucose sensor in clinical trials. Another analytical application testing by He et al. using hemin‐functionalized GQD and GOx in serum samples also saw high accuracy and reliability for hydrogen peroxide detection in biological examples as well as specificity [88]. Hemin‐functionalized GQDs showed a linear response to hydrogen peroxide in the range of 1–300 μM (hemin‐GQD with GOx exhibited a linear range from 9 to 300 μM) with a detection limit of 0.1 μM. These developments in glucose‐biosensors are promising with greatly increased accuracy and selectivity. However, shortcomings of these approaches still exist, such as photobleaching, complication of conjugated poly‐electrolyte synthesis, fluorophore classification, and most importantly, cytotoxicity. Additionally, many conventional point‐of‐care (POC) devices for glucose do not meet criterion recommended by the American Diabetes Association, whereby the accuracy of a blood glucose POC assay needs to be below 5% of the measured value [89]. As such, improved accuracy, reliability, and toxicity in human systems are always required to be considered when developing new glucose sensors, and considerations of practicality, technicality, and economical aspects are needed to meet requirements for potential marketing.
Research for developing CD‐ and GQD‐based sensors for DA are still in early stages and therefore the full potential of GQD biosensors has not yet been fully realized. One promising approach to that includes the use of photoelectrochemical (PEC) properties of both GQD and TiO2 particles to develop sensors for DA. Yan et al. [90] synthesized GQD‐TiO2 nanocomposites, which were shown to be highly sensitive to DA whereby the addition of 5 μM of DA enhanced the photocurrent of GQD‐TiO2 by 2.8 times. The mechanism for the determination of DA by PEC is based on excitation of TiO2 particles with visible light, which generates electron‐hole pairs; the excited electrons in the conduction band are transferred to GQDs allowing them to participate in PEC reactions to produce a photocurrent. When DA is present, the oxidized products of DA by photogenerated holes are able to avoid electron‐hole recombination, thus increasing the photocurrent intensity. Results showed that the photocurrent response of the developed sensor increases linearly within DA ranges of 0.02–105 μM with acceptable accuracy and precision. Compared to DA biosensors incorporating metal oxides, graphene, Au nanoclusters, Ag2Se QDs, and CNT‐cobalt NPs, fabricated GQD‐TiO2 sensors showed wider linear response, lower detection limit, and higher sensitivity. Application testing of GQD‐TiO2 in human plasma showed high recovery percentages, high specificity, and long‐term stability, ascertaining the practical application of GQD‐TiO2 biosensors in clinical analysis.
8.3.2 Carbon Nanotube‐Based Biosensors for Cancer Biomarker Detection
CNTs can be used for both constructing a sensitive sensing layer of the sensor and for fabricating labels for signal amplification in sandwich‐type biosensors due to increasing the surface area of the electrode, which is essential for immobilizing capture biomolecules [91–93]. CNTs are widely used to construct sensors for cancer and disease biomarker detection, and are usually functionalized, typically with hydroxy and carboxy groups, in order to facilitate composite formation or immobilization of target complementer capture aptamers, proteins, or antibodies, ribonucleic acids (RNAs), proteins, and antigens present in serum are extensively used tumor markers. Immunoassays based on the antibody−antigen, aptamer−protein, and protein−protein interaction are the most important analytical techniques in the quantitative detection of tumor markers.
Prostate‐specific antigen (PSA) is an androgen‐regulated serine protease. The total‐prostate‐specific antigen (T‐PSA) level significantly increases in serum during prostate cancer; therefore, PSA is an important prostate cancer biomarker. An electrochemical immunosensor using microelectrode arrays modified with SWCNTs was developed by Okuno et al. [94] for label‐free detection of T‐PSA, and later on for label‐free detection of biomarkers [95, 96]. In order to prepare the working electrode of the sensor, conventional photolithography, and metal lift‐off processes were used to pattern Pt on SiO2/Si substrate. SWNCTs were synthesized on Pt electrodes by the thermal chemical vapor deposition (CVD) method, and T‐PSA monoclonal antibodies were immobilized on SWCNTs using 1‐pyrenebutanoic acid succinimidyl ester as the linker (Figure 8.5). The sensor exhibited high selectivity with successful suppression of the nonspecific adsorption. Osteopontin (OPN) is a phosphoprotein that cancer cells use to facilitate their expansion, and OPN is a potential prostate cancer biomarker. A transparent chemiresistor‐type immunosensor was developed by Sharma et al. [97] to detect OPN. The electrodes of the biosensor were fabricated using conventional photolithography and lift‐off process of two Au/indium tin oxide (ITO) electrodes on a glass substrate. Carboxy functionalized SWCNTs were deposited between the two electrodes by dielectrophoresis, and monoclonal antibodies specific to OPN were covalently immobilized on SWCNTs via the 1‐Ethyl‐3‐(3‐dimethylaminopropyl)‐carbodiimide (EDC)/N‐hydroxysuccinimide (NHS) coupling reaction. The selective and sensitive biosensor possess great potential as sensing platform of protein biomarkers, along with simultaneous optical measurements. Lerner et al. [98] fabricated an OPN biosensor by attaching genetically engineered monoclonal antibodies specific to OPN to CNT field‐effect transistor (FET) using the EDC/NHS coupling reaction. The sensor exhibited an antigen‐specific, concentration‐dependent response and high sensitivity for OPN.

Figure 8.5 Illustration of the sensor experimental set‐up with SWCNT‐modified Pt microelectrode as the working electrode (a) and the label‐free electrochemical immunosensor design (b) [94].
Source: Copyright 2007. Reproduced with permission from Elsevier.
Galectin‐3, a β‐galactoside‐binding protein, is an important cancer biomarker, because the high level of circulating galectin‐3 is correlated with an increased potential for malignancy in several types of cancer. Park et al. [99] constructed a FET biosensor, which is based on D‐(+)‐galactose‐conjugated SWCNTs as chemical probes to detect galectin‐3. The sensing platform was prepared by drop casting SWCNTs immobilized with D‐(+)‐galactose on a Mo electrode prepatterned SiO2 substrate, printed using conventional photolithography. The receptor tyrosine kinase human epidermal growth factor receptor‐3 (HER‐3) belongs to the HER family and normally presents in human adults. However, the abnormally high level of HER‐3 indicates a risk of cancer. Asav et al. [100] constructed a highly sensitive HER‐3 biosensor by immobilizing the anti‐HER‐3 antibody on the surface of a screen‐printed carbon electrode (SPCE) modified with carboxy functionalized SWCNTs. The sensor exhibited reduced cost and functional simplicity, and was successfully applied to the HER‐3 analysis of artificial serum samples. A structurally similar biosensor was developed by Yang et al. [101] for the detection of alpha‐fetoprotein (AFP), a reliable biomarker for HCC, employing wheat‐germ agglutinin (WGA) lectin as molecular recognition element. Carboxylated SWCNTs were drop‐coated on SPCE and WGA was coupled covalently to SWCNTs using EDC/NHS. MicroRNAs (miRNAs) are a class of small non‐protein‐coding single‐strand RNAs molecules of 18–30 nucleotides. Deregulations of miRNAs are associated with various human cancers thus they are important biomarkers in body fluids. An extremely sensitive and selective biosensor for the detection of miRNA‐122a was constructed by Ramnani et al. [102] by integrating an extremely sensitive carbon nanotube‐field‐effect transistor (CNT‐FET) transducer and a highly selective biorecognition element of Carnation Italian ringspot virus p19 binding protein. SWCNTs were deposited between two gold electrodes of FET using 3‐aminopropyltriethoxysilane (APTES)‐assisted assembly of SWCNTs from enriched CNT solution. The CNT‐FET device was functionalized with p19 through 1‐pyrenebutanoic acid succinimidyl ester, and treated with Tween 20 to block nonspecific binding. A let‐7 miRNA sensor based on CNT enhanced label‐free detection and on hairpin (HP) DNA probe triggered solid‐phase rolling‐circle amplification (RCA) was developed by Tian et al. [103]. A glassy carbon electrode (GCE) was modified by drop casting carboxy functionalized CNTs on the electrode surface, followed by immobilizing amino functionalized HP DNA probes through chemical bonding. Target miRNAs, which unfold the HP probe, and circulate probe (CP) DNAs were deposited in the following step, and polymerization was initiated by phi29 DNA polymerize enzyme. The sensor exhibited ultrasensitive detection limit and excellent specificity for resolving lung cancer‐related let‐7 miRNA family members. Li et al. [104] constructed a sensitive miRNA‐24 sensor by drop‐casting carboxylated MWCNTs on GCE and immobilizing aminated capture probe ss‐DNAs on the electrode surface using EDC/NHS coupling.
The detection performance of a biosensor can be increased by combining CNTs with organic polymers, metal oxide NPs, and metal NPs. These functional composites promote electron transfer due to sinergistic effects and introduce a lot of binding sites for capturing biomolecules. A miRNA‐141 (prostate cancer biomarker) sensor based on MWCNT nanostructures conducting polymer film was fabricated by Tran et al. [105]. Oxidized MWCNTs were deposited on a GCE by drop casting and the GCE/ox‐MWCNT electrode was modified by co‐electrooxidation of 5‐hydroxy‐1,4‐naphthoquinone (JUG) and 3‐(5‐Hydroxy‐1,4‐dioxo‐1,4‐dihydronaphthalen‐2(3)‐yl) propanoic acid (JUGA) monomers. Amino‐modified 22‐mer probe DNAs were immobilized on the electrode surface using EDC/NHS binding for miRNA capture. Ox‐MWCNTs enhanced the copolymer electro‐activity, and contributed to the low detection limit and high sensitivity of the sensor. Antibodies developed against cancer are important biomarkers. Choudhary et al. [106] developed a biosensor for simultaneous detection of lung cancer biomarkers (anti‐MAGE A2 and anti‐MAGE A11) using carboxyl functionalized SWCNT–chitosan (CS) composite. The composite was casted on graphite (GR) electrode and biomarker specific antigens were immobilized on the composite surface separately using the EDC/NHS method. Simultaneous detection of anti‐MAGE A2 and anti‐MAGE A11 was carried out using multichannel electrochemical analyzer having two working electrodes in one reaction cell. Carcinoembryonic antigen (CEA) is a key biomarker for early diagnosis of cervical carcinomas, and pancreatic, colorectal, gastric, and lung cancer. A microelectrode array immunosensor based on CS‐MWCNT‐thionine (THI) hybrid film was developed by Xu et al. [107] for the detection of CEA. The CS‐MWCNT‐THI film was electrochemically deposited onto the gold electrode. Anti‐CEA was immobilized on the surface of this hybrid film, then bovine serum albumin (BSA) was deposited to block non‐specific adsorption. CS provided active sites for anti‐CEA immobilization. The good electrochemical property of the immunosensor was based on the synergistic effect between MWCNT and THI and the nanoporous structure of the film. A conducting paper (CP) sensor based on conducting polymer‐CNT composite was developed by Kumar et al. [108] for the detection of CEA. The working electrode of the sensor was prepared by dip coating a Whatman paper in an aqueous solution of carboxy functionalized CNT and PEDOT:PSS. After formic acid (FA) treatment, anti‐CEA monoclonal antibodies were immobilized on the electrode surface via the formation of covalent bonds between the carboxylic groups of CNTs and the NH2‐terminal of antibodies via EDC/NHS coupling reactions. The tumor suppressor gene TP53 is mutated in most type of human cancers, therefore TP53 mutation is an important early diagnostic cancer marker. Fayazfar et al. [88] developed a highly sensitive DNA biosensor based on AuNP/vertically aligned MWCNT array platform for detecting the TP53 mutation. The working electrode of the sensor was prepared by synthesizing well‐aligned MWCNTs on Ni‐deposited Ta plate by CVD, electrodepositing AuNPs, and finally immobilizing 26‐mer thiolated capture DNAs on AuNPs. The synergistic interactions of the vertically aligned MWCNT array and AuNPs at modified electrode improved the density of the probe DNA attachment, which strongly increased the sensitivity of the sensor. The fabrication of the immunosensor is shown in Figure 8.6.

Figure 8.6 Illustration of the fabrication of DNA immunosensor [88].
Source: Copyright 2014. Reproduced with permission from Elsevier.
The determination of tumor suppressor protein p53 (AGp53) is of great significance for early diagnosis of cancer, because more than 50% of human cancers are related to the mutated p53. An enzyme electrocatalytic sandwich‐type immunosensor was developed by Wang et al. [109] for the detection of AGp53. A GCE was modified first by depositing carboxylated MWCNT‐Nylon 6 (PA6)‐polythionine (PTH) composite nanofibers using electrospinning and electropolymerization. Anti‐AGp53 capture antibodies were immobilized on the electrode surface for target AGp53 capture, and signal amplification was introduced by depositing horseradish peroxidase (HRP)‐conjugated secondary polyclonal anti‐AGp53. Matrix metalloproteinase‐3 (MMP‐3) is a potential cancer biomarker, because elevated expression of MMP‐3 is associated with squamous cell carcinoma of the head and neck, and adrenal tumors. A SWCNT forest‐primary antibody sensor platform was designed by Munge et al. [110] in order to sensitively detect MMP‐3. MMP‐3 antibodies were immobilized on SWCNTs using EDC/NHS. Bioconjugate labels were prepared by immobilizing secondary MMP‐3 antibodies and biotinylated HRP on polystyrene beads coated with streptavidin. An ultrasensitive PEC CEA biosensor based on carboxylated MWCNT‐Congo red (CR)‐C60 hybrid labels and poly(p‐aminobenzoic acid) (PABA)‐carboxylated MWCNT nanocomposite‐modified ITO electrode with immobilized CEA antibodies was constructed by Hu et al. [111] (Figure 8.7). The sensor exhibited good performance in human serum samples and demonstrated promising application possibilities of fullerene‐based nanocomposites in developing highly sensitive, environmentally friendly, and cost‐effective PEC biosensors.

Figure 8.7 Illustration of the sandwich‐type immunosensor [111]. (a) Photo of the light source, (b) schematics of the photoelectrochemical cell, and (c) schematics of the fabrication of the working electrode. (See color plate section for the color representation of this figure.)
Source: Copyright 2013. Reproduced with permission from American Chemical Society.
8.3.3 Carbon Nanotube‐Based Biosensors for Disease Biomarker Detection
Myoglobin (Mgb) is an important biomarker of cardiovascular disease, and is one of the very early markers of acute myocardial infarction. Khan et al. [112] constructed a label‐free biosensor for rapid, sensitive, and selective detection of Mgb. The immunoelectrode was prepared by depositing monoclonal anti‐Mgb antibodies onto the screen‐printed‐MWCNTs electrode by adsorption technique, followed by blocking step with BSA to avoid nonspecific binding of antigen. Netrin1, abundantly expressed in atherosclerotic lesions, is also a cardiovascular biomarker. A sensitive netrin 1 biosensor was fabricated by Xu et al. [113] by modifying a GCE consecutively with carboxyl functionalized MWCNT‐CS composite film, THI, gold NPs, and immobilizing netrin 1 antibodies on the electrode surface to capture netrin 1. Myeloperoxidase (MPO) enzyme overexpression is a sensitive predictor for myocardial infarction. A cheap and disposable MPO biosensor was designed by Herrasti et al. [114]. In order to construct the sensor, streptavidin‐coated magnetic microparticles were modified with biotinylated anti‐MPO antibodies (MP‐anti‐MPO), and carboxylated SWCNTs were deposited onto MP surfaces for signal amplification. CNT/MP complexes were concentrated on a SPCE using a magnet for electrochemical measurement. Liu et al. [115] fabricated a sensitive and disposable MPO‐sensor based on modified ITO. The electrode was prepared by electropolymerizing a poly(o‐phenylenediamine) (PoPD)‐MWCNT‐EMIMBr composite film on ITO, depositing AuNPs on the film, and finally immobilizing anti‐MPO on AuNPs. The cholesterol level in blood is a biomarker for cardiovascular diseases. Elevated cholesterol levels may occur with hypothyroidism, nephrotic syndrome, diabetes, and various liver diseases. Navamani et al. [116] prepared a fast and sensitive cholesterol sensor by casting a film containing Nafion, carboxylated MWCNT, cholesterol oxidase (ChOx), and poly‐N‐vinyl‐2‐pyrrolidone (PVP) encapsulated ZnS NPs onto a GCE electrode. ZnS NPs and MWCNTs acted as effective mediators between the enzyme and GCE.
Neuromyelitis optica (NMO, also known as Devic's disease) is a disease of the central nerve system. The disease's specific antibody, a highly specific biomarker in human blood, targets aquaporin‐4 (AQP4), a transmembrane protein expressed in the central nerve system. Son et al. [117] constructed a CNT‐FET functionalized with AQP4 extracellular loop peptides for the rapid detection of AQP4 antibody in human serum without pretreatment. The CNT‐FET was fabricated via the photolithography process and phenylalanine modified loop peptides were immobilized on the CNT surface via pi–pi interactions. Lyme disease is caused by the bacteria Borrelia burgdorferi, and Lyme antigens present in body fluids are key biomarkers of bacterial infection. Lerner et al. [118] developed a sensitive and rapid biosensor based on antibody‐functionalized single‐walled carbon nanotube‐field‐effect transistor (SWCNT‐FET) for the detection of Lyme antigens. The SWCNT‐FET was fabricated by standard photolithography and CVD techniques. SWCNTs were functionalized using 4‐carboxybenzene diazonium salt, and monoclonal Lyme antigen antibodies were immobilized on modified SWCNTs using EDC/NHS.
Alzheimer's disease is becoming an increasing health problem along with the aging of the world population. α‐1 antitrypsin (AAT), a protease inhibitor, is a recognized biomarker of Alzheimer's disease. Zhu et al. [119] developed an aptamer‐antigen‐antibody sandwich‐type AAT biosensor based on 3,4,9,10‐perylenetetracarboxylic acid (PTCA)/CNT as sensing platform and alkaline phosphatase (ALP)‐labeled AAT antibody functionalized Ag NPs as a signal enhancer. The working electrode of the sensor was constructed by drop casting 3,4,9,10‐perylenetetracarboxylic acid‐carbon nanotube (PTCA‐CNT) on SPCE and immobilizing AAT specific amino‐terminated 37‐mer DNA aptamers, using EDC/NHS, on the modified electrode surface. Ag NPs were functionalized with alkaline phosphatase‐α‐1 antitrypsin (ALP‐AAT) using EDC/NHS. Amyloid‐β (Aβ) is one of the biomarkers for early diagnosis of Alzheimer's disease. Oh et al. [120] developed a SWCNT‐film‐based metal semiconductor FET for Aβ detection in human serum. The gold top gate was deposited in the middle of the SWCNT channel, and Aβ antibodies were immobilized on the gold layer using an antibody binding protein. Acetylcholine is a neurotransmitter, and monitoring the level of acetylcholine and its precursor choline in serum is very important to detect neurodegenerative diseases such as Alzheimer's and neuromuscular diseases. A bienzymatic choline biosensor was constructed by Pundir et al. [121] by electrodepositing carboxylated MWCNT and ZrO2 NPs on GCE, and co‐immobilizing acetylcholinesterase (AChE) and choline oxidase (ChlO) on the electrode surface.
8.3.4 Graphene‐Based Biosensors for Cancer Biomarker Detection
Accurate and fast detection of cancer markers in body fluids is of utmost importance for early diagnosis of cancer. To this end, graphene‐based sensor platforms are continuously being modified and have shown to be promising new sensing materials for the development of early cancer detection [122–126].
The cyclin A2 protein is a prognostic indicator in early‐stage cancers; thus, its detection is of crucial importance for both clinical diagnosis and treatment of cancer. A sensitive and selective electrochemical cyclin A2 sensor, based on label‐free electrochemical impedance detection, was developed by Feng et al. [127] by modifying GCE with meso‐tetra(4‐carboxyphenyl)porphyrin (TCPP) modified chemically converted graphene (CCG) and immobilizing hexapeptide P0 (RWIMYF) and Tween 20 on the surface. P0 and Tween 20 served as the specific binding site for cyclin A2 and protection agent for nonspecific binding, respectively. An ultrasensitive chemiresistor‐type biosensor based on layer‐by‐layer (LbL) self‐assembled graphene composite was fabricated by Zhang and Cui [128] for real‐time detection of a PSA. The LbL graphene film composite was prepared on a flexible polyethylene terephthalate (PET) substrate with lithographically patterned gold electrodes by immersing the substrate into charged suspensions of poly(diallyldiamine chloride) (PDDA), PSS, and graphene to obtain a multilayer with a sequence of [PDDA + PSS]2 + [PDDA + graphene]5. After self‐assembly, PSA capture antibodies were immobilized on the top graphene layer and nonspecific binding sites were blocked with BSA. Sensor performances were further increased by Zhang et al. [129] by suspending the graphene multilayer between gold electrodes of the sensor. Improved sensitivity was attributed to the strong suppression of electrical flicker noise. The messenger RNA (mRNA) biomarker PCA3 is related to prostate cancer. A high performance and relatively simple PCA3 optical biosensor were constructed by Vilela et al. [130]. The sensor is based on NaYF4:Yb,Er upconversion nanoparticles (UCNPs) as emitters and GO as the fluorescence quencher. Aminohexyl‐functionalized 25‐mer ssDNAs targeting PCA3 were attached to poly(acrylic acid) (PAA) covered UCNPs via EDC/NHS coupling. In the presence of the target PCA3, UCNPs did not interact with GO due to target‐capture DNA hybridization, therefore retained their fluorescence signal. The detection strategy of the sensor is shown in Figure 8.8.

Figure 8.8 Illustration of the GO and UCNPs based optical immunosensor [130].
Source: Copyright 2017. Reproduced with permission from American Chemical Society.
Li et al. [131] constructed a biosensor based on tunable graphene composites for a lung cancer tumor marker (annexin A2 (ANXA2), enolase 1 (ENO1), and vascular endothelial growth factor (VEGF)) detection. The composite film with a sequence of [PDDA + graphene]2 + [PDDA + TiO2] + [PDDA + graphene]2 was prepared by depositing PDDA, graphene, and TiO2 layers on a shape‐memory polymer polyolefin using the self‐assembly technique. It was tuned by the shrink polymer and titania NP layer. Lung cancer biomarkers capture antibody and BSA were deposited on the surface of the composite for capturing biomarkers and blocking nonspecific binding sites, respectively. Li et al. [132] developed another biosensor for the multiplex detection of the three lung cancer tumor markers. The FET sensor was based on suspended single crystalline graphene prepared by low pressure chemical vapor deposition. Antibodies were immobilized on graphene using poly‐L‐lysine. The suspended structure largely improved sensor performances, compared to polycrystalline GR flakes, due to the absence of grain boundary and substrate scattering. Thrombin is an important protease and hormone, and a biomarker of pulmonary metastases. Li et al. [133] constructed a label free optical sensing platform for thrombin detection, which was based on [Ru(2,2′‐bipyridine)2(2‐(2‐methoxylphenyl)‐imidazo[4,5‐f][1,10]phenanthroline)]2+ (RuOMO), GO, and a thrombin specific aptamer pair. Thrombin detection was achieved by restoration of the fluorescence of RuOMO prequenched by GO.
An ultrasensitive immunosensor was constructed by Akter et al. [134] for the detection of the breast cancer biomarker carbohydrate antigen 15‐3 (CA 15‐3) using non‐covalent functionalized GO/1‐pyrenecarboxylic acid (Py‐COOH) as sensor probe and carboxylated MWCNT‐supported ferritin as labels. The working electrode of the sensor was fabricated by depositing GO/Py‐COOH on cysteamine (Cys) self‐assembled monolayer (SAM) modified gold electrode. Anti‐CA 15‐3 antibodies were immobilized on both GO/Py‐COOH and MWCNT/ferritin through amide bond formation. The sensor sensitivity was enhanced due to the synergistic effect of GO/Py‐COOH/antibody platform and the antibody‐MWCNT‐Ferritin label. The HER ErbB2 is a biomarker for early detection of breast cancer. Ali et al. [135] constructed a microfluidic ErbB2 immunosensor based on porous graphene foam (GF) electrode modified with electrospun carbon‐doped TiO2 nanofibers and ErbB2 antibodies, immobilized using EDC/NHS. The sensor exhibited femtomolar sensitivity and high selectivity for ErbB2. The HER2 protein is a prognostic biomarker for breast cancer. Tabasi et al. [136] developed a HER2 specific aptamer‐based immunosensor for HER2 detection. The sensor was fabricated by depositing an RGO‐CS film on GCE and covalently immobilizing amino‐terminated aptamers via glutaraldehyde linking. Methylene blue (MB) was used to probe biointerface events. The sensor exhibited high sensitivity and selectivity. HER3 is implicated in tumorigenesis of numerous cancers including breast cancer. Rajesh et al. [137] fabricated a sensitive HER3 biosensor, which is based on graphene FET decorated with antibody‐functionalized platinum nanoparticles (PtNPs). PtNPs were attached to graphene using the bifunctional 1‐methyl pyrene amine linker, and thiol‐containing single‐chain variable fragment antibodies (scFv) were immobilized on PtNPs.
Folic acid protein (FAP) is over‐expressed in many human epithelial‐derived tumors, therefore it is an important tumor marker. He et al. [138] fabricated a FAP biosensor by electrophoretic deposition of RGO onto a gold electrode and post‐functionalizing deposited RGO with FA. The sensing performance of the sensor is based on the strong binding affinity of FAP to FA. The overexpression of MMPs is related to tumor invasion and metastasis, therefore the detection of MMPs is of great importance for cancer diagnosis. Song et al. [139] developed a fluorescence turn‐on sensor for MMP‐2 detection in serum samples by assembling amino‐terminated fluorescein isothiocyanate‐labeled peptide (Pep‐FITC) onto the GO surface through covalent binding using EDC/NHS. The pre‐quenched fluorescence of the FITC was restored upon contact with MMP‐2, because MMP‐2 selectively cleaved the peptide and FITC were released from the GO surface. The cytokeratin‐19 fragment CYFRA‐21‐1 is a proteinaceous biomarker of oral cancer. Its concentration in saliva of oral cancer patients is elevated. Kumar et al. [140] constructed a noninvasive biosensor based on zirconia decorated RGO to detect CYFRA‐21‐1 in saliva. The sensor was prepared by electrophoretic deposition of APTES functionalized nanostructured ZrO2 decorated RGO onto ITO electrode and immobilizing anti‐CYFRA‐21‐1 antibodies on the thin nanocomposite film using EDC/NHS.
8.3.5 Graphene‐Based Biosensors for Disease Biomarker Detection
D‐amino acids are biomarkers of various diseases, because normally only L‐amino acids are involved in physiological functions. D‐Tyrosine (Tyr), for example, is a renal biomarker. Martín et al. [141] constructed a biosensor based on a dual electrochemical and enzymatic approach for the analysis of D and L tyrosine and methionine. The analysis involved the D‐amino acid bio‐sensing using a class‐enzyme, D‐amino acid oxidase (DAAO), that selectively reacts with the D‐enantiomers, and the direct electrochemical sensing of the L‐enantiomer. The sensor platform was prepared by casting reduced graphene oxide nanoribbons (RGONRs) onto SPCE. Amyloid beta (Aβ) peptide is a blood‐brain‐barrier‐penetrating biomarker of Alzheimer's disease. Kim et al. [142] developed an effective wafer‐scale RGO patterning method based on dry etching in conventional micro‐electro‐mechanical systems (MEMS) equipment, and constructed an ultrahigh‐sensitivity Aβ40 sensor. For Aβ detection, Aβ antibodies were immobilized on RGO by EDC/NHS coupling. The mRNA biomarker BACE‐1 is related to Alzheimer's disease. Vilela et al. [130] constructed a high‐performance and relatively simple beta‐secretase 1 (BACE‐1) optical fluorescence turn‐on biosensor based on BACE‐1 complementer capture ssDNAs immobilized on NaYF4:Yb,Er UCNPs as emitters and GO as the fluorescence quencher. Insulin is a peptide hormone and abnormalities in insulin secretion and activity lead to various types of diabetes and increased risk factors for various diseases including kidney failure, myocardial infarction, and neurodegenerative disease. Yagati et al. [143] constructed an insulin sensor based on Ag nanoflower (AgNF) decorated RGO modified micro‐disk electrode arrays. Anti‐insulin antibodies were anchored on the electrode to capture insulin using 3‐mercaptopropionic acid (MPA) linkers and EDC/NHS activation. The fabrication procedure is shown in Figure 8.9. The sensor exhibited fast response, high selectivity and sensitivity, and low detection limit.

Figure 8.9 Illustration of the fabrication of the immunosensor and binding of insulin [143].
Source: Copyright 2016. Reproduced with permission from Elsevier.
Lactate is an important biomarker of several diseases. Increased lactate level in serum is an indirect marker of anaerobic glucose breakdown, and a potential marker of MS, cerebral ischemia, and malignancy in brain tumor. Manna et al. [144] developed a sensor to detect L‐lactate in serum by depositing p‐nitrophenyl functionalized RGO on GCE, generating a surface confined redox mediator (RGO‐PhNHOH), and immobilizing L‐lactate dehydrogenase (LDH) on the electrode surface. The sensor exhibited high sensitivity and selectivity, and rapid response. The glucose detection in blood and urine is essential for the diagnosis of diabetes. Glucose sensors, in general, are based on the application of glucose oxidase (GlOx) immobilized on the modified sensor surface or incorporated into the thin layer modifying the bare electrode. Electrospun fiber membranes often show superior performance in sensor construction compared to casted film membranes due to very high porosity and large surface area. Su et al. [145] fabricated a glucose sensor by electrospinning a mixture of PVA, CS, GO, and GlOx directly onto a platinum electrode and depositing a thin layer of nafion onto the modified electrode surface for anti‐interference effects. Zhang et al. [146] casted graphene decorated MnCo2O4 composite nanofibers onto GCE obtained by calcinating electrospun RGO/Mn(Ac)2/Co(Ac)2/PVP composite fibers at high temperature. Liang et al. [147] used single graphene coated silk fibers for sensor construction. A composite film of these fibers was prepared by vacuum filtration of a mixed solution of GO and silk fibers, followed by chemical reduction with ascorbic acid. Pt nanospheres were electrochemically deposited onto the film surface and GlOx was immobilized on the electrode by cross‐linking to predeposited BSA, using glutaraldehyde vapors. LbL assembly of functionalized graphene sheets provides a route to control layer thickness and allows the incorporation of multiple components, including GlOx, into graphene layers. Liu et al. [148] fabricated a glucose sensor by preparing a graphene platelet‐GlOx nanostructure on GCE, synthesized through self‐assembly of GlOx and CS functionalized graphene platelets by electrostatic attraction. Gu et al. [149] prepared a graphene multilayer on GCE, by self‐assembling amine‐terminated ionic liquid (IL) modified RGO, and sulfonic acid (SA) functionalized RGO sheets, followed by immobilizing GlOx on the top layer. A glucose biosensor based on nitrogen doped graphene (NGR) and GlOx was fabricated by Barsan et al. [150] by LbL self‐assembling positively charged CS containing GlOx and NGR with negatively charged PSS on a gold electrode. Yan et al. [151] constructed a glucose sensor based on a (RGO/PDDA‐PB/GlOx/PDDA‐PB)3 multilayer film by assembling poly(diallyldimethylammonium chloride) protected Prussian blue NPs (PDDA‐PB), GlOx, and RGO onto a GCE. Zeng et al. [152] prepared a multilayer film on GCE by alternating deposition of poly(ethyleneimine) (PEI), pyrene‐grafted poly(acrylic acid) modified RGO (PAA‐RGO), and GlOx. The glucose sensor was obtained by depositing a multilayer of (PEI/PAA‐rGO)3(PEI/GlOx)5. Compared to the two‐dimensional graphene powder, graphene aerogel (GA) possesses much higher electrical conductivity since the constituent graphene sheets are chemically bonded. A sensitive glucose sensor based on GA/Au‐NPs hybrid material synthesized through a hydrothermal route from GO and HAuCl4 was fabricated by Wang et al. [153] by casting the GA/Au‐NPs onto a GCE and immobilizing GlOx in the aerogel framework.
8.4 Conclusions and Perspectives
Carbon nanomaterials have gained increasing interest in recent years due to their distinctive and tunable electrical and PL properties, chemical inertness, high photostability, biocompatibility, and nanoscale size. These properties have allowed carbon nanomaterials to provide a significant potential across many biomedical applications, especially in constructing chemical vapor sensors and biosensors. CNTs, graphenes, and CNDs are widely used to construct sensors for cancer and disease diagnosis. The application of other carbon nanomaterials like nanodiamonds, fullerenes, and carbon nanohorns is still in early stages and therefore their potential in sensor construction is not understood yet. Many efforts in the application of carbon nanomaterials for both kinds of sensors have been done in recent years to develop increasingly more accurate, reliable, selective, and low cost sensors aiding better diagnosis and monitoring of diseases. Both chemical vapor sensors and biosensors detecting disease biomarkers provide an alternative way for the diagnosis of various diseases, compared to classical techniques involving invasive biopsies, expensive and complex labeling processes, and time‐consuming analysis. The collection of exhaled breath and skin headspace are frequently used as patient‐friendly sources of VOCs and their chemical fingerprinting can be analyzed for early detection and monitoring of diseases by chemical vapor sensors. Serum, urine, and tears contain a relatively large variation of various biomarkers in low concentration, and biosensors are important tools for detecting biomarkers in body fluids. The sensitivity of chemoresistive sensors and biosensors now reached the limit of detecting biomarkers present in either breath or body fluids in very low concentration, respectively. Non‐invasive sampling techniques in medical practice are getting more and more important for the diagnosis of human diseases. Most of the sensor developments thus far have considered the detection of a single target. However, simultaneous measurement of multiple biomarkers can improve the diagnostic value, because many of the disease markers are indicative of multiple diseases. Besides, the biocompatibility and toxicology for those materials used in designed biosensors have not been comprehensively tested or addressed in human systems despite showing low toxicity in mice models (in vivo testing). Furthermore, developed biomarker‐based biosensors require further considerations into meeting the ideal criterion of sensors, practicality, and economical aspects. Similarly, the characterization in terms of kinetics and analytical performance of developed biomarker sensors should be further studied to allow greater comparison between variously developed biosensors. Research for the development of high selective biosensors that are able to detect biomarkers without significant interference from other compounds found in human plasma will continue in the future. Finally, the point‐of‐care sensing in clinical practice requires fast, sensitive, selective, stable, portable, and cheap sensors, and future sensor researches are expected to move into this direction.
Acknowledgments
This work was supported by the IH 150100003 ARC Research Hub for Graphene Enable Industry Transformation, School of Chemical Engineering and the University of Adelaide. This was also supported under the framework of international cooperation program managed by National Research Foundation of Korea (NRF‐2016K1A3A7A08952122).
References
- 1 Konvalina, G. and Haick, H. (2014). Sensors for breath testing: from nanomaterials to comprehensive disease detection. Accounts of Chemical Research 47 (1): 66–76.
- 2 Oleksandr, K., Brett, L.A., and Alexander, S. (2007). Carbon nanotube sensors for exhaled breath components. Nanotechnology 18 (37): 375502–375508.
- 3 Broza, Y.Y. and Haick, H. (2013). Nanomaterial‐based sensors for detection of disease by volatile organic compounds. Nanomedicine 8 (5): 785–806.
- 4 Cheng, Z., Li, Q., Li, Z. et al. (2010). Suspended graphene sensors with improved signal and reduced noise. Nano Letters 10 (5): 1864–1868.
- 5 Tripathi, K.M., Bhati, A., Singh, A. et al. (2016). From the traditional way of pyrolysis to tunable photoluminescent water soluble carbon nano‐onions for cell imaging and selective sensing of glucose. RSC Advances 6 (44): 37319–37329.
- 6 Broza, Y.Y., Zuri, L., and Haick, H. (2014). Combined volatolomics for monitoring of human body chemistry. Scientific Reports 4: 4611–4616.
- 7 Broza, Y.Y., Mochalski, P., Ruzsanyi, V. et al. (2015). Hybrid volatolomics and disease detection. Angewandte Chemie International Edition 54 (38): 11036–11048.
- 8 Nakhleh, M.K., Jeries, V., Gharra, A. et al. (2014). Detecting active pulmonary tuberculosis with a breath test using nanomaterial‐based sensors. European Respiratory Journal 43 (5): 1522–1525.
- 9 Peng, G., Hakim, M., Broza, Y.Y. et al. (2010). Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. British Journal of Cancer 103 (4): 542–551.
- 10 Costello, B.d.L., Amann, A., Al‐Kateb, H. et al. (2014). A review of the volatiles from the healthy human body. Journal of Breath Research 8 (1): 014001–014029.
- 11 Pauling, L., Robinson, A.B., Teranishi, R. et al. (1971). Quantitative analysis of urine vapor and breath by gas‐liquid partition chromatography. Proceedings of the National Academy of Sciences 68 (10): 2374–2376.
- 12 Lord, H., Yu, R., Segal, A. et al. (2002). Breath analysis and monitoring by membrane extraction with sorbent interface. Analytical Chemistry 74 (21): 5650–5657.
- 13 Miekisch, W., Schubert, J.K., and Noeldge‐Schomburg, G.F.E. (2004). Diagnostic potential of breath analysis – focus on volatile organic compounds. Clinica Chimica Acta 347 (1–2): 25–39.
- 14 Peng, G., Tisch, U., Adams, O. et al. (2009). Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nature Nanotechnology 4 (10): 669–673.
- 15 Tung, T.T., Nine, M.J., Krebsz, M. et al. (2017). Recent advances in sensing applications of graphene assemblies and their composites. Advanced Functional Materials . 27 (46): 1702891.
- 16 Pasinszki, T., Krebsz, M., Tung, T.T. et al. (2017). Carbon nanomaterial based biosensors for non‐invasive detection of cancer and disease biomarkers for clinical diagnosis. Sensors 17 (8): 1919.
- 17 Feng, L., Wu, L., and Qu, X. (2013). New horizons for diagnostics and therapeutic applications of graphene and graphene oxide. Advanced Materials 25 (2): 168–186.
- 18 Tisch, U., Schlesinger, I., Ionescu, R. et al. (2012). Detection of Alzheimer's and Parkinson's disease from exhaled breath using nanomaterial‐based sensors. Nanomedicine 8 (1): 43–56.
- 19 Tripathi, K.M., Schlesinger, I., Ionescu, R. et al. (2016). Recent advances in engineered graphene and composites for detection of volatile organic compounds (VOCs) and non‐invasive diseases diagnosis. Carbon 110: 97–129.
- 20 Konvalina, G. and Haick, H. (2013). Sensors for breath testing: from nanomaterials to comprehensive disease detection. Accounts of Chemical Research 47 (1): 66–76.
- 21 Haick, H., Hakim, M., Patrascu, M. et al. (2009). Sniffing chronic renal failure in rat model by an array of random networks of single‐walled carbon nanotubes. ACS Nano 3 (5): 1258–1266.
- 22 Choi, S.‐J., Choi, C., Kim, S.‐J. et al. (2015). Facile synthesis of hierarchical porous WO3 nanofibers having 1D nanoneedles and their functionalization with non‐oxidized graphene flakes for selective detection of acetone molecules. RSC Advances 5 (10): 7584–7588.
- 23 Chen, Y., Zhang, Y., Pan, F. et al. (2016). Breath analysis based on surface‐enhanced raman scattering sensors distinguishes early and advanced gastric cancer patients from healthy persons. ACS Nano 10 (9): 8169–8179.
- 24 Peng, G., Trock, E., and Haick, H. (2008). Detecting simulated patterns of lung cancer biomarkers by random network of single‐walled carbon nanotubes coated with nonpolymeric organic materials. Nano Letters 8 (11): 3631–3635.
- 25 Wang, F. and Swager, T.M. (2011). Diverse chemiresistors based upon covalently modified multiwalled carbon nanotubes. Journal of the American Chemical Society 133 (29): 11181–11193.
- 26 Ionescu, R., Broza, Y., Shaltieli, H. et al. (2011). Detection of multiple sclerosis from exhaled breath using bilayers of polycyclic aromatic hydrocarbons and single‐wall carbon nanotubes. ACS Chemical Neuroscience 2 (12): 687–693.
- 27 Tang, Z., Liu, Y., and Duan, Y. (2015). Development of solid‐phase microextraction fibers based on multi‐walled carbon nanotubes for pre‐concentration and analysis of alkanes in human breath. Journal of Chromatography A 1425: 34–41.
- 28 Xia, X., Guo, S., Zhao, W. et al. (2014). Carboxyl functionalized gold nanoparticles in situ grown on reduced graphene oxide for micro‐gravimetric ammonia sensing. Sensors and Actuators B: Chemical 202: 846–853.
- 29 Some, S., Xu, Y., Kim, Y. et al. (2013). Highly sensitive and selective gas sensor using hydrophilic and hydrophobic graphenes. Scientific Reports 3: 1868.
- 30 Pang, C., Lee, G.‐Y., Kim, T.‐I. et al. (2012). A flexible and highly sensitive strain‐gauge sensor using reversible interlocking of nanofibres. Nature Materials 11 (9): 795–801.
- 31 Kahn, N., Lavie, O., Paz, M. et al. (2015). Dynamic nanoparticle‐based flexible sensors: diagnosis of ovarian carcinoma from exhaled breath. Nano Letters 15 (10): 7023–7028.
- 32 Yang, Y., Asiri, A.M., Tang, Z. et al. (2013). Graphene based materials for biomedical applications. Materials Today 16 (10): 365–373.
- 33 Tripathi, K.M., Sonker, A.K., Bhati, A. et al. (2016). Large‐scale synthesis of soluble graphitic hollow carbon nanorods with tunable photoluminescence for the selective fluorescent detection of DNA. New Journal of Chemistry 40 (2): 1571–1579.
- 34 Luo, P.G., Sahu, A., Yang, S.‐T. et al. (2013). Carbon “quantum” dots for optical bioimaging. Journal of Materials Chemistry B 1 (16): 2116–2127.
- 35 Baker, S.N. and Baker, G.A. (2010). Luminescent carbon nanodots: emergent nanolights. Angewandte Chemie International Edition 49 (38): 6726–6744.
- 36 Tyagi, A., Tripathi, K.M., Singh, N. et al. (2016). Green synthesis of carbon quantum dots from lemon peel waste: applications in sensing and photocatalysis. RSC Advances 6 (76): 72423–72432.
- 37 Li, H., Kang, Z., Liu, Y. et al. (2012). Carbon nanodots: synthesis, properties and applications. Journal of Materials Chemistry 22 (46): 24230–24253.
- 38 Tripathi, K.M., Tran, T.S., Tung, T.T. et al. (2017). Water soluble fluorescent carbon nanodots from biosource for cells imaging. Journal of Nanomaterials 2017: 10: 7029731
- 39 Tripathi, K.M., Tran, T.S., Kim, Y.J. et al. (2017). Green fluorescent onion‐like carbon nanoparticles from flaxseed oil for visible light induced photocatalytic applications and label‐free detection of Al(III) ions. ACS Sustainable Chemistry & Engineering 5 (5): 3982–3992.
- 40 Tripathi, K.M., Bhati, A., Singh, A. et al. (2017). Sustainable changes in the contents of metallic micronutrients in first generation gram seeds imposed by carbon nano‐onions: life cycle seed to seed study. ACS Sustainable Chemistry & Engineering 5 (4): 2906–2916.
- 41 Zheng, M., Li, Y., Zhang, Y. et al. (2016). Solvatochromic fluorescent carbon dots as optic noses for sensing volatile organic compounds. RSC Advances 6 (86): 83501–83504.
- 42 Dolai, S., Bhunia, S.K., and Jelinek, R. (2017). Carbon‐dot‐aerogel sensor for aromatic volatile organic compounds. Sensors and Actuators B: Chemical 241: 607–613.
- 43 Wang, R., Li, G., Dong, Y. et al. (2013). Carbon quantum dot‐functionalized aerogels for NO2 gas sensing. Analytical Chemistry 85 (17): 8065–8069.
- 44 Bhattacharyya, D., Sarswat, P.K., and Free, M.L. (2017). Quantum dots and carbon dots based fluorescent sensors for TB biomarkers detection. Vacuum 146: 606–613.
- 45 Gavgani, J.N., Dehsari, H.S., Hasani, A. et al. (2015). A room temperature volatile organic compound sensor with enhanced performance, fast response and recovery based on N‐doped graphene quantum dots and poly(3,4‐ethylenedioxythiophene)‐poly(styrenesulfonate) nanocomposite. RSC Advances 5 (71): 57559–57567.
- 46 Gavgani, J.N., Hasani, A., Nouri, M. et al. (2016). Highly sensitive and flexible ammonia sensor based on S and N co‐doped graphene quantum dots/polyaniline hybrid at room temperature. Sensors and Actuators B: Chemical 229: 239–248.
- 47 Ellis, J.E. and Star, A. (2016). Carbon nanotube‐based gas sensors toward breath analysis. ChemPlusChem 81 (12): 1248–1265.
- 48 Begum, A., Tripathi, K.M., and Sarkar, S. (2014). Water‐induced formation, characterization, and photoluminescence of carbon nanotube‐based composites of gadolinium(III) and platinum(II) dithiolenes. Chemistry – A European Journal 20 (50): 16657–16661.
- 49 Tripathi, S., Sonkar, S.K., and Sarkar, S. (2011). Growth stimulation of gram (Cicer arietinum) plant by water soluble carbon nanotubes. Nanoscale 3 (3): 1176–1181.
- 50 Tripathi, K.M., Begum, A., Sonkar, S.K. et al. (2013). Nanospheres of copper(iii) 1,2‐dicarbomethoxy‐1,2‐dithiolate and its composite with water soluble carbon nanotubes. New Journal of Chemistry 37 (9): 2708–2715.
- 51 Miller, D.R., Akbar, S.A., and Morris, P.A. (2014). Nanoscale metal oxide‐based heterojunctions for gas sensing: a review. Sensors and Actuators B: Chemical 204: 250–272.
- 52 Kaushik, A., Kumar, R., Arya, S.K. et al. (2015). Organic–inorganic hybrid nanocomposite‐based gas sensors for environmental monitoring. Chemical Reviews 115 (11): 4571–4606.
- 53 Ariga, K., Minami, K., and Shrestha, L.K. (2016). Nanoarchitectonics for carbon‐material‐based sensors. Analyst 141 (9): 2629–2638.
- 54 Salavagione, H.J., Díez‐Pascual, A.M., Lázaro, E. et al. (2014). Chemical sensors based on polymer composites with carbon nanotubes and graphene: the role of the polymer. Journal of Materials Chemistry A 2 (35): 14289–14328.
- 55 Nag, S., Sachan, A., Castro, M. et al. (2016). Spray layer‐by‐layer assembly of POSS functionalized CNT quantum chemo‐resistive sensors with tuneable selectivity and ppm resolution to VOC biomarkers. Sensors and Actuators B: Chemical 222: 362–373.
- 56 Chatterjee, S., Castro, M., and Feller, J.F. (2013). An e‐nose made of carbon nanotube based quantum resistive sensors for the detection of eighteen polar/nonpolar VOC biomarkers of lung cancer. Journal of Materials Chemistry B 1 (36): 4563–4575.
- 57 Nag, S., Castro, M., Choudhary, V. et al. (2017). Sulfonated poly(ether ether ketone) [SPEEK] nanocomposites based on hybrid nanocarbons for the detection and discrimination of some lung cancer VOC biomarkers. Journal of Materials Chemistry B 5 (2): 348–359.
- 58 Zilberman, Y., Tisch, U., Shuster, G. et al. (2010). Carbon nanotube/hexa‐peri‐hexabenzocoronene bilayers for discrimination between nonpolar volatile organic compounds of cancer and humid atmospheres. Advanced Materials 22 (38): 4317–4320.
- 59 Zilberman, Y., Tisch, U., Pisula, W. et al. (2009). Spongelike structures of hexa‐peri‐hexabenzocoronene derivatives enhance the sensitivity of chemiresistive carbon nanotubes to nonpolar volatile organic compounds of cancer. Langmuir 25 (9): 5411–5416.
- 60 Shirsat, M.D., Sarkar, T., Kakoullis, J. et al. (2012). Porphyrins‐functionalized single‐walled carbon nanotubes chemiresistive sensor arrays for VOCs. The Journal of Physical Chemistry C Nanomaterials and Interfaces 116 (5): 3845–3850.
- 61 Liu, S.F., Moh, L.C.H., and Swager, T.M. (2015). Single‐walled carbon nanotube–metalloporphyrin chemiresistive gas sensor arrays for volatile organic compounds. Chemistry of Materials 27 (10): 3560–3563.
- 62 Eamsa‐ard, T., Tisch, U. Pisula, W. et al. (2016). Screening and discrimination of Hepatocellular carcinoma patients by testing exhaled breath with smart devices using composite polymer/carbon nanotube gas sensors. in 2016 9th Biomedical Engineering International Conference (BMEiCON).
- 63 Tisch, U., Aluf, Y., Ionescu, R. et al. (2012). Detection of asymptomatic nigrostriatal dopaminergic lesion in rats by exhaled air analysis using carbon nanotube sensors. ACS Chemical Neuroscience 3 (3): 161–166.
- 64 Gelperin, A. and Johnson, A.T.C. (2008). Nanotube‐based sensor arrays for clinical breath analysis. Journal of Breath Research 2 (3): 037015.
- 65 Bayn, A., Nol, P., Tisch, U. et al. (2013). Detection of volatile organic compounds in brucella abortus‐seropositive bison. Analytical Chemistry 85 (22): 11146–11152.
- 66 Du, X., Skachko, I., Barker, A. et al. (2008). Approaching ballistic transport in suspended graphene. Nature Nanotechnology 3 (8): 491–495.
- 67 Geim, A.K. and Novoselov, K.S. (2007). The rise of graphene. Nature Materials 6 (3): 183–191.
- 68 Varghese, S.S., Lonkar, S., Singh, K. et al. (2015). Recent advances in graphene based gas sensors. Sensors and Actuators B: Chemical 218: 160–183.
- 69 Yavari, F. and Koratkar, N. (2012). Graphene‐based chemical sensors. The Journal of Physical Chemistry Letters 3 (13): 1746–1753.
- 70 Tung, T.T., Castro, M., Feller, J.‐F. et al. (2013). Hybrid film of chemically modified graphene and vapor‐phase‐polymerized PEDOT for electronic nose applications. Organic Electronics 14 (11): 2789–2794.
- 71 Tung, T.T., Pham‐Huu, C., Janowska, I. et al. (2015). Hybrid films of graphene and carbon nanotubes for high performance chemical and temperature sensing applications. Small 11 (28): 3485–3493.
- 72 Choi, S.‐J., Fuchs, F., Demadrille, R. et al. (2014). Fast responding exhaled‐breath sensors using WO3 hemitubes functionalized by graphene‐based electronic sensitizers for diagnosis of diseases. ACS Applied Materials & Interfaces 6 (12): 9061–9070.
- 73 Tung, T.T., Castro, M., Kim, T.‐Y. et al. (2012). Graphene quantum resistive sensing skin for the detection of alteration biomarkers. Journal of Materials Chemistry 22: 21754–21766.
- 74 Tung, T.T., Castro, M., Pillin, I. et al. (2014). Graphene–Fe3O4/PIL–PEDOT for the design of sensitive and stable quantum chemo‐resistive VOC sensors. Carbon 74: 104–112.
- 75 Tung, T.T., Castro, M., Kim, T.Y. et al. (2014). High stability silver nanoparticles–graphene/poly(ionic liquid)‐based chemoresistive sensors for volatile organic compounds' detection. Analytical and Bioanalytical Chemistry 406 (16): 3995–4004.
- 76 Saxena, R. and Srivastava, S. (2016). Non‐invasive toluene sensor for early diagnosis of lung cancer. AIP Conference Proceedings 1724 (1): 020079.
- 77 Zhang, X.‐F., Zhang, Z.‐W., He, Y.‐L. et al. (2015). Sniffing lung cancer related biomarkers using an oxidized graphene SAW sensor. Frontiers of Physics 11 (2): 116801.
- 78 Sone, S., Nakayama, T., Honda, T. et al. (2007). Long‐term follow‐up study of a population‐based 1996–1998 mass screening programme for lung cancer using mobile low‐dose spiral computed tomography. Lung Cancer 58 (3): 329–341.
- 79 Yoo, E.‐H. and Lee, S.‐Y. (2010). Glucose biosensors: an overview of use in clinical practice. Sensors 10 (5): 4558–4576.
- 80 Brooks, D.J. (2010). Imaging approaches to Parkinson disease. Journal of Nuclear Medicine 51 (4): 596–609.
- 81 Erden, P.E. and Kılıç, E. (2013). A review of enzymatic uric acid biosensors based on amperometric detection. Talanta 107: 312–323.
- 82 Bacon, M., Bradley, S.J., and Nann, T. (2014). Graphene quantum dots. Particle & Particle Systems Characterization 31 (4): 415–428.
- 83 Sun, H., Wu, L., Wei, W. et al. (2013). Recent advances in graphene quantum dots for sensing. Materials Today 16 (11): 433–442.
- 84 Wang, S., Cole, I.S., Zhao, D. et al. (2016). The dual roles of functional groups in the photoluminescence of graphene quantum dots. Nanoscale 8 (14): 7449–7458.
- 85 Wang, S., Chen, Z.‐G., Cole, I. et al. (2015). Structural evolution of graphene quantum dots during thermal decomposition of citric acid and the corresponding photoluminescence. Carbon 82: 304–313.
- 86 Li, Y.‐H., Zhang, L., Huang, J. et al. (2013). Fluorescent graphene quantum dots with a boronic acid appended bipyridinium salt to sense monosaccharides in aqueous solution. Chemical Communications 49 (45): 5180–5182.
- 87 Razmi, H. and Mohammad‐Rezaei, R. (2013). Graphene quantum dots as a new substrate for immobilization and direct electrochemistry of glucose oxidase: application to sensitive glucose determination. Biosensors and Bioelectronics 41: 498–504.
- 88 Fayazfar, H., Afshar, A., Dolati, M. et al. (2014). DNA impedance biosensor for detection of cancer, TP53 gene mutation, based on gold nanoparticles/aligned carbon nanotubes modified electrode. Analytica Chimica Acta 836: 34–44.
- 89 Lawal, A.T. (2016). Synthesis and utilization of carbon nanotubes for fabrication of electrochemical biosensors. Materials Research Bulletin 73: 308–350.
- 90 Yan, Y., Liu, Q., Du, X. et al. (2015). Visible light photoelectrochemical sensor for ultrasensitive determination of dopamine based on synergistic effect of graphene quantum dots and TiO 2 nanoparticles. Analytica Chimica Acta 853: 258–264.
- 91 Feng, T., Wang, Y., and Qiao, X. (2017). Recent advances of carbon nanotubes‐based electrochemical immunosensors for the detection of protein cancer biomarkers. Electroanalysis 29 (3): 662–675.
- 92 Tîlmaciu, C.‐M. and Morris, M.C. (2015). Carbon nanotube biosensors. Frontiers in Chemistry 3: 59.
- 93 Justino, C.I., Rocha‐Santos, T.A., and Duarte, A.C. (2013). Advances in point‐of‐care technologies with biosensors based on carbon nanotubes. TrAC Trends in Analytical Chemistry 45: 24–36.
- 94 Okuno, J., Maehashi, K., Kerman, K. et al. (2007). Label‐free immunosensor for prostate‐specific antigen based on single‐walled carbon nanotube array‐modified microelectrodes. Biosensors and Bioelectronics 22 (9): 2377–2381.
- 95 Münzer, A.M., Michael, Z.P., and Star, A. (2013). Carbon nanotubes for the label‐free detection of biomarkers. ACS Nano 7 (9): 7448–7453.
- 96 Zhang, J., Kruss, S., Hilmer, A.J. et al. (2014). A rapid, direct, quantitative, and label‐free detector of cardiac biomarker troponin T using near‐infrared fluorescent single‐walled carbon nanotube sensors. Advanced Healthcare Materials 3 (3): 412–423.
- 97 Sharma, A., Hong, S., Singh, R. et al. (2015). Single‐walled carbon nanotube based transparent immunosensor for detection of a prostate cancer biomarker osteopontin. Analytica Chimica Acta 869: 68–73.
- 98 Lerner, M.B., D, Souza, J., Pazina, T. et al. (2012). Hybrids of a genetically engineered antibody and a carbon nanotube transistor for detection of prostate cancer biomarkers. ACS Nano 6 (6): 5143–5149.
- 99 Park, Y.K., Bold, B., Lee, W.K. et al. (2011). D‐(+)‐galactose‐conjugated single‐walled carbon nanotubes as new chemical probes for electrochemical biosensors for the cancer marker galectin‐3. International Journal of Molecular Sciences 12 (5): 2946–2957.
- 100 Asav, E. and Sezgintürk, M.K. (2014). A novel impedimetric disposable immunosensor for rapid detection of a potential cancer biomarker. International Journal of Biological Macromolecules 66: 273–280.
- 101 Yang, H., Li, Z., Wei, X. et al. (2013). Detection and discrimination of alpha‐fetoprotein with a label‐free electrochemical impedance spectroscopy biosensor array based on lectin functionalized carbon nanotubes. Talanta 111: 62–68.
- 102 Ramnani, P., Gao, Y., Ozsoz, M. et al. (2013). Electronic detection of microRNA at attomolar level with high specificity. Analytical Chemistry 85 (17): 8061–8064.
- 103 Tian, Q., Wang, Y., Deng, R. et al. (2015). Carbon nanotube enhanced label‐free detection of microRNAs based on hairpin probe triggered solid‐phase rolling‐circle amplification. Nanoscale 7 (3): 987–993.
- 104 Li, F., Peng, J., Wang, J. et al. (2014). Carbon nanotube‐based label‐free electrochemical biosensor for sensitive detection of miRNA‐24. Biosensors and Bioelectronics 54: 158–164.
- 105 Tran, H., Piro, B., Reisberg, S. et al. (2013). Label‐free and reagentless electrochemical detection of microRNAs using a conducting polymer nanostructured by carbon nanotubes: application to prostate cancer biomarker miR‐141. Biosensors and Bioelectronics 49: 164–169.
- 106 Choudhary, M., Singh, A., Kaur, S. et al. (2014). Enhancing lung cancer diagnosis: electrochemical simultaneous bianalyte immunosensing using carbon nanotubes–chitosan nanocomposite. Applied Biochemistry and Biotechnology 174 (3): 1188–1200.
- 107 Xu, H., Wang, Y., Wang, L. et al. (2016). A label‐free microelectrode array based on one‐step synthesis of chitosan–multi‐walled carbon nanotube–thionine for ultrasensitive detection of carcinoembryonic antigen. Nanomaterials 6 (7): 132.
- 108 Kumar, S., Willander, M., Sharma, J.G. et al. (2015). A solution processed carbon nanotube modified conducting paper sensor for cancer detection. Journal of Materials Chemistry B 3 (48): 9305–9314.
- 109 Wang, X., Gao, C., Shu, G. et al. (2015). The enzyme electrocatalytic immunosensor based on functional composite nanofibers for sensitive detection of tumor suppressor protein p53. Journal of Electroanalytical Chemistry 756: 101–107.
- 110 Munge, B.S., Fisher, J., Millord, L.N. et al. (2010). Sensitive electrochemical immunosensor for matrix metalloproteinase‐3 based on single‐wall carbon nanotubes. Analyst 135 (6): 1345–1350.
- 111 Hu, C., Zheng, J., Su, X. et al. (2013). Ultrasensitive all‐carbon photoelectrochemical bioprobes for zeptomole immunosensing of tumor markers by an inexpensive visible laser light. Analytical Chemistry 85 (21): 10612–10619.
- 112 Khan, R., Pal, M., Kuzikov, A.V. et al. (2016). Impedimetric immunosensor for detection of cardiovascular disorder risk biomarker. Materials Science and Engineering: C 68: 52–58.
- 113 Xu, W., He, J., Gao, A. et al. (2015). Immunoassay for netrin 1 via a glassy carbon electrode modified with multi‐walled carbon nanotubes, thionine and gold nanoparticles. Microchimica Acta 182 (13–14): 2115–2122.
- 114 Herrasti, Z., Martínez, F., and Baldrich, E. (2014). Carbon nanotube wiring for signal amplification of electrochemical magneto immunosensors: application to myeloperoxidase detection. Analytical and Bioanalytical Chemistry 406 (22): 5487–5493.
- 115 Liu, B., Lu, L., Li, Q. et al. (2011). Disposable electrochemical immunosensor for myeloperoxidase based on the indium tin oxide electrode modified with an ionic liquid composite film containing gold nanoparticles, poly (o‐phenylenediamine) and carbon nanotubes. Microchimica Acta 173 (3–4): 513.
- 116 Navamani, J., Palanisamy, R., Gurusamy, R. et al. (2012). Development of nanoprobe for the determination of blood cholesterol. Journal of Biosensors and Bioelectronics 3: 1–8.
- 117 Son, M., Kim, D., Park, K.S. et al. (2016). Detection of aquaporin‐4 antibody using aquaporin‐4 extracellular loop‐based carbon nanotube biosensor for the diagnosis of neuromyelitis optica. Biosensors and Bioelectronics 78: 87–91.
- 118 Lerner, M.B., Dailey, J., Goldsmith, B.R. et al. (2013). Detecting Lyme disease using antibody‐functionalized single‐walled carbon nanotube transistors. Biosensors and Bioelectronics 45: 163–167.
- 119 Zhu, G. and Lee, H.J. (2017). Electrochemical sandwich‐type biosensors for α− 1 antitrypsin with carbon nanotubes and alkaline phosphatase labeled antibody‐silver nanoparticles. Biosensors and Bioelectronics 89: 959–963.
- 120 Oh, J., Yoo, G., Chang, Y.W. et al. (2013). A carbon nanotube metal semiconductor field effect transistor‐based biosensor for detection of amyloid‐beta in human serum. Biosensors and Bioelectronics 50: 345–350.
- 121 Pundir, S., Chauhan, N., Narang, J. et al. (2012). Amperometric choline biosensor based on multiwalled carbon nanotubes/zirconium oxide nanoparticles electrodeposited on glassy carbon electrode. Analytical Biochemistry 427 (1): 26–32.
- 122 Campuzano, S., Yáñez‐Sedeño, P., and Pingarrón, J.M. (2016). Diagnostics strategies with electrochemical affinity biosensors using carbon nanomaterials as electrode modifiers. Diagnostics 7 (1): 2.
- 123 Cruz, S., Girão, A.F., Gonçalves, G. et al. (2016). Graphene: the missing piece for cancer diagnosis? Sensors 16 (1): 137.
- 124 Patil, A.V., Fernandes, F.B., Bueno, P.R. et al. (2015). Graphene‐based protein biomarker detection. Bioanalysis 7 (6): 725–742.
- 125 Wang, L., Xiong, Q., Xiao, F. et al. (2017). 2D nanomaterials based electrochemical biosensors for cancer diagnosis. Biosensors and Bioelectronics 89: 136–151.
- 126 Bollella, P., Fusco, G., Tortolini, C. et al. (2017). Beyond graphene: electrochemical sensors and biosensors for biomarkers detection. Biosensors and Bioelectronics 89: 152–166.
- 127 Feng, L., Wu, L., Wang, J. et al. (2012). Detection of a prognostic indicator in early‐stage cancer using functionalized graphene‐based peptide sensors. Advanced Materials 24 (1): 125–131.
- 128 Zhang, B. and Cui, T. (2011). An ultrasensitive and low‐cost graphene sensor based on layer‐by‐layer nano self‐assembly. Applied Physics Letters 98 (7): 073116.
- 129 Zhang, B., Li, Q., and Cui, T. (2012). Ultra‐sensitive suspended graphene nanocomposite cancer sensors with strong suppression of electrical noise. Biosensors and Bioelectronics 31 (1): 105–109.
- 130 Vilela, P., El‐Sagheer, A., Millar, T. et al. (2016). Graphene oxide‐upconversion nanoparticle based optical sensors for targeted detection of mRNA biomarkers present in Alzheimer's disease and prostate cancer. ACS Sensors 2 (1): 52–56.
- 131 Li, P., Zhang, B., and Cui, T. (2015). TiO2 and shrink induced tunable nano self‐assembled graphene composites for label free biosensors. Sensors and Actuators B: Chemical 216: 337–342.
- 132 Li, P., Zhang, B., and Cui, T. (2015). Towards intrinsic graphene biosensor: a label‐free, suspended single crystalline graphene sensor for multiplex lung cancer tumor markers detection. Biosensors and Bioelectronics 72: 168–174.
- 133 Li, J., Hu, X., Shi, S. et al. (2016). Three label‐free thrombin aptasensors based on aptamers and [Ru (bpy) 2 (o‐mopip)] 2+. Journal of Materials Chemistry B 4 (7): 1361–1367.
- 134 Akter, R., Jeong, B., Choi, J.‐S. et al. (2016). Ultrasensitive Nanoimmunosensor by coupling non‐covalent functionalized graphene oxide platform and numerous ferritin labels on carbon nanotubes. Biosensors and Bioelectronics 80: 123–130.
- 135 Ali, M.A., Mondal, K., Jiao, Y. et al. (2016). Microfluidic immuno‐biochip for detection of breast cancer biomarkers using hierarchical composite of porous graphene and titanium dioxide nanofibers. ACS Applied Materials & Interfaces 8 (32): 20570–20582.
- 136 Tabasi, A., Noorbakhsh, A., and Sharifi, E. (2017). Reduced graphene oxide‐chitosan‐aptamer interface as new platform for ultrasensitive detection of human epidermal growth factor receptor 2. Biosensors and Bioelectronics 95: 117–123.
- 137 Gao, Z., Vishnubhotla, R., Ducos, P. et al. (2016). Genetically engineered antibody functionalized platinum nanoparticles modified CVD‐graphene nanohybrid transistor for the detection of breast cancer biomarker, HER3. Advanced Materials Interfaces 3 (17): 1600124.
- 138 He, L., Wang, Q., Mandler, D. et al. (2016). Detection of folic acid protein in human serum using reduced graphene oxide electrodes modified by folic‐acid. Biosensors and Bioelectronics 75: 389–395.
- 139 Song, E., Cheng, D., Song, Y. et al. (2013). A graphene oxide‐based FRET sensor for rapid and sensitive detection of matrix metalloproteinase 2 in human serum sample. Biosensors and Bioelectronics 47: 445–450.
- 140 Kumar, S., Sharma, J.G., Maji, S. et al. (2016). Nanostructured zirconia decorated reduced graphene oxide based efficient biosensing platform for non‐invasive oral cancer detection. Biosensors and Bioelectronics 78: 497–504.
- 141 Martín, A., Batalla, P., Hernández‐Ferrer, J. et al. (2015). Graphene oxide nanoribbon‐based sensors for the simultaneous bio‐electrochemical enantiomeric resolution and analysis of amino acid biomarkers. Biosensors and Bioelectronics 68: 163–167.
- 142 Kim, J., Chae, M.‐S., Lee, S.M. et al. (2016). Wafer‐scale high‐resolution patterning of reduced graphene oxide films for detection of low concentration biomarkers in plasma. Scientific Reports 6: 31276.
- 143 Yagati, A.K., Choi, Y., Park, J. et al. (2016). Silver nanoflower–reduced graphene oxide composite based micro‐disk electrode for insulin detection in serum. Biosensors and Bioelectronics 80: 307–314.
- 144 Manna, B. and Raj, C.R. (2016). Covalent functionalization and electrochemical tuning of reduced graphene oxide for the bioelectrocatalytic sensing of serum lactate. Journal of Materials Chemistry B 4 (26): 4585–4593.
- 145 Su, X., Ren, J., Meng, X. et al. (2013). A novel platform for enhanced biosensing based on the synergy effects of electrospun polymer nanofibers and graphene oxides. Analyst 138 (5): 1459–1466.
- 146 Zhang, Y., Liu, S., Li, Y. et al. (2015). Electrospun graphene decorated MnCo2O4 composite nanofibers for glucose biosensing. Biosensors and Bioelectronics 66: 308–315.
- 147 Liang, B., Fang, L., Hu, Y. et al. (2014). Fabrication and application of flexible graphene silk composite film electrodes decorated with spiky Pt nanospheres. Nanoscale 6 (8): 4264–4274.
- 148 Liu, S., Tian, J., Wang, L. et al. (2011). Self‐assembled graphene platelet–glucose oxidase nanostructures for glucose biosensing. Biosensors and Bioelectronics 26 (11): 4491–4496.
- 149 Gu, H., Yu, Y., Liu, X. et al. (2012). Layer‐by‐layer self‐assembly of functionalized graphene nanoplates for glucose sensing in vivo integrated with on‐line microdialysis system. Biosensors and Bioelectronics 32 (1): 118–126.
- 150 Barsan, M.M., David, M., Florescu, M. et al. (2014). A new self‐assembled layer‐by‐layer glucose biosensor based on chitosan biopolymer entrapped enzyme with nitrogen doped graphene. Bioelectrochemistry 99: 46–52.
- 151 Yan, J., Zhong, T., Qi, W. et al. (2015). The application of assembled inorganic and organic hybrid nanoarchitecture of Prussian blue/polymers/graphene in glucose biosensing. Journal of Inorganic and Organometallic Polymers and Materials 25 (2): 275–281.
- 152 Zeng, G., Xing, Y., Gao, J. et al. (2010). Unconventional layer‐by‐layer assembly of graphene multilayer films for enzyme‐based glucose and maltose biosensing. Langmuir 26 (18): 15022–15026.
- 153 Wang, B., Yan, S., and Shi, Y. (2015). Direct electrochemical analysis of glucose oxidase on a graphene aerogel/gold nanoparticle hybrid for glucose biosensing. Journal of Solid State Electrochemistry 19: 307–314.
